Abstract
Gene replacement therapies, like organ and cell transplantation, are likely to introduce neoantigens that elicit rejection via humoral and/or effector T-cell immune responses. Nonetheless, thanks to an ever-growing body of preclinical studies; it is now well accepted that gene transfer protocols can be specifically designed and optimized for induction of antigen-specific immune tolerance. One approach is to specifically express a gene in a tissue with a tolerogenic microenvironment such as the liver or thymus. Another strategy is to transfer a particular gene into hematopoietic stem cells or immunological precursor cells thus educating the immune system to recognize the therapeutic protein as “self.” In addition, expression of the therapeutic protein in protolerogenic antigen-presenting cells such as immature dendritic cells and B cells has proven to be promising. All three approaches have successfully prevented unwanted immune responses in preclinical studies aimed at the treatment of inherited protein deficiencies, e.g., lysosomal storage disorders and hemophilia, and of type 1 diabetes and multiple sclerosis. In this review, we focus on current gene transfer protocols that induce tolerance, including gene delivery vehicles and target tissues, and discuss successes and obstacles in different disease models.
The Challenge of Inducing Antigen-Specific Immune Tolerance
Gene replacement therapy, like organ or cell transplantation, and protein/enzyme replacement therapies share the risk for immune-mediated rejection. The immune system may be induced by the novel antigen(s) to reverse therapy by specific antibody and/or T-cell responses. Another parallel can be drawn with autoimmune diseases, where self-antigens are accidently targeted by antibodies or T cells. Whereas in any of these circumstances the unwanted immune response can potentially be eliminated by general immune suppression, this creates risks for opportunistic infections and typically involves use of drugs with various side effects. Induction of antigen-specific immune tolerance is therefore the preferred choice, which is more likely to succeed when the specifically targeted antigen(s) is known, as general effects on the immune system can be minimized. During the past decade, a number of studies have supported the notion that gene transfer can be a powerful method for inducing antigen-specific tolerance, provided that the requisite-specific vectors, the correct selection of the routes of administration, and target cells are optimized for tolerance induction (Figure 1).
Figure 1.
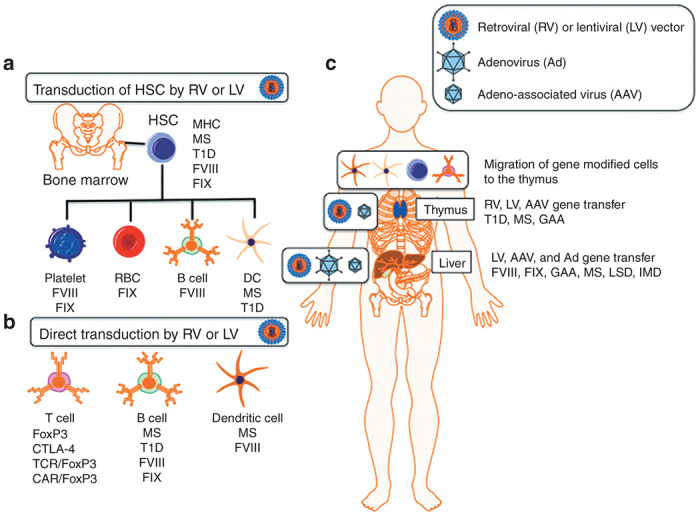
Overview of gene therapy vectors and target cells and tissues for inducing transgene-specific tolerance. (a) Hematopoietic stem cells (HSC) transduced ex vivo with a retroviral vector (RV) or lentiviral vector (LV) to express an antigen and are transferred to the donor with conditioning to promote engraftment. (b) Differentiated T cells, B cells, and dendritic cells (DC) are transduced ex vivo with a RV or LV. Antigen-specific expanded effector CD4+ T cells are cotransduced with FoxP3 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) to generate Treg. Naive CD4+ T cells can be converted into Treg by cotransduction with an antigen-specific T-cell receptor (TCR) or chimeric antigen receptor (CAR) along with FoxP3. B cells are transduced with a RV or LV expressing a transgene-IgG heavy chain fusion protein and transferred back to the donor. DC are cotransduced with a RV or LV expressing the immunosuppressive cytokine IL-10 and a transgene ex vivo and transferred back to the donor. (c) The thymus and liver are the main target organs for tolerance by in vivo gene transfer. Gene-modified HSC and differentiated lineages including DC and T cells are capable of migrating to the thymus and induce nTregs. Direct thymic gene transfer using adeno-associated virus (AAV), RV, or LV results in effector deletion and nTreg induction. Hepatocyte-restricted transgene expression from adenovirus (Ad), AAV, RV, and LV transduction promotes the induction of antigen-specific iTreg. FVIII, factor VIII; FIX, factor IX; GAA, acid-alpha glucosidase; IMD, inherited metabolic disorder; LSD, lysosomal storage disease; MHC, major histocompatibility complex; MS, multiple sclerosis; T1D, type 1 diabetes.
The Potential for Immune Responses During Gene Therapy
Gene therapy for the correction of monogenic diseases aims at correcting the cause of a disease at the molecular level by delivering a functional copy of a disease-associated defective gene. Viral vectors have emerged as very efficacious delivery vehicles of therapeutic genes to cells ex vivo and organs in vivo. These vectors have their respective strengths and limitations and are extensively reviewed elsewhere.1 One drawback is that the immune system may target antigens associated with the gene transfer vehicle itself. Hence, gene transfer protocols need to be designed to minimize immune responses against the vector itself.
Recently, several new systems have been developed for gene editing, which provides a means for site-specific correction of mutated genes or site-specific insertion of a therapeutic gene with an improved safety profile and the ability to maintain both endogenous tissue and temporal expression. These approaches rely on a synthetic DNA-binding proteins coupled to a dimer-dependent endonuclease (ZFNs and TALENS) or a guide RNA associated with an endonuclease (CRISPR-Cas9) to provide a targeted double-strand break within the mutated gene.2 A functional version of the gene, delivered with the endonucleases, can then be edited into the double-strand break using the homologous recombination repair pathway. Next-generation approaches may also combine viral vectors as a delivery platform for site-specific gene editing. Once again, the potential for immune responses against these recombination-mediating proteins or the delivered proteins remain of concern.
In general, immune responses directed against the therapeutic gene product provide a major obstacle to long-term disease correction with gene therapy, especially when the gene product is completely absent. In the case of an absent protein, the newly expressed therapeutic protein is seen by the immune system as nonself, resulting in the activation of both humoral (antibody) and cell-mediated (cytotoxic T lymphocyte) responses. The humoral immune response is often dependent on antigen-specific T-helper lymphocytes (CD4+ T cells) to activate B lymphocytes that recognize the same antigen and license their maturation to start producing antibodies. Cell-mediated immunity is directed through the activation of antigen-specific cytotoxic T cells, which are typically major histocompatibility complex (MHC) class I–restricted CD8+. Although CD8+ T cells can be activated in the absence of CD4+ T-helper cells, the speed and strength of activation as well as the generation of a good memory CD8+ T response is heavily dependent on help by CD4+ T cells. Other subsets of CD4+ T cells exist that can dampen or suppress humoral and cell-mediated responses and are dubbed regulatory T cells (Treg). In order to prevent unwanted immune responses against a therapeutic protein, many gene transfer approaches have been developed that selectively activate antigen-specific Treg and induce a state of tolerance.
As explained above, immunological tolerance can be induced at a nonspecific (global immune suppression) or specific antigen-specific level depending on multiple cell intrinsic and extrinsic factors. This review defines tolerance as an active process that maintains unresponsiveness even when repeatedly exposed to antigen (as opposed to immunological ignorance of the antigen). This often involves Treg induction as part of the tolerance mechanism. From a therapeutic standpoint, nonspecific immune suppression is not an optimal approach as it may disrupt normal immune surveillance and responses to antigens, including and not limited to bacterial and viral pathogens and malignant cells. What types of therapies would benefit from inducing antigen-specific tolerance? Gene transfer–based immune tolerance induction protocols can be developed for inherited protein deficiencies, transplant antigens, autoimmune diseases, and allergies.3 The focus of this review is to provide an overview on different approaches to promote antigen-specific tolerance through genetic modification or gene transfer to cells and tissues.
