Abstract
The present investigation examined the spatiotemporal expression of estrogen receptors (ER-α and ER-β) and progesterone receptor (PR) in the periimplantation mouse uterus (days 1–8). ER-α messenger RNA (mRNA) was detected at much higher levels in the periimplantation uterus compared with that of ER-β mRNA, the levels of which were very low in all uterine cells during this period. Results of in situ hybridization demonstrated expression of ER-α mRNA primarily in the luminal and glandular epithelia on days 1 and 2 of pregnancy. On days 3 and 4, the accumulation was localized primarily in stromal cells in addition to its presence in the epithelium. Following implantation on day 5, the accumulation of this mRNA was more condensed in the luminal and glandular epithelia, but declined in the subluminal epithelial stroma at the sites of implanting embryos. On days 6–8, the accumulation of ER-α mRNA was primarily localized in the secondary decidual zone (SDZ) with more intense localization in the subepithelial cells at the mesometrial pole. In contrast, signals were very low to undetectable in the primary decidual zone (PDZ), and no signals were detected in implanting embryos. The undifferentiated stroma underneath the myometrium also showed positive signals. The immunolocalization of ER-α protein correlated with the mRNA localization. Western blot analysis showed down-regulation of ER-α in day 8 decidual cell extracts consistent with the down-regulation of ER-α mRNA in decidual cells immediately surrounding the embryo on this day. The expression pattern of PR was also dynamic in the periimplantation uterus. On day 1, the accumulation of PR mRNA was very low to undetectable, whereas only a modest level of accumulation in the epithelium was noted on day 2. On days 3 and 4, the accumulation of this mRNA was detected in both the epithelium and stroma. In contrast, the expression was restricted only to the stroma with increased signals at the sites of implantation on day 5. On days 6–8, PR mRNA accumulation increased dramatically throughout the deciduum. The localization of immunoreactive PR correlated with the mRNA distribution in the periimplantation uterus. Taken together, the results demonstrate that the expression of ER-α, ER-β, and PR is differentially regulated in the periimplantation mouse uterus. This compartmentalized expression of ER and PR provides information regarding the sites of coordinated effects of estrogen and progesterone in the preparation of the uterus for implantation and decidualization during early pregnancy.
THE UTERUS is composed of heterogeneous cell-types that undergo dynamic changes to support embryo development and implantation. These changes are primarily dependent on coordinate interactions mediated by ovarian estrogen and progesterone (P4). Estrogen stimulates proliferation of both uterine epithelial and stromal cells in neonatal mice, but this proliferative action of estrogen is restricted to epithelial cells in the adult mouse uterus (1, 2). In contrast, while P4 is inhibitory to estrogen-mediated proliferation of the luminal and glandular epithelial cells (2, 3), P4 alone or a combined treatment of P4 and estrogen leads to uterine stromal cell proliferation. Synchronized development of the preimplantation embryo to the blastocyst stage and differentiation of the uterus to the receptive state are essential to successful implantation (4, 5). In the mouse, preovulatory ovarian estrogen secretion causes proliferation of the luminal and glandular epithelial cells during the first 2 days of pregnancy. On day 3, P4 from newly formed corpora lutea initiates stromal cell proliferation, which is further potentiated by preimplantation ovarian estrogen secretion on day 4 of pregnancy (the day of implantation) (2). On this day, epithelial cells cease to proliferate and become differentiated. This preimplantation ovarian estrogen secretion is also necessary for the increased endometrial capillary permeability at the location of the blastocyst, a prerequisite event in the initiation of implantation and subsequent decidualization of stromal cells. At the beginning of decidualization, the stromal cells immediately surrounding the implanting blastocyst proliferate and form the primary decidual zone (pdz) late on day 5. This is followed by the cessation of proliferation of stromal cells in the pdz and proliferation of stromal cells outside the pdz by day 6, forming the secondary decidual zone (sdz) (reviewed in Ref. 6).
The mechanism(s) by which estrogen initiates implantation in the P4-primed uterus is not clearly understood. It is thought that the estrogen and/or P4-mediated events are accomplished by the expression of a unique set of genes in the uterus. While female sex steroid hormones directly regulate several genes including vitellogenin, PRL, uteroglobin, ovalbumin, progesterone receptor, and lactoferrin in target cells because of the presence of the steroid-responsive elements in their promoter sequences (reviewed in Ref. 7), these steroids also modulate the expression of several growth factors and their receptors in the uterus in a spatiotemporal manner (8-15). Steroid hormone actions in target cells are normally mediated by binding to nuclear receptors, which are ligand-inducible transcription factors. These transcription factors modulate the expression of their target genes upon binding to the appropriate ligands (16-19). Many of the known physiological actions of estrogens are considered to be mediated within the target cells primarily by two nuclear estrogen receptors (ER), ER-α, and ER-β. Molecular analysis of these two receptors revealed that they share approximately 95% and 55% homology in the DNA binding domain and the hormone binding domain, respectively. In contrast, the sequence dissimilarity in the transactivation domains (AF-1 and AF-2) has been suspected for differential gene activation by these two receptors (reviewed in Ref. 20). Both of these receptors exhibit high affinity binding to estradiol in the same estrogen response element (ERE) (21, 22). Furthermore, because these receptor subtypes can form heterodimers in vitro (23), it is suggested that they can also act together to regulate gene transcription. The relative distribution and expression of these receptor subtypes vary considerably within tissue- or cell-types. For example, ER-α has a broad spectrum of expression, whereas ER-β shows restricted pattern of expression with high levels in the ovary, prostate, lung, epididymis, and hypothalamus (21, 24). However, the biological significance of these differential expression largely remains undefined. The disruption of ER-α gene causes infertility and defects in the reproductive tract and gonads in addition to many other abnormalities including behavior and breast development in females (25-27). Targeting of the ER-β gene in the mouse has revealed a role for ER-β in ovulation efficiency. However, this gene is not required for fertility, lactation, or sexual differentiation (28).
Traditionally P4 is considered as the hormone of pregnancy. During early pregnancy, this hormone coordinates a series of complex events that ultimately leads to the synchronized development of the embryo and differentiation of uterus for implantation. P4 acts through progesterone receptor (PR), a complex binding protein composed of two isoforms, termed PRA and PRB (29), originating from a single gene (30, 31). PRA lacks 164 amino acids from the N-terminal region of the full-length receptor, PRB (32). The relationship between the two isoforms and their biological activity still remain unclear. The consensus is that PR is induced by estrogen via the ER. Thus, many of the effects of P4 may be attributed to the combined effects of estrogen and P4. However, recent studies demonstrate that P4 is essential for the induction of uterine decidualization because this process fails to occur in PR (−/−) mouse uteri (33). In contrast, ER-α (−/−) mouse uteri exhibit decidualization only in the presence of P4 (34, 35). These results suggested that estrogenic influence via ER-α is minimal for the induction of decidualization process. Thus, although various complex uterine responses to ovarian steroids are mediated by differential effects of these steroids, to our knowledge no comprehensive information regarding spatiotemporal expression of these receptors in the mouse uterus during the periimplantation period is available. This basic information is important to ascertain whether implantation and decidualization defects resulting from targeting of several genes are due to altered uterine responsiveness to steroids and/or altered uterine expression of ER and/or PR. Thus, we examined spatiotemporal expression of ER-α, ER-β, and PR in the periimplantation mouse uterus. The results clearly demonstrate that these receptors are differentially expressed in the uterus in a spatiotemporal manner.
