Proteome analysis of two types of lipid droplets in Dunaliella bardawil uncovers enzymes involved in β-carotene biosynthesis and lipid droplet biogenesis.
Abstract
The halotolerant green alga Dunaliella bardawil is unique in that it accumulates under stress two types of lipid droplets: cytoplasmatic lipid droplets (CLD) and β-carotene-rich (βC) plastoglobuli. Recently, we isolated and analyzed the lipid and pigment compositions of these lipid droplets. Here, we describe their proteome analysis. A contamination filter and an enrichment filter were utilized to define core proteins. A proteome database of Dunaliella salina/D. bardawil was constructed to aid the identification of lipid droplet proteins. A total of 124 and 42 core proteins were identified in βC-plastoglobuli and CLD, respectively, with only eight common proteins. Dunaliella spp. CLD resemble cytoplasmic droplets from Chlamydomonas reinhardtii and contain major lipid droplet-associated protein and enzymes involved in lipid and sterol metabolism. The βC-plastoglobuli proteome resembles the C. reinhardtii eyespot and Arabidopsis (Arabidopsis thaliana) plastoglobule proteomes and contains carotene-globule-associated protein, plastid-lipid-associated protein-fibrillins, SOUL heme-binding proteins, phytyl ester synthases, β-carotene biosynthesis enzymes, and proteins involved in membrane remodeling/lipid droplet biogenesis: VESICLE-INDUCING PLASTID PROTEIN1, synaptotagmin, and the eyespot assembly proteins EYE3 and SOUL3. Based on these and previous results, we propose models for the biogenesis of βC-plastoglobuli and the biosynthesis of β-carotene within βC-plastoglobuli and hypothesize that βC-plastoglobuli evolved from eyespot lipid droplets.
Lipid droplets are the least characterized organelles in both mammalian and plant cells, and they were considered until a few years ago as passive storage compartments for triglycerides (TAG), sterol esters, and some pigments. However, recent studies have shown that they have diverse metabolic functions (Goodman, 2008; Farese and Walther, 2009; Murphy, 2012). Proteomic analyses in plants and some microalgae have shown that lipid droplets in the cytoplasm and in the chloroplast contain a large diversity of proteins including both structural proteins and many enzymes, indicating that they take an active metabolic role in the synthesis, degradation, and mobilization of glycerolipids, sterols, and pigments as well as in regulatory functions that have not yet been clarified (Schmidt et al., 2006; Ytterberg et al., 2006; Nguyen et al., 2011; Lundquist et al., 2012b; Eugeni Piller et al., 2014). A major limitation for determining the proteomes of lipid droplets, particularly in microalgae, is the purity and the homogeneity of the preparation. Green microalgae, for example, may contain three distinct pools of lipid droplets in one cell: the cytoplasmatic lipid droplets (CLD), the major neutral lipid pool, which are induced under stress conditions such as nitrogen limitation or at the stationary growth phase (Wang et al., 2009); plastoglobules, which are smaller lipid droplets within the chloroplast that have been shown to change in size and number under stress conditions and seem to be involved in stress resistance, metabolite transport, and the regulation of photosynthetic electron transport (Bréhélin et al., 2007; Besagni and Kessler, 2013); and the eyespot structure, part of the visual system in green algae, composed of one or several layers of lipid droplets, characterized by their orange color resulting from a high content of β-carotene (Kreimer, 2009). Disruption of microalgal cells, which is required for the isolation of the lipid droplets, usually involves harsh treatments such as sonication, mixing with glass beads, or use of a French press that breaks not only the cell membrane but also the chloroplast. Therefore, it is almost impossible to separate the different lipid droplet classes by the subsequent density gradient centrifugation, making it difficult to assign the origin of identified proteins. The other major difficulty is contamination by proteins released during cell lysis and fractionation, which associate and copurify with lipid droplets. These include cytoplasmic, chloroplastic, and mitochondrial proteins (Moellering and Benning, 2010; James et al., 2011; Nguyen et al., 2011; Nojima et al., 2013). Purification of isolated lipid droplets from loosely associated proteins is possible by treatments with detergents, high salt, and chaotropic agents (Jolivet et al., 2004; Nguyen et al., 2011); however, the danger in such treatments is that they also remove native loosely associated proteins from the lipid droplets.
In this work, we tried to circumvent these problems by choosing a special algal species that is suitable for controlled cell lysis and fractionation and by utilizing two different contamination filters.
The alga we selected, Dunaliella bardawil, is unique in that it accumulates large amounts of two different types of lipid droplets, CLD and β-carotene-rich (βC) plastoglobuli, under stress conditions (Davidi et al., 2014). The lack of a rigid cell wall in this alga allows lysis of the plasma membrane by a gentle osmotic shock, releasing CLD but leaving the chloroplast intact (Katz et al., 1995). This enables the recovery of large quantities of the two types of highly purified lipid droplets by differential lysis. In a recent study, we described the isolation and lipid compositions of these two lipid pools and showed that they have similar TAG compositions but different lipid-associated major proteins (Davidi et al., 2014).
The high nutritional and pharmacological value of β-carotene for humans has promoted intensive research aimed to clarify its biosynthesis and regulation in plants and also led to attempts to increase β-carotene levels by genetic manipulations in crop plants such as tomato (Solanum lycopersicum; Rosati et al., 2000; Giorio et al., 2007) or by the creation of Golden rice (Oryza sativa; Ye et al., 2000). However, the capacity of plants to store β-carotene is limited, and in this respect, D. bardawil is an exceptional example of an organism that can accumulate large amounts of this pigment, up to 10% of its dry weight. This is enabled by the compartmentation and storage of this lipophilic pigment in specialized plastoglobules. Also, the unusual isomeric composition, consisting of around 50% 9-cis- and 50% all-trans-isomers (Ben-Amotz et al., 1982, 1988), is probably of major importance in this respect, due to the better solubility of the cis-isomer in lipids, which enables the storage of high concentrations exceeding 50% of the lipid droplets. The localization of carotenoid biosynthesis in plants appears to be tissue specific: in green tissues, it takes place in chloroplast membranes, probably within the inner chloroplast envelope membrane (Joyard et al., 2009), whereas in carotenoid-accumulating fruits, such as tomato or bell pepper (Capsicum annuum), it takes place in specialized organelles derived from chromoplasts (Siddique et al., 2006; Barsan et al., 2010). In green microalgae, there are at least two types of carotenoid-accumulating organelles: CLD and eyespot. Algae such as Haematococcus pluvialis and Chlorella zofigiensis accumulate carotenoids within CLD. In H. pluvialis, the major pigment, astaxanthin, is synthesized initially in the chloroplast as β-carotene and then transferred to CLD, where it is oxidized and hydroxylated to astaxanthin (Grünewald et al., 2001). The eyespot, which is composed of one or several layers of small β-carotene-containing lipid droplets, has been shown by proteomic analysis to include part of the β-carotene biosynthesis enzymes, indicating that β-carotene is probably synthesized within these lipid droplets (Schmidt et al., 2006). Similarly, plant chromoplasts also contain carotenoid biosynthesis enzymes (Schmidt et al., 2006; Ytterberg et al., 2006; Schapire et al., 2009). D. bardawil and Dunaliella salina are unique in that they accumulate large amounts of β-carotene within βC-plastoglobuli. A special focus in this work was the identification of the β-carotene biosynthesis machinery in D. bardawil. It is not known if the synthesis takes place inside the lipid βC-plastoglobuli or in chloroplast envelope membranes. Since D. bardawil also contains β-carotene and xanthophylls at the photosynthetic system, it is interesting to know whether the β-carotene that accumulates under stress in βC-plastoglobuli is produced by the constitutive carotenoid biosynthetic pathway or by a different stress-induced enzymatic system.
RESULTS AND DISCUSSION
Protein Extraction
In this work, we introduced two filters for contamination to analyze the proteomes of these lipid droplets: one involves the addition of synthetic lipid dispersion to control cells during cell fractionation (Davidi et al., 2012). The rationale for this filter was that contaminating proteins released from other organelles could be identified in the isolated synthetic lipid droplets (contamination filter). We also determined the proteome of an isolated thylakoid membrane preparation and compared the enrichment of the protein of lipid droplets relative to the thylakoid membrane proteins (enrichment filter; Lundquist et al., 2012b).
Isolation of two types of lipid droplets from D. bardawil was performed as described in our recent article (Davidi et al., 2014). In brief, cells deprived from nitrogen for 2 d were lysed by an osmotic shock and separated to CLD and chloroplasts. Chloroplasts were washed and lysed by sonication to release the βC-plastoglobuli. Three independent preparations of CLD and βC-plastoglobuli (two samples of each, a total of six repeats) were purified by Suc density gradient centrifugation. The purity of the two preparations was verified by the absence of chlorophyll, by negative western analysis tests for chloroplast major proteins, and by the lack of cross-contaminations by the different major lipid-associated proteins or by β-carotene (Davidi et al., 2014). Proteins were precipitated in 80% (v/v) acetone at −20°C and suspended and extracted in 50 mm ammonium bicarbonate (AmBc), and the insoluble pellet was reextracted with 1% SDS. For thylakoid proteins, chloroplast membranes of noninduced cells were washed several times, lipids were extracted by acetone precipitation, and the pellet was extracted with SDS as above. All protein extracts were digested with trypsin. The samples containing SDS were cleaned using detergent-removal columns (Pierce). The digested peptides were analyzed by nanoliquid chromatography-tandem mass spectrometry. Semiquantitative comparisons were conducted by spectral counting.
In order to analyze the lipid droplet proteomes, we constructed a proteome database of D. salina CCAP 19/18/ D. bardawil, which was based on protein, EST, and complementary DNA (cDNA) sequences available at the National Center for Biotechnology Information (NCBI) and from the Joint Genome Institute (JGI) D. salina sequencing program (provided by Jon Magnuson, John Cushman, and Jurgen Polle). The D. salina/D. bardawil proteome database comprises 83,694 proteins (more than 50 amino acids) with 20,068 annotated proteins (average length of 318 amino acids). Functional annotation of the proteins was achieved by running all sequences in the Blast2GO program.
A total of 570 proteins were identified in all our lipid droplet samples. Proteins with at least two peptides in at least two biological repeats were analyzed. A total of 305 and 92 unique proteins were identified in βC-plastoglobuli and CLD fractions, respectively, of which 154 and 13 were contained in both the AmBc and SDS extracts (see scheme in Fig. 1).
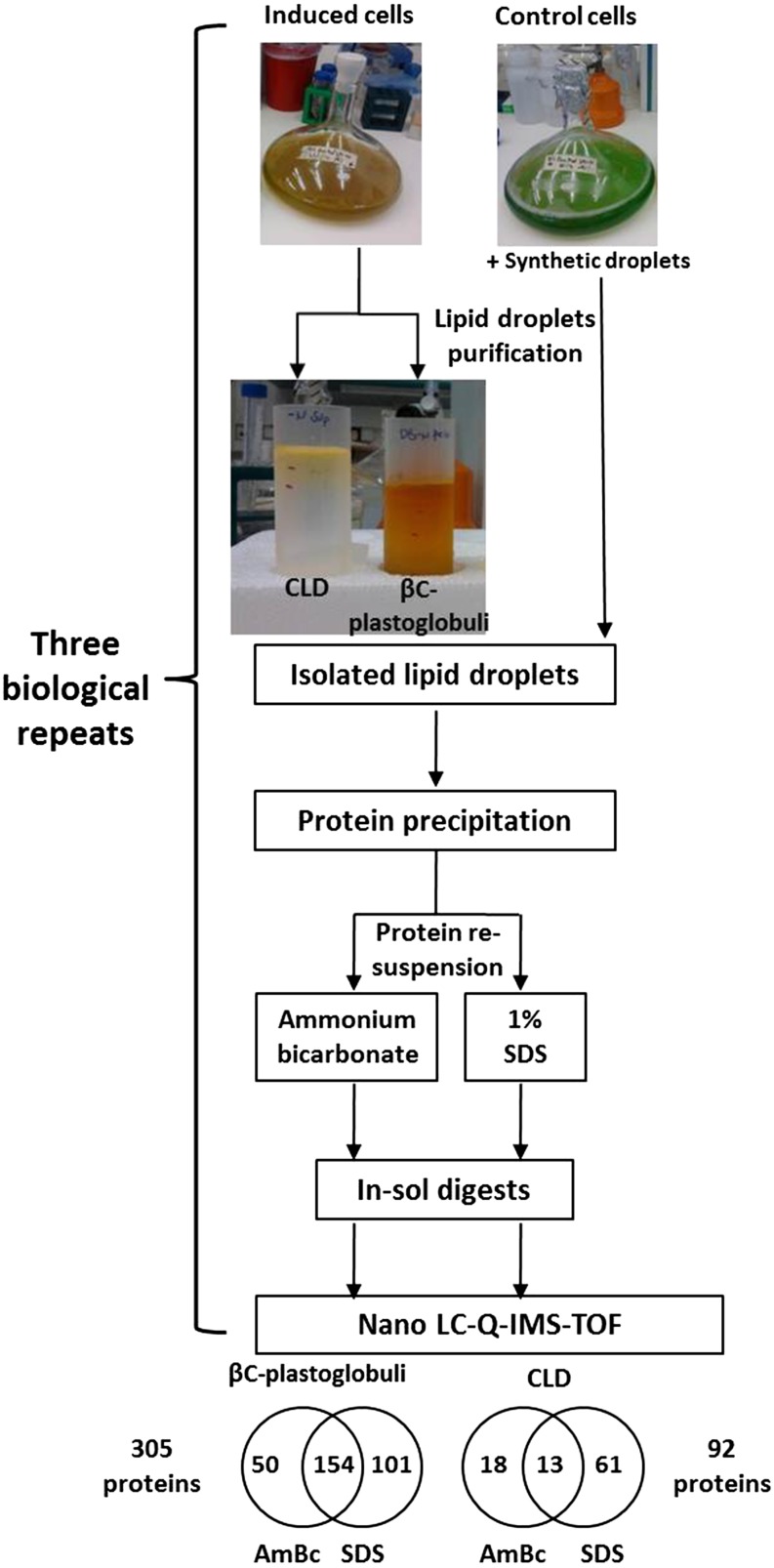
Figure 1.