Background: B- and T-Cell Development
Newly generated T lymphocytes undergo maturation and selection within the thymus. In an initial round of selection, immature T cells (CD4+ and CD8+) are deleted due to a lack of survival signals from the absence of engagement of peptide displayed on the MHC proteins and the T-cell receptor (TCR). Those T cells that receive a threshold level of TCR signaling are further selected for their level of TCR signaling. In this phase, T cells with too strong TCR signaling either undergo receptor editing to modulate affinity or are eliminated through induction of apoptosis. Those T cells that have a moderate level of TCR signaling are retained, complete maturation and released into the circulation. To avoid developing autoimmune responses to tissue-restricted antigens (i.e., antigens not normally expressed in the thymus), medullary thymic epithelial cells express the transcription factor AIRE (autoimmune regulator) that can globally activate mRNA expression. Thus, developing T cells are exposed to most self-proteins, and those with too high of a TCR affinity are eliminated. To underscore the importance of autoimmune regulator in self-tolerance, mice and humans with defective autoimmune regulator suffer from severe autoimmunity.4 Similar to T cells, B cells undergo maturation within the bone marrow and later in the spleen and lymph nodes, where highly autoreactive B cells either undergo receptor editing or are deleted.
Active Immune Suppression by Regulatory T Cells
Although thymic selection is effective, some self-reactive T cells escape the selection process. These cells are kept in check via a second level of T-cell immune regulation, which employs a subset of CD4+ T cells called regulatory T cells (Tregs). These Tregs, which for this review are minimally defined by the markers CD4+CD25+FoxP3+ (Figure 2), act in an antigen-specific and nonspecific manner to dampen immune responses directly through cell–cell interactions and indirectly by release of immunosuppressive cytokines and sequestration of growth factors. The FoxP3 transcription factor has been identified as a master regulator of Tregs suppressive function and as with autoimmune regulator, severe autoimmunity is associated in mice and humans defective for FoxP3.5,6 There are two main subsets of Tregs defined as “central or natural” Tregs (nTregs) developed within the thymus and “peripheral or induced” Tregs (iTregs) developed from peripheral CD4+ effector T cells, which are induced to express FoxP3.7 Although studies indicate there are little differences in suppressive function between nTreg and iTreg, there are some indications that nTreg exhibit more stable FoxP3 expression, whereas iTreg are considered to be more plastic and can lose FoxP3 expression and revert back to effectors.8 Phenotypic differentiation of nTreg and iTreg can be difficult. Some reports indicate that Helios or Neuropilin-1 are exclusively expressed in nTregs and that nTregs are more hypomethylated in the Treg-specific demethylated region. Although there are other classes of suppressive T cells such as T regulatory 1 (Tr1) cells defined as IL-10 secreting CD4+ (Figure 2) and CD8+ T cells,9,10 CD8+ FoxP3+ T cells,11 and IL-10+ regulatory B cells (Breg),12–15 such cells and their role in antigen-specific tolerance through gene transfer are beyond the scope of this review.
Figure 2.
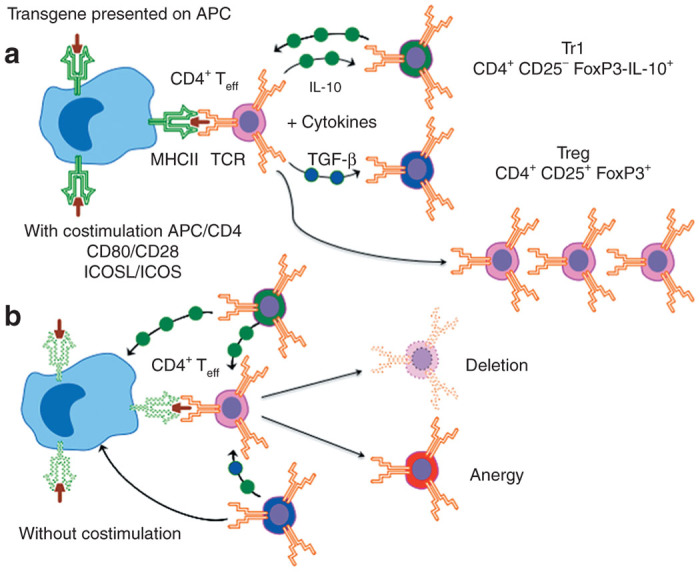
A simplified model for the activation of either antigen-specific Tr1, Treg, or T effector (Teff) cells in the context of TCR engagement of a MHC-II–presented epitope on an APC. (a) In the case where there are costimulatory signals between the APC and Teff (CD80/CD28 and ICOSL/ICOS) and the absence of immunosuppressive cytokines, Teff become activated and expand. In the presence of excessive IL-10, Teff can become Tr1 (CD4+CD25−FoxP3−IL-10+) regulatory T cells, and excessive TGF-β promote the induction of iTreg (CD4+CD25+FoxP3+). Tr1 and iTreg can indirectly suppress APC and Teff. In addition, iTreg can also directly suppress APC and Teff through contact inhibition. (b) In the absence of costimulatory signals or suppression by Tr1 and Treg, Teff cells are either eliminated or become anergic. TGF, transforming growth factor.
Tolerance Induction by Hepatocyte-Restricted Transgene Expression
Liver tolerance and the hepatic environment
One pathway to specific tolerance induction is to express the antigen in a tissue that is prone to activation of immune regulatory pathways. The potential of using the liver as an organ for promoting tolerance was initiated from early transplant studies conducted in MHC-mismatched animals and humans.16–18 Further evidence came from basic anatomy and physiology studies that places the liver immediately downstream of blood flow from the gut, where the liver is routinely exposed to copious amounts of foreign antigens derived from food and bacteria. The fact that liver transplants are well tolerated compared with other single organ transplants, that multiple organ transplants from the same donor are better tolerated when the liver is transplanted, and that eating a meal does not routinely induce severe inflammation in the liver suggested that there is a mechanism in place for dampening immune responses. Indeed, many pathogenic viruses and parasites are able to exploit this mechanism and develop protection from immune-mediated clearance as seen with chronic infections of hepatitis B and C viruses and malaria, which initially infects human hepatocytes.19
The liver primarily consists of the following cell types: hepatocytes, resident macrophages (Kupffer cells), specialized endothelial cells, liver sinusoidal endothelial cells, and hepatic stellate cells. Each of these liver cells has been associated with contributing to tolerance independently and most likely act synergistically to skew local immune responses toward tolerance.20–32 Thus, it is possible to induce transgene-specific tolerance by liver gene transfer and expressing the transgene in this microenvironment. When designing a tolerogenic, liver-directed gene transfer protocol, several critical factors have to be considered, including: immunological microenvironment of the liver, restricted transgene expression to hepatocytes, achieving adequate levels of transgene expression, the relative immunogenicity of the gene transfer vehicle and transgene, and optimal induction of Treg.