Materials and Methods
Animals and tissue preparation
CD-1 mice (Charles River Laboratories, Inc., Raleigh, NC) were housed in the animal care facility at the University of Kansas Medical Center in accordance with NIH standards for the care and use of experimental animals. Adult females were mated with fertile males of the same strain to induce pregnancy (day 1 = vaginal plug). Mice on days 1–8 were killed at 0830–0900 h. On days 5 and 6, implantation sites were identified by monitoring the localized uterine vascular permeability at the site of blastocyst after iv injection of Chicago Blue B dye solution (1% in saline). Implantation sites were demarcated by discrete blue bands along the uterus (4, 5). On days 7 and 8, implantation sites were distinct, and their identification did not require any special manipulation.
Hybridization probes
All of the complementary DNAs (cDNAs) used were specific to the mouse. A 342-bp cDNA fragment (nt 659-1000, GenBank Accession No. M38651) of ER-α was obtained by RT-PCR. For RT reaction, day 4 pregnant mouse uterine total RNA was used. The RT-PCR derived fragment was subcloned into pCR-Script (SK)+ vector, and the identity of the clone was confirmed by nucleotide sequencing. The subcloning and vectors for mouse ER-β, PR, and the ribosomal protein L7 (rpL7) have been previously described (10, 35, 36). For Northern blot hybridization, antisense 32P-labeled complementary RNA (cRNA) probes were generated using SP6 polymerase. For in situ hybridization, sense and antisense 35S-labeled cRNA probes were generated using appropriate RNA polymerases. The probes had specific activities of 2 × 109 dpm/μg.
Northern blot hybridization
Total RNAs were extracted from whole uteri pooled from 7–10 mice on the indicated day of pregnancy by a modified guanidine thiocyanate procedure (37, 38). Polyadenylated [poly (A+)] RNAs were isolated from total RNAs by oligo(dT)-cellulose column chromatography (39). Poly (A+) RNA (2.0 μg) was denatured, separated by formaldehyde-agarose gel electrophoresis, and transferred to nylon membranes. RNA was cross-linked to the membranes by UV irradiation (Spectrolinker, XL-1500; Spectronics Corp., Westbury, NY) and the blots were prehybridized, hybridized, and washed as previously described by us (9, 38). Except for ER-β, the blots were hybridized and washed under stringent condition as described by us (40). The stripping of hybridized probe for subsequent rehybridization was achieved as described (9, 14). The hybrids were detected by autoradiography (38) and the autoradiographic exposure times are indicated in the figure legends.
In situ hybridization
In situ hybridization was performed as previously described (8, 10, 11). In brief, frozen sectioned were mounted onto poly-l-lysine coated slides and fixed in 4% paraformaldehyde in PBS for 15 min at 4 C. Sections were prehybridized followed by hybridization with 35S-labeled antisense or sense cRNA probes for 4 h at 45 C. After hybridization and washing, the sections were incubated with RNase-A (20 μg/ml) at 37 C for 20 min. RNase-A resistant hybrids were detected by autoradiography using Kodak NTB-2 liquid emulsion (Eastman Kodak Co., Rochester, NY). The slides were poststained with hematoxylin and eosin. The reddish brown grains indicate the sites of messenger RNA (mRNA) accumulation. This color shade is the result of lateral light scattering from the eosin staining under dark-field microscopy. Sections hybridized with the sense probes served as negative controls.
Antibodies
The affinity-purified rabbit polyclonal antibody, C1355 for rat ER-α isoform was obtained from Dr. M. A. Shupnik, University of Virginia Medical Center (Charlottesville, VA). This antibody was raised against a peptide for the last 14 amino acids in the C-terminal end of rat ER-α (41). Mouse monoclonal antihuman PR was purchased from Zymed Laboratories, Inc. (catalog no. ZS08–0172; Zymed Laboratories, Inc., San Francisco, CA). This antibody was developed against a peptide in the N-terminal proline-rich region of human PR. Both ER-α and PR antibodies cross-react with corresponding mouse proteins. These antibodies were used for protein analysis using either immunostaing and/or Western blotting experiments.
Immunohistochemical staining
The localization of nuclear ER-α and PR was achieved by immunohistochemistry. Small pieces of mouse uteri recovered on days 4, 5, and 8 of pregnancy were fixed in 10% neutral buffered formalin for 24 h at 4 C followed by dehydration in ascending grades of ethanol, cleared in xylene, and embedded in paraffin. Paraffin sections (6 μm) where placed onto poly-l-lysine-coated slides. Sections were deparaffinized in xylene and hydrated in descending grades of ethanol. After washing 2 times (5 min each) in PBS, slides were placed in a plastic Coplin jar filled with 10 mm citrate buffer (pH 6.0), and irradiated for 8 min in microwave oven (antigen retrieval) followed by cooling to room temperature. Sections were washed twice in PBS. Nonspecific reaction was blocked by incubating the sections in 10% nonimmune goat serum (for ER-α) or rabbit serum (for PR) for 10 min. Sections were incubated with ER-α primary antibody (1:10,000 in PBS) and PR antibody (ready-to-use) in a humidified chamber at 4 C for 18 h. After incubation, sections were washed twice in PBS followed by incubation in secondary antibodies, goat antirabbit (for ER-α), or rabbit antimouse (for PR) for 10 min. Sections were washed thoroughly in PBS and incubated with 0.23% periodic acid for 30 sec to block endogenous peroxidase activity. Sections were washed again in PBS and stained using a Zymed Laboratories, Inc. Histostain-SP kit (Zymed Laboratories, Inc. San Francisco, CA). Red deposits indicted the sites of positive immunostaining.
Western blot analysis
Proteins were extracted from the whole uterus on day 4, and from the separated decidua and the uterus minus decidua on day 8 of pregnancy by homogenization in buffer containing 50 mm Tris (pH 7.4), 1 mm EDTA, 150 mm NaCl and proteinase inhibitors (1 μg/ml phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 1 μg/ml leupeptin). The homogenates were centrifuged at 2000 × g for 15 min at 4 C. The supernatants were separated and their protein concentrations were measured. The supernatants (50 μg protein) were boiled for 5 min in SDS sample buffer [0.06 m Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 0.7 m β-mercaptoethanol] containing 0.01% bromophenol blue dye. After centrifugation, the samples were run on 7.5% SDS-PAGE gels under reducing condition and transferred onto nitrocellulose membranes. The membranes were blocked with 5% carnation milk in TBS [10 mm Tris-HCl (pH 8.0) and 150 mm NaCl] plus 0.05% Tween-20 for overnight at 4 C and then incubated in 5% milk containing antibodies (1:7500) to ER-α for overnight at 4 C or 2 h at room temperature. After incubation, membranes were washed three times (10 min each) with 5% milk, incubated with goat antirabbit IgG conjugated with horseradish peroxidase (1:5000) in 5% milk for 1 h, and washed 3 times (5 min each) in TBS. The bands were detected by ECL kit (Amersham Pharmacia Biotech, Arlington Heights, IL).