Scheme of the isolation and identification of the CLD and βC-plastoglobuli proteomes. Lipid droplets were prepared from D. bardawil cells cultured without nitrogen for 2 d and from nitrogen-sufficient cells supplemented with synthetic globules. Thylakoids were isolated from nitrogen-sufficient cells. Proteins were precipitated in acetone and resuspend first in 50 mm AmBc and next in 1% SDS. Each sample was digested with trypsin. Peptide digests were analyzed by nanoliquid chromatography quadrupole ion mobility time-of-flight mass spectrometry (LC-Q-IMS-TOF). A total of 570 proteins were identified in both lipid pools. Proteins with at least two peptides in at least two biological repeats were analyzed. A total of 305 and 92 unique proteins were identified in βC-plastoglobuli and CLD, respectively, 154 and 13 of which were identified by both experimental methods in βC-plastoglobuli and CLD, respectively. In-sol, In solution.
In order to identify contaminating proteins, we added a novel control based on supplementation of synthetic lipid droplets to control cells during the preparation: control D. bardawil cells (nitrogen sufficient) were lysed as described above in the presence of added triolein synthetic lipid droplets. These lipid droplets were isolated by Suc density gradient centrifugation; their proteins were extracted and analyzed as above. This protein preparation served as a contamination filter.
The proteins of βC-plastoglobuli and CLD fractions were compared with triolein synthetic droplet proteins. Common proteins also contained in the latter fraction yielding a similar number of peptides were eliminated as contaminants. The protein abundance of all residual proteins in all lipid droplet fractions was next compared with the protein abundance of the corresponding proteins in the chloroplast membrane proteome (Thylakoids). For βC-plastoglobuli, proteins with enrichment ratios of less than 10 (lipid droplet/Thylakoids < 10) and having less than four identified peptides, or, alternatively, having less than 5-fold enrichment and 10 peptides, were excluded. For CLD proteins, proteins having enrichment values of less than 10 and at least two identified peptides were excluded. These criteria are more stringent than the criteria set previously for defining the core plastoglobule proteome (Lundquist et al., 2012b). This filter removed many chloroplast-derived proteins from the plastoglobule proteome as well as a lot of enzymes such as Fru-bisP aldolase, previously identified as a plastoglobule protein but later removed by the more stringent criteria set for core proteins (Lundquist et al., 2012b). Another indication that the filters are effective is the fact that the number of common proteins in the two proteomes decreased from 38 to eight after applying the filtration. The risk of applying such stringent filters is that it may exclude minor potentially important proteins such as protein kinases or proteins involved in signaling. A total of 124 and 42 proteins in the βC-plastoglobuli and CLD fractions passed both filters (see scheme in Fig. 2; all core sequences are available in Supplemental Fig. S1 [CLD] and Supplemental Fig. S2 [βC-plastoglobuli]). Very few chloroplast-derived proteins still escaped the filter, such as ferredoxins and a chloroplast precursor in the CLD list, for unknown reasons. Ignoring these few proteins as well as predicted proteins or proteins with no assigned function resulted in a list of 84 and 28 proteins in the βC-plastoglobuli and CLD proteomes, respectively. The finding that only eight common proteins were identified in both fractions (Fig. 2) suggests that the two proteomes are distinct and have different origins.
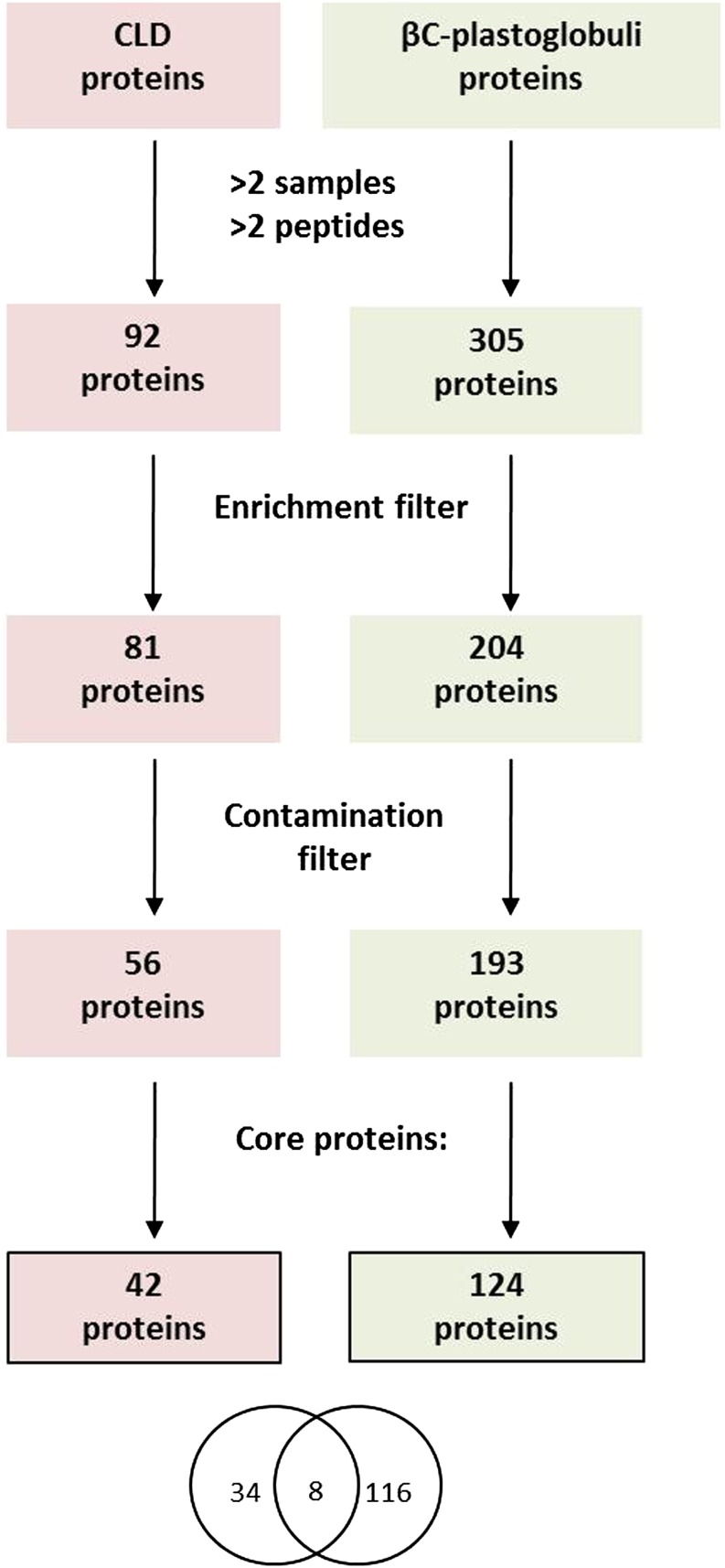
Figure 2.
Definition of core proteomes. A total of 305 and 92 unique proteins were identified in βC-plastoglobuli and CLD, respectively. These proteins were passed through two sequential filters: the enrichment filter and the contamination filter. The enrichment filter excluded protein with a lipid droplet to thylakoid ratio less than 2. The contamination filter excluded proteins appearing on the synthetic globule proteome. Totals of 193 and 56 proteins in βC-plastoglobuli and CLD, respectively, passed both filters. Core proteins were defined as having a lipid droplet to thylakoid ratio greater than 10 and more than two peptides or a lipid droplet to thylakoid ratio greater than 4 and more than nine peptides. The final core proteomes comprise 42 and 124 core proteins in CLD and βC-plastoglobuli, respectively.
Core CLD and βC-Plastoglobuli Proteomes
Functional category diagrams of the two lipid droplet proteomes (Fig. 3) show that they differ in their major functional categories: in CLD, the major category is lipid-metabolizing enzymes, whereas in βC-plastoglobuli, it is secondary metabolism enzymes.
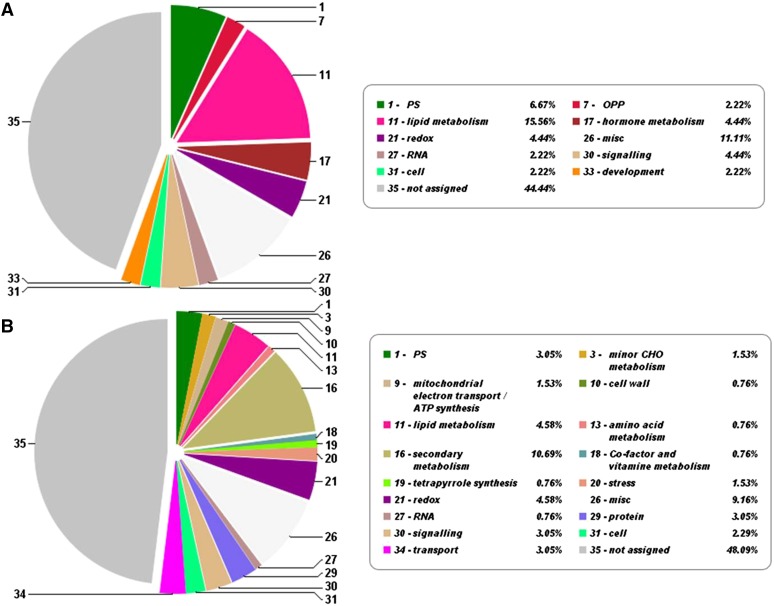
Figure 3.
Distribution of protein functional categories. Functional categories are shown for CLD (A) and βC-plastoglobuli (B) core proteomes according to Mercator analysis. CHO, Carbohydrate; misc, miscellaneous; OPP, oxidative pentose phosphate; PS, photosynthesis; redox, oxidation reduction. Numbers represent the protein category according to Mercator, and percentage values represent the number of proteins in this category as a percentage of total proteins.
Tables I and II summarize the predicted proteins identified in the CLD and βC-plastoglobuli fractions, respectively. Tables III and IV show comparisons between the proteomes of D. bardawil CLD and βC-plastoglobuli and between the proteomes of two Chlamydomonas reinhardtii cytoplasmic droplets (Moellering and Benning, 2010; Nguyen et al., 2011), Arabidopsis (Arabidopsis thaliana) plastoglobule full and core proteomes (Lundquist et al., 2012b), C. reinhardtii eyespot (Schmidt et al., 2006), and bell pepper chromoplasts (Siddique et al., 2006).
Table I. CLD proteome.