Tolerance induction to transgene products by in vivo viral vector gene transfer to hepatocytes
Published protocols that were successful in tolerance induction typically used in vivo gene transfer mediated by a viral vector, with expression restricted to hepatocytes. Among these, adeno-associated viral (AAV) vectors have been used to induce tolerance to a large number of transgene products. AAV vectors have the advantages of the availability of serotypes with strong tropism to hepatocytes and of limited innate immunogenicity.33,34 When initial inflammatory responses are low, activation signals to the immune system may be avoided, thereby increasing the chance for transgene expression to induce tolerance. Similarly, limited induction of IFN I (IFNα/β) preserves transgene expression and reduces antiviral responses.35 A minimal level of transgene expression is required for tolerance induction, for example, ~1% of normal coagulation factor IX levels in murine models.32 For therapeutic transgenes that have low levels of expression, such as factor VIII protein, it may be possible to employ codon optimization of the cDNA encoding the transgene product to augment protein expression to a level that promotes tolerance.36,37 One of the key mechanistic features of tolerance induction by hepatic gene transfer is the induction of transgene product-specific CD4+CD25+FoxP3+ Treg.38–40 Induced Treg actively suppress antibody and CD8+ T-cell responses against the transgene product.31,38,41 Treg induction is required for induction and maintenance of tolerance and correlated to the level of transgene expression.38,39,42 Although secreted antigens may also be presented in the thymus, peripheral Treg induction, a transforming growth factor-β–dependent process, is likely a major source for the generation of transgene product-specific Treg.21 The costimulatory molecule glucocorticoid-induced tumor necrosis factor receptor ligand has recently been identified as another factor required for efficient induction of Treg following AAV liver gene transfer.43 For suppression of CD8+ T-cell responses, hepatic expression of the suppressive cytokine IL-10 is required, which occurs in Kupffer cells and in Treg such as the aforementioned FoxP3+ Treg or type 1 regulatory (Tr1) T cells, which are IL-10–induced CD4+CD25−FoxP3− cells expressing transforming growth factor-β and large amounts of their hallmark cytokine IL-10.21,27 Activation of CD8+ T cells may be further reduced by the development of vector genomes devoid of immune stimulatory CpG motifs, as innate immunity to AAV vectors in the liver is TLR9 dependent.44,45 In addition to Treg induction, deletion of effector T cells, via induction of activation-induced cell death/programmed cell death, has been shown to be required for effective tolerance induction.32,46,47
The importance of hepatocyte-restricted expression for tolerance is underscored in a set of studies evaluating gene transfer with a lentiviral vector (LV), an alternative platform for hepatic tolerance induction. LV pseudotyped with the VSVg envelope protein efficiently transduce antigen-presenting cells (APC) in the liver48 and fail to induce tolerance even when using tissue-specific promoters. Remarkably, when transgene expression is detargeted in APC by a complementary microRNA target to microRNA 142-3p, a microRNA specifically expressed in hematopoietic cells, tolerance is induced for cytosolic GFP49 and secreted hFIX protein for conventional LV50 and integrase-defective LV.51 Hence, although we understand some of the factors needed for tolerance, certain questions such as, what are the tolerogenic APCs in the liver, remain unanswered. Additional details on the mechanism for tolerance by liver gene transfer have been extensively reviewed elsewhere.16,52,53
Illustrating the broad applicability of the approach, liver gene transfer has resulted in the induction of robust transgene tolerance to a variety of cytosolic and secreted transgene products in small and large animal disease models, including hemophilia A,36,37,54–57 hemophilia B,32,39,42,58,59 Pompe disease,60–62 allo-MHC for skin graft,63 and experimental autoimmune encephalomyelitis.64 Liver-induced tolerance extends to extrahepatic tissues, such as muscle, brain, and central nervous system as demonstrated in supplemental gene transfer to the muscle,65 and to brain/central nervous system in Niemann–Pick disease,66 central nervous system in experimental autoimmune encephalomyelitis,64 and muscle in Pompe disease.62 Hence, in an optimized protocol, immune tolerance induced by hepatic gene transfer may be dominant over activation of immune responses elsewhere, a phenomenon that can be exploited for treatment of disease that requires gene transfer to multiple organs or for development of immune modulatory gene therapy. Such a protocol may involve coadministration of a liver-targeted vector with a second vector targeting other tissues or simultaneous or sequential administration of two vectors via different routes. There are now a growing number of published studies demonstrating long-term correction of a variety of inherited metabolic and lysosomal storage disorders following liver gene transfer67,68 and supporting evidence in nonhuman primates that AAV8 liver-directed α-galactosidase A promotes tolerance.69
Although there have been ample studies in different disease models showing that liver gene transfer can prophylactically induce transgene tolerance, there have been limited studies on the potential for hepatic gene transfer to reverse an ongoing immune response. In the case of hemophilia A and B, patients with severe forms of disease are at risk to develop inhibitory antibodies against FVIII and FIX proteins during the course of recombinant protein therapy. It is unknown what the impact on patients with inhibitors would be following liver gene transfer. In the case of hemophilia B and/or Pompe disease, a subset of patients who develop inhibitory antibodies during enzyme replacement therapy to FIX and GAA proteins develop acute anaphylaxis. One could predict that liver gene transfer would either exacerbate or suppress the ongoing immune response. Three recent studies addressed this important question in a canine hemophilia A70 and murine hemophilia B models.42,71 Importantly, each of these studies indicated that liver gene transfer with an AAV or LV could reverse preexisting inhibitors, provide therapeutic factor expression, and protect against anaphylaxis and pathogenic antibody responses. Inhibitor reversal was dependent on the active suppression of induced antigen-specific Treg that rapidly eliminated antibody-secreting plasma cells and suppressed the activation of memory B cells. Thus, beyond therapeutic protein expression, liver-directed gene transfer might hold promise as a novel approach to treating autoimmune disease and severe allergies.
AAV vectors, which are largely maintained in episomal form, have now been successfully used in clinical trials for liver gene transfer. Due to size limitations, it is difficult to include extensive endogenous enhancer and promoter elements within the vector to maintain regulated, spatial, and temporal therapeutic transgene expression. Although strict control of transgene expression is not as critical for secreted zymogens, such as FVIII and FIX in hemophilia, other therapeutic transgenes may require strict regulation. Taking advantage of new gene-editing tools, Li et al.72 demonstrated robust gene correction in the liver of young hemophilia mice using AAV vectors to deliver a specific ZFN and hF9 cDNA sequence. This study was followed by similar results by Anguela et al.73 in adult hemophilia mice. Such an approach is also able to direct site-specific integration into so-called “safe harbor” regions within a chromosome and provided stable transgene expression with minimal genotoxicity, such as a reduced risk for insertional mutagenesis, and may pave the way as a next-generation therapeutic for treating monogenic disorders.
Taking Advantage of Age-Dependent Development of the Immune System—Neonatal and IN UTERO Gene Transfer
Another approach that has seen some success in tolerance induction is liver gene transfer either in utero or neonatally. The idea is that expressing a transgene when the immune system is immature or in the early stages of development will promote tolerance, most likely in a mechanism that incorporates the transgene as a self-protein. Additionally, this approach would also avoid any potential immune responses directed against the delivery vector. Naturally, such an approach is more effective with a gene delivery system that provides stable integration of the transgene cassette (such as retrovirus, lentivirus, or site-directed integration) as episomal vectors will become diluted and eventually lost as the liver grows to adult size. Most successes with neonatal gene transfer tolerance are in murine models, as mice have a very immature immune system at birth and in some reported canine studies.55,74–78 Although there have been many neonatal gene transfer studies conducted in rats and large animal models (cats, dogs, and nonhuman primates), tolerance induction is often not as robust as seen in mice.79–82
Thymic Gene Transfer—Negative Selection and NTREG Induction
Given the role of the thymus in the negative selection of autoreactive T cells and induction of nTreg, it is not surprising that direct thymic gene transfer has been considered as a means of inducing antigen-specific tolerance.83 Such studies conducted in mice demonstrated induction of specific tolerance to viral antigens,84–86 reduction in the occurrence of type 1 diabetes in nonobese diabetes mice,85 protection against the development but not progression of experimental autoimmune encephalomyelitis,87 and resistance to the therapeutic hGAA protein.88 Alternatively, Hadeiba et al.89 have demonstrated that CCR9-expressing plasmacytoid dendritic cells (DCs) can be peripherally loaded with an antigen and migrate to the thymus to promote tolerance. Although such an approach may be feasible in animal models, the exact mechanism that determines whether a T cell becomes an effector or regulatory T cell following encounter with MHC-II–presented antigen is not completely understood.90–92 Therefore, some “fine tuning” may be required in designing a gene transfer approach that can reliably promote induction of antigen-specific nTreg.
Hematopoietic Stem Cell Gene Transfer for Transplant Tolerance, Treatment of Inherited Protein Deficiencies, and Autoimmune Disease
Hematopoietic stem cells (HSCs) represent an attractive target cell for genetic modification for tolerance induction. Defined protocols have been established for the collection, culturing, transduction, and transfer/engraftment into a recipient. In most instances, autologous cells can be used, reducing potential host versus graft disease. Using specific regulatory elements such as tissue-specific promoters and microRNA targets, it is possible to strictly control transgene to a particular cell lineage.93–97 As platelets, lymphocytes (B and T cells), and most of our professional APC are derived from HSC, it is possible to direct the expression of a transgene product to promote the generation of nTreg from antigen presentation in the thymus or peripheral induction of CD4+ effector T cells to iTreg. Later sections will discuss approaches of direct gene modification of differentiated B and T cells and professional APCs. Therefore, it is not surprising that similar to the liver, hematopoitic stem cell gene transfer has been used for expressing therapeutic proteins and for inducing transgene-specific tolerance.