Results
Northern blot analysis of ER-α, ER-β, and PR mRNAs in the periimplantation mouse uterus
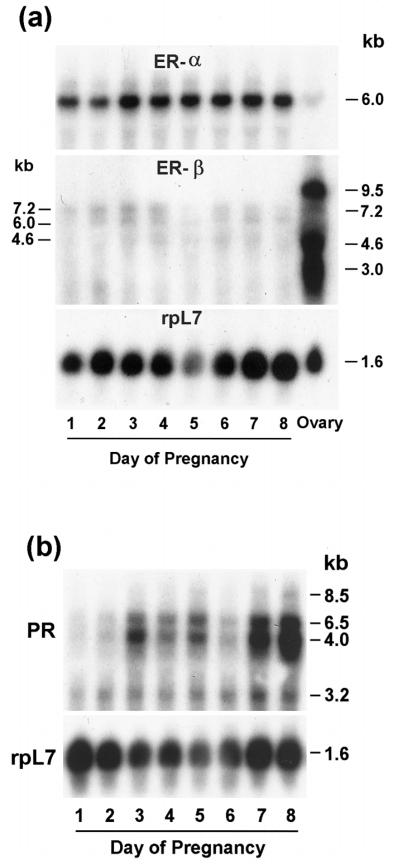
The steady-state levels of ER-α, ER-β, and PR mRNAs in the uterus on days 1–8 of pregnancy were examined by Northern blot hybridization using mouse-specific cRNA probes (Fig. 1, a and b). Consistent with the previous report (22), a single transcript (~6.0 kb) for ER-α mRNA was detected at higher abundance in the uterus on days 1–8 of pregnancy, whereas the abundance of this mRNA was very low in the adult mouse ovary (Fig. 1a). In general, the levels of ER-α mRNA did not show much variation in the uterus during this period. In contrast, the abundance for ER-β mRNA in the periimplantation uterus was extremely low, although some expression was noted on days 2–4 and days 6–7. As detected before for ER-β (22), three major transcripts (~9.5, 4.6, and 3.0 kb) and a minor transcript (~7.2 kb) were detected in the ovary, whereas three transcripts (~7.2, 6.0, and 4.6 kb) were detected in the pregnant uterus (Fig. 1a). Consistent with previous reports (22, 35, 42), ER-β mRNA expression was high in the ovary (Fig. 1b). Consistent with our previous observation (36), four different transcripts (~8.5, 6.5, 4.0, and 3.2 kb) of PR were detected in the periimplantation uterus. The analysis of the levels of these transcripts revealed that the expression was low on days 1 and 2 followed by substantial increases on days 3–5. The levels of expression declined on day 6, but again increased on days 7 and 8. The integrity and loading of RNA samples were examined by rehybridizing the same blots to a mouse rpL7 cRNA probe.
FIG. 1.
a, Northern blot hybridization of ER-α, ER-β, and rpL7 mRNAs in the periimplantation mouse uterus (days 1–8) and ovary. b, Northern blot hybridization of PR and rpL7 mRNAs in the periimplantation uterus (days 1–8). Poly (A)+ RNA (2 μg) was separated by formaldehyde-agarose gel electrophoresis, transferred and UV cross-linked to nylon membrane, and hybridized as described in Materials and Methods. Hybridization was performed using 32P-labeled cRNA probes sequentially to (a) ER-α, ER-β, and rpL7 or (b) PR and rpL7. Autoradiographic exposures were 2 h for ER-α, 5 days for ER-β, 6 h for PR, and 1.5 h for rpL7.
In situ hybridization of ER-α, ER-β, and PR mRNAs in the periimplantation mouse uterus
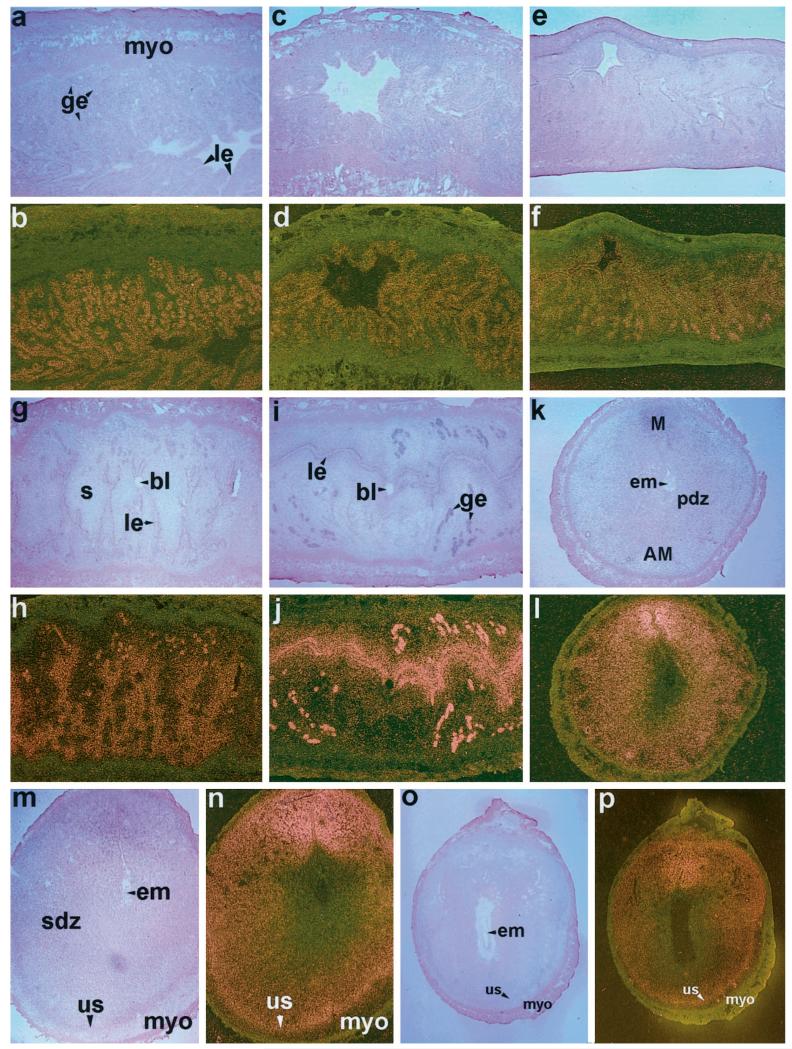
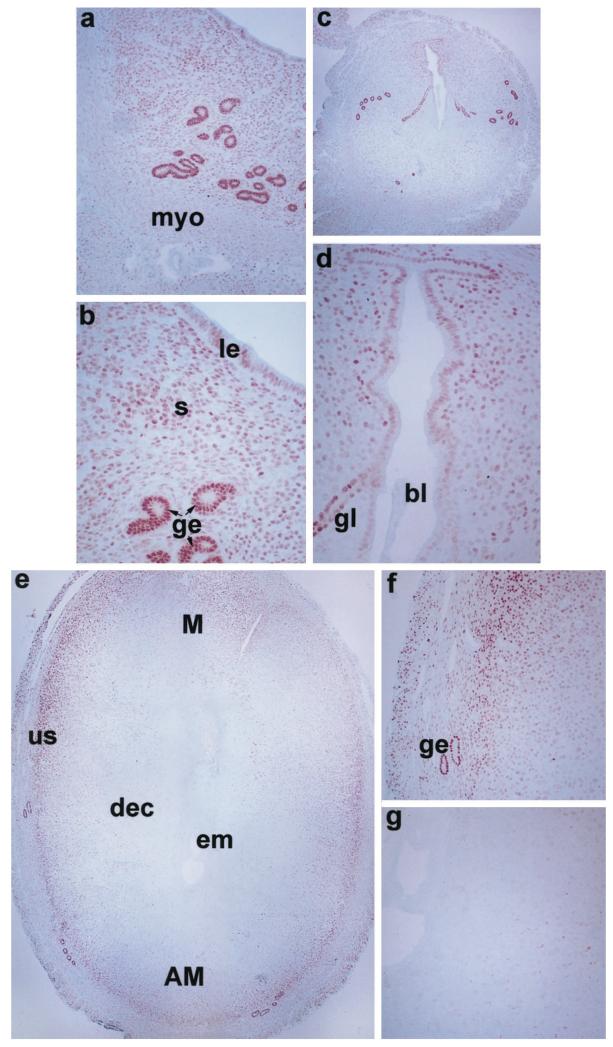