| Name/Functional Category | Sequence Description | Mass | Sequence Length | No. of Peptides | Fold Change |
|---|---|---|---|---|---|
| kD | |||||
| Structural | |||||
| AEW43285.1 | MLDP | 31 | 282 | 11 | 4,866,667 |
| isotig01487.2 | SOUL heme-binding protein | 43 | 384 | 7 | 8 |
| isotig16296 | SOUL heme-binding protein | 44 | 393 | 22 | 87 |
| isotig01487.2 | SOUL heme-binding protein | 43 | 384 | 7 | 8 |
| Membrane remodeling | |||||
| CL1Contig7649 | Vesicle-inducing protein in plastids (VIPP1) | 36 | 330 | 8 | 7 |
| Lipid metabolism | |||||
| isotig13730 | Diacylglyceryl-trimethylhomo-Ser synthesis protein | 79 | 694 | 12 | 1,360,000 |
| F62NEKU02F32Z3 | Cyclopropane-fatty acyl-phospholipid synthase | 17 | 154 | 6 | 730,000 |
| isotig07478 | Acetyl-CoA synthetase-like protein | 73 | 662 | 14 | 1,950,000 |
| isotig15711 | Acetyl-CoA synthetase-like protein | 76 | 690 | 6 | 610,000 |
| isotig06859 | Acyl carrier protein (ACP) | 16 | 150 | 4 | 41 |
| CL8760Contig1 | Glycerophosphodiester phosphodiesterase | 42 | 377 | 5 | 570,000 |
| isotig02168.1 | Acylglycerol lipase | 49 | 450 | 2 | 200,000 |
| Sterol biosynthesis | |||||
| isotig07413 | Cycloartenol synthase | 88 | 780 | 20 | 1,666,667 |
| isotig02727 | Squalene epoxidase | 58 | 532 | 7 | 520,000 |
| isotig06167.2 | Oxysterol-binding family protein | 48 | 426 | 2 | 625,000 |
| isotig14763 | NAD-dependent steroid dehydrogenase | 40 | 371 | 2 | 200,000 |
| Carotenoid metabolism | |||||
| isotig08132.2 | Retinol dehydrogenase12 | 41 | 373 | 5 | 690,000 |
| isotig14327 | Retinol dehydrogenase14 | 34 | 309 | 9 | 1,130,000 |
| Quinone metabolism | |||||
| CL1Contig605 | NADPH:quinone reductase zinc-dependent oxidoreductase | 39 | 364 | 6 | 590,000 |
| Glycolysis | |||||
| isotig00097.1 | Glyceraldehyde-3-phosphate dehydrogenase | 42 | 389 | 9 | 29 |
| Amino acid metabolism | |||||
| isotig16293 | Saccharopine dehydrogenase | 47 | 436 | 17 | 98 |
| Catabolism | |||||
| CL2520Contig1 | α/β-Hydrolase | 50 | 436 | 3 | 455,000 |
| isotig03580 | Amidase signature enzyme | 28 | 250 | 21 | 2,200,000 |
| Oxidation/reduction | |||||
| isotig10793 | FAD NAD-binding oxidoreductase | 26 | 251 | 8 | 1,305,000 |
| isotig16031 | Short-chain dehydrogenase | 41 | 371 | 8 | 805,000 |
| Ca/endoplasmic reticulum (ER) | |||||
| isotig15122 | EF hand | 20 | 180 | 3 | 240,000 |
| isotig04172.2 | Calreticulin precursor | 48 | 429 | 14 | 55 |
| Stress related | |||||
| CL1Contig4905.1 | Hypersensitive-induced response protein | 33 | 299 | 5 | 190,000 |
| Nucleic acids | |||||
| F60QV6V01CU6OC | RNP-1 like RNA-binding protein | 20 | 165 | 3 | 39 |
| isotig01568 | Endonuclease exonuclease phosphatase | 42 | 371 | 6 | 610,000 |
| Protein modification | |||||
| isotig14032.2 | Prenyl-Cys methylesterase | 53 | 487 | 13 | 1,400,000 |
| Plastid | |||||
| F62NEKU02FTJB3 | CP12 domain-containing protein1 | 17 | 160 | 4 | 30 |
| isotig09740 | AIG2 family protein | 47 | 433 | 8 | 1,055,000 |
| isotig17627 | N-Acylethanolamine amidohydrolase | 66 | 623 | 17 | 442 |
| CX160991.1 | Chloroplast precursor | 19 | 180 | 2 | 65 |
| CL1Contig7761 | Ferredoxin | 16 | 143 | 2 | 61 |
| F97XPG002IZM8C | Ferredoxin | 13 | 125 | 2 | 29 |
| Unknown | |||||
| CL6181Contig1 | Hypothetical protein | 23 | 207 | 4 | 405,000 |
| isotig16390 | Predicted protein | 26 | 247 | 4 | 91 |
| CL1Contig3525 | Predicted protein | 47 | 438 | 2 | 23 |
| isotig16101 | Protein (DUF1350) | 55 | 511 | 4 | 11 |
| CL1331Contig1 | Protein (DUF500) | 35 | 319 | 6 | 355,000 |
Table II. βC-plastoglobuli proteome.
| Name/Functional Category | Sequence Description | Mass | Sequence Length | No. of Peptides | Fold Change |
|---|---|---|---|---|---|
| kD | |||||
| Structural | |||||
| isotig16296 | Carotene globule protein (CGP) | 44 | 206 | 40 | 13 |
| CL10820Contig1 | SOUL heme-binding protein | 29 | 265 | 4 | 48,333 |
| CL17536Contig1 | SOUL heme-binding protein | 40 | 353 | 22 | 36 |
| isotig01491 | SOUL heme-binding protein | 37 | 332 | 13 | 71 |
| isotig16144 | SOUL heme-binding protein | 49 | 434 | 24 | 13 |
| isotig01487.2 | SOUL heme-binding protein | 43 | 137 | 22 | 13 |
| CL4597Contig1 | SOUL3-like protein | 29 | 261 | 3 | 55,666 |
| isotig09899 | Plastid-lipid-associated protein (PAP)-fibrillin family protein | 29 | 268 | 10 | 293,333 |
| isotig15498 | PAP-fibrillin family protein | 31 | 279 | 5 | 72,000 |
| isotig04183.2 | PAP-fibrillin family protein | 31 | 282 | 7 | 240,000 |
| isotig15898 | Eyespot assembly protein (EYE3), ABC1 | 118 | 1,095 | 21 | 780,000 |
| isotig17235 | Eyespot assembly protein (EYE3), ABC1 | 129 | 1,231 | 35 | 1,193,330 |
| CL745Contig4 | ABC1 | 35 | 315 | 9 | 286,667 |
| isotig17483 | ABC1 | 96 | 876 | 34 | 1,083,330 |
| CL1Contig10490.1 | ABC1 | 66 | 634 | 18 | 573,333 |
| isotig16860 | ABC1 | 96 | 887 | 4 | 119,500 |
| isotig06232 | ABC1 | 63 | 567 | 23 | 653,333 |
| isotig16432 | ABC1 | 73 | 653 | 43 | 1,483,330 |
| isotig17448 | ABC1 | 91 | 824 | 19 | 436,667 |
| isotig14084 | ABC1 | 81 | 734 | 13 | 333,333 |
| Carotenoid metabolism | |||||
| isotig15955 | Phytoene desaturase | 66 | 596 | 29 | 4 |
| CL4186Contig1 | Lycopene cyclase (LCY) | 67 | 605 | 22 | 65 |
| isotig16154 | LCY | 74 | 692 | 25 | 833,333 |
| isotig16249 | ζ-Carotene desaturase (ζCDS) | 65 | 587 | 22 | 12 |
| CL7381Contig1 | ζCDS | 43 | 388 | 4 | 80,000 |
| 302140351 | ζCDS | 64 | 576 | 3 | 81,666 |
| CL1Contig352.2 | Carotene isomerase | 39 | 367 | 12 | 426,667 |
| contig23620 | Carotene isomerase | 29 | 269 | 7 | 21 |
| isotig11982 | Carotene biosynthesis-related protein | 24 | 229 | 3 | 58,000 |
| isotig04110 | Zeaxanthin epoxidase | 47 | 440 | 12 | 326,667 |
| isotig14327 | Retinol dehydrogenase14 | 34 | 309 | 6 | 143,333 |
| isotig06854 | Pheophorbide A oxygenase | 66 | 593 | 6 | 115,000 |
| Tocopherol biosynthesis | |||||
| isotig16794.2 | Tocopherol vitamin E cyclase1 (VTE1) | 50 | 601 | 15 | 9 |
| CL1Contig3478 | γ-Tocopherol methyltransferase | 37 | 349 | 6 | 136,667 |
| Lipid metabolism | |||||
| isotig07478 | Acetyl synthetase | 73 | 267 | 15 | 296,666 |
| isotig15851 | Phytyl ester synthase (PES) | 87 | 781 | 26 | 926,667 |
| isotig04015 | Phytyl ester synthase (PES) | 100 | 918 | 11 | 450,000 |
| CL1Contig10166.1 | Phytyl ester synthase (PES) | 102 | 936 | 24 | 580,000 |
| CL4703Contig1 | Esterase/lipase/thioesterase family protein | 57 | 509 | 6 | 180,000 |
| GBSVTBZ01B1VF9 | Glycolipid transfer protein | 17 | 155 | 4 | 69,500 |
| isotig16199.1 | 3-β-Hydroxysteroid dehydrogenase isomerase | 31 | 285 | 11 | 12 |
| isotig17627 | N-Acylethanolamine amidohydrolase | 66 | 519 | 16 | 84 |
| Membrane remodeling | |||||
| CL1Contig6738 | Plant synaptotagmin | 51 | 480 | 4 | 29,666 |
| CL1Contig7649 | VIPP1 | 36 | 330 | 17 | 7 |
| Hydrolases/lyases | |||||
| isotig08376 | α/β-Hydrolase | 21 | 187 | 5 | 130,000 |
| CL1Contig4447 | Hydrolase-like protein | 36 | 331 | 15 | 14 |
| isotig03580 | Amidase signature enzyme | 28 | 250 | 16 | 546,667 |
| isotig08435 | RNase p protein component | 22 | 198 | 3 | 72,000 |
| isotig19825 | Peptidase M48 | 41 | 373 | 5 | 123,333 |
| isotig13720 | Signal peptide peptidase | 81 | 769 | 5 | 105,000 |
| Chlorophyll/cofactors/vitamins | |||||
| CL1Contig605 | NADPH:quinone reductase zinc-dependent oxidoreductase | 39 | 364 | 8 | 176,667 |
| isotig17345.1 | Methylenetetrahydrofolate reductase | 44 | 411 | 4 | 47 |
| CL2130Contig1 | Divinyl protochlorophyllide a 8-vinyl reductase | 47 | 431 | 7 | 170,000 |
| CL5220Contig1 | Ubiquinone menaquinone biosynthesis methyltransferase | 44 | 406 | 8 | 263,333 |
| isotig13689 | MPBQ/MSBQ methyltransferase2 | 37 | 328 | 13 | 19 |
| Signaling | |||||
| isotig20674 | GTP-binding protein | 23 | 207 | 8 | 240,000 |
| isotig06897.1 | Rab2 family small GTPase | 25 | 221 | 11 | 213,333 |
| isotig16758 | Rab11 family small GTPase | 25 | 225 | 7 | 119,500 |
| CL77Contig4 | Extracellular calcium-sensing receptor | 43 | 409 | 10 | 4 |
| isotig04273 | Rho GTPase-activating protein | 49 | 467 | 3 | 97,000 |
| Protein kinases/phosphatases | |||||
| isotig14775 | Tyr phosphatase family | 49 | 451 | 9 | 220,000 |
| Methyltransferases | |||||
| isotig04348.2 | S-Adenosyl-l-Met-dependent methyltransferase | 40 | 372 | 8 | 143,333 |
| CL1Contig335 | Generic methyltransferase | 61 | 167 | 26 | 30 |
| Oxidoreductases | |||||
| isotig20240 | Glc-methanol-choline oxidoreductase | 67 | 625 | 18 | 17 |
| isotig16059.1 | Thiol-disulfide oxidoreductase DCC | 52 | 473 | 5 | 155,000 |
| isotig14675 | Oxidoreductase-like protein | 50 | 470 | 20 | 18 |
| CL1Contig4961.1 | Amine oxidase | 62 | 563 | 15 | 346,667 |
| isotig16623 | Amine oxidase | 63 | 582 | 17 | 666,667 |
| isotig06585 | Plastid terminal oxidase | 52 | 449 | 4 | 88,666 |
| isotig14010 | Short-chain dehydrogenase | 36 | 330 | 18 | 37 |
| isotig13659 | Aldo/keto-reductase | 41 | 376 | 20 | 13 |
| CL5790Contig1 | Rossmann fold NAD-binding protein | 28 | 268 | 3 | 33,000 |
| CL1Contig7258 | Rossmann fold NAD-binding protein | 66 | 617 | 21 | 5 |
| CL4625Contig2 | NAD(P)-binding protein | 35 | 339 | 6 | 180,000 |
| isotig07081 | NADH dehydrogenase | 71 | 642 | 28 | 14 |
| isotig16044 | NADH dehydrogenase | 60 | 570 | 13 | 5 |
| Amino acid metabolism | |||||
| isotig16293 | Saccharopine dehydrogenase | 47 | 2,681 | 22 | 31 |
| isotig14128 | Saccharopine dehydrogenase | 50 | 457 | 10 | 176,667 |
| isotig17511 | Saccharopine dehydrogenase | 51 | 479 | 20 | 28 |
| isotig15874 | Aryl-alcohol dehydrogenase | 42 | 386 | 30 | 26 |
| isotig01812.1 | AMP-dependent synthetase and ligase | 84 | 782 | 11 | 8 |
| Stress related | |||||
| isotig16513 | Glutathione S-transferase | 48 | 430 | 13 | 400,000 |
| isotig14874 | Harpin-binding protein1 | 29 | 267 | 5 | 103,333 |
| CL1Contig8605 | DNAJ-like protein | 49 | 445 | 4 | 69,500 |
| CL1Contig5346 | N-Acetylmuramoyl-l-Ala amidase | 34 | 303 | 10 | 260,000 |
| isotig16141 | Early light-induced protein | 20 | 187 | 5 | 120,000 |
| Transport | |||||
| isotig15740 | ABC transporter | 96 | 876 | 8 | 29 |
| isotig16443 | Mitochondrial carrier protein | 46 | 429 | 7 | 185,000 |
| CL5834Contig2 | Nuclear transport factor2 | 19 | 173 | 7 | 170,000 |
| isotig13261 | Arsenical pump-driving ATPase-like | 82 | 758 | 4 | 69,500 |
| ER/Ca | |||||
| CL3422Contig1 | Cytochrome P450 | 60 | 552 | 9 | 175,000 |
| Cell wall | |||||
| isotig18214 | UDP-GlcNAc pyrophosphorylase | 145 | 1,362 | 22 | 6 |
| Photosynthesis | |||||
| HO703428.1 | Chlorophyll a/b-binding protein | 30 | 277 | 3 | 92,000 |
| isotig02840 | Chlorophyll a/b-binding protein | 32 | 301 | 10 | 6 |
| 383930352 | Cytochrome F | 31 | 287 | 14 | 7 |
| isotig16365 | Thioredoxin family protein | 42 | 383 | 17 | 14 |
| Unknown | |||||
| isotig14695 | α/β-Fold family protein | 43 | 394 | 4 | 88,666 |
| isotig16434 | SLR1470 gene product | 31 | 274 | 11 | 7 |
| isotig13386 | Membrane protein | 62 | 593 | 11 | 196,667 |
| isotig08678.2 | Predicted protein | 27 | 243 | 3 | 57,666 |
| isotig05803 | Predicted protein | 33 | 297 | 5 | 80,000 |
| isotig16288 | Predicted protein | 30 | 273 | 9 | 303,333 |
| isotig15794 | Predicted protein | 31 | 295 | 6 | 180,000 |
| isotig06249 | Predicted protein | 51 | 475 | 5 | 206,667 |
| isotig08618 | Predicted protein | 9 | 82 | 4 | 96,333 |
| CL1Contig10414 | Predicted protein | 15 | 138 | 5 | 11 |
| isotig14932 | Predicted protein | 27 | 245 | 3 | 58,000 |
| isotig15746.1 | Predicted protein | 36 | 325 | 7 | 206,667 |
| isotig14316 | Hypothetical protein | 30 | 273 | 10 | 343,333 |
| isotig11579 | Hypothetical protein | 25 | 225 | 6 | 190,000 |
| contig00323 | Hypothetical protein | 35 | 314 | 9 | 200,000 |
| CL4228Contig1 | Hypothetical protein | 23 | 211 | 15 | 543,333 |
| isotig16545 | Hypothetical protein | 32 | 300 | 13 | 416,667 |
| contig16530.1 | Hypothetical protein | 28 | 259 | 4 | 96,333 |
| CL1Contig9415.1 | Hypothetical protein | 23 | 214 | 8 | 16 |
| isotig15720 | Hypothetical protein | 35 | 322 | 19 | 27 |
| isotig15617.1 | Hypothetical protein | 42 | 376 | 13 | 5 |
| isotig21337 | Hypothetical protein | 12 | 113 | 3 | 88,666 |
| isotig06777 | Hypothetical protein | 111 | 1,009 | 13 | 4 |
| isotig20329.2 | Hypothetical protein | 47 | 415 | 4 | 11 |
| CL6726Contig1 | Protein (DUF393) | 27 | 244 | 5 | 166,333 |
| isotig16101 | Protein (DUF1350) | 55 | 511 | 22 | 36 |
| isotig13969.1 | Protein (DUF1997) | 38 | 344 | 9 | 15 |
| isotig14136 | Protein (DUF4336) | 56 | 502 | 11 | 5 |
Table III. Comparison of CLD with C. reinhardtii CLD.
