The modern post-genome sequencing era is becoming increasingly replete with genetic assignments for disease. Often in relation to liver, the findings can be complex, necessitating evaluations of very large datasets to deliver insights to the molecular mechanisms. However, there also are times when a single patient yields significant new insights to physiology and disease. Such an example is the recent work by a multicenter group of talented Dutch investigators in the current issue of HEPATOLOGY1. These investigators pursued the unexpected clinical finding of very high plasma bile acid levels in a child without apparent liver disease, ultimately leading to the identification of a new condition affecting hepatic transport and enterohepatic cycling of bile acids.
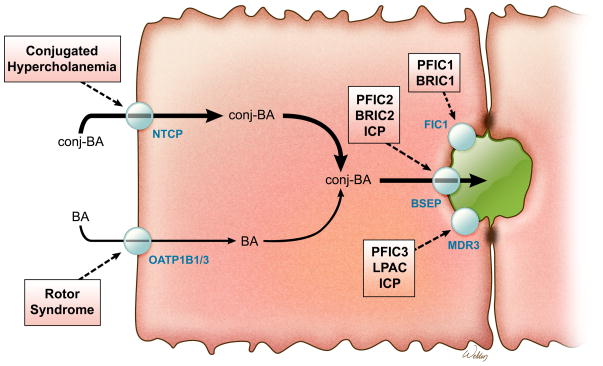
Hepatic bile formation depends on the synthesis, canalicular secretion and efficient intestinal reabsorption of bile acids. In fact, only about 5% of the biliary bile acids are newly synthesized, and most have already been secreted by the liver into small intestine and returned via the enterohepatic circulation. Many of the players important for the enterohepatic circulation of bile acids are known and inherited defects in those genes can have profound effects on hepatic function (Figure 1; clinical characteristics summarized in Table 1). The list of genes (and associated disease states) includes: ATP8B1/FIC1 (Progressive familial intrahepatic cholestasis type 1; PFIC1), ABCB11/BSEP (PFIC2), ABCB4/MDR3 (PFIC3), SLCO1B1/OATP1B1 and SLCO1B3/OATP1B3 (Rotor Syndrome), and SLC10A2/ASBT (Primary bile acid malabsorption; PBAM).2 Notably absent from this list is SLC10A1/NTCP, the presumed primary transporter for hepatic uptake of conjugated bile acids3. Polymorphisms in SLC10A1 that interfere with bile acid transport have been characterized in vitro, but clinical data was unavailable for subjects carrying these variants.4 Loss of NTCP is predicted to result in increased plasma conjugated bile acid levels (hypercholanemia), yet previous reports of hypercholanemia-associated inherited disorders found causal mutations in genes other than SLC10A1.5,6 These negative findings, coupled with fact that the hepatocyte sinusoidal membrane also expresses other sodium-independent bile acid transporters, raised the possibility that an isolated NTCP gene defect may be asymptomatic, with limited impact on bile acid homeostasis. The present identification and characterization of a phenotypic loss-of-function SLC10A1 mutation addresses those lingering doubts and clearly establishes a primary role for NTCP in hepatic bile acid clearance.
Fig 1.
Schematic view of transporters involved in inherited hypercholanemic disorders. BA, bile acid; BSEP, bile salt export pump; FIC1, P-type ATPase mutated in progressive familial intrahepatic cholestasis type 1; ICP, intrahepatic cholestasis of pregnancy; LPAC, low-phospholipid-associated cholelithiasis; MDR, multidrug resistance protein; NTCP, Na+-taurocholate cotransporting polypeptide; PFIC, progressive intrahepatic cholestasis.
Table 1.