To examine uterine cellular localization of ER-α, ER-β, and PR mRNAs during the periimplantation period, in situ hybridization was performed (Figs. 2, 3, and 4, respectively). On days 1 and 2, distinct autoradiographic signals of ER-α mRNA were evident primarily in the luminal and glandular epithelia (Fig. 2, a–d). On day 3, accumulation of this mRNA was noted in the subluminal stromal cells in addition to its localization in epithelial cells (Fig. 2, e and f). On day 4, the pattern of accumulation was similar to that of day 3, but the signals were more widespread and intense (Fig. 2, g and h). The expression pattern did not alter at the site of blastocyst before implantation on this day. Following implantation on day 5, the autoradiographic signals increased further in the glandular and luminal epithelia, and in the subluminal epithelial stroma at the mesometrial pole. However, the signals were less intense in the decidualizing stroma at the site of implantation at the antimesometrial pole (Fig. 2, i and j). On days 6–8, autoradiographic signals were primarily detected in the secondary decidual zone (sdz) and signals were more intense at the mesometrial pole, the presumptive site of placentation (Fig. 2, k–p). ER-α mRNA accumulation was very low or undetectable at the primary decidual zone (pdz) immediately surrounding the implanting embryo. Furthermore, the undifferentiated stroma (us) just above the myometrium showed positive signals during days 7 and 8 of pregnancy (Fig. 2, m–p).
FIG. 2.
In situ hybridization of ER-α mRNA in the periimplantation mouse uterus. Brightfield and darkfield photomicrographs of representative longitudinal sections of uteri on days 1–5 (a–j) at 40×, and cross-sections of uteri on days 6–7 (k–n) at 40× and day 8 (o, p) at 20× are shown. le, Luminal epithelium; ge, glandular epithelium; myo, myometrium; s, stroma; bl, blastocyst; pdz, primary decidual zone; em, embryo; M, mesometrial pole; AM, antimesometrial pole; sdz, secondary decidual zone; us, undifferentiated stroma. These experiments were repeated three times with similar results. Sections hybridized with 35S-labeled sense probe did not show any positive signals (data not shown).
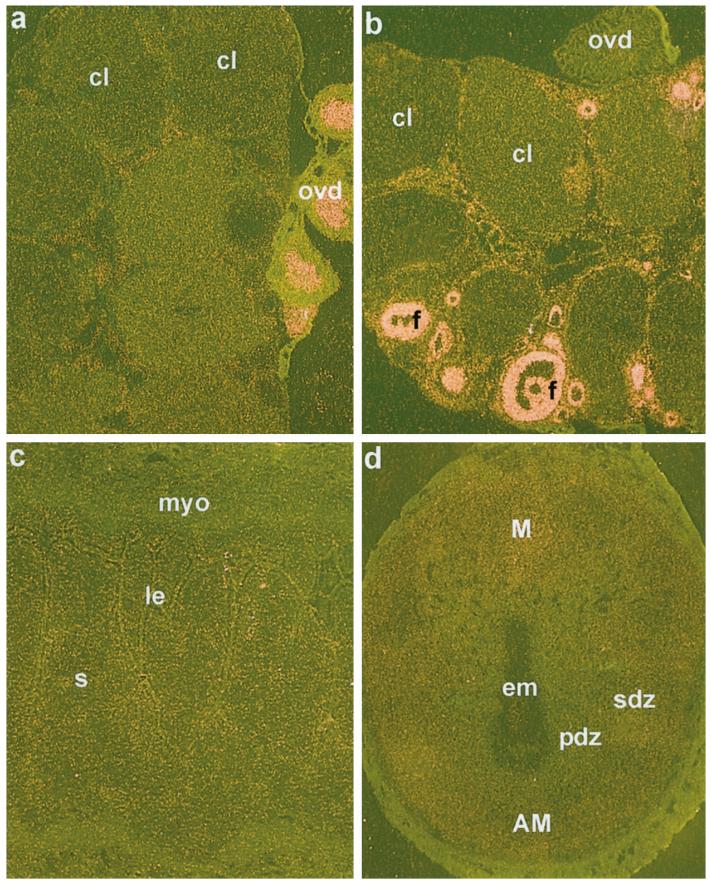
FIG. 3.
In situ hybridization of ER-α and ER-β mRNAs in the ovary and ER-β mRNA in the uterus on days 4 and 8 of pregnancy. Darkfield photomicrographs of representative ovary section hybridized with cRNA probes for ER-α (a), ER-β (b) are shown at 40×. Darkfield photomicrographs of representative uterine sections on days 4 (c) and 8 (d) of pregnancy hybridized with ER-β probe are shown at 40× and 25×, respectively. cl, Corpus luteum; ovd, oviduct; f, follicles; le, luminal epithelium; s, stroma; myo, myometrium; pdz, primary decidual zone; sdz, secondary decidual zone; em, embryo; M, mesometrial pole; AM, antimesometrial pole. These experiments were repeated three times with similar results. Sections hybridized with 35S-labeled sense probe did not show any positive signals (data not shown).