Arabidopsis plastoglobules were added as a reference.
| Protein Name | D. bardawil CLD | C. reinhardtii CLDa | C. reinhardtii CLDb | Arabidopsis Core Plastoglobulesc |
|---|---|---|---|---|
| MLDP | AEW43285.1 | 338,214 | 192,823 | |
| Diacylglyceryl-trimethylhomo-Ser synthesis protein | isotig13730 | 77,062 | 77,062 | |
| Cyclopropane-fatty acyl-phospholipid synthase | F62NEKU02F32Z3 | 399,825 | 119,132 | |
| Acetyl synthetase-like protein | isotig07478 | 380,622 | ||
| Acetyl-CoA synthetase-like protein | isotig15711 | 377,723 | 123,147 | |
| Cycloartenol synthase | isotig07413 | 196,409 | 116,558 | |
| Squalene epoxidase | isotig02727 | 381,157 | ||
| Retinol dehydrogenase14 | isotig14327 | 390,185 | ||
| Retinol dehydrogenase12-like | isotig08132.2 | 176,680 | 176,680 | |
| NADPH:quinone reductase zinc-dependent oxidoreductase | CL1Contig605 | |||
| Saccharopine dehydrogenase | isotig16293 | |||
| Glyceraldehyde-3-phosphate dehydrogenase | isotig00097.1 | 140,618 | 140,618 | |
| Calreticulin precursor | isotig04172.2 | 78,954 | ||
| Prenyl-Cys methylesterase | isotig14032.2 | 343,002 | ||
| Unknown function | isotig16390 | |||
| Protein (DUF1350) | isotig16101 | 121,991 | AT3G43540.1 | |
| NAD-dependent steroid dehydrogenase-like | isotig14763 | 58,501 | 58,501 | |
| α/β-Hydrolase fold protein | CL2520Contig1 | 330,619 | 173,167 | |
| RNP-1-like RNA-binding protein | F60QV6V01CU6OC | 184,151 | 184,151 | |
| Chloroplast precursor | CX160991.1 | |||
| Ferredoxin | CL1Contig7761 | 159,161 | ||
| SOUL heme-binding protein | isotig01487.2 |
Table IV. Comparison of βC-plastoglobuli with C. reinhardtii eyespot, Arabidopsis plastoglobules, and bell pepper chromoplasts.
C. reinhardtii CLD were added as reference.
| Protein Name | D. bardawil βC-Plastoglobuli | C. reinhardtii CLDa | Eyespotb | Arabidopsis Core Plastoglobulesc | Arabidopsis Full Plastoglobulesc | Bell Pepper Chromoplastd |
|---|---|---|---|---|---|---|
| SOUL domain-containing protein | isotig16144 | C_970031 | AT3G10130.1 | AT3G10130.1 | ||
| ABC1 | isotig17483 | AT3G24190.1 | AT3G24190.1 | |||
| ABC1 | isotig06232 | AT5G05200.1 | AT5G05200.1 | |||
| ABC1 | isotig16432 | C_230061 | AT4G31390.1 | AT4G31390.1 | ||
| ABC1 | isotig17448 | C_110160 | AT1G79600.1 | AT1G79600.1 | ||
| EYE3 | isotig17235 | g6053.t1* | ||||
| EYE3 | isotig15898 | Cre02.g105600.t2* | ||||
| PDS | isotig15955 | C_490019 | AT4G14210.1 | AAK64084.1 | ||
| ζCDS | isotig16249 | AAB35386.1 | ||||
| ζCDS | 302140351 | |||||
| Carotene biosynthesis-related protein | isotig11982 | |||||
| Zeaxanthin epoxidase | isotig04110 | |||||
| Lycopene β-cyclase | CL4186Contig1 | Q42435.1 | ||||
| Retinol dehydrogenase14 | isotig14327 | 145,585 | AT1G03630.1 | |||
| Tocopherol cyclase | isotig16794.2 | AT4G32770.1 | AT4G32770.1 | |||
| 3-β-Hydroxysteroid dehydrogenase isomerase | isotig16199.1 | C_100060 | AT2G34460.1 | AT2G34460.1 | ||
| Acetyl synthetase-like protein | isotig07478 | 123,147 | C_7940001 | |||
| Acyltransferase-like protein chloroplastic-like | isotig15851 | AT1G54570.1 | AT1G54570.1 | |||
| PAP-fibrillin family protein | isotig04183.2 | C_250022 | AT2G46910.1 | AT2G46910.1 | CAA65784.1 | |
| PAP-fibrillin family protein | isotig15498 | C_580038 | ||||
| Harpin-binding protein1 | isotig14874 | C_2460003 | AT3G23400.1 | AT3G23400.1 | AAR26481.1 | |
| Rab11 family small GTPase | isotig16758 | NP_563750.2 | ||||
| Rab2 family small GTPase | isotig06897.1 | 148,836 | ||||
| Protein kinase domain-containing protein | isotig14084 | AT3G07700.3 | AT3G07700.1 | |||
| AMP-dependent synthetase and ligase | isotig01812.1 | AAL29212.1 | ||||
| Saccharopine dehydrogenase | isotig17511 | AT5G39410.1 | ||||
| Saccharopine dehydrogenase-like protein | isotig14128 | C_970001 | AT1G50450.1 | |||
| Type II calcium-dependent NADH dehydrogenase | isotig07081 | 133,334 | ||||
| Short-chain dehydrogenase | isotig14010 | C_2440006 | BAB93004.1 | |||
| NADH dehydrogenase | isotig16044 | C_820024 | AT5G08740.1 | AT5G08740.1 | ||
| Rossmann fold NAD-binding domain-containing protein | CL5790Contig1 | 118,820 | ||||
| Rossmann fold NAD-binding domain-containing protein | CL1Contig7258 | AT4G18810.1 | ||||
| NAD(P)-binding protein | CL4625Contig2 | AT1G32220.1 | AT1G32220.1 | |||
| NADPH:quinone reductase zinc-dependent oxidoreductase | CL1Contig605 | AT4G13010.1 | ||||
| Peptidase M48 | isotig19825 | C_240088 | AT3G27110.1 | AT3G27110.1 | ||
| Signal peptide peptidase | isotig13720 | AT1G73990.1 | ||||
| UDP-GlcNAc pyrophosphorylase | isotig18214 | C_1400008 | ||||
| Ubiquinone menaquinone biosynthesis methyltransferase | CL5220Contig1 | C_390049 | ||||
| Generic methyltransferase | CL1Contig335 | C_290078 | ||||
| γ-Tocopherol methyltransferase | CL1Contig3478 | 119,132 | C_220002 | AT4G33110.1 | ||
| MPBQ/MSBQ methyltransferase2 | isotig13689 | 129,760 | AT3G63410.1 | |||
| S-Adenosyl-l-Met-dependent methyltransferase | isotig04348.2 | AT2G41040.1 | AT2G41040.1 | |||
| N-Acetylmuramoyl-l-Ala amidase | CL1Contig5346 | 184,328 | C_80056 | |||
| Aldo/keto-reductase | isotig13659 | C_190016 | AT1G06690.1 | AT1G06690.1 | ||
| Thiol-disulfide oxidoreductase DCC | isotig16059.1 | C_140123 | ||||
| Plastid terminal oxidase | isotig06585 | |||||
| Amine oxidase | CL1Contig4961.1 | C_230123 | ||||
| Pheophorbide a oxygenase | isotig06854 | AT2G24820.1 | AAL32300.1 | |||
| Glutathione S-transferase | isotig16513 | AT5G44000.1 | NP_199315.1 | |||
| Divinyl protochlorophyllide a 8-vinyl reductase | CL2130Contig1 | C_1330031 | ||||
| Chlorophyll a/b-binding protein chloroplastic-like | isotig02840 | 130,414 | C_530002 | AT4G10340.1 | ||
| Cytochrome P450 | CL3422Contig1 | AT5G07990.1 | ||||
| Extracellular calcium-sensing receptor | CL77Contig4 | C_1010018 | AT5G23060.1 | |||
| Cytochrome F | 383930352 | NP_958358 | ATCG00540.1 | |||
| Chlorophyll a/b binding | HO703428.1 | 184,810 | C_10030 | AT2G40100.1 | ||
| DNAJ-like protein | CL1Contig8605 | C_490015 | AT1G80030.1 | |||
| Mitochondrial carrier domain-containing protein | isotig16443 | 159,938 | C_1540001 | |||
| GTP-binding protein | isotig20674 | 81,259 | C_10830001 | |||
| Thioredoxin family protein | isotig16365 | AT5G03880.1 | ||||
| Protein DUF1350 | isotig16101 | 121,991 | C_1670026 | AT3G43540.1 | AT5G47860.1 | |
| Protein DUF393 | CL6726Contig1 | AT1G52590.1 | ||||
| Predicted protein (C. reinhardtii) | CL1Contig10414 | C_210162 | ||||
| Hypothetical protein | contig16530.1 | |||||
| Hypothetical protein | isotig16545 | C_370103 | ||||
| Hypothetical protein | isotig14316 | |||||
| Hypothetical protein | isotig15617.1 | C_10188 | ||||
| Hypothetical protein | isotig06777 | C_190173 | ||||
| Hypothetical protein | CL1Contig9415.1 | 148,810 | C_1250029 | |||
| Hypothetical protein | isotig15720 | C_1550001 | ||||
| Hypothetical protein | isotig21337 | C_120189 |
As clearly seen from the comparisons, the D. bardawil CLD and βC-plastoglobuli proteomes resemble different proteomes: D. bardawil CLD mostly resemble cytoplasmic droplets from C. reinhardtii, whereas D. bardawil βC-plastoglobuli resemble Arabidopsis plastoglobules and C. reinhardtii eyespot proteomes.
For example, the βC-plastoglobuli contain PAP-fibrillins, SOUL heme-binding proteins, Activity of bc1 complex (ABC1-kinase) kinase proteins, VTE1, and PESs, which may be considered as protein markers of plastoglobules in plants (Nacir and Bréhélin, 2013). Other proteins that have also been identified in other plastoglobules include acyltransferase, peptidase M48, aldo-keto-reductase, harpin-binding protein, and Rossmann fold NAD(P)-binding domain protein. We also identified in the D. bardawil βC-plastoglobuli proteome most β-carotene biosynthesis enzymes, including phytoene desaturase (PDS), lycopene cyclase (LCY), and ζCDS, part of which were identified previously in eyespot and bell pepper chromoplast proteomes, which also accumulate carotenoids (Schmidt et al., 2006; Siddique et al., 2006; Ytterberg et al., 2006). In addition, we identified several unique proteins in the D. bardawil βC-plastoglobuli: the major lipid-associated protein CGP (Katz et al., 1995; Davidi et al., 2014), the eyespot assembly protein EYE3 (Boyd et al., 2011), the vesicle-inducing plastid protein VIPP1, and plant synaptotagmin. The proteome includes several enzymes involved in the synthesis and/or degradation of lipids, carotenoids, terpenoids, quinones, enzymes involved in carbohydrate and energy metabolism, stress-related proteins, protein kinases and phosphatases, as well as signaling proteins, suggesting diverse metabolic and regulatory roles (Tables II and IV). A comprehensive Kyoto Encyclopedia of Genes and Genomes metabolic map showing the identified enzymes in the relevant metabolic pathways is depicted in Supplemental Figure S3A.