HSC gene modification for inducing tolerance was inspired by the observation of immunological tolerance to donor MHC proteins following the generation of mixed donor–host chimerism following HSC transplantation.98,99 Although this approach could induce tolerance to donor cells and tissues,100 use of allogenic HSC often resulted in graft versus host disease and engraftment failure. Therefore, to prevent graft versus host disease, investigators found a way to generate molecular chimerism by autologous HSC gene transfer.101–106 Gene transfer to HSC has successfully induced tolerance for tissue transplantation, desensitized allergic responses, protected against autoimmune diseases, and provided tolerance and therapeutic protein expression in a variety of disease models.102,107–110
One of the limiting factors for successful tolerance induction of gene-modified HSCs is efficient engraftment into the host. Efficient engraftment in early HSC transplantations often required complete myeloablation of the host bone marrow compartment by total body irradiation. Milder nonmyeloablation conditioning regimens using chemicals or low-dose radiation often failed to promote sufficient levels of engraftment to induce tolerance but instead were hyporesponsive,102,107 with the level of antigen expression determining hyporesponsiveness or tolerance. Newly developed nonmyeloablative regimens and gene transfer platforms can now provide sufficient engraftment and transgene expression for successful tolerance induction from gene-modified HSC following transplantation.111–114
Genetic modification of HSCs has been used for the induction of tolerance toward skin grafts using the cytosolic reporter gene GFP115,116 and MHC-II.117 In terms of controlling autoimmunity, gene transfer to HSC has been successful in preventing onset and controlling early disease progression in an experimental autoimmune encephalomyelitis mouse model for multiple sclerosis118 and recently has been shown to be effective using nonmyeloablative conditioning to effectively halt disease progression.112 Additional success has been obtained in controlling progression of type 1 diabetes in a nonobese diabetes mouse model.109 HSC gene modification has also been reported to control allergic responses in a mouse model.95,111,119 Several small and large animal disease models have shown long-term correction and tolerance using HSC gene transfer protocols including hemophilia A,120–125 hemophilia B,126–129 and Pompe disease.130,131 The recently reported safety and efficacy of LV gene transfer to HSCs in two clinical trials for Wiskott–Aldrich syndrome132 and metachromatic leukodystrophy133 provide optimism for the translation of some of the above studies into new clinical trials.
B-Cell Gene Transfer for Tolerance Induction
In addition to producing antibodies, B cells are also APCs, particularly for memory CD4+ T cells. Interestingly, it is possible to harness the ability of B cells to process and present antigen, not only to promote immune responses but also for tolerogenic antigen presentation. Specifically, stable retroviral gene transfer to primary B cells of a transgene fused in frame to the immunoglobulin G (IgG)-1 heavy chain leads to the induction of antigen-specific iTreg and tolerance.134–136 In this method, tolerance induction was shown to be dependent on endogenous processing and MHC-II presentation of the fusion gene product,135 B7 expression on B cells,137 and on CD4+CD25+FoxP3+ Treg.138,139 Expression of the immunosuppressive cytokine IL-10 may be required in gene-modified B cells (possibly through induction of regulatory Tr1 cells), as suggested by one study or indirectly required in cells of the recipient of the B-cell therapy as suggested by others.140–142 It is interesting to note that multiple MHC-II epitopes dubbed “Tregitopes” have been identified within the Fc fragment of IgG that expand nTreg and promote global tolerance.143 Tregitopes have been used to promote antigen-specific tolerance,144–148 likely through a bystander suppression mechanism.
Gene-modified B cells expressing antigens fused to IgG has provided antigen-specific tolerance in autoimmune models149 including multiple sclerosis,150,151 rheumatoid arthritis,152 and type 1 diabetes.150 In addition to autoimmune disease, retroviral gene transfer of an in-frame fusion of FVIII or FIX to IgG1 heavy chain to B cells is capable of promoting tolerance and controlling inhibitors in murine hemophilia A and B.153,154 As seen with liver gene transfer of FIX protein, FIX-IgG–expressing B cells are capable of partially reversing ongoing anti-FIX immune responses and can protect against anaphylaxis.
T-Cell Gene Modification and Tolerance
Following the identification of FoxP3 as a master regulator for Treg,5,155,156 it was demonstrated that forced expression of FoxP3 by retroviral gene transfer to effector CD4+ T cell produced cells with similar suppressive functions to Treg.157 A typical protocol consists of expanding CD4+ effector T cells ex vivo followed by transduction with a retroviral vector expressing FoxP3 and has been successful in inducing tolerance in graft versus host disease158 and autoimmune disease.159,160 Monoclonal expanded CD4+ effector T cells are more efficient than polyclonal cells following FoxP3 transduction for inducing antigen-specific tolerance. Additional forced expression of other Treg surface markers such as CTLA-4 can further improve suppressive function. Additionally, it is possible to generate antigen-specific Treg by gene transfer of an antigen-specific TCR161 to nTreg or combining gene transfer of an antigen-specific TCR and FoxP3 to naive CD4+ T cells.162 Chimeric antigen receptors have also been tested recently as a means for generating antigen-specific Treg and provided protection in a murine multiple sclerosis model.163 It is not clear if signaling through the chimeric antigen receptors is activating Tregs upon encountering antigen or providing a means of enriching the local concentration of Treg. Future studies are required to define the mechanism of tolerance with chimeric antigen receptor–modified Treg.
DC Gene Modification
DCs as professional APCs are capable of presenting antigen on MHC-II and depending on their maturation state can activate either CD4+ effector T cells or Tregs.164 Therefore, gene transfer of an antigen to immature DCs is a potential approach to inducing antigen-specific Treg. Given the fact that DCs contain sensors for viral pathogen–associated molecular patterns, finding a means to transduce DC with a viral vector without inducing maturation has proven challenging.165,166 Nonetheless, a successful protocol was reported generating FVIII-specific Treg by ex vivo transduction of tolerogenic DC that express either FVIII or FVIII and IL-10.167 A second approach used in vivo gene transfer of LV with a DC-restricted promoter to promote central and peripheral antigen-specific tolerance.168 Although direct modification of DCs is successful for inducing antigen-specific tolerance, gene transfer to a precursor such as HSCs, coupled with antigen-restricted expression to a DC lineage, may prove more effective.169
Conclusions
The induction of transgene-specific tolerance through gene transfer or gene modification is possible using a variety of cells and tissues and gene delivery vehicles. Tolerance is typically dependent on the induction of antigen-specific Treg. Preclinical studies conducted in rodent and canine disease models have demonstrated robust tolerance induction, the ability to transfer tolerance by adoptive transfer of Treg to naive animals, and the ability to suppress ongoing immune responses. The fact that liver gene transfer can induce Treg in a “hostile” proinflammatory setting and mediate suppression in the midst of an ongoing immune response has broader implications beyond therapies aimed at treating monogenic disorders and offer therapeutic approaches to treat autoimmune and hypersensitivity disorders. Clinical studies evaluating ex vivo gene delivery to HSC and in vivo gene transfer to muscle and liver have so far demonstrated no immune responses directed against the therapeutic gene product, suggesting that humans may respond similarly as seen in preclinical studies. Indeed, most immunological complications in patients have been associated with immune responses directed against the gene delivery vector and vector-associated genotoxicity. Although beyond the scope of this review, it is also important to note that including a transient immune suppression protocol that spares Treg can augment tolerance mediated by gene transfer, especially when using highly immunogenic vectors, delivery routes, or transgenes,170–172 Conversely, careful consideration should be placed on avoiding immune suppression protocols that effect Treg, as this can induce unwanted transgene immune responses.173 The advent of new tools for site-specific modification of chromosomes may greatly reduce the risks for insertional mutagenesis and pave the way for a transition from gene therapy to one of gene editing.