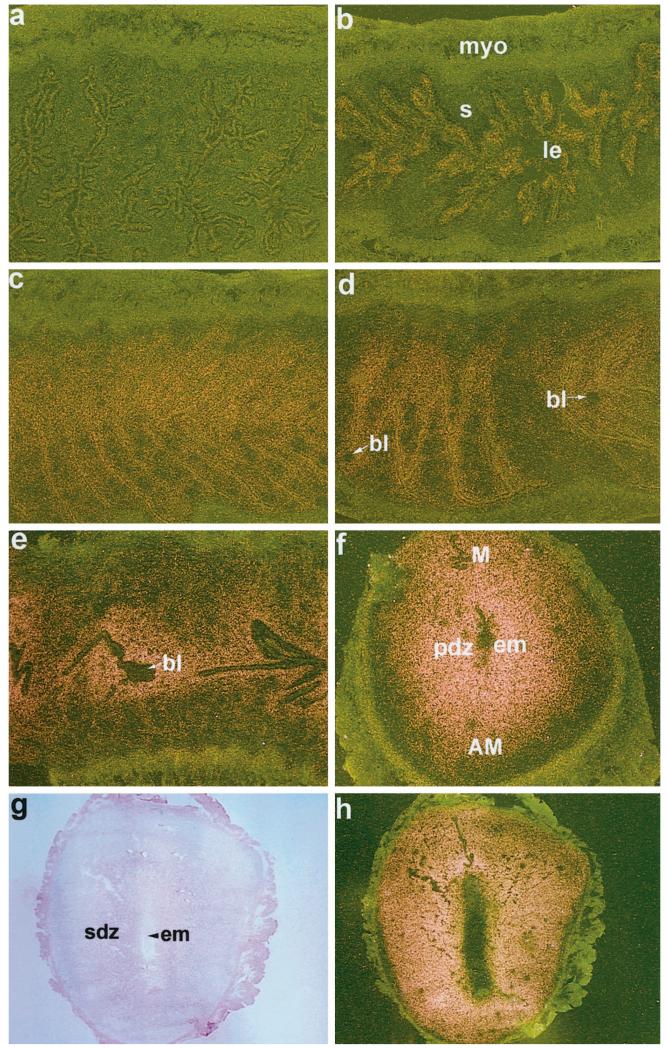
FIG. 4.
In situ hybridization of PR mRNA in the uterus on days 1–7 of pregnancy. Darkfield photomicrographs of representative longitudinal sections of uteri on days 1–5 (a–e) at 40 × and cross-section of uterus on day 6 (f) at 40× are shown. Brightfield and darkfield photomicrographs of uterine cross-section on day 7 (g and h, respectively) at 25× are shown. le, Luminal epithelium; s, stroma; myo, myometrium; bl, blastocyst; pdz, primary decidual zone; em, embryo; M, mesometrial pole; AM, antimesometrial pole; sdz, secondary decidual zone. These experiments were repeated three times with similar results. Sections hybridized with 35S-labeled sense probe did not show any positive signals (data not shown).
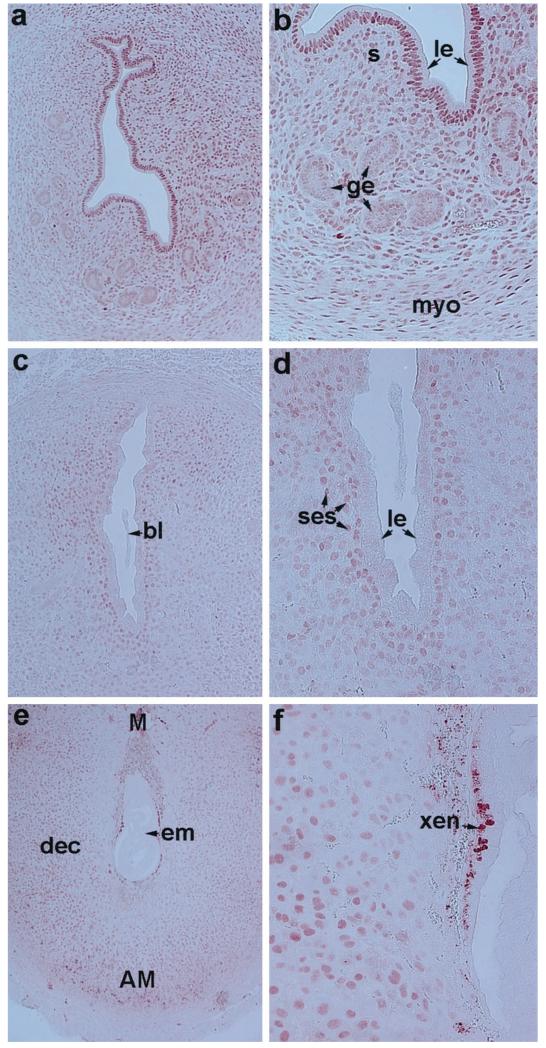
The expression of ER-α mRNA was very low in the ovary (Fig. 3a), although epithelial cells in the oviduct lumen attached to the ovarian sections showed strong signals for ER-α mRNA (Fig. 3a). On the contrary, as observed previously (22, 35, 42), the accumulation of ER-β mRNA was intense in ovarian follicles at all stages of development (Fig. 3b). However, ER-β mRNA was not detected in oviductal cells (Fig. 3b). Consistent with previous findings by us and others (24, 35), uterine expression of ER-β mRNA in general was extremely low during early pregnancy compared with ER-α mRNA (see Fig. 3, c and d, vs. Fig. 2).
The accumulation of PR mRNA was very low to undetectable on day 1 of pregnancy followed by low level accumulation in the epithelium on day 2 (Fig. 4, a and b). In contrast, the expression of PR mRNA was significantly upregulated in both the epithelium and the subepithelial stroma on days 3 and 4 (Fig. 4, c and d). The presence of blastocysts in the uterus before the onset of implantation did not alter the expression pattern (Fig. 4d). After implantation on day 5, the accumulation of mRNA was no longer detectable in the luminal epithelium, but was strikingly up-regulated in the subepithelial stroma at the site of implantation (Fig. 4e). With the progression of implantation on days 6 and 7 of pregnancy, the accumulation of PR mRNA increased dramatically throughout the decidual bed both at the mesometrial and the antimesometrial poles (Fig. 4, f–h). The expression pattern of PR mRNA on day 8 was similar to that of day 7 (data not shown).