CLD, in contrast, contain a much smaller and mostly different protein composition: a different major lipid-droplet-associated protein (MLDP), characteristic of green algae (Davidi et al., 2012), and several glycerolipid and sterol biosynthesis enzymes identified earlier in cytoplasmic droplets of C. reinhardtii, including diacylglyceryl trimethyl homo-Ser synthesis protein (betaine lipid synthase), a protein marker of CLD in green algae involved in the synthesis of trimethylhomo-Ser diacylglycerol (DGTS), cyclopropane-fatty-acyl-phospholipid synthase, acetyl-CoA synthase, squalene epoxidase, and NAD-dependent steroid dehydrogenase (Tables I and III). Supplemental Figure S3B depicts the identified enzymes in the relevant metabolic pathways.
Sequence Analysis and Comparisons with Other Gene Families
Major Structural Proteins: CGP, MLDP, and Fibrillins
CGP is the major plastoglobule-associated protein in D. bardawil (Katz et al., 1995). It differs in sequence from sequenced green algae MLDPs, fibrillins, and plant oleosins, suggesting that it has a different origin. However, we identified several ortholog proteins whose functions are not known in other microalgae and plants (Fig. 4A). As we noted earlier (Davidi et al., 2014), the sequence of CGP reveals partial homology to SOUL heme-binding proteins. The βC-plastoglobuli proteome also contained four PAP-fibrillin sequences, which show clear similarity to plastoglobulins in plants and algae (PAP-FIBRILLIN1 [FBN1], FBN7, and FBN8; Fig. 4B). Two of the four fibrillins most closely resemble homologs in the eyespot proteome of C. reinhardtii. In contrast to plant and green algae such as C. reinhardtii, in which fibrillins are the major lipid-associated proteins (Ytterberg et al., 2006; Singh and McNellis, 2011; Lundquist et al., 2012b), in the D. bardawil proteome they are minor constituents compared with CGP. In earlier work, we found that proteolysis of CGP destabilizes βC-plastoglobuli, suggesting that CGP may have a similar role to fibrillins in stabilizing the plastoglobules (Katz et al., 1995; Youssef et al., 2010; Singh and McNellis 2011). We did not identify in our proteomes homologs of green algal oleosins (Huang et al., 2013), Chlorella spp. caleosin (Lin et al., 2012), Nannochloropsis spp. hydrophobic lipid droplet surface protein (Vieler et al., 2012), or the avocado (Persea americana) lipid droplet-associated proteins (Horn et al., 2013).
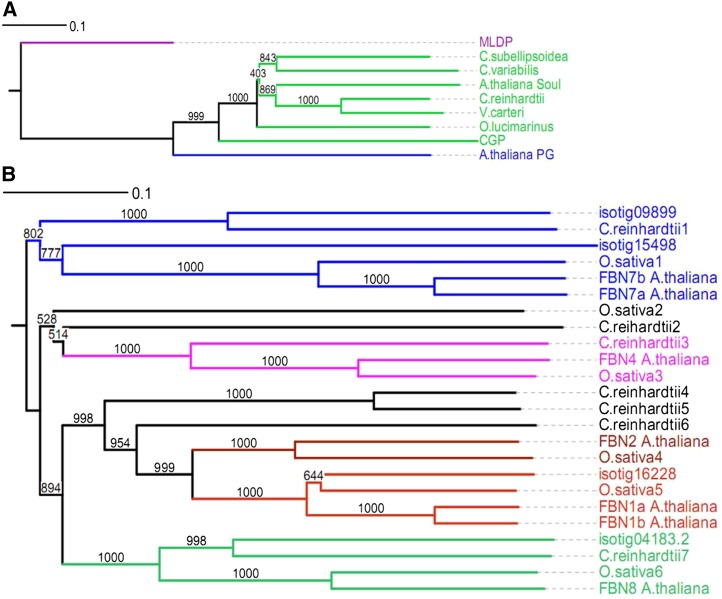
Figure 4.
Phylogenetic tree of CGP and PAP-fibrillin in βC-plastoglobuli. A, Phylogenic tree of CGP compared with sequences from other green algae (Coccomyxa subellipsoidea, C. reinhardtii, Vovlox carteri, Ostreococcus lucimarinus, and Chlorella variabilis) and plants (Arabidopsis). MLDP was added as a reference. Sequence names followed by NCBI accession numbers are as follows: C.subellipsoidea (EIE18519.1), C. reinhardtii (XP_001691398.1), V. carteri (XP_002947474.1), O. lucimarinus (XP_001418356.1), C. variabilis (EFN56543.1), A. thaliana_PG (ABG48434.1), A. thaliana_Soul (NP_001190345.1). B, Phylogenetic tree of PAP-fibrillin of βC-plastoglobuli together with proteins from Arabidopsis, rice, and C. reinhardtii. Sequence names followed by NCBI accession numbers are as follows: C.reinhardtii1 (XP_001698259.1), C.reinhardtii2 (XP_001693298.1), C.reinhardtii3 (XP_001702245.1), C. reinhardtii4 (XP_001698968.1), C. reinhardtii5 (XP_001698965.1), C. reinhardtii6 (XP_001692028.1), C. reinhardtii7 (XP_001690132.1), FBN1a_A. thaliana (AT4G04020.1), FBN1b_A. thaliana (AT4G22240.1), FBN2_A. thaliana (AT2G35490.1), FBN4_A. thaliana (AT3G23400.1), FBN7a_A. thaliana (AT3G58010.1), FBN7b_A.thaliana (AT2G42130.4), FBN8_A. thaliana (AT2G46910.1), O. sativa1 (NP_001054180.1), O. sativa2 (EEE61457.1), O. sativa3 (NP_001068210.1), O. sativa4 (Q7XBW5.1), O. sativa5 (AAO72593.1), O. sativa6 (EEE51252.1).
ABC1 Kinases
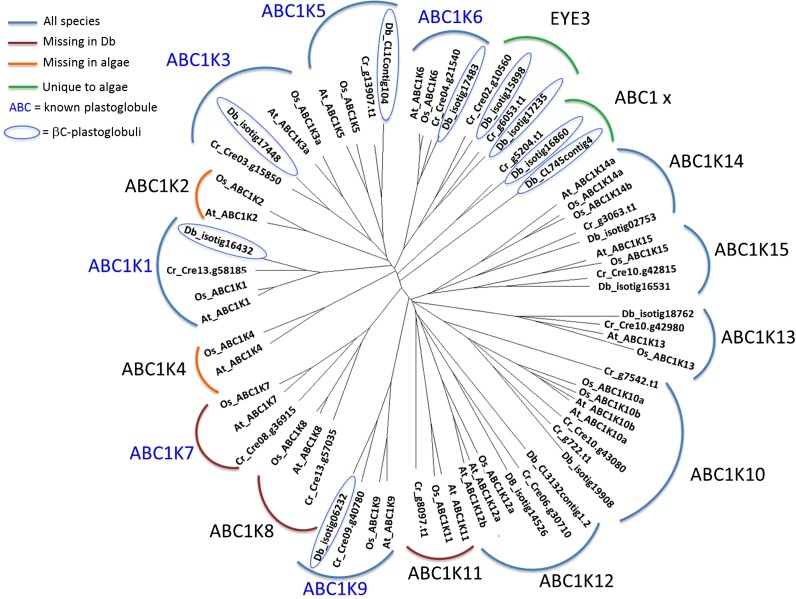
ABC1 kinases, belonging to the atypical protein kinase superfamily, are ubiquitous proteins in plant and algal plastoglobules (Lundquist et al., 2012a), and until recently their function was not known (Nacir and Bréhélin, 2013). However, recent studies showed that the function of the plastoglobule ABC1 kinase ABCK3 may be the regulation of chloroplast prenylquinone metabolism and also regulation of the activity of the tocopherol cyclase VTE1 (Manara et al., 2013), likely by phosphorylation (Martinis et al., 2013), and that the ABC1 kinase complex ABCK1/3 contributes to plastoglobule function in prenyl-lipid metabolism, stress response, and thylakoid remodeling (Lundquist et al., 2013). We identified nine distinct ABC1 kinase sequences in the D. bardawil βC-plastoglobuli proteome. Phylogenetic analysis shows that five of these proteins belong to ABC1 subgroups K1, K3, K5, K6, and K9, identified as plastoglobule proteins in plants and algae (Fig. 5). Two other sequences closely resemble proteins identified as eyespot assembly protein EYE3 in C. reinhardtii (ABC1-EYE3; Fig. 5). EYE3 is a Ser/Thr kinase belonging to the ABC1 superfamily. A recent study localized EYE3 in eyespot lipid droplets in C. reinhardtii and proposed that this protein is involved in pigment granule biogenesis (Boyd et al., 2011). The identification of two homologs of EYE3 in high abundance (21 and 35 peptides) and high enrichment in βC-plastoglobuli suggests that they are integral core components of βC-plastoglobuli in D. bardawil and may be involved in their biogenesis or structural stabilization.
Figure 5.
Phylogenetic tree of ABC1 proteins from βC-plastoglobuli. Nine proteins with ABC1 kinase annotation were located in the βC-plastoglobuli. Members of each ABC1 protein family (K1–K15) from rice (Os), Arabidopsis (At), and C. reinhardtii (Cr) were used to find orthologs. The sequences were then aligned with both ClustalW (version 2.1) and Muscle (version 3.8.31), and the best alignment was chosen for phylogenetic analysis. Phylogenetic analysis was performed with neighbor joining in ClustalW and ProML (Maximum Likelihood) in Phylip (version 3.69; as described in “Materials and Methods”). Db, D. bardawil.
Two additional sequences, which have homologs in C. reinhardtii, have not been categorized previously as plastoglobular proteins (ABC1-X).
SOUL Heme-Binding Proteins
SOUL heme-binding proteins were identified in higher plant plastoglobuli (Ytterberg et al., 2006; Lundquist et al., 2012b) and in green algae eyespot (Schmidt et al., 2006; Kreimer, 2009) proteomes, whose function is unknown. In the D. bardawil plastoglobule proteome, we identified five distinct SOUL heme-binding protein sequences, which do not resemble CGP but show clear homology to plastoglobule and eyespot proteins in other algae (Tables II and IV; Supplemental Fig. S2 in Davidi et al., 2014). Of particular interest is a SOUL3 heme-binding protein, homolog of a SOUL3 recently identified in the eyespot of C. reinhardtii, proposed to act in the organization and cellular positioning of the eyespot (Schulze et al., 2013). Interestingly, one SOUL heme-binding protein was identified also in the CLD proteome.
Acyltransferases and Lipases
Different lipid-metabolizing enzymes were identified in CLD and in the βC-plastoglobuli proteome. Of particular interest for us are enzymes that can contribute to TAG biosynthesis, which was the focus of our recent study (Davidi et al., 2014). We have shown that the synthesis of CLD precedes that of βC-plastoglobuli and that they are made primarily by the de novo synthesis of TAG at the ER, whereas βC-plastoglobuli are made in part from the degradation of chloroplast membrane lipids and in part from the transfer of TAG or of fatty acids from CLD (Davidi et al., 2014). The identification of different lipid-metabolizing enzymes in these lipid droplets can shed light on these processes.
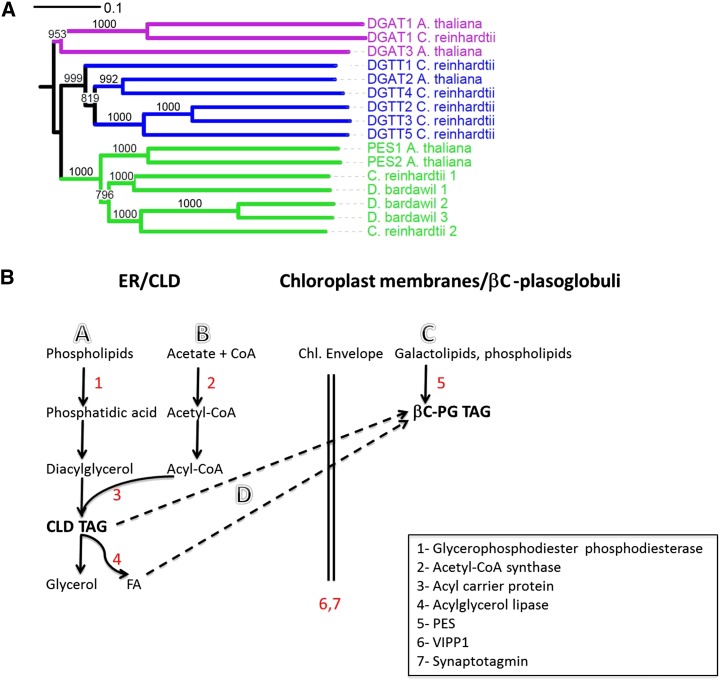
We did not identify any homologs of diacylglycerol acyltransferase (DGAT) or of phospholipid diacylglycerol acyltransferase (PDAT), which are the terminal enzymes in TAG biosynthesis in plants and algae, in the Dunaliella spp. lipid droplet proteomes. In this respect, D. bardawil seems to differ from C. reinhardtii, which was reported to contain PDAT, glycerol-3-phosphate acyltransferase, and lysophospatidic acid acyltransferase (Nguyen et al., 2011). However, in the βC-plastoglobuli proteome, we identified three proteins with close homology to PES from Arabidopsis (Lippold et al., 2012) that belong to the esterase/lipase/thioestesase family (Fig. 6A). These enzymes are induced under stress conditions such as nitrogen deprivation and have a dual function in the degradation of polar lipids and their conversion to TAG. According to our analysis, proteins identified previously in the Arabidopsis plastoglobule proteome as DGAT3 and DGAT4 (Lundquist et al., 2012b) have closer sequence homology to PES than to DGAT. PES homologs were identified also in the lipid droplet proteome from C. reinhardtii (Moellering and Benning, 2010; Nguyen et al., 2011), but it is not clear if they originate from cytoplasmatic or plastidic lipid droplets. In view of the finding of PES homologs in the βC-plastoglobuli proteome, but not in the CLD proteome, it is tempting to speculate that these putative PES enzymes are involved in the synthesis of TAG in the chloroplast from the degradation of chloroplast membrane lipids, which creates the βC-plastoglobuli.