Acknowledgments
This article is supported by the National Institutes of Health grants P01 HD078810 (R.W.H. and C.T.), R01 AI51390 (R.W.H.), and a Bayer Hemophilia Early Career Investigator Award (D.M.M.).
The authors declare no conflict of interest.
References
- Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserfall CH, Herzog RW. Gene therapy approaches to induce tolerance in autoimmunity by reshaping the immune system. Curr Opin Investig Drugs. 2009;10:1143–1150. [PubMed] [Google Scholar]
- Su MA, Giang K, Zumer K, Jiang H, Oven I, Rinn JL. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest. 2008;118:1712–1726. doi: 10.1172/JCI34523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y. Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity. 2013;38:153–165. doi: 10.1016/j.immuni.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode I, Xu H, Ildstad ST. Regulatory B cells: the new “it” cell. Transplant Proc. 2014;46:3–8. doi: 10.1016/j.transproceed.2013.08.075. [DOI] [PubMed] [Google Scholar]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5:173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- Sriwatanawongsa V, Davies HS, Calne RY. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat Med. 1995;1:428–432. doi: 10.1038/nm0595-428. [DOI] [PubMed] [Google Scholar]
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood. 2008;112:3175–3185. doi: 10.1182/blood-2008-05-159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BE, Martino AT, Sack BK, Cao O, Liao G, Terhorst C. Nonredundant roles of IL-10 and TGF-β in suppression of immune responses to hepatic AAV-factor IX gene transfer. Mol Ther. 2011;19:1263–1272. doi: 10.1038/mt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yin W, Sun R, Wei H, Tian Z. Liver type I regulatory T cells suppress germinal center formation in HBV-tolerant mice. Proc Natl Acad Sci USA. 2013;110:16993–16998. doi: 10.1073/pnas.1306437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- Breous E, Somanathan S, Vandenberghe LH, Wilson JM. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E, Somanathan S, Wilson JM. BALB/c mice show impaired hepatic tolerogenic response following AAV gene transfer to the liver. Mol Ther. 2010;18:766–774. doi: 10.1038/mt.2009.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- Charles R, Chou HS, Wang L, Fung JJ, Lu L, Qian S. Human hepatic stellate cells inhibit T-cell response through B7-H1 pathway. Transplantation. 2013;96:17–24. doi: 10.1097/TP.0b013e318294caae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, Pulendran B. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol. 2013;190:2009–2016. doi: 10.4049/jimmunol.1201937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L, Herzog RW. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci USA. 2006;103:4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GL, Martino AT, Aslanidi GV, Jayandharan GR, Srivastava A, Herzog RW. Innate immune responses to AAV vectors. Front Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Bertin TK, Rogers GL, Cela RG, Zolotukhin I, Palmer DJ. Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs. adeno-associated viral vectors in vivo. Mol Ther. 2013;21:796–805. doi: 10.1038/mt.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack BK, Merchant S, Markusic DM, Nathwani AC, Davidoff AM, Byrne BJ. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One. 2012;7:e37671. doi: 10.1371/journal.pone.0037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NJ, Buckley SM, Waddington SN, Vandendriessche T, Chuah MK, Nathwani AC. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117:798–807. doi: 10.1182/blood-2010-05-282707. [DOI] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O, Herzog RW. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni A, Brown BD, Cantore A, Sergi LS, Naldini L, Roncarolo MG. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino AT, Nayak S, Hoffman BE, Cooper M, Liao G, Markusic DM. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS One. 2009;4:e6376. doi: 10.1371/journal.pone.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markusic DM, Hoffman BE, Perrin GQ, Nayak S, Wang X, LoDuca PA. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G, van Driel B, Magelky E, O’Keeffe MS, de Waal Malefyt R, Engel P. Glucocorticoid-induced TNF receptor family-related protein ligand regulates the migration of monocytes to the inflamed intestine. FASEB J. 2014;28:474–484. doi: 10.1096/fj.13-236505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BE, Herzog RW. Covert warfare against the immune system: decoy capsids, stealth genomes, and suppressors. Mol Ther. 2013;21:1648–1650. doi: 10.1038/mt.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust SM, Bell P, Cutler BJ, Ashley SN, Zhu Y, Rabinowitz JE. CpG-depleted adeno-associated virus vectors evade immune detection. J Clin Invest. 2013;123:2994–3001. doi: 10.1172/JCI68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust SM, Bell P, Zhu Y, Sanmiguel J, Wilson JM. The role of apoptosis in immune hyporesponsiveness following AAV8 liver gene transfer. Mol Ther. 2013;21:2227–2235. doi: 10.1038/mt.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- van Til NP, Markusic DM, van der Rijt R, Kunne C, Hiralall JK, Vreeling H. Kupffer cells and not liver sinusoidal endothelial cells prevent lentiviral transduction of hepatocytes. Mol Ther. 2005;11:26–34. doi: 10.1016/j.ymthe.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- Mátrai J, Cantore A, Bartholomae CC, Annoni A, Wang W, Acosta-Sanchez A. Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology. 2011;53:1696–1707. doi: 10.1002/hep.24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski E, Herzog RW. Tolerance induction by viral in vivo gene transfer. Clin Med Res. 2005;3:234–240. doi: 10.3121/cmr.3.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoDuca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Cela RG, Suzuki M, Lee B, Lipshutz GS. Neonatal helper-dependent adenoviral vector gene therapy mediates correction of hemophilia A and tolerance to human factor VIII. Proc Natl Acad Sci USA. 2011;108:2082–2087. doi: 10.1073/pnas.1015571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata A, Mimuro J, Mizukami H, Kashiwakura Y, Takano K, Ohmori T. Liver-restricted expression of the canine factor VIII gene facilitates prevention of inhibitor formation in factor VIII-deficient mice. J Gene Med. 2009;11:1020–1029. doi: 10.1002/jgm.1391. [DOI] [PubMed] [Google Scholar]
- Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C. A murine model for induction of long-term immunologic tolerance to factor VIII does not require persistent detectable levels of plasma factor VIII and involves contributions from Foxp3+ T regulatory cells. Blood. 2009;114:677–685. doi: 10.1182/blood-2009-03-202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, Li M. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther. 2010;18:2048–2056. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Bird A, Young SP, Kishnani PS, Chen YT, Koeberl DD. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Kulis MD, Young SP, Hobeika AC, Li S, Bird A. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol Ther. 2010;18:353–360. doi: 10.1038/mt.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Sun B, Osada T, Rodriguiz R, Yang XY, Luo X. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine pompe disease. Hum Gene Ther. 2012;23:460–472. doi: 10.1089/hum.2011.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham EC, Tay SS, Wang C, Rtshiladze M, Wang ZZ, McGuffog C. Gene therapy for tolerance: high-level expression of donor major histocompatibility complex in the liver overcomes naive and memory alloresponses to skin grafts. Transplantation. 2013;95:70–77. doi: 10.1097/TP.0b013e318278d39a. [DOI] [PubMed] [Google Scholar]
