Abstract
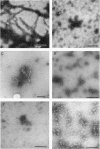
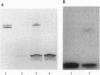
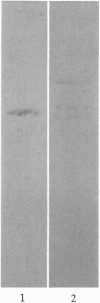
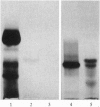
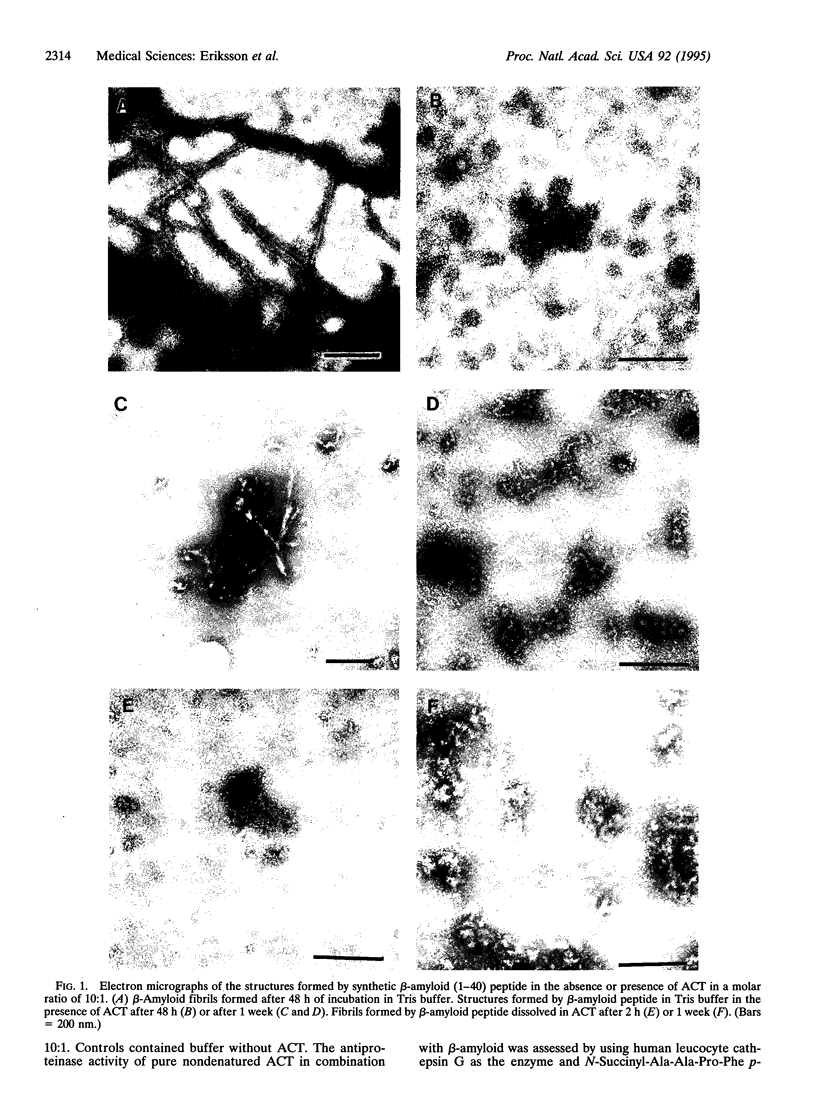
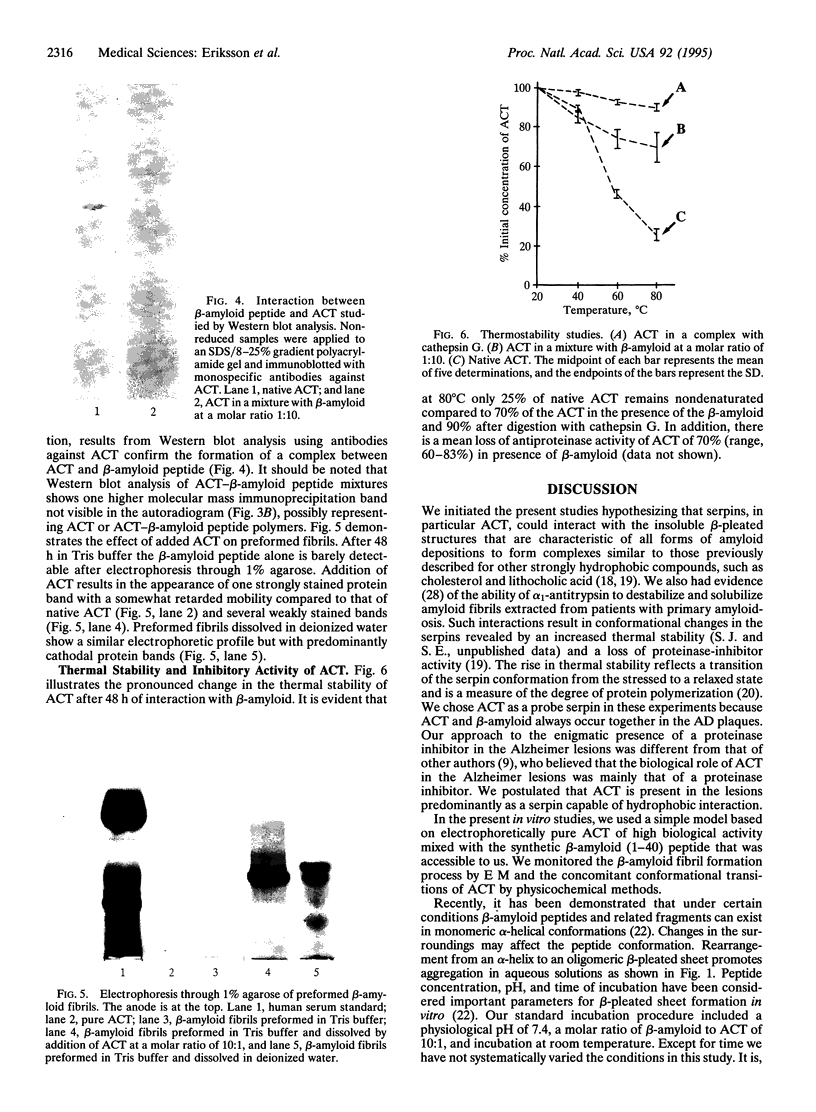
The major component of the cerebral plaques in Alzheimer disease is the beta-amyloid peptide, but serine proteinase inhibitors like alpha 1-antichymotrypsin (ACT) are also present. Their role in the pathogenesis of amyloid formation is unsettled. In addition to their function as proteinase inhibitors, serine proteinase inhibitors can interact with various hydrophobic compounds, a reaction accompanied by a transition from the stressed to the relaxed conformation. We report here on the ability of ACT to regulate the formation of beta-amyloid fibrils in vitro. In a molar ratio of 1:10 (ACT to beta-amyloid) ACT inhibits beta-amyloid fibril formation. Furthermore, ACT promotes rapid disaggregation of beta-amyloid fibrils when added in the same molar ratio to preformed beta-amyloid fibrils. These processes are accompanied by increased thermostability of ACT and loss of its biological activity, consistent with a conformational transition of ACT from the stressed to the relaxed state. The influence of ACT on beta-amyloid fibril formation may be an example of a hydrophobic interaction between the beta-amyloid peptide and the hydrophobic domain C terminal to the reactive center of ACT.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Cathepsin G. Methods Enzymol. 1981;80(Pt 100):561–565. doi: 10.1016/s0076-6879(81)80044-4. [DOI] [PubMed] [Google Scholar]
- Barrow C. J., Zagorski M. G. Solution structures of beta peptide and its constituent fragments: relation to amyloid deposition. Science. 1991 Jul 12;253(5016):179–182. doi: 10.1126/science.1853202. [DOI] [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Carrell R. W., Evans D. L., Stein P. E. Mobile reactive centre of serpins and the control of thrombosis. Nature. 1991 Oct 10;353(6344):576–578. doi: 10.1038/353576a0. [DOI] [PubMed] [Google Scholar]