Figure 6.
Phylogenetic tree of PES and DGAT in βC-plastoglobuli and schemes of proposed TAG biosynthesis in CLD and βC-plastoglobuli. A, Phylogenic tree of PES and DGAT showing that the three isotigs in D. bardawil βC-plastoglobuli show higher homology to PES than to DGAT. D. bardawil 1 to 3 refer to isotigs isotig15851, CL1Contig10166.1, and isotig04015, respectively. Sequence names followed by organism and NCBI accession numbers are as follows: PES1_AT1G54570 (Arabidopsis; NP_564662.1), PES2_AT3G26840 (Arabidopsis; NP_566801.1), DGAT1 (Arabidopsis; NP_179535.1), DGAT2_AT3G51520 (Arabidopsis; NP_566952.1), DGAT1_Chlamy (C. reinhardtii; from Boyle et al. [2012]), DGTT1_Chlamy (C. reinhardtii; AFB73929.1), DGTT2_Chlamy (C. reinhardtii; XM_001694852), DGTT4_Chlamy (C. reinhardtii; XM_001693137), DGTT5_Chlamy (C. reinhardtii; XM_001701615), Chlamy_Cre08.g365950 (C. reinhardtii; XP_001696047.1), Chlamy_Cre12.g521650 (C. reinhardtii; XP_001696915.1). B, Proposed TAG biosynthesis and mobilization in CLD and βC-plastoglobules in D. bardawil. Paths are as follows: A, fatty acid (FA) recycling from phospholipids; B, de novo synthesis; C, fatty acid recycling from galactolipids; and D, CLD TAG recycling.
The βC-plastoglobuli proteome also contains an acyltransferase, an acyl carrier protein, and a glycolipid transfer protein involved in the exchange of glycolipids between inner and outer membrane leaflets (Mattjus, 2009). The localization of these enzymes in lipid-metabolizing pathways is depicted in Supplemental Figure S3A.
The CLD proteome contains different enzymes involved in the early stages of lipid biosynthesis, such as acetyl-CoA synthase, acyl carrier protein, cyclopropane fatty acyl phospholipid synthase, which modifies acyl chains of phospholipids by the methylation of unsaturated double bonds, and BTA1, involved in the synthesis of trimethylhomo-Ser diacylglycerol. All these enzymes were also identified in a C. reinhardtii lipid droplet proteome (Moellering and Benning, 2010; Nguyen et al., 2011; Table III). Two enzymes involved primarily in lipid degradation, glycerophosphodiester phosphodiesterase and acylglycerol lipase, were also identified. These results suggest that CLD are involved in a broad range of lipid biosynthesis and degradation reactions, whereas βC-plastoglobuli are more specifically involved in the degradation of chloroplast membrane lipids and their conversion to TAG during nitrogen deprivation.
Several enzymes identified in CLD may be involved in the degradation of microsomal membrane lipids and of TAG and in the mobilization of fatty acids from CLD into βC-plastoglobuli: glycerophosphodiester phosphodiesterase is a broad-specificity hydrolase that can hydrolyze phosphate ester bonds in phosphatidylcholine, phosphatidylethanolamine, or phosphatidylglycerol to phosphatidic acid, which can be converted into TAG. Acylglycerol lipase and the acyl carrier protein may be involved in the hydrolysis and exchange of fatty acids derived from polar phospholipids or TAG and their transfer from CLD into βC-plastoglobuli. Acetyl-CoA synthase proteins are involved in the early stages of de novo fatty acid biosynthesis (see the localization of these enzymes in lipid-metabolizing pathways in Supplemental Fig. S3B). Based on the identification of these enzyme homologs and on our recent studies of TAG biosynthesis in these lipid bodies (Davidi et al., 2014), we propose that TAG biosynthesis in CLD is made in part from the recycling of fatty acids from membrane phospholipids and in part from the de novo synthesis of fatty acids, whereas βC-plastoglobuli TAG are produced in part by recycling fatty acids released from chloroplast membrane lipids and in part from fatty acids derived from CLD TAG. A hypothetical scheme summarizing these pathways is shown in Figure 6B.
Sterol Biosynthesis
Three enzymes in sterol biosynthesis were identified in CLD: squalene epoxidase and cycloartenol synthase, central enzymes in the early stages of sterol biosynthesis, and NAD-dependent steroid dehydrogenase, involved in cholesterol biosynthesis. Homologs of these enzymes were identified previously also in lipid droplets from C. reinhardtii (Moellering and Benning, 2010; Nguyen et al., 2011; Table III). These results suggest that at least part of the sterol biosynthesis in Dunaliella spp. takes place in the CLD. The localization of sterol biosynthesis in plants is not entirely clear: a recent study that tried to localize sterol biosynthesis in Arabidopsis suggests that it is localized in at least three cellular domains: in the ER, at the plasma membrane, and in lipid droplets, which may be homologous to Dunaliella spp. CLD (Silvestro et al., 2013).
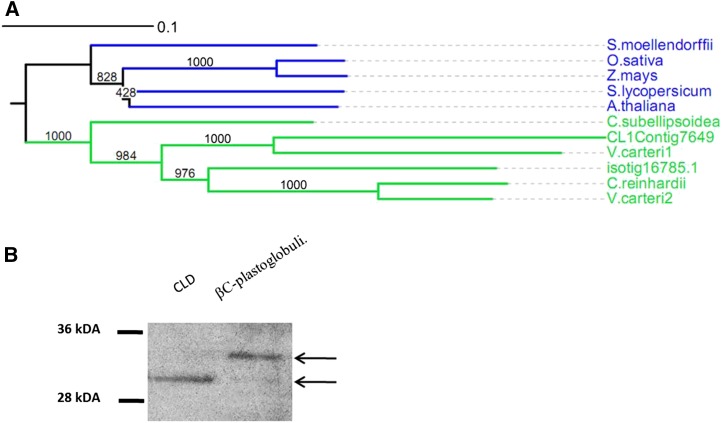
VIPP1
One of the proteins that were identified in both CLD and in βC-plastoglobuli is VIPP1, which has not been identified previously in lipid droplets. VIPP1 is a chloroplast membrane-associated protein that is involved in chloroplast envelope and thylakoid membrane biogenesis and stabilization (Vothknecht et al., 2012; Zhang and Sakamoto, 2013). VIPP1 probably evolved from the bacterial phage-shock protein PspA (Westphal et al., 2001), is essential for thylakoid membrane formation (Kroll et al., 2001), and is involved in the transport of proteins across chloroplast thylakoid membranes (Lo and Theg, 2012). Recent studies have shown that VIPP1 and its bacterial homolog PspA associate tightly with membrane lipids and identified the domains in the proteins responsible for their oligomerization and association with chloroplast membranes (Otters et al., 2013). The two D. bardawil VIPP1 homologs clearly resemble proteins in other green algae from the Volvocales order (Fig. 7A). In order to verify the existence of VIPP1 in lipid droplets in D. bardawil, we analyzed by western analysis the presence of proteins cross-reacting with anti-VIPP1 antibodies. As shown in Figure 7B, protein bands cross-reacting with anti-VIPP1 were indeed identified in protein extracts from both purified CLD and βC-plastoglobuli. Interestingly, two different proteins were identified: whereas CLD contain only one protein of about 30 kD, βC-plastoglobuli seem to contain a major larger protein of about 32 kD. Both proteins seem to be derived from gene CL1Contig7649 by our proteome analysis. These results may suggest that VIPP1 has alternative splicing sites leading to two proteins: a 30-kD protein dominant in CLD and a 32-kD protein dominant in the βC-plastoglobuli.
Figure 7.
Expression and phylogenetic tree of VIPP1 in CLD and βC-plastoglobuli. A, Phylogenetic tree of VIPP1 from D. bardawil CLD and βC-plastoglobuli together with orthologs from plant and green algal VIPP1. Sequence names followed by NCBI accession numbers are as follows: C. reinhardtii (XP_001693830.1), O. sativa (NP_001045073.1), A. thaliana (NP_564846.1), Z. mays (Zea mays; ACG32836.1), S. moellendorffii (Selaginella moellendorffii; XP_002970544.1), S. lycopersicum (Solanum lycopersicum; XP_004250100.1), V. carteri1 (XP_002949072.1), V. carteri2 (XP_002948865.1), C. subellipsoidea (XP_005643904.1). B, Western-blot analysis of protein from CLD and βC-plastoglobuli with VIPP1 antibodies (dilution, 1:1,000) showing one band in CLD and two bands in βC-plastoglobuli.
Proteins Potentially Involved in βC-Plastoglobuli Formation and Stabilization
In a previous study, we showed that the formation of βC-plastoglobuli was preceded by close associations between CLD and chloroplast envelope membranes and by discontinuous envelope membrane staining, which could indicate structural reorganization. If cytoplasmic droplet-derived lipids indeed contribute to the formation of βC-plastoglobuli, it would involve a massive transfer of lipids from CLD to βC-plastoglobuli through the chloroplast envelope membranes. Such a process would possibly involve structural reorganizations in chloroplast envelope membranes. In this study, we identified four potential candidate proteins that might be involved in such an intriguing process: VIPP1, synaptotagmin, SOUL3, and EYE3. Synaptotagmin is a calcium sensor that mediates neurotransmitter release in mammalian synapses by the fusion of neurotransmitter-storing vesicles with the outer cell membranes (Chapman, 2008) and the endosome recycling and trafficking of plant virus genomes in plants (Lewis and Lazarowitz, 2010). The identification of a homolog of this protein in D. bardawil βC-plastoglobuli may indicate that it is involved in their biogenesis and/or interactions with chloroplast membranes.
The identification of VIPP1 in both CLD and βC-plastoglobuli may also provide a clue to clarify how they interact with chloroplast envelope membranes leading to transmembrane lipid transfer.
SOUL3 and EYE3, as mentioned above, were localized in eyespot lipid droplets and proposed to be involved in the biogenesis, stabilization, and targeting of these lipid droplets in C. reinhardtii. They may have a similar function in βC-plastoglobuli in D. bardawil.