- Lüth S, Huber S, Schramm C, Buch T, Zander S, Stadelmann C. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118:3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BE, Dobrzynski E, Wang L, Hirao L, Mingozzi F, Cao O. Muscle as a target for supplementary factor IX gene transfer. Hum Gene Ther. 2007;18:603–613. doi: 10.1089/hum.2007.042. [DOI] [PubMed] [Google Scholar]
- Passini MA, Bu J, Fidler JA, Ziegler RJ, Foley JW, Dodge JC. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc Natl Acad Sci USA. 2007;104:9505–9510. doi: 10.1073/pnas.0703509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah CS, Soustek MS, Todd AG, McCall A, Smith BK, Corti M. Adeno-associated virus-mediated gene therapy for metabolic myopathy. Hum Gene Ther. 2013;24:928–936. doi: 10.1089/hum.2013.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne BJ, Falk DJ, Clément N, Mah CS. Gene therapy approaches for lysosomal storage disease: next-generation treatment. Hum Gene Ther. 2012;23:808–815. doi: 10.1089/hum.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietupski JB, Hurlbut GD, Ziegler RJ, Chu Q, Hodges BL, Ashe KM. Systemic administration of AAV8-α-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression. Mol Ther. 2011;19:1999–2011. doi: 10.1038/mt.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn JD, Ozelo MC, Sabatino DE, Franck HW, Merricks EP, Crudele JM. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116:5842–5848. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni A, Cantore A, Della Valle P, Goudy K, Akbarpour M, Russo F. Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol Med. 2013;5:1684–1697. doi: 10.1002/emmm.201302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011;475:217–221. doi: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguela XM, Sharma R, Doyon Y, Miller JC, Li H, Haurigot V. Robust ZFN-mediated genome editing in adult hemophilic mice. Blood. 2013;122:3283–3287. doi: 10.1182/blood-2013-04-497354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Gao C, Sands MS, Cai SR, Nichols TC, Bellinger DA. Neonatal or hepatocyte growth factor-potentiated adult gene therapy with a retroviral vector results in therapeutic levels of canine factor IX for hemophilia B. Blood. 2003;101:3924–3932. doi: 10.1182/blood-2002-10-3050. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu L, Haskins ME, Parker Ponder K. Neonatal gene transfer with a retroviral vector results in tolerance to human factor IX in mice and dogs. Blood. 2004;103:143–151. doi: 10.1182/blood-2003-06-2181. [DOI] [PubMed] [Google Scholar]
- Sabatino DE, Mackenzie TC, Peranteau W, Edmonson S, Campagnoli C, Liu YL. Persistent expression of hF.IX After tolerance induction by in utero or neonatal administration of AAV-1-F.IX in hemophilia B mice. Mol Ther. 2007;15:1677–1685. doi: 10.1038/sj.mt.6300219. [DOI] [PubMed] [Google Scholar]
- Xu L, Mei M, Ma X, Ponder KP. High expression reduces an antibody response after neonatal gene therapy with B domain-deleted human factor VIII in mice. J Thromb Haemost. 2007;5:1805–1812. doi: 10.1111/j.1538-7836.2007.02629.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Falahati R, Zhang J, Flebbe-Rehwaldt L, Gaensler KM. Role of antigen-specific regulatory CD4+CD25+ T cells in tolerance induction after neonatal IP administration of AAV-hF.IX. Gene Ther. 2013;20:987–996. doi: 10.1038/gt.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppen J, van Til NP, van der Rijt R, Hiralall JK, Kunne C, Elferink RP. Immune response to lentiviral bilirubin UDP-glucuronosyltransferase gene transfer in fetal and neonatal rats. Gene Ther. 2006;13:672–677. doi: 10.1038/sj.gt.3302681. [DOI] [PubMed] [Google Scholar]
- Ponder KP, Wang B, Wang P, Ma X, Herati R, Wang B. Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-Ig. Mol Ther. 2006;14:5–13. doi: 10.1016/j.ymthe.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Ponder KP. Immunology of neonatal gene transfer. Curr Gene Ther. 2007;7:403–410. doi: 10.2174/156652307782151434. [DOI] [PubMed] [Google Scholar]
- Xu L, Mei M, Haskins ME, Nichols TC, O’donnell P, Cullen K. Immune response after neonatal transfer of a human factor IX-expressing retroviral vector in dogs, cats, and mice. Thromb Res. 2007;120:269–280. doi: 10.1016/j.thromres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Durkin HG, Waksman BH. Thymus and tolerance. Is regulation the major function of the thymus? Immunol Rev. 2001;182:33–57. doi: 10.1034/j.1600-065x.2001.1820103.x. [DOI] [PubMed] [Google Scholar]
- Marshall DJ, Park BH, Korostoff JM, Gaulton GN. Manipulation of the immune response by foreign gene expression in the thymus. Leukemia. 1995;9 suppl. 1:S128–S132. [PubMed] [Google Scholar]
- Marodon G, Fisson S, Levacher B, Fabre M, Salomon BL, Klatzmann D. Induction of antigen-specific tolerance by intrathymic injection of lentiviral vectors. Blood. 2006;108:2972–2978. doi: 10.1182/blood-2006-03-010900. [DOI] [PubMed] [Google Scholar]
- Gottrand G, Taleb K, Ragon I, Bergot AS, Goldstein JD, Marodon G. Intrathymic injection of lentiviral vector curtails the immune response in the periphery of normal mice. J Gene Med. 2012;14:90–99. doi: 10.1002/jgm.1650. [DOI] [PubMed] [Google Scholar]
- Siatskas C, Seach N, Sun G, Emerson-Webber A, Silvain A, Toh BH. Thymic gene transfer of myelin oligodendrocyte glycoprotein ameliorates the onset but not the progression of autoimmune demyelination. Mol Ther. 2012;20:1349–1359. doi: 10.1038/mt.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Q, Moreland RJ, Gao L, Taylor KM, Meyers E, Cheng SH. Induction of immune tolerance to a therapeutic protein by intrathymic gene delivery. Mol Ther. 2010;18:2146–2154. doi: 10.1038/mt.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- Edelmann SL, Marconi P, Brocker T. Peripheral T cells re-enter the thymus and interfere with central tolerance induction. J Immunol. 2011;186:5612–5619. doi: 10.4049/jimmunol.1004010. [DOI] [PubMed] [Google Scholar]
- Coleman MA, Bridge JA, Lane SW, Dixon CM, Hill GR, Wells JW. Tolerance induction with gene-modified stem cells and immune-preserving conditioning in primed mice: restricting antigen to differentiated antigen-presenting cells permits efficacy. Blood. 2013;121:1049–1058. doi: 10.1182/blood-2012-06-434100. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schroeder JA, Kuether EL, Zhang G, Shi Q. Platelet gene therapy by lentiviral gene delivery to hematopoietic stem cells restores hemostasis and induces humoral immune tolerance in FIX(null) mice. Mol Ther. 2014;22:169–177. doi: 10.1038/mt.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi U, Gattringer M, Farkas AM, Hock K, Pilat N, Iacomini J. The site of allergen expression in hematopoietic cells determines the degree and quality of tolerance induced through molecular chimerism. Eur J Immunol. 2013;43:2451–2460. doi: 10.1002/eji.201243277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Fahs SA, Wilcox DA, Kuether EL, Morateck PA, Mareno N. Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunity. Blood. 2008;112:2713–2721. doi: 10.1182/blood-2008-02-138214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A. Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat Biotechnol. 2007;25:1457–1467. doi: 10.1038/nbt1372. [DOI] [PubMed] [Google Scholar]
- Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- Sundt TM, 3rd, Sachs DH. Applications of molecular biology to transplantation tolerance. Immunol Today. 1988;9:342–344. doi: 10.1016/0167-5699(88)91334-5. [DOI] [PubMed] [Google Scholar]
- Bagley J, Tian C, Sachs DH, Iacomini J. Induction of T-cell tolerance to an MHC class I alloantigen by gene therapy. Blood. 2002;99:4394–4399. doi: 10.1182/blood.v99.12.4394. [DOI] [PubMed] [Google Scholar]
- Sykes M, Sachs DH, Nienhuis AW, Pearson DA, Moulton AD, Bodine DM. Specific prolongation of skin graft survival following retroviral transduction of bone marrow with an allogeneic major histocompatibility complex gene. Transplantation. 1993;55:197–202. doi: 10.1097/00007890-199301000-00037. [DOI] [PubMed] [Google Scholar]