Analysis of immunoreactive ER-α and PR in the periimplantation mouse uterus
To examine the cellular distribution of nuclear ER-α and PR in the pregnant mouse uterus, immunohistochemistry was performed during the preimplantation (day 4 morning) and postimplantation (days 5 and 8) period. Consistent with in situ hybridization results, immunoreactive ER-α protein was detected in same uterine cell types that expressed ER-α mRNA (Fig. 5). On day 4, accumulation was high in the glandular epithelium (Fig. 5, a and b). The luminal epithelium and the subepithelial stroma also showed distinct, albeit at low levels, nuclear staining. On day 5, the high levels of immunostaining persisted in the glandular epithelium. In addition, mostly the luminal epithelium and subluminal epithelial stroma at the mesometrial pole were positive for ER-α (Fig. 5, c and d). On day 8, ER-α was detected primarily in stromal cells adjacent to the luminal epithelium at the mesometrial pole, and also in the undifferentiated stromal cells underneath the myometrium both at the mesometrial and antimesometrial poles (Fig. 5, e–g). Furthermore, some glands situated within the undifferentiated stroma remained strongly positive for ER-α (Fig. 5f). Immunostaining was not detected in the implanting embryo or in decidualizing stromal cells immediately surrounding the embryo (Fig. 5g). With respect to PR, nuclear immunostaining was detected predominantly in the luminal epithelium and in the subepithelial stroma on day 4 of pregnancy (Fig. 6, a and b). In contrast to ER-α, nuclear accumulation of PR on this day in the gland was below the level of detection (Fig. 6, a and b). On day 5, immunostaining was primarily restricted to the subepithelial stroma at the sites of implantation (Fig. 6, c and d). The luminal and glandular epithelia were devoid of immunostaining. On day 8, immunoreactive PR was distributed throughout the decidual bed both at the mesometrial and the antimesometrial poles (Fig. 6, e and f). The staining for PR was absent in the embryo proper. However, the positive staining for PR was detected in the region of the extraembryonic endoderm (xen) (Fig. 6f).
FIG. 5.
Immunohistochemistry of ER-α in the uterus on days 4, 5, and 8 of pregnancy. Brightfield photomicrographs of representative uterine sections are shown. Red deposits indicate positive nuclear immunostaining for ER-α. a, Day 4, 100×; b, day 4, 200×; c, day 5, 40×; d, day 5, 200×; e, day 8, 40×; f and g, two areas of (e) at 100×. myo, Myometrium; le, luminal epithelium; ge, glandular epithelium; s, stroma; bl, blastocyst; em, embryo; gl, gland; dec, decidual cells; us, undifferentiated stroma; M, mesometrial pole; AM, antimesometrial pole. These experiments were repeated three times with similar results.
FIG. 6.
Immunohistochemistry of PR in the periimplantation mouse uterus. Brightfield photomicrographs of representative uterine sections are shown. Red deposits indicate positive nuclear immunostaining for PR. a, Day 4, 100×; b, day 4, 200×; c, day 5, 100×; d, day 5, 200×; (e) day 8, 40×; and (f) day 8, 200×. le, Luminal epithelium; ge, glandular epithelium; s, stroma; myo, myometrium; bl, blastocyst; em, embryo; ses, subepithelial stroma; dec, decidual cells; M, mesometrial pole; AM, antimesometrial pole; xen, extraembryonic endoderm. These experiments were repeated three times with similar results.
Western blot analysis of ER-α
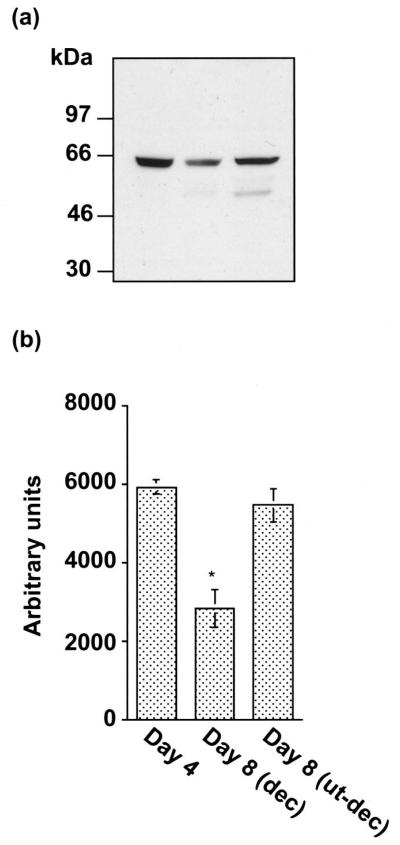
Western blotting experiments for ER-α were performed to determine the size and the levels of accumulation of this protein during pregnancy in the whole uterus on day 4 and the separated decidua and uterus minus decidua on day 8 of pregnancy (Fig. 7). As detected before (43), the same polyclonal antibody detected a major band (66 kDa) for full-length ER-α in uterine tissues (Fig. 7a). However, a minor band (~50 kDa) was also detected in the uterus on day 8 of pregnancy. The identity of this minor band is not known. The densitometric quantitation of the major band revealed that the expression of ER-α was down-regulated in the decidua on day 8 of pregnancy (Fig. 6b).
FIG. 7.
a, Western blot analysis of ER-α in extracts of whole uterus on day 4 of pregnancy, and the separated decidua and the uterus minus decidua on day 8 of pregnancy. Immunoblotting with the primary antibody for ER-α was performed as described in Materials and Methods. b, The quantitation of ER-α protein levels was examined by densitometric scanning (Personal Densitometer SI, Molecular Dynamics, Inc., Sunnyvale, CA) of the immunoblots. Results are mean ± SEM from three independent experiments. *, Significantly different from other groups (P < 0.05).
Discussion
Cell-specific localization of ER-α, ER-β, and PR by autoradiographic ligand binding and immunohistochemistry has been demonstrated in the uterus of various species (44–49). However, interactions between ER and PR with respect to ovarian steroid hormone effects in uterine biology during early pregnancy are poorly understood. This investigation demonstrates uterine expression of ER-α, ER-β, and PR with respect to periimplantation events in the mouse. Although ovarian steroids primarily direct uterine cell proliferation and differentiation in a spatiotemporal manner during the periimplantation period, the molecular mechanisms involved in these processes are poorly understood. In the mouse, implantation occurs at 2300 h on day 4 of pregnancy. An extremely low level of estrogen is required to initiate this process in a P4-primed uterus, suggesting that estrogen mediated effects in the uterus are locally amplified by various growth factors. This suggestion is consistent with the temporal and cell-specific expression of several growth factors and their receptors in the uterus and/or embryo at the time of implantation (reviewed in Refs. 10 and 12). The expression of ER-α in the uterine epithelium on day 1 of pregnancy under the influence of preovulatory estrogen is correlated with epithelial cell proliferation, whereas stromal cell expression of both ER-α and PR under the influence of rising P4 and preimplantation ovarian estrogen secretion on day 4 is associated with stromal cell proliferation. In contrast, expression of ER-α and PR in luminal epithelial cells is correlated with cessation of their proliferation because differentiation of these cells are essential for interactions with blastocyst trophectoderm for implantation.
Cross-tissue recombination of the uterine epithelium and stroma between the wild-type and PR null mice suggests that stromal cell PR is important in mediating the inhibitory effects of P4 on epithelial cell proliferation (50). A similar approach using the wild-type and ER-α mutant mice proposes that estrogen positively controls epithelial cell proliferation via stromal cell ER-α in a paracrine manner (51). Our observation of differential expression of ER-α and PR in the luminal and glandular epithelial cells on day 4 of pregnancy suggests that these two epithelial cell types behave differently as potential targets to P4 and estrogen with respect to mesenchymal interactions for preparation of the uterus to implantation (Fig. 5, a and b, vs. Fig. 6, a and b). This is consistent with the expression of amphiregulin (a P4 responsive gene) in both the luminal and glandular epithelia and leukemia inhibitory factor (estrogen-regulated gene) primarily in the glandular epithelium on day 4 of pregnancy (9, 52, 53). On day 5, the absence of epithelial PR with persistent ER-α may be responsible for down-regulation of epithelial expression of amphiregulin because estrogen antagonizes P4 induction of amphiregulin (9).