- Chandra T., Stackhouse R., Kidd V. J., Robson K. J., Woo S. L. Sequence homology between human alpha 1-antichymotrypsin, alpha 1-antitrypsin, and antithrombin III. Biochemistry. 1983 Oct 25;22(22):5055–5061. doi: 10.1021/bi00291a001. [DOI] [PubMed] [Google Scholar]
- Christensson A., Laurell C. B., Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990 Dec 27;194(3):755–763. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Aebersold R., Ziltener H., Schrader J. W., Hood L. E., Kent S. B. Automated chemical synthesis of a protein growth factor for hemopoietic cells, interleukin-3. Science. 1986 Jan 10;231(4734):134–139. doi: 10.1126/science.3079915. [DOI] [PubMed] [Google Scholar]
- Come J. H., Fraser P. E., Lansbury P. T., Jr A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., McLachlan D. R., Abraham C. R., Kirschner D. A. Alpha 1-antichymotrypsin binding to Alzheimer A beta peptides is sequence specific and induces fibril disaggregation in vitro. J Neurochem. 1993 Jul;61(1):298–305. doi: 10.1111/j.1471-4159.1993.tb03568.x. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gollin P. A., Kalaria R. N., Eikelenboom P., Rozemuller A., Perry G. Alpha 1-antitrypsin and alpha 1-antichymotrypsin are in the lesions of Alzheimer's disease. Neuroreport. 1992 Feb;3(2):201–203. doi: 10.1097/00001756-199202000-00020. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Halverson K., Fraser P. E., Kirschner D. A., Lansbury P. T., Jr Molecular determinants of amyloid deposition in Alzheimer's disease: conformational studies of synthetic beta-protein fragments. Biochemistry. 1990 Mar 20;29(11):2639–2644. doi: 10.1021/bi00463a003. [DOI] [PubMed] [Google Scholar]