- Wong W, Morris PJ, Wood KJ. Syngeneic bone marrow expressing a single donor class I MHC molecule permits acceptance of a fully allogeneic cardiac allograft. Transplantation. 1996;62:1462–1468. doi: 10.1097/00007890-199611270-00014. [DOI] [PubMed] [Google Scholar]
- Bracy JL, Sachs DH, Iacomini J. Inhibition of xenoreactive natural antibody production by retroviral gene therapy. Science. 1998;281:1845–1847. doi: 10.1126/science.281.5384.1845. [DOI] [PubMed] [Google Scholar]
- Bracy JL, Iacomini J. Induction of B-cell tolerance by retroviral gene therapy. Blood. 2000;96:3008–3015. [PubMed] [Google Scholar]
- Gunthart M, Kearns-Jonker M. Gene therapy for the induction of chimerism and transplant tolerance. Curr Gene Ther. 2007;7:411–420. doi: 10.2174/156652307782793522. [DOI] [PubMed] [Google Scholar]
- Horn PA, Figueiredo C, Kiem HP. Gene therapy in the transplantation of allogeneic organs and stem cells. Curr Gene Ther. 2007;7:458–468. doi: 10.2174/156652307782793513. [DOI] [PubMed] [Google Scholar]
- Coleman MA, Steptoe RJ. Induction of antigen-specific tolerance through hematopoietic stem cell-mediated gene therapy: the future for therapy of autoimmune disease? Autoimmun Rev. 2012;12:195–203. doi: 10.1016/j.autrev.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Leventhal J, Miller J, Abecassis M, Tollerud DJ, Ildstad ST. Evolving approaches of hematopoietic stem cell-based therapies to induce tolerance to organ transplants: the long road to tolerance. Clin Pharmacol Ther. 2013;93:36–45. doi: 10.1038/clpt.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi U, Pilat N, Gattringer M, Linhart B, Klaus C, Schwaiger E. Persistent molecular microchimerism induces long-term tolerance towards a clinically relevant respiratory allergen. Clin Exp Allergy. 2012;42:1282–1292. doi: 10.1111/j.1365-2222.2012.04049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasa Z, Chung JY, Chan J, Toh BH, Alderuccio F. Nonmyeloablative conditioning generates autoantigen-encoding bone marrow that prevents and cures an experimental autoimmune disease. Am J Transplant. 2012;12:2062–2071. doi: 10.1111/j.1600-6143.2012.04068.x. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Giannoni F, Lee CC, Wherley J, Sumiyoshi T, Martinez M. Nonmyeloablative conditioning regimen to increase engraftment of gene-modified hematopoietic stem cells in young rhesus monkeys. Mol Ther. 2012;20:1033–1045. doi: 10.1038/mt.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frecha C, Costa C, Nègre D, Amirache F, Trono D, Rio P. A novel lentiviral vector targets gene transfer into human hematopoietic stem cells in marrow from patients with bone marrow failure syndrome and in vivo in humanized mice. Blood. 2012;119:1139–1150. doi: 10.1182/blood-2011-04-346619. [DOI] [PubMed] [Google Scholar]
- Tian C, Bagley J, Kaye J, Iacomini J. Induction of T cell tolerance to a protein expressed in the cytoplasm through retroviral-mediated gene transfer. J Gene Med. 2003;5:359–365. doi: 10.1002/jgm.363. [DOI] [PubMed] [Google Scholar]
- Andersson G, Denaro M, Johnson K, Morgan P, Sullivan A, Houser S. Engraftment of retroviral EGFP-transduced bone marrow in mice prevents rejection of EGFP-transgenic skin grafts. Mol Ther. 2003;8:385–391. doi: 10.1016/s1525-0016(03)00210-7. [DOI] [PubMed] [Google Scholar]
- Jindra PT, Tripathi S, Tian C, Iacomini J, Bagley J. Tolerance to MHC class II disparate allografts through genetic modification of bone marrow. Gene Ther. 2013;20:478–486. doi: 10.1038/gt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Haviernik P, Wolfraim LA, Bunting KD, Scott DW. Bone marrow transplantation combined with gene therapy to induce antigen-specific tolerance and ameliorate EAE. Mol Ther. 2006;13:42–48. doi: 10.1016/j.ymthe.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gattringer M, Baranyi U, Pilat N, Hock K, Klaus C, Buchberger E. Engraftment of retrovirally transduced Bet v 1-GFP expressing bone marrow cells leads to allergen-specific tolerance. Immunobiology. 2013;218:1139–1146. doi: 10.1016/j.imbio.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering CB, Gangadharan B, Dukart HZ, Spencer HT. Hematopoietic stem cells encoding porcine factor VIII induce pro-coagulant activity in hemophilia A mice with pre-existing factor VIII immunity. Mol Ther. 2007;15:1093–1099. doi: 10.1038/sj.mt.6300146. [DOI] [PubMed] [Google Scholar]
- Ide LM, Iwakoshi NN, Gangadharan B, Jobe S, Moot R, McCarty D. Functional aspects of factor VIII expression after transplantation of genetically-modified hematopoietic stem cells for hemophilia A. J Gene Med. 2010;12:333–344. doi: 10.1002/jgm.1442. [DOI] [PubMed] [Google Scholar]
- Ramezani A, Hawley RG. Correction of murine hemophilia A following nonmyeloablative transplantation of hematopoietic stem cells engineered to encode an enhanced human factor VIII variant using a safety-augmented retroviral vector. Blood. 2009;114:526–534. doi: 10.1182/blood-2009-01-199653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A, Zweier-Renn LA, Hawley RG. Factor VIII delivered by haematopoietic stem cell-derived B cells corrects the phenotype of haemophilia A mice. Thromb Haemost. 2011;105:676–687. doi: 10.1160/TH10-11-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Kuether EL, Chen Y, Schroeder JA, Fahs SA, Montgomery RR. Platelet gene therapy corrects the hemophilic phenotype in immunocompromised hemophilia A mice transplanted with genetically manipulated human cord blood stem cells. Blood. 2014;123:395–403. doi: 10.1182/blood-2013-08-520478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du LM, Nurden P, Nurden AT, Nichols TC, Bellinger DA, Jensen ES. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat Commun. 2013;4:2773. doi: 10.1038/ncomms3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AH, Stephan MT, Sadelain M. Stem cell-derived erythroid cells mediate long-term systemic protein delivery. Nat Biotechnol. 2006;24:1017–1021. doi: 10.1038/nbt1227. [DOI] [PubMed] [Google Scholar]
- Bigger BW, Siapati EK, Mistry A, Waddington SN, Nivsarkar MS, Jacobs L. Permanent partial phenotypic correction and tolerance in a mouse model of hemophilia B by stem cell gene delivery of human factor IX. Gene Ther. 2006;13:117–126. doi: 10.1038/sj.gt.3302638. [DOI] [PubMed] [Google Scholar]
- Chen H, Yao H, Huang L, Shen Q, Jia W, Xue J. Expression of human factor IX gene in murine plasma through lentiviral vector-infected haematopoietic stem cells. Clin Exp Pharmacol Physiol. 2006;33:1196–1201. doi: 10.1111/j.1440-1681.2006.04511.x. [DOI] [PubMed] [Google Scholar]
- Chang AH, Stephan MT, Lisowski L, Sadelain M. Erythroid-specific human factor IX delivery from in vivo selected hematopoietic stem cells following nonmyeloablative conditioning in hemophilia B mice. Mol Ther. 2008;16:1745–1752. doi: 10.1038/mt.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Til NP, Stok M, Aerts Kaya FS, de Waard MC, Farahbakhshian E, Visser TP. Lentiviral gene therapy of murine hematopoietic stem cells ameliorates the Pompe disease phenotype. Blood. 2010;115:5329–5337. doi: 10.1182/blood-2009-11-252874. [DOI] [PubMed] [Google Scholar]
- Douillard-Guilloux G, Richard E, Batista L, Caillaud C. Partial phenotypic correction and immune tolerance induction to enzyme replacement therapy after hematopoietic stem cell gene transfer of alpha-glucosidase in Pompe disease. J Gene Med. 2009;11:279–287. doi: 10.1002/jgm.1305. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- Skupsky J, Su Y, Lei TC, Scott DW. Tolerance induction by gene transfer to lymphocytes. Curr Gene Ther. 2007;7:369–380. doi: 10.2174/156652307782151443. [DOI] [PubMed] [Google Scholar]
- El-Amine M, Melo M, Kang Y, Nguyen H, Qian J, Scott DW. Mechanisms of tolerance induction by a gene-transferred peptide-IgG fusion protein expressed in B lineage cells. J Immunol. 2000;165:5631–5636. doi: 10.4049/jimmunol.165.10.5631. [DOI] [PubMed] [Google Scholar]