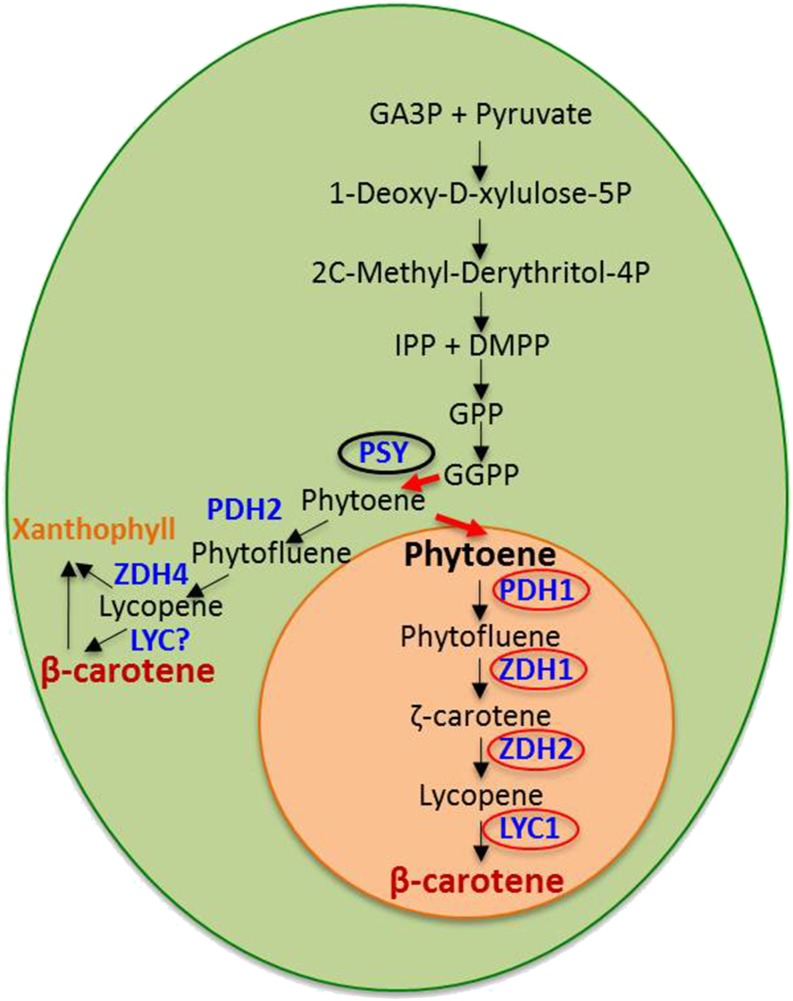
β-Carotene Biosynthesis Enzymes
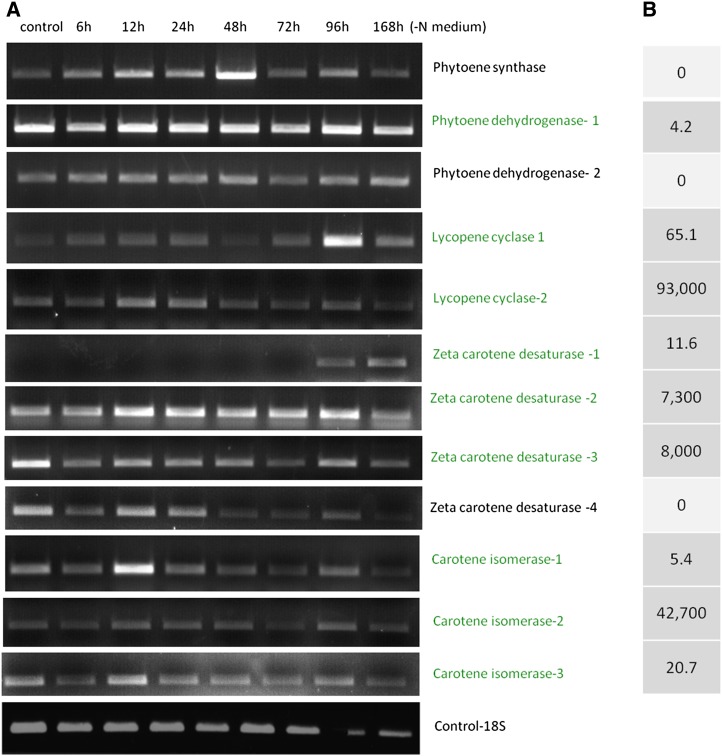
We identified in the D. bardawil proteome one phytoene synthase (PSY) gene, two PDS genes, two LCY genes, four ζCDS genes, and three carotene isomerase genes. In order to clarify the possible involvement of these gene products in β-carotene biosynthesis, we also tested the changes in mRNA expression levels of these genes during nitrogen deprivation in high-light conditions, which induce β-carotene accumulation (Fig. 8A). Interestingly, we found different subcellular localizations and mRNA expression patterns for PSY and for the two PDS genes: PSY was not identified in βC-plastoglobuli and seemed to be restricted to chloroplast membranes; PDS1 was enriched 4-fold in βC-plastoglobuli, whereas PDS2, like PSY, seems to be excluded from βC-plastoglobuli (Fig. 8B). The expression of PSY was greatly increased 6 to 48 h following stress induction, whereas PDS1 appears to be highly expressed continuously, and so is PDS2, but at a lower level. These results suggest that the synthesis of phytoene takes place in chloroplast membranes and that it is activated under stress induction. In contrast, phytoene desaturation takes place in parallel in plastoglobules and in chloroplast membranes by different enzymes. LCY1 and LCY2 both seem to be localized exclusively in plastoglobules (65- and 95,000-fold enrichment, respectively), and appear to be differentially induced after 12 to 24 h (LCY2) or after 96 h (LCY1). The four ζCDS genes reveal the most diverse pattern of expression: ζCDS1 and ζCDS2, localized in βC-plastoglobuli, seem to be differentially expressed 96 to 168 h (ζCDS1) or 12 to 96 h (ζCDS2) after induction, whereas ζCDS3, localized in the βC-plastoglobuli, and ζCDS4, localized in chloroplast membranes, seem to be suppressed during induction.
Figure 8.
mRNA expression and protein enrichment levels of β-carotene biosynthetic enzymes. A, mRNA expression of β-carotene biosynthetic enzymes in D. bardawil nitrogen-deprived (−N) cells. Enzyme names in black are located in chloroplast membranes; enzyme names in green are located in βC-plastoglobuli. Expression of 18S was added as a control. B, Protein enrichment levels. Numbers indicate the protein fold change increase in βC-plastoglobuli compared with chloroplast membranes (fold change calculations are described in “Materials and Methods”).
This complex pattern of localization and expression suggests that part of the β-carotene biosynthesis enzymes is located in chloroplast membranes, whereas others are contained in βC-plastoglobuli, suggesting two parallel biosynthetic pathways.
The finding of only one PSY gene in chloroplast membranes and not in βC-plastoglobuli, and all subsequent enzymes in the βC-plastoglobuli, suggests that the initial stages of β-carotene biosynthesis in D. bardawil, up to phytoene, takes place in chloroplast membranes, whereas all subsequent parts of the biosynthetic pathway occur in the βC-plastoglobuli. This finding is consistent with the fact that phytoene is the first intermediate in the pathway that is lipophilic and would preferentially dissolve in lipid droplets as compared with membranes. Therefore, its transfer from chloroplast membranes to βC-plastoglobuli should be kinetically favored. The finding that phytoene is the only biosynthetic intermediate that accumulates in βC-plastoglobuli at early stages of induction (figure 2A in Davidi et al., 2014) is also in agreement with this hypothesis. The finding of several isoforms of phytoene dehydrogenase (PDH) and ζCDS, one localized in chloroplast membranes and the other in the βC-plastoglobuli, is consistent with the idea of two pathways for β-carotene biosynthesis: a constitutive pathway in chloroplast membranes, for the biosynthesis of β-carotene and light-harvesting accessory xanthophylls, and the inductive pathway in βC-plastoglobuli, for the stress-induced massive accumulation of β-carotene. We also identified several putative carotene isomerases, which may promote the isomerization of all-trans- to 9-cis-β-carotene or of one of its precursors. A model summarizing this proposed biosynthesis pathway is depicted in Figure 9.
Figure 9.
Proposed scheme of β-carotene biosynthesis in D. bardawil. Two pathways for β-carotene biosynthesis in D. bardawil are a constitutive pathway in the chloroplast (green) and an inducible pathway in the βC-plastoglobuli (orange). DMPP, Dimethylallyl diphosphate; GA3P, glyceraldehyde 3-phosphate; GGPP, geranylgeranyl diphosphate; GPP, geranyl pyrophosphate; IPP, isopentenyl diphosphate; ZDH, ζ-carotene dehydrogenase.
Origin of βC-Plastoglobuli
The question of how βC-plastoglobuli evolved in D. bardawil is still a mystery, but the comparison of the βC-plastoglobuli proteome with previously published proteomes of other lipid droplets in microalgae and plants may provide a clue to this interesting question. Logical possible origins are the two types of lipid droplets in green algae chloroplasts: plastoglobules and eyespot lipid droplets. According to their pigment contents, βC-plastoglobuli resemble eyespot lipid droplets, since both contain a similar mixture of 9-cis- and all-trans-isomers of β-carotene. Also, comparison of the proteomes of βC-plastoglobuli with other lipid droplet proteomes shows the highest resemblance to the eyespot (Table IV): notably, βC-plastoglobuli, like the eyespot, contains EYE3 as major proteins, SOUL3 heme-binding protein, similar PAP-fibrillins, β-carotene biosynthesis enzymes, and many other proteins without identified functions, culminating in about 40 homologous proteins. However, βC-plastoglobuli also closely resemble the Arabidopsis plastoglobule in their proteomes. A possible reason why the number of core proteins in these lipid droplets is significantly higher than in plant plastoglobules or in C. reinhardtii eyespot globules may be that they combine the functions of both, such as lipid metabolism and β-carotene biosynthesis, respectively.
Did βC-plastoglobuli evolve from the amplification of the eyespot? In this respect, it is noteworthy that, in contrast to all other Dunaliella spp. that we studied (including Dunaliella tertilecta, Dunaliella parva, and Dunaliella acidophila), all of which have clearly defined eyespots, visible in light and electron micrographs, we never detected a clear eyespot structure in D. bardawil. Based on the above considerations, we propose that D. bardawil βC-plastoglobuli have evolved from the disintegration and amplification of the eyespot.
In summary, our work shows that CLD and βC-plastoglobuli in D. bardawil have different proteomes, suggesting that they have different functions. Of special note are the different lipid-metabolizing enzymes, which are consistent with different TAG biosynthesis mechanisms reported in our earlier work (Davidi et al., 2014), the identification of distinct β-carotene biosynthesis enzymes in βC-plastoglobuli and in chloroplast membranes, suggesting branching of the inductive metabolic pathway for β-carotene biosynthesis in the βC-plastoglobuli from phytone, and the identification of VIPP1, synaptotagmin, and EYE3, possibly involved in βC-plastoglobuli biogenesis. This work also provides indications that βC-plastoglobuli in D. bardawil evolved from eyespot lipid droplets.
MATERIALS AND METHODS
Strain and Growth Conditions
Dunaliella bardawil is an isolated species (Ben-Amotz et al., 1989) deposited at the American Type Culture Collection (no. 30861). Culturing conditions, growth media, and nitrogen limitation induction were as described previously (Davidi et al., 2014).
Preparation of Lipid Droplets and Thylakoid Membranes
Isolation of CLD and βC-plastoglobuli lipid droplets was performed essentially as described previously (Davidi et al., 2014). Thylakoid membranes were isolated as described previously (Finel et al., 1984), and synthetic lipid droplets were obtained as described previously (Davidi et al., 2012). Three biological repeats were prepared from each sample.
Immunoblotting
Proteins from isolated lipid droplets were precipitated in 80% (v/v) acetone (Davidi et al., 2012). Then, the proteins were analyzed by 12% (w/v) SDS-PAGE, blotted to nitrocellulose, immunoblotted with anti-VIPP1 antibodies (a gift from Michael Schroda, Molekulare Biotechnologie und Systembiologie Technische Universität) in a 1:1,000 dilution, and visualized by the horseradish peroxidase-based enhanced chemiluminescence system (homemade, using γ-caproic acid and luminol from Sigma-Aldrich).
cDNA Preparation
Cells precultured for 48 h in complete growth medium were collected by centrifugation, washed once, and cultured in nitrogen-deficient medium. After 0, 6, 12, 24, 32, 48, 72, 96, and 168 h, samples of 10 mL containing 1 to 2 × 107 cells were taken for RNA isolation. The cells were collected by centrifugation, immediately flash frozen in liquid nitrogen, and stored at −80°C for further use. Total RNA was isolated using the Tri Reagent procedure according to the manufacturer’s protocol (Molecular Research Center). Independent RNA isolations were conducted for each growth period. Template cDNA was synthesized using 0.1 µg of total RNA in a total volume of 20 µL using the SuperScript kit (Invitrogen). Gene expression of β-carotene enzymes in nitrogen-deprived cells was examined using the following primers: for PSY, 5′-GCGATGCATACAAACC-3′ and 5′-TGTCATCAGTCCCACAGTGC-3′; for PDH (1), 5′-GGCTTGCACATCTTCTTTG-3′ and 5′-TCAGCACAATTTGCTTGAGG-3′; for PDH (2), 5′-TTGATTTCCTTGACCTTCGG-3′ and 5′-ATGATGGACTCACAGCCCTC-3′; for ζCDS (1), 5′-TAAAGAAGGCTTTCAGGCCA-3′ and 5′-GACCACCCAGGATCTTAGCA-3′; for ζCDS (2), 5′-CTTGCTGGTCAAGGATCACA-3′ and 5′-GTGAGCTGAGGGGTGGTAAA-3′; for ζCDS (3), 5′-CATTGGAGGGTGACTCTGGT-3′ and 5′-ACGTCATCGGCGTTTTATTC-3′; for ζCDS (4), 5′-AGCCAAACATCTCAGCGAGT-3′ and 5′-AAGGGTATCATTGTGAGCCG-3′; for LCY (1), 5′-TTCGAACGAAGCATCAAGTG-3′ and 5′-GACAAGAAGTTCGCACACGA-3′; for LCY (2), 5′-GACTCCAGGCAGCAAACTTC-3′ and 5′-AACTCATGGGCAATGACCTC-3′; for carotene isomerase (1), 5′-GTTAGCAGAAGGCTTGACGG-3′ and 5′-CCTCAAACACACTCGCTTCA-3′; for carotene isomerase (2), 5′-GTACGACCTATGGAAGGGCA-3′ and 5′-TGATCAACCCTCTCCGAATC-3′; and for carotene isomerase (3), 5′-CACCTGAGGCACTAACAGCA-3′ and 5′-ACCGGTCGTATTGTTTAGCG-3′. All transcripts were compared with the expression of the 18S control gene.