Inherited Disorders Associated with Altered Transport of Bile Acids
| Protein (gene symbol) | Function | Disease | Clinical Presentation |
|---|---|---|---|
|
| |||
| Liver | |||
|
| |||
| NTCP (SLC10A1) | Sinusoidal membrane uptake of bile acids | Conjugated Hypercholanemia | Index patient in this report—no liver phenotype. |
|
| |||
|
OATP1B1 (SLCO1B1) OATP1B3 (SLCO1B3) |
Sinusoidal membrane uptake of organic anion conjugates and unconjugated bile acids | Rotor Syndrome | Jaundice, benign conjugated hyperbilirubinemia, delayed clearance of cholephilic anions such as BSP, ICG |
|
| |||
| FIC1 (ATP8B1) | Canalicular membrane aminophospholipid flipping | PFIC1 | Progressive cholestasis, elevated serum bile acids, pruritus, normal serum GGT, pancreatitis, intestinal malabsorption, hearing loss |
| BRIC1 | Periodic attacks of cholestasis, cholelithiasis, normal serum GGT, hepatosplenomegaly | ||
|
| |||
| BSEP (ABCB11) | Canalicular export of bile acids | PFIC2 | Progressive cholestasis, pruritus, jaundice, giant cell formation, lobular and portal fibrosis, hepatobiliary malignancy, normal serum GGT |
| BRIC2 | Periodic attacks of cholestasis, cholelithiasis, normal serum GGT, hepatosplenomegaly | ||
| ICP | Cholestasis in third trimester of pregnancy, pruritus, fetal loss and prematurity, normal serum GGT | ||
|
| |||
| MDR3 (ABCB4) | Canalicular export of phosphatidylcholine | PFIC3 | Cholestasis, extensive bile duct proliferation and periportal fibrosis, elevated serum GGT |
| LPAC | Cholelithiasis, intrahepatic hyperechoic foci, biliary cirrhosis, elevated serum GGT | ||
| ICP | Cholestasis in third trimester of pregnancy, fetal loss and prematurity, elevated serum GGT | ||
|
| |||
| MRP2 (ABCC2) | Canalicular export of organic anion conjugates | Dubin-Johnson Syndrome | Jaundice, benign conjugated hyperbilirubinemia |
|
| |||
| TJP2 | Maintain tight cellular junctions | Progressive cholestatic liver disease | Severe progressive cholestasis, normal GGT |
|
| |||
| Intestinal | |||
|
| |||
| ASBT (SLC10A2) | Apical membrane uptake of bile acids | PBAM | Chronic diarrhea, steatorrhea, fat-soluble vitamin malabsorption |
ABC, ATP-binding cassette; ASBT, apical sodium-dependent bile acid transporter; BSEP, bile salt export pump; BSP, bromsulphalein; FIC1, P-type ATPase mutated in progressive familial intrahepatic cholestasis type 1; GGT, gamma glutamyl transpeptidase; ICG, indocyanine green; ICP, intrahepatic cholestasis of pregnancy; LPAC, low-phospholipid-associated cholelithiasis; MDR, multidrug resistance protein; MRP, multidrug resistance–associated protein; NTCP, Na+-taurocholate cotransporting polypeptide; PBAM, primary bile acid malabsorption; TJP2, tight junction protein 2.
In brief, the authors describe the 4th child of consanguineous Afghan parents, who presented in the first year of life with growth retardation, hypotonia, and substantial impairments in achieving developmental milestones. Starting at approximately 9 months of age, this anicteric child underwent a comprehensive workup for her developmental delay and neurocognitive impairments, which excluded a variety of potential diagnoses but did not reveal a cause. Abdominal imaging was normal and her lab work, including liver function tests, was unremarkable. The only substantive lab abnormalities were a low 25-hydroxy vitamin D level, which was associated with reduced bone density, and mildly reduced levels of fat-soluble vitamins, A and K (as evidenced by a mildly prolonged PT). However as part of this workup, total and fractionated bile acids were measured in plasma. This yielded the surprising finding of markedly elevated plasma bile acid levels, 445 μM (normal <16 μM), nearly all of which were conjugated primary bile acids. By 2 years of age, the patient’s plasma bile acid levels had risen to 1531 μM, yet during this time the child was without jaundice, pruritus, or steatorrhea. Additional plasma bile acid measurements were obtained at 3, 4, and 5 years of age, over which time the total bile acid levels tended downward to 494 μM, and the proportion of conjugated secondary bile acids increased. Urine bile acid levels were also higher than normal, but not specifically quantitated. Plasma levels of C4 (7α-hydroxy-4-cholesten-3-one), a marker of hepatic bile acid synthesis, were normal, as were plasma levels of Fibroblast growth factor-19 (FGF19), an ileal-derived enterokine involved in regulating hepatic bile acid synthesis. Levels of autotaxin activity, a marker for pruritus in cholestasis, were also normal in this patient.
At 3 years of age, the authors sequenced the NTCP gene and identified a homozygous nonsynonymous variant (NTCPR252H) that could explain the conjugated hypercholanemia in this patient. This rare single nucleotide polymorphism (SNP, rs147226818) has been identified previously, and is present in less than 0.1% of European and African ancestry alleles (Exome variant server, evs.gs.washington.edu). The R252 residue is highly conserved in NTCP. In silico analysis predicted that R252H is likely a damaging variant (PolyPhen2 score, 0.975). This was directly validated using cell-based assays, demonstrating that the NTCPR252H variant is poorly trafficked to the plasma membrane (even after treatment with known molecular chaperones), reducing taurocholate uptake by more than 9-fold.