- Lei TC, Su Y, Scott DW. Tolerance induction via a B-cell delivered gene therapy-based protocol: optimization and role of the Ig scaffold. Cell Immunol. 2005;235:12–20. doi: 10.1016/j.cellimm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Litzinger MT, Su Y, Lei TC, Soukhareva N, Scott DW. Mechanisms of gene therapy for tolerance: B7 signaling is required for peptide-IgG gene-transferred tolerance induction. J Immunol. 2005;175:780–787. doi: 10.4049/jimmunol.175.2.780. [DOI] [PubMed] [Google Scholar]
- Skupsky J, Zhang AH, Su Y, Scott DW. B-cell-delivered gene therapy induces functional T regulatory cells and leads to a loss of antigen-specific effector cells. Mol Ther. 2010;18:1527–1535. doi: 10.1038/mt.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukhareva N, Jiang Y, Scott DW. Treatment of diabetes in NOD mice by gene transfer of Ig-fusion proteins into B cells: role of T regulatory cells. Cell Immunol. 2006;240:41–46. doi: 10.1016/j.cellimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ahangarani RR, Janssens W, Carlier V, Vanderelst L, Vandendriessche T, Chuah M. Retroviral vectors induce epigenetic chromatin modifications and IL-10 production in transduced B cells via activation of toll-like receptor 2. Mol Ther. 2011;19:711–722. doi: 10.1038/mt.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer F, Heinen TJ, Wunderlich FT, Yogev N, Buch T, Roers A. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J Immunol. 2008;181:5748–5759. doi: 10.4049/jimmunol.181.8.5748. [DOI] [PubMed] [Google Scholar]
- Su Y, Zhang AH, Noben-Trauth N, Scott DW. B-cell gene therapy for tolerance induction: host but not donor B-cell derived IL-10 is necessary for tolerance. Front Microbiol. 2011;2:154. doi: 10.3389/fmicb.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112:3303–3311. doi: 10.1182/blood-2008-02-138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens LP, Najafian N, Mingozzi F, Elyaman W, Mazer B, Moise L. In vitro and in vivo studies of IgG-derived Treg epitopes (Tregitopes): a promising new tool for tolerance induction and treatment of autoimmunity. J Clin Immunol. 2013;33 suppl. 1:S43–S49. doi: 10.1007/s10875-012-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Rossi R, De Groot AS, Scott DW. Regulatory T cell epitopes (Tregitopes) in IgG induce tolerance in vivo and lack immunogenicity per se. J Leukoc Biol. 2013;94:377–383. doi: 10.1189/jlb.0912441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DJ, Basner-Tschakarjan E, Chen Y, Davidson RJ, Buchlis G, Yazicioglu M. Modulation of CD8+ T cell responses to AAV vectors with IgG-derived MHC class II epitopes. Mol Ther. 2013;21:1727–1737. doi: 10.1038/mt.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens LP, Tassone R, Mazer BD, Ramachandiran V, Scott DW, De Groot AS. Tregitope update: mechanism of action parallels IVIg. Autoimmun Rev. 2013;12:436–443. doi: 10.1016/j.autrev.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Cousens LP, Su Y, McClaine E, Li X, Terry F, Smith R. Application of IgG-derived natural Treg epitopes (IgG Tregitopes) to antigen-specific tolerance induction in a murine model of type 1 diabetes. J Diabetes Res. 2013;2013:621693. doi: 10.1155/2013/621693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AH, Li X, Onabajo OO, Su Y, Skupsky J, Thomas JW. B-cell delivered gene therapy for tolerance induction: role of autoantigen-specific B cells. J Autoimmun. 2010;35:107–113. doi: 10.1016/j.jaut.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo ME, Qian J, El-Amine M, Agarwal RK, Soukhareva N, Kang Y. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J Immunol. 2002;168:4788–4795. doi: 10.4049/jimmunol.168.9.4788. [DOI] [PubMed] [Google Scholar]
- Xu B, Scott DW. A novel retroviral gene therapy approach to inhibit specific antibody production and suppress experimental autoimmune encephalomyelitis induced by MOG and MBP. Clin Immunol. 2004;111:47–52. doi: 10.1016/j.clim.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Satpute SR, Soukhareva N, Scott DW, Moudgil KD. Mycobacterial Hsp65-IgG-expressing tolerogenic B cells confer protection against adjuvant-induced arthritis in Lewis rats. Arthritis Rheum. 2007;56:1490–1496. doi: 10.1002/art.22566. [DOI] [PubMed] [Google Scholar]
- Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105:4865–4870. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moghimi B, Zolotukhin I, Morel LM, Cao O, Herzog RW. Immune tolerance induction to factor IX through B cell gene transfer - TLR9 signaling delineates between tolerogenic and immunogenic B cells. Mol Ther. 2014. [DOI] [PMC free article] [PubMed]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- Albert MH, Liu Y, Anasetti C, Yu XZ. Antigen-dependent suppression of alloresponses by Foxp3-induced regulatory T cells in transplantation. Eur J Immunol. 2005;35:2598–2607. doi: 10.1002/eji.200526077. [DOI] [PubMed] [Google Scholar]
- Loser K, Hansen W, Apelt J, Balkow S, Buer J, Beissert S. In vitro-generated regulatory T cells induced by Foxp3-retrovirus infection control murine contact allergy and systemic autoimmunity. Gene Ther. 2005;12:1294–1304. doi: 10.1038/sj.gt.3302567. [DOI] [PubMed] [Google Scholar]
- Peng J, Dicker B, Du W, Tang F, Nguyen P, Geiger T. Converting antigen-specific diabetogenic CD4 and CD8 T cells to TGF-beta producing non-pathogenic regulatory cells following FoxP3 transduction. J Autoimmun. 2007;28:188–200. doi: 10.1016/j.jaut.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Elinav E, Adam N, Waks T, Eshhar Z. Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology. 2009;136:1721–1731. doi: 10.1053/j.gastro.2009.01.049. [DOI] [PubMed] [Google Scholar]
- Wright GP, Notley CA, Xue SA, Bendle GM, Holler A, Schumacher TN. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci USA. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornete M, Piccirillo CA. Functional crosstalk between dendritic cells and Foxp3(+) regulatory T cells in the maintenance of immune tolerance. Front Immunol. 2012;3:165. doi: 10.3389/fimmu.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert JM, Halary F. Viral and non-viral methods to genetically modify dendritic cells. Curr Gene Ther. 2012;12:127–136. doi: 10.2174/156652312800099580. [DOI] [PubMed] [Google Scholar]
- Tan PH, Beutelspacher SC, Xue SA, Wang YH, Mitchell P, McAlister JC. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood. 2005;105:3824–3832. doi: 10.1182/blood-2004-10-3880. [DOI] [PubMed] [Google Scholar]
- Su RJ, Epp A, Feng J, Roy J, Latchman Y, Wu X. Suppression of the immune response to FVIII in hemophilia A mice by transgene modified tolerogenic dendritic cells. Mol Ther. 2011;19:1896–1904. doi: 10.1038/mt.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresch C, Edelmann SL, Marconi P, Brocker T. Lentiviral-mediated transcriptional targeting of dendritic cells for induction of T cell tolerance in vivo. J Immunol. 2008;181:4495–4506. doi: 10.4049/jimmunol.181.7.4495. [DOI] [PubMed] [Google Scholar]
- de Andrade Pereira B, Fraefel C, Hilbe M, Ackermann M, Dresch C. Transcriptional targeting of DCs with lentiviral vectors induces antigen-specific tolerance in a mouse model of multiple sclerosis. Gene Ther. 2013;20:556–566. doi: 10.1038/gt.2012.73. [DOI] [PubMed] [Google Scholar]
- Liu CL, Ye P, Yen BC, Miao CH. In vivo expansion of regulatory T cells with IL-2/IL-2 mAb complexes prevents anti-factor VIII immune responses in hemophilia A mice treated with factor VIII plasmid-mediated gene therapy. Mol Ther. 2011;19:1511–1520. doi: 10.1038/mt.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Sarkar D, Perrin GQ, Moghimi B, Hoffman BE, Zhou S. Prevention and reversal of antibody responses against factor IX in gene therapy for hemophilia B. Front Microbiol. 2011;2:244. doi: 10.3389/fmicb.2011.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR, Favaro P, Finn JD. Strategies to modulate immune responses: a new frontier for gene therapy. Mol Ther. 2009;17:1492–1503. doi: 10.1038/mt.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]