The general consensus is that estrogen induces progesterone receptors (PR) in the uterus (36, 54). Furthermore, studies in primates and cats have demonstrated that uterine expression of ER-α and PR is increased by estrogen, whereas P4 reduces their expression (55, 56). In addition, it has recently been demonstrated that estrogen has dual effects on the expression of PR, i.e. it decreases PR levels in the luminal epithelium but increases the levels of PR in the stroma and myometrium (57). The significance of our present observation of discoordinate expression of ER-α and PR in the luminal epithelium on day 1 of pregnancy under the influence of preovulatory estrogen and P4 is not clear. On this day, several estrogen-regulated genes have been shown to be induced (13, 52, 53, 58, 59), whereas progesterone-regulated genes are not inducible, probably because of the absence of PR. Thus, the heightened expression of ER-α, but not PR, in the epithelium suggests a differential regulation of PR. Furthermore, up-regulation of PR, as opposed to down-regulation of ER-α, in the decidua on days 7 and 8 of pregnancy may suggest that PR could be regulated by some non-ER-mediated pathway. This suggestion is in agreement with the recent demonstration of induction of decidualization in ER-α null mice with up-regulation of uterine PR (35). The down-regulation of the ER-α, together with very low levels ER-β expression, in the deciduum also suggests that ERs have limited functions in this process. This is consistent with the observation that progesterone, but not estrogen, is an absolute requirement for sustained decidualization in the rodent, although estrogen is essential for the initiation of the implantation process (reviewed in Ref. 6). In contrast, low levels of ER-α mRNA in the SDZ at the antimesometrial pole on day 8 are associated with diminishing cell proliferation.
Adequate levels of P4 and stromal expression of PR are necessary for optimal decidualization (60, 61). It has recently been implicated that Hoxa-10 is involved in stromal cell responsiveness to P4 (62). Hoxa-10 is expressed in the stroma on day 4 and in decidualizing stroma on day 5 of pregnancy, suggesting that this gene is important for stromal cell proliferation and subsequent decidualization. Indeed, the null mutation of this gene causes decidualization defect in the mouse (63, 64). Recent results also show that P4-dependent stromal cell proliferation is compromised in Hoxa-10 (−/−) uteri, whereas E2-dependent epithelial cell proliferation is normal (62). However, reduced stromal responsiveness to P4 and decidualization defect in Hoxa-10 (−/−) mice are not the result of reduced uterine expression of PR, suggesting that Hoxa-10’s role in uterine functions is downstream of PR. P4 effects via PR is an absolute requirement for sustained decidualization in the rodent (reviewed in Ref. 6) because this response is prevented by blocking P4 actions by neutralizing antibody or PR antagonists (60, 65, 66). The failure of decidualization in PR null mice is also consistent with these observations (33).
In conclusion, the results of this investigation have demonstrated a differential expression pattern of nuclear ER-α, ER-β, and PR in the mouse uterus during early pregnancy and provide insights to our understanding regarding the roles of these receptors in uterine biology during implantation and decidualization.
Footnotes
This research was supported in part by grants from the National Institute of Environmental Health Sciences (ES-07814 to Sa.K.D.), the National Institute of Child Health and Human Development (HD-12304 and HD-29968 to Su.K.D. and HD-35114 to B.C.P.), and the core support from NICHD center grants (HD-02528 and HD-33994).
References
- 1.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after estrogen treatment: an autoradiographic study. J Endocrinol. 1973;56:133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 2.Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-Myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–1690. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 3.Martin L, Das RM, Finn CA. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–554. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 4.Psychoyos A. Endocrine control of egg implantation. In: Greep RO, Astwood EG, Geiger SR, editors. Handbook of Physiology. American Physiological Society; Washington DC: 1973. pp. 187–215. [Google Scholar]
- 5.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey SK. Implantation. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery, and Technology. Lippincott-Raven; New York: 1996. pp. 421–434. [Google Scholar]
- 7.Orimo A, Inoue S, Ikeda K, Noji S, Muramatsu M. Molecular cloning, structure, and expression of mouse estrogen-responsive finger protein Efp. J Biol Chem. 1995;270:24406–24413. doi: 10.1074/jbc.270.41.24406. [DOI] [PubMed] [Google Scholar]
- 8.Das SK, Wang X-N, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 9.Das SK, Chakraborty I, Paria BC, Wang X-N, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 10.Das SK, Das N, Wang J, Lim H, Schryver B, Plowman GD, Dey SK. Expression of betacellulin and epiregulin genes in the mouse uterus temporally by the blastocyst solely at the site of its apposition is coincident with the “window” of implantation. Dev Biol. 1997;190:178–190. doi: 10.1006/dbio.1997.8694. [DOI] [PubMed] [Google Scholar]
- 11.Lim H, Dey SK, Das SK. Differential expression of the erbB2 gene in the periimplantation mouse uterus: potential mediator of signaling by epidermal growth factor (EGF)-like growth factors. Endocrinology. 1997;138:1328–1337. doi: 10.1210/endo.138.3.4991. [DOI] [PubMed] [Google Scholar]
- 12.Lim H, Das SK, Dey SK. erbB genes in the mouse uterus: cell-specific signaling by EGF family of growth factors during implantation. Dev Biol. 1998;204:97–110. doi: 10.1006/dbio.1998.9072. [DOI] [PubMed] [Google Scholar]
- 13.Das SK, Tsukamura H, Paria BC, Andrews GK, Dey SK. Differential expression of epidermal growth factor receptor (EGF-R) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology. 1994;134:971–81. doi: 10.1210/endo.134.2.7507841. [DOI] [PubMed] [Google Scholar]
- 14.Wang XN, Das SK, Damm D, Klagsbrun M, Abraham JA, Dey SK. Differential regulation of heparin-binding EGF-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology. 1994;135:1264–1271. doi: 10.1210/endo.135.3.8070372. [DOI] [PubMed] [Google Scholar]
- 15.Kapur S, Tamada H, Dey SK, Andrews GK. Expression of insulin-like growth factor-1 (IGF-1) and its receptor in the periimplantation mouse uterus, and cell-specific regulation of IGF-1 gene expression by estradiol and progesterone. Biol Reprod. 1992;46:208–219. doi: 10.1095/biolreprod46.2.208. [DOI] [PubMed] [Google Scholar]
- 16.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green S, Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988;4:309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- 18.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/ thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 19.Beato M, Herrlich P, Schultz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 20.Kuiper GG, Gustafsson JA. The novel estrogen receptor-β subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 22.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor. Mol Endocrinol. 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- 23.Petterson K, Grandien K, Kuiper GGJM, Gustafsson JA. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 24.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 25.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson J-A, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor α. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrader WT, O’Malley BW. Progesterone-binding components of chick oviduct. IV. Characterization of purified subunits. J Biol Chem. 1972;247:51–59. [PubMed] [Google Scholar]