This first clinical description of an isolated NTCP-deficiency delivers unique insights to human physiology and the fate of “wandering bile acids”. Those include: i) Conclusive support for the long presumed primary role of NTCP in hepatic bile acid clearance, as evidenced by the striking elevation of plasma bile acids in the absence of apparent liver disease. ii) Support for a limited role of alternative hepatic bile acid uptake mechanisms. Although non-NTCP hepatic uptake mechanisms are sufficient to maintain normal enterohepatic cycling of bile acids in some lower vertebrates7, that was not the case in this patient. Persistent but low levels of hepatic uptake and enterohepatic cycling of bile acids were apparently maintained, as evidenced by the downward trend in plasma bile acid levels over time, without apparent changes in synthesis, and increased proportion of conjugated secondary bile acids. Candidates for this residual hepatic activity include OATP1B1/1B3, which transport both conjugated and unconjugated bile acids. With a functioning NTCP, OATP1B1/1B3-deficiency does not affect plasma bile acid levels8, yet in the absence of NTCP, OATP1B1/1B3 may be play a larger but still limited role in bile acid clearance. The other candidate for the residual hepatic uptake activity is OSTα-OSTβ, which can function as a bidirectional transporter. The findings suggest that the transport capacity of these alternative systems is insufficient to compensate for loss of NTCP, although alterations in their expression or activity secondary to altered hepatic bile acid homeostasis in this patient cannot be excluded. iii) Insights regarding the regulation of hepatic bile acid synthesis in humans. Reducing hepatic uptake of bile acids by blocking their return from the intestine, for example by use of a bile acid sequestrant, typically leads to a dramatic induction of de novo bile acid synthesis. Remarkably, reducing hepatic uptake of bile acids at the sinusoidal membrane appeared to have little effect on their synthesis in this patient. Although hepatic bile acid levels were not measured, these findings support the concept that mechanisms other than bile acid return to the liver regulates synthesis in humans, such as signaling via the FGF19-FGFR4 pathway.9,10 iv) The etiology of cholestasis-associated pruritus. The striking absence of pruritus in this patient further supports the argument that a factor other than conjugated bile acids, such as lysophosphatidic acid (a product of the circulating enzyme autotaxin), is the offending pruritogen in cholestatic patients.11
The study also raises many new questions, not least of which is whether hypercholanemia is a disease or not. With regard to the health of the liver in this patient, we do not know if liver histology is normal or if hepatic secretion of other biliary constituents such as cholesterol, phospholipids or conjugated xenobiotics is impacted. One may also postulate that the undiagnosed extrahepatic manifestations in this child (muscular weakness, neurocognitive impairments) may have a basis in the high bile acid levels in the circulation, and presumably in her developing brain. Several studies suggest that cholestasis during the newborn period results in substantial impairments in neurocognitive function, including expressive language, more so in girls than boys.12,13 The relationship between isolated conjugated hypercholanemia and the spectrum of this child’s growth and cognitive impairments is unclear and perhaps unrelated, but should be explored as a rational new area for investigation.
Plasma bile acid levels are often elevated in cholestatic liver disease. However, little is known regarding the long-term clinical consequences of conjugated hypercholanemia in the absence of liver disease. In addition to their potentially cytotoxic detergent properties, bile acids act as metabolic regulators and activate a variety of nuclear and G protein-coupled receptors in tissues beyond the liver and gastrointestinal tract.14,15 Kidney16, heart17,18, vascular19, and endocrine tissues20,21 are but a few of the extrahepatic organs and systems whose functions could be adversely affected by persistently high circulating levels of bile acids. Careful follow-up of this index patient and any future cases is warranted and will provide additional insights.
The authors should be applauded for pursuing this unexpected finding of severe hypercholanemia and ultimately identifying the likely cause. With characterization of the molecular defect in this patient, the authors confirm that NTCP is critical for efficient hepatic clearance of bile acids, but not necessarily for hepatic function. This report of a solitary patient with a case of wandering bile acids advances our understanding of both normal physiology and disease, and will complement advances emerging from large scale genome and exome sequencing efforts of patients with hepatobiliary disorders. These findings are an important step in the long journey towards understanding the broader role for bile acids in health and disease.
Acknowledgments
This work was supported by NIH research grants DK056239 (S.J.K.) and DK047987 (P.A.D).
