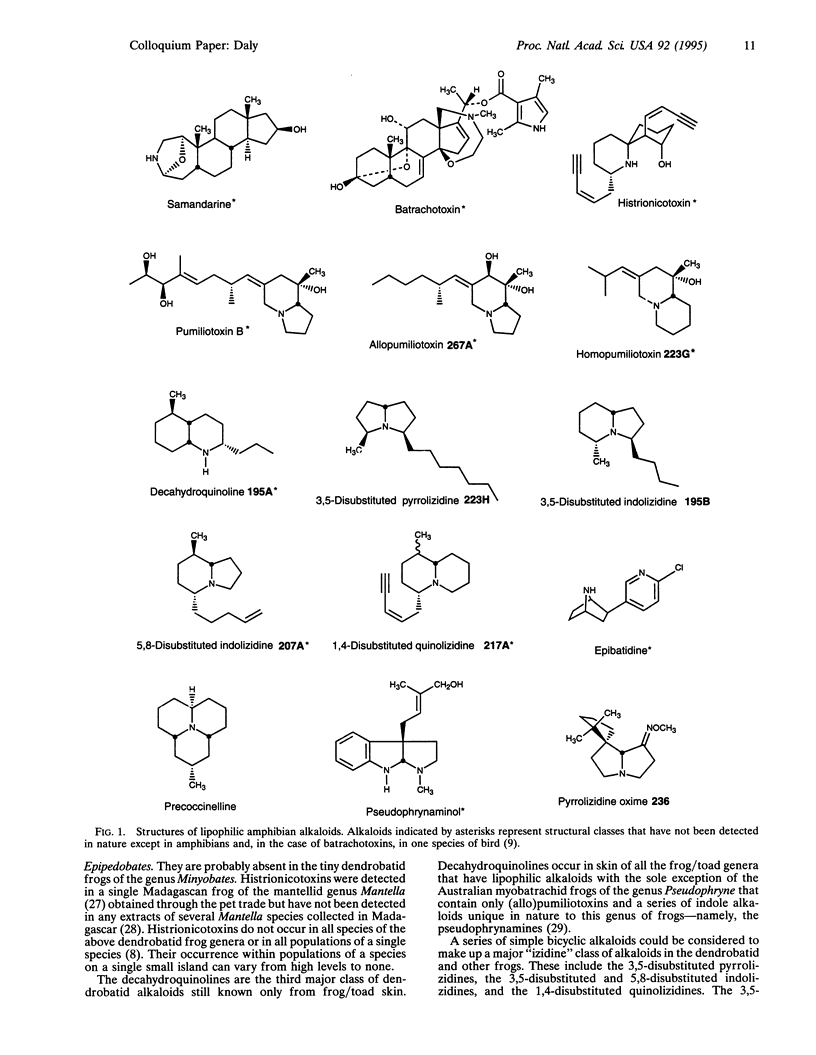
Abstract
Poisons are common in nature, where they often serve the organism in chemical defense. Such poisons either are produced de novo or are sequestered from dietary sources or symbiotic organisms. Among vertebrates, amphibians are notable for the wide range of noxious agents that are contained in granular skin glands. These compounds include amines, peptides, proteins, steroids, and both water-soluble and lipid-soluble alkaloids. With the exception of the alkaloids, most seem to be produced de novo by the amphibian. The skin of amphibians contains many structural classes of alkaloids previously unknown in nature. These include the batrachotoxins, which have recently been discovered to also occur in skin and feathers of a bird, the histrionicotoxins, the gephyrotoxins, the decahydroquinolines, the pumiliotoxins and homopumiliotoxins, epibatidine, and the samandarines. Some amphibian skin alkaloids are clearly sequestered from the diet, which consists mainly of small arthropods. These include pyrrolizidine and indolizidine alkaloids from ants, tricyclic coccinellines from beetles, and pyrrolizidine oximes, presumably from millipedes. The sources of other alkaloids in amphibian skin, including the batrachotoxins, the decahydroquinolines, the histrionicotoxins, the pumiliotoxins, and epibatidine, are unknown. While it is possible that these are produced de novo or by symbiotic microorganisms, it appears more likely that they are sequestered by the amphibians from as yet unknown dietary sources.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balandrin M. F., Klocke J. A., Wurtele E. S., Bollinger W. H. Natural plant chemicals: sources of industrial and medicinal materials. Science. 1985 Jun 7;228(4704):1154–1160. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- Bevins C. L., Zasloff M. Peptides from frog skin. Annu Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- Brower L. P., van Brower J., Corvino J. M. Plant poisons in a terrestrial food chain. Proc Natl Acad Sci U S A. 1967 Apr;57(4):893–898. doi: 10.1073/pnas.57.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W., Caceres J., Moni R. W., Gusovsky F., Moos M., Jr, Seamon K. B., Milton K., Myers C. W. Frog secretions and hunting magic in the upper Amazon: identification of a peptide that interacts with an adenosine receptor. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10960–10963. doi: 10.1073/pnas.89.22.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W., Garraffo H. M., Pannell L. K., Spande T. F., Severini C., Erspamer V. Alkaloids from Australian frogs (Myobatrachidae): pseudophrynamines and pumiliotoxins. J Nat Prod. 1990 Mar-Apr;53(2):407–421. doi: 10.1021/np50068a020. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Gusovsky F., Myers C. W., Yotsu-Yamashita M., Yasumoto T. First occurrence of tetrodotoxin in a dendrobatid frog (Colostethus inguinalis), with further reports for the bufonid genus Atelopus. Toxicon. 1994 Mar;32(3):279–285. doi: 10.1016/0041-0101(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Highet R. J., Myers C. W. Occurrence of skin alkaloids in non-dendrobatid frogs from Brazil (Bufonidae), Australia (Myobatrachidae) and Madagascar (Mantellinae). Toxicon. 1984;22(6):905–919. doi: 10.1016/0041-0101(84)90182-x. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Myers C. W., Warnick J. E., Albuquerque E. X. Levels of batrachotoxin and lack of sensitivity to its action in poison-dart frogs (Phyllobates). Science. 1980 Jun 20;208(4450):1383–1385. doi: 10.1126/science.6246586. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Myers C. W., Whittaker N. Further classification of skin alkaloids from neotropical poison frogs (Dendrobatidae), with a general survey of toxic/noxious substances in the amphibia. Toxicon. 1987;25(10):1023–1095. doi: 10.1016/0041-0101(87)90265-0. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Secunda S. I., Garraffo H. M., Spande T. F., Wisnieski A., Cover J. F., Jr An uptake system for dietary alkaloids in poison frogs (Dendrobatidae). Toxicon. 1994 Jun;32(6):657–663. doi: 10.1016/0041-0101(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Secunda S. I., Garraffo H. M., Spande T. F., Wisnieski A., Nishihira C., Cover J. F., Jr Variability in alkaloid profiles in neotropical poison frogs (Dendrobatidae): genetic versus environmental determinants. Toxicon. 1992 Aug;30(8):887–898. doi: 10.1016/0041-0101(92)90387-k. [DOI] [PubMed] [Google Scholar]
- Dumbacher J. P., Beehler B. M., Spande T. F., Garraffo H. M., Daly J. W. Homobatrachotoxin in the genus Pitohui: chemical defense in birds? Science. 1992 Oct 30;258(5083):799–801. doi: 10.1126/science.1439786. [DOI] [PubMed] [Google Scholar]
- Eisner T., Meinwald J. Defensive secretions of arthropods. Science. 1966 Sep 16;153(3742):1341–1350. doi: 10.1126/science.153.3742.1341. [DOI] [PubMed] [Google Scholar]
- Eisner T., Wiemer D. F., Haynes L. W., Meinwald J. Lucibufagins: Defensive steroids from the fireflies Photinus ignitus and P. marginellus (Coleoptera: Lampyridae). Proc Natl Acad Sci U S A. 1978 Feb;75(2):905–908. doi: 10.1073/pnas.75.2.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erspamer V., Melchiorri P., Falconieri Erspamer G., Montecucchi P. C., de Castiglione R. Phyllomedusa skin: a huge factory and store-house of a variety of active peptides. Peptides. 1985;6 (Suppl 3):7–12. doi: 10.1016/0196-9781(85)90343-2. [DOI] [PubMed] [Google Scholar]
- Flier J., Edwards M. W., Daly J. W., Myers C. W. Widespread occurrence in frogs and toads of skin compounds interacting with the ouabain site of Na+, K+-ATPase. Science. 1980 May 2;208(4443):503–505. doi: 10.1126/science.6245447. [DOI] [PubMed] [Google Scholar]
- Garraffo H. M., Caceres J., Daly J. W., Spande T. F., Andriamaharavo N. R., Andriantsiferana M. Alkaloids in Madagascan frogs (Mantella): pumiliotoxins, indolizidines, quinolizidines, and pyrrolizidines. J Nat Prod. 1993 Jul;56(7):1016–1038. doi: 10.1021/np50097a005. [DOI] [PubMed] [Google Scholar]
- Porto A. M., Gros E. G. Biosynthesis of the bufadienolide marinobufagin in toads Bufo paracnemis from cholesterol-20-14C. Experientia. 1971 May 15;27(5):506–506. doi: 10.1007/BF02147562. [DOI] [PubMed] [Google Scholar]
- Richter K., Egger R., Negri L., Corsi R., Severini C., Kreil G. cDNAs encoding [D-Ala2]deltorphin precursors from skin of Phyllomedusa bicolor also contain genetic information for three dermorphin-related opioid peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4836–4839. doi: 10.1073/pnas.87.12.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer P. J. Some marine ecological phenomena: chemical basis and biomedical potential. Science. 1990 Apr 13;248(4952):173–177. doi: 10.1126/science.2183350. [DOI] [PubMed] [Google Scholar]
- Spande T. F., Garraffo H. M., Yeh H. J., Pu Q. L., Pannell L. K., Daly J. W. A new class of alkaloids from a dendrobatid poison frog: a structure for alkaloid 251F. J Nat Prod. 1992 Jun;55(6):707–722. doi: 10.1021/np50084a002. [DOI] [PubMed] [Google Scholar]
- Whittaker R. H., Feeny P. P. Allelochemics: chemical interactions between species. Science. 1971 Feb 26;171(3973):757–770. doi: 10.1126/science.171.3973.757. [DOI] [PubMed] [Google Scholar]