- 30.Conneely OM, Maxwell BL, Toft DO, Schrader WT, O’Malley BW. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987;149:493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- 31.Kastner P, Krust A, Turcotte B, Strupp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993;7:1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- 33.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 34.Curtis SW, Clark J, Myers P, Korach KS. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor α knockout mouse uterus. Proc Natl Acad Sci USA. 1999;96:3646–3651. doi: 10.1073/pnas.96.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paria BC, Tan J, Lubahn DB, Dey SK, Das SK. Uterine decidual response occurs in estrogen receptor-α deficient mice. Endocrinology. 1999;140:2704–2710. doi: 10.1210/endo.140.6.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das SK, Tan J, Johnson DC, Dey SK. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen and xenoestrogen. Endocrinology. 1998;139:2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han JH, Stratowa C, Rutter WJ. Isolation of full-length putative rat lysophospholipase cDNA using improved methods for mRNA isolation and cDNA cloning. Biochemistry. 1987;26:1617–1625. doi: 10.1021/bi00380a020. [DOI] [PubMed] [Google Scholar]
- 38.Das SK, Flanders KC, Andrews GK, Dey SK. Expression of transforming growth factor-β isoforms (β2 and β3) in the mouse uterus: analysis of the periimplantation period and effects of ovarian steroids. Endocrinology. 1992;130:3459–3466. doi: 10.1210/endo.130.6.1375903. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 40.Tamada H, Das SK, Andrews GK, Dey SK. Cell-type-specific expression of transforming growth factor-α in the mouse uterus during the periimplantation period. Biol Reprod. 1991;45:365–372. doi: 10.1095/biolreprod45.2.365. [DOI] [PubMed] [Google Scholar]
- 41.Friend KE, Resnick EM, Ang LW, Shupnik MA. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol Cell Endocrinol. 1997;131:147–155. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 42.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchner NA, Garlick C, Ben-Jonathan N. Cellular distribution and gene regulation of estrogen receptors α and β in the rat pituitary gland. Endocrinology. 1998;139:3976–3983. doi: 10.1210/endo.139.9.6181. [DOI] [PubMed] [Google Scholar]
- 44.Hiroi H, Inoue S, Watanabe T, Goto W, Orimo A, Momoeda M, Tsutsumi O, Taketani Y, Muramatsu M. Differential immunolocalization of estrogen receptor α and β in rat ovary and uterus. J Mol Endocrinol. 1999;22:37–44. doi: 10.1677/jme.0.0220037. [DOI] [PubMed] [Google Scholar]
- 45.Hild-Petito S, Verhage HG, Fazleabas AT. Immunocytochemical localization of estrogen and progestin receptors in the baboon (Papio anubis) uterus during implantation and pregnancy. Endocrinology. 1992;130:2343–2353. doi: 10.1210/endo.130.4.1372241. [DOI] [PubMed] [Google Scholar]
- 46.Okulicz WC, Scarrell R. Estrogen receptor alpha and progesterone receptor in the rhesus endometrium during the late secretory phase and menses. Proc Soc Exp Biol Med. 1998;218:316–321. doi: 10.3181/00379727-218-44298. [DOI] [PubMed] [Google Scholar]
- 47.Brenner RM, McClellan MC, West NB, Novy MJ, Haluska GJ, Sternfeld MD. Estrogen and progestin receptors in the macaque endometrium. Ann NY Acad Sci. 1991;622:149–166. doi: 10.1111/j.1749-6632.1991.tb37859.x. [DOI] [PubMed] [Google Scholar]
- 48.Li W, Boomsma RA, Verhage HG. Immunocytochemical analysis of estrogen and progestin receptors in uteri of steroid-treated and pregnant cat. Biol Reprod. 1992;47:1073–1081. doi: 10.1095/biolreprod47.6.1073. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita S, Newbold RR, McLachlan JA, Korach KS. Developmental pattern of estrogen receptor expression in female mouse genital tracts. Endocrinology. 1989;125:2888–2896. doi: 10.1210/endo-125-6-2888. [DOI] [PubMed] [Google Scholar]
- 50.Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, Cunha GR. Stormal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- 51.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen YC, Leder P. Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proc Natl Acad Sci USA. 1992;89:8240–8244. doi: 10.1073/pnas.89.17.8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci USA. 1991;88:11408–11412. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 55.West N, Brenner R. Progesterone mediated suppression of estradiol receptors in cynomolgus macaque cervix, endometrium and oviduct during sequential estradiol progesterone treatment. J Steroid Biochem. 1985;22:29–37. doi: 10.1016/0022-4731(85)90138-4. [DOI] [PubMed] [Google Scholar]
- 56.Verhage H, Boomsma R, Murray M, Jaffe R. Subcellular compartmentalization of the progesterone receptor in cat uteri following the acute administration of progesterone. Biol Reprod. 1983;28:545–550. doi: 10.1095/biolreprod28.3.545. [DOI] [PubMed] [Google Scholar]
- 57.Tibbetts TA, Mendoza-Meneses M, O’Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod. 1998;59:1143–1152. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- 58.McMaster MT, Teng CT, Dey SK, Andrews GK. Lactoferrin in the mouse uterus: analysis of the preimplantation period and regulation by ovarian steroids. Mol Endocrinol. 1991;5:101–111. doi: 10.1210/mend.6.1.1738363. [DOI] [PubMed] [Google Scholar]
- 59.Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Pimental RA, Wegner CC, Dey SK, Carson DD. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995;136:3639–3647. doi: 10.1210/endo.136.8.7628404. [DOI] [PubMed] [Google Scholar]
- 60.Parandoosh Z, Crombie DL, Tetzke TA, Hayes JS, Heap RB, Wang MW. Progesterone and oestrogen receptors in the decidualized mouse uterus and effects of different types of anti-progesterone treatment. J Reprod Fertil. 1995;105:215–220. doi: 10.1530/jrf.0.1050215. [DOI] [PubMed] [Google Scholar]
- 61.Villee CA, Armstrong EG, Jr, Talley DJ, Hoshiai H. Decidual cell function: role of steroid hormones and their receptors. In: Glasser SR, Bullock DW, editors. Cellular and Molecular Aspects of Implantation. Plenum; New York: 1981. pp. 241–252. [Google Scholar]
- 62.Lim H, Ma L, Ma W, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–1017. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- 63.Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanism of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 64.Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in Mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–154. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- 65.Crombie DL, Mukherjee R, McDonnell DP, Hayes JS, Wang MW. Creatine kinase activity as an indicator of unopposed estrogen action in the mouse uterus associated with anti-progesterone treatment. J Steroid Biochem Mol Biol. 1994;49:123–129. doi: 10.1016/0960-0760(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z, Funk C, Glasser SR, Mullholland J. Progesterone regulation of heparin-binding epidermal growth factor-like growth factor gene expression during sensitization and decidualization in the rat uterus: effects of antiprogestin, ZK 98,299. Endocrinology. 1994;135:1256–1263. doi: 10.1210/endo.135.3.8070371. [DOI] [PubMed] [Google Scholar]