Abbreviations
- ABC
ATP-binding cassette
- ASBT
apical sodium-dependent bile acid transporter
- BSEP
bile salt export pump
- FIC1
P-type ATPase mutated in progressive familial intrahepatic cholestasis type 1
- MDR
multidrug resistance protein
- NTCP
Na+-taurocholate cotransporting polypeptide
- OATP
organic anion transporting polypeptide
- OST
organic solute transporter
- PBAM
primary bile acid malabsorption
- PFIC
progressive familial intrahepatic cholestasis
Footnotes
Conflict of Interest
Potential conflict of interest: Dr. Karpen has no conflicts of interest. Dr. Dawson has consulted for Lumena Pharmaceuticals.
References
- 1.Vaz FM, Paulusma CC, Huidekoper H, de Ru M, Lim C, Koster J, Ho-Mok K, et al. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: conjugated hypercholanemia without a clear clinical phenotype. Hepatology. 2014 doi: 10.1002/hep.27240. [DOI] [PubMed] [Google Scholar]
- 2.Oude Elferink RP, Paulusma CC, Groen AK. Hepatocanalicular transport defects: pathophysiologic mechanisms of rare diseases. Gastroenterology. 2006;130:908–925. doi: 10.1053/j.gastro.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 3.Dawson PA, Lan T, Rao A. Bile acid transporters. Journal of lipid research. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem. 2004;279:7213–7222. doi: 10.1074/jbc.M305782200. [DOI] [PubMed] [Google Scholar]
- 5.Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KD, Heubi JE, Shah S, Lavine JE, Suskind D, Al-Edreesi M, Potter C, et al. Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology. 2013;144:945–955. e946. doi: 10.1053/j.gastro.2013.02.004. quiz e914–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fricker G, Wossner R, Drewe J, Fricker R, Boyer JL. Enterohepatic circulation of scymnol sulfate in an elasmobranch, the little skate (Raja erinacea) Am J Physiol. 1997;273:G1023–1030. doi: 10.1152/ajpgi.1997.273.5.G1023. [DOI] [PubMed] [Google Scholar]
- 8.van de Steeg E, Stranecky V, Hartmannova H, Noskova L, Hrebicek M, Wagenaar E, van Esch A, et al. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. The Journal of clinical investigation. 2012;122:519–528. doi: 10.1172/JCI59526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Galman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. Journal of internal medicine. 2011;270:580–588. doi: 10.1111/j.1365-2796.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 11.Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, Reiners KS, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391–1400. doi: 10.1002/hep.25748. [DOI] [PubMed] [Google Scholar]
- 12.Caudle SE, Katzenstein JM, Karpen SJ, McLin VA. Language and motor skills are impaired in infants with biliary atresia before transplantation. The Journal of pediatrics. 2010;156:936–940. 940 e931. doi: 10.1016/j.jpeds.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Caudle SE, Katzenstein JM, Karpen S, McLin V. Developmental assessment of infants with biliary atresia: differences between boys and girls. Journal of pediatric gastroenterology and nutrition. 2012;55:384–389. doi: 10.1097/MPG.0b013e318259ed20. [DOI] [PubMed] [Google Scholar]
- 14.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nature reviews Gastroenterology & hepatology. 2014;11:55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 15.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell metabolism. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fickert P, Krones E, Pollheimer MJ, Thueringer A, Moustafa T, Silbert D, Halilbasic E, et al. Bile acids trigger cholemic nephropathy in common bile-duct-ligated mice. Hepatology. 2013;58:2056–2069. doi: 10.1002/hep.26599. [DOI] [PubMed] [Google Scholar]
- 17.Desai MS, Shabier Z, Taylor M, Lam F, Thevananther S, Kosters A, Karpen SJ. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology. 2010;51:2097–2107. doi: 10.1002/hep.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainer PP, Primessnig U, Harenkamp S, Doleschal B, Wallner M, Fauler G, Stojakovic T, et al. Bile acids induce arrhythmias in human atrial myocardium--implications for altered serum bile acid composition in patients with atrial fibrillation. Heart. 2013;99:1685–1692. doi: 10.1136/heartjnl-2013-304163. [DOI] [PubMed] [Google Scholar]
- 19.Khurana S, Raina H, Pappas V, Raufman JP, Pallone TL. Effects of deoxycholylglycine, a conjugated secondary bile acid, on myogenic tone and agonist-induced contraction in rat resistance arteries. PloS one. 2012;7:e32006. doi: 10.1371/journal.pone.0032006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dufer M, Horth K, Wagner R, Schittenhelm B, Prowald S, Wagner TF, Oberwinkler J, et al. Bile acids acutely stimulate insulin secretion of mouse beta-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes. 2012;61:1479–1489. doi: 10.2337/db11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap-bile acids in metabolic control. Nature reviews. Endocrinology. 2014 doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]