- Janciauskiene S., Eriksson S. In vitro complex formation between cholesterol and alpha 1-proteinase inhibitor. FEBS Lett. 1993 Feb 1;316(3):269–272. doi: 10.1016/0014-5793(93)81306-k. [DOI] [PubMed] [Google Scholar]
- Janciauskiene S., Eriksson S. The interaction of hydrophobic bile acids with the alpha 1-proteinase inhibitor. FEBS Lett. 1994 Apr 25;343(2):141–145. doi: 10.1016/0014-5793(94)80306-4. [DOI] [PubMed] [Google Scholar]
- Johansson J., Gröndal S., Sjövall J., Jörnvall H., Curstedt T. Identification of hydrophobic fragments of alpha 1-antitrypsin and C1 protease inhibitor in human bile, plasma and spleen. FEBS Lett. 1992 Mar 9;299(2):146–148. doi: 10.1016/0014-5793(92)80234-8. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lomas D. A., Evans D. L., Stone S. R., Chang W. S., Carrell R. W. Effect of the Z mutation on the physical and inhibitory properties of alpha 1-antitrypsin. Biochemistry. 1993 Jan 19;32(2):500–508. doi: 10.1021/bi00053a014. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Smith K. F., Nealis A. S., Haris P. I., Chapman D., Bauer C. J., Harrison R. A. Secondary structure changes stabilize the reactive-centre cleaved form of SERPINs. A study by 1H nuclear magnetic resonance and Fourier transform infrared spectroscopy. J Mol Biol. 1992 Dec 20;228(4):1235–1254. doi: 10.1016/0022-2836(92)90329-i. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Woods A. S., Cotter R. J., Gowing E., Ball M. J. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J. Physiological production of the beta-amyloid protein and the mechanism of Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- Seubert P., Oltersdorf T., Lee M. G., Barbour R., Blomquist C., Davis D. L., Bryant K., Fritz L. C., Galasko D., Thal L. J. Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature. 1993 Jan 21;361(6409):260–263. doi: 10.1038/361260a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]