Protein Extraction for Proteomic Analysis
Proteins were extracted from isolated CLD, βC-plastoglobuli, synthetic lipid droplet, and thylakoid membrane precipitation in 80% acetone overnight at 4°C. Precipitated proteins were pelleted by centrifugation and suspended first in 50 mm AmBc (collected as AmBc samples), then the undissolved protein were suspended in 1% SDS (collected as SDS samples). Proteins were quantified using a bicinchoninic acid kit (Pierce). All samples were subjected to in-solution tryptic digestion. Proteins were first reduced using dithiothreitol (Sigma-Aldrich) to a final concentration of 5 mm and incubated for 30 min at 60°C followed by alkylation with 10 mm iodoacetemide (Sigma-Aldrich) in the dark for 30 min at 21°C. Proteins were then digested using trypsin (Promega) at a ratio of 1:50 (trypsin:protein, w/w) for 16 h at 37°C. Digestions were stopped by the addition of formic acid to a concentration of 1%. The samples were lyophilized and stored at −80°C until further analysis.
Liquid Chromatography
Liquid chromatography/mass spectrometry-grade solvents were used for all chromatographic steps. Each sample was dissolved in 97:3 water:acetonitrile and loaded using splitless nano-ultraperformance liquid chromatography (10,000-p.s.i. nanoAcquity device; Waters) in high-pH/low-pH reverse-phase two-dimensional liquid chromatography mode. Samples were loaded onto a C18 Xbridge column (0.3 × 50 mm, 5-μm particles; Waters). Buffers used were 20 mm ammonium formate, pH 10 (A), and acetonitrile (B). For the cytoplasmic samples, peptides were fractionated using a three-fraction regime. For the pure and chloroplast globules, a seven-fraction method was used. The seven-fraction method included a step gradient of 10.8% B, 13.8% B, 15.8% B, 17.8% B, 20.1% B, 23.4% B, and 65% B. The three-fraction approach induced steps of 13.1% B, 17.7% B, and 65% B. Buffers used in the low-pH reverse phase were water + 0.1% formic acid (A) and acetonitrile + 0.1% formic acid (B). Desalting of samples was performed online using a reverse-phase C18 trapping column (180 µm i.d., 20 mm length, 5 µm particle size; Waters). Peptides were separated using a C18 T3 HSS nano-column (75 µm i.d., 200 mm length, 1.8 µm particle size; Waters) at 0.4 µL min−1 and eluted from the column using the following gradient (all v/v): 5% to 30% B in 50 min, 30% to 95% B in 5 min, maintained at 95% for 7 min, and then back to the initial conditions.
Mass Spectrometry
The nanoliquid chromatograph was coupled online through a nanoESI emitter (7 cm length, 10-mm tip; New Objective) to a quadrupole ion mobility time-of-flight mass spectrometer (Synapt G2 HDMS; Waters) tuned to at least 20,000 mass resolution (full width at one-half height) for both MS1 and MS2. Data were acquired using Masslynx version 4.1 in HDMSE positive ion mode. Ions were separated in the T-Wave ion mobility chamber and transferred into the collision cell, as described (Tenzer et al., 2013). Wave velocity and height were set to 300 m s−1 and 0.2 V, respectively. Collision energy was alternated from low to high throughout the acquisition time. In low-energy (MS1) scans, the collision energy was set to 5 eV; it was ramped from 27 to 50 eV for high-energy scans. Mass range was set to 50 to 2,000 Thomsons, with a scan rate set to 1 Hz. A reference compound (Glu-Fibrinopeptide B; Sigma) was infused continuously for external calibration using a LockSpray and scanned every 30 s.
Data Processing, Searching, and Analysis
Raw data processing and database searching were performed using Proteinlynx Global Server (IdentityE) version 2.5.2. Database searching was carried out using the Ion Accounting algorithm described by Li and Godzik (2006).
Data were searched against the Dunaliella salina/D. bardawil proteome Weizmann Institute of Science (WIS) combined target and reversed (decoy) database and the list of common laboratory contaminants (www.crapome.org). Trypsin was set as the protease, and one missed cleavage was allowed. Fixed modification was set to carbamidomethylation of Cys, and variable modification was set to oxidation of Met.
All identifications were imported to Scaffold version 3.6. A minimum of two peptides per protein and a protein false discovery rate of 1% were set as minimum identification criteria.
Using Scaffold, the normalized spectral counts were calculated for each protein. Student’s t test was used for statistical evaluation. Fold changes were calculated based on the normalized spectral counts. The average normalized spectral count of each sample was calculated and divided by the average normalized spectral count of the thylakoid membrane samples. The result was designated as fold enrichment.
Data Set Construction
D. salina ESTs were downloaded from GenBank, limiting the search by the taxid: 3046. A total of 6,811 sequences were found and cleaned using Seqclean (http://sourceforge.net/projects/seqclean/) and then trimmed with Sequencher (version 4.10; Gene Codes). The mRNA sequences from GenBank (106 sequences) and the JGI reads (the good_ESTs files from the following libraries: CGFP, CGFS, CGFY, CBZO, CBZP, CBZS, and CBZT) were added to the cleaned ESTs, and redundancy was reduced with CD-HIT-EST (version 4.5.4). The sequences were then assembled using TGICL version 2.1 (http://compbio.dfci.harvard.edu/tgi/software/). The resulting sequences, both the assembled contigs (38,156) and the nonassembled singletons (54,122), were translated in six frames, and the longest open reading frame (from stop to stop) was taken. CD-HIT was run on the proteins with a cutoff of 90%, and protein sequences 50 amino acids or longer were taken in a data set called wis90 (76,843 sequences). The JGI assembled reads (454Isotigs.gte50.fasta, 22,234 sequences) were translated, and the longest open reading frame was taken. CD-HIT was run as above, and a minimum length of 50 amino acids resulted in 20,884 sequences in the jgi90 data set. An in-house Perl script was run to split accidentally joined transcripts in both the wis90 and jgi90 sets, and the resulting transcripts were then combined in a final data set, together with the D. bardawil proteins from the NCBI, and CD-HIT at 90% was performed again. This resulted in the final data set of 83,694 sequences, called D. salina/bardawil proteome WIS.
General Annotation
Annotation was performed on the final protein data set with Blast2GO using the default parameters (Conesa et al., 2005). A total of 20,068 sequences were annotated.
Annotation of Mass Spectrometry Results
The mass spectrometry results were further annotated using Mercator (Lohse et al., 2014) and WebMGA (Kegg and Kog; Wu et al., 2011).
Specific Proteins/Protein Families
Proteins of interest were studied further. The sequences were analyzed with BLASTP at the NCBI (Altschul et al., 1997) to find similar proteins in other species. In extended protein families (ABC, PAP-fibrillin, acyl carrier, esterase, and lipase), members were first characterized in D. bardawil, and then those sequences were used to find orthologs in Arabidopsis (Arabidopsis thaliana), japonica rice (Oryza sativa), and Chlamydomonas reinhardtii. The Arabidopsis orthologs were found by BLASTP at The Arabidopsis Information Resource (www.arabidopsis.org; Lamesch et al., 2012), and the other species were found by using Arabidopsis as input into Greenphyl version 3 (http://www.greenphyl.org/cgi-bin/get_homologs.cgi; Rouard et al., 2011).
The sequences were then aligned with both ClustalW (version 2.1; Larkin et al., 2007) and Muscle (version 3.8.31; Edgar, 2004), and the better alignment was chosen for phylogenetic analysis. In cases where only one region was properly aligned, the alignment was cut manually into blocks (ABC1 and PAP-fibrillin). Phylogenetic analysis was performed with neighbor joining in ClustalW and ProML (Maximum Likelihood) in Phylip (version 3.69; Felsenstein, 2005).
Comparison with Other Data Sets
The core lipid droplet lists were compared with C. reinhardtii eyespot (Schmidt et al., 2006), two C. reinhardtii CLD (Moellering and Benning, 2010; Nguyen et al., 2011), one Arabidopsis plastoglobule (Lundquist et al., 2012b), and one chromoplast (Siddique et al., 2006) proteome collections using Proteinortho version 2.3 (Lechner et al., 2011). The best reciprocal BLAST hit for each collection compared with the βC-plastoglobuli or CLD was taken.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. CLD core proteome sequences list.
Supplemental Figure S2. βC-plastoglobuli core proteome sequences list.
Supplemental Figure S3. Metabolic diagram of βC-plastoglobuli and CGP metabolic pathways diagram showing identified enzymes in βC-plastoglobuli and CLD.
Supplementary Material
Acknowledgments
We thank Dr. Irit Orr (Biological Service Unit at the Weizmann Institute) for help in preparing the proteome database and Dr. Alexandra Gabashvili (The Israel Center for Personalized Medicine Proteomics Unit) for help in the preparation of samples for proteomic analysis.
Glossary
- TAG
triglyceride
- CLD
cytoplasmatic lipid droplets
- βC
β-carotene-rich
- AmBc
ammonium bicarbonate
- cDNA
complementary DNA
- NCBI
National Center for Biotechnology Information
- JGI
Joint Genome Institute
- PES
phytyl ester synthase
Footnotes
This work was supported by the Ruth and Herman Albert Scholars Program for New Scientists (to Y.L.), the Charles and Louise Gartner Fund, and the Alternative Energy Research Initiative Center at the Weizmann Institute (to U.P.).
Articles can be viewed without a subscription.
References
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsan C, Sanchez-Bel P, Rombaldi C, Egea I, Rossignol M, Kuntz M, Zouine M, Latché A, Bouzayen M, Pech JC (2010) Characteristics of the tomato chromoplast revealed by proteomic analysis. J Exp Bot 61: 2413–2431 [DOI] [PubMed] [Google Scholar]
- Ben-Amotz A, Katz A, Avron M (1982) Accumulation of β-carotene in halotolerant algae: purification and characterization of β-carotene-rich globules from Dunaliella bardawil. J Phycol 18: 529–537 [Google Scholar]
- Ben-Amotz A, Lers A, Avron M (1988) Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol 86: 1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A, Shaish A, Avron M (1989) Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol 91: 1040–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besagni C, Kessler F (2013) A mechanism implicating plastoglobules in thylakoid disassembly during senescence and nitrogen starvation. Planta 237: 463–470 [DOI] [PubMed] [Google Scholar]
- Boyd JS, Mittelmeier TM, Lamb MR, Dieckmann CL (2011) Thioredoxin-family protein EYE2 and Ser/Thr kinase EYE3 play interdependent roles in eyespot assembly. Mol Biol Cell 22: 1421–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle NR, Page MD, Liu B, Blaby IK, Casero D, Kropat J, Cokus SJ, Hong-Hermesdorf A, Shaw J, Karpowicz SJ, et al. (2012) Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J Biol Chem 287: 15811–15825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bréhélin C, Kessler F, van Wijk KJ (2007) Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci 12: 260–266 [DOI] [PubMed] [Google Scholar]
- Chapman ER. (2008) How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem 77: 615–641 [DOI] [PubMed] [Google Scholar]
- Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676 [DOI] [PubMed] [Google Scholar]
- Davidi L, Katz A, Pick U (2012) Characterization of major lipid droplet proteins from Dunaliella. Planta 236: 19–33 [DOI] [PubMed] [Google Scholar]
- Davidi L, Shimoni E, Khozin-Goldberg I, Zamir A, Pick U (2014) Origin of β-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol 164: 2139–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugeni Piller L, Glauser G, Kessler F, Besagni C (2014) Role of plastoglobules in metabolite repair in the tocopherol redox cycle. Front Plant Sci 5: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV Jr, Walther TC (2009) Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139: 855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (2005) PHYLIP (Phylogeny Inference Package), Version 3.6. Department of Genome Sciences, University of Washington, Seattle [Google Scholar]
- Finel M, Pick U, Selman-Reimer S, Selman BR (1984) Purification and characterization of a glycerol-resistant CF0-CF1 and CF1-ATPase from the halotolerant alga Dunaliella bardawil. Plant Physiol 74: 766–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorio G, Stigliani AL, D’Ambrosio C (2007) Agronomic performance and transcriptional analysis of carotenoid biosynthesis in fruits of transgenic HighCaro and control tomato lines under field conditions. Transgenic Res 16: 15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JM. (2008) The gregarious lipid droplet. J Biol Chem 283: 28005–28009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald K, Hirschberg J, Hagen C (2001) Ketocarotenoid biosynthesis outside of plastids in the unicellular green alga Haematococcus pluvialis. J Biol Chem 276: 6023–6029 [DOI] [PubMed] [Google Scholar]
- Horn PJ, James CN, Gidda SK, Kilaru A, Dyer JM, Mullen RT, Ohlrogge JB, Chapman KD (2013) Identification of a new class of lipid droplet-associated proteins in plants. Plant Physiol 162: 1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NL, Huang MD, Chen TL, Huang AH (2013) Oleosin of subcellular lipid droplets evolved in green algae. Plant Physiol 161: 1862–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GO, Hocart CH, Hillier W, Chen H, Kordbacheh F, Price GD, Djordjevic MA (2011) Fatty acid profiling of Chlamydomonas reinhardtii under nitrogen deprivation. Bioresour Technol 102: 3343–3351 [DOI] [PubMed] [Google Scholar]
- Jolivet P, Roux E, D’Andrea S, Davanture M, Negroni L, Zivy M, Chardot T (2004) Protein composition of oil bodies in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem 42: 501–509 [DOI] [PubMed] [Google Scholar]
- Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, Rolland N (2009) Chloroplast proteomics and the compartmentation of plastidial isoprenoid biosynthetic pathways. Mol Plant 2: 1154–1180 [DOI] [PubMed] [Google Scholar]
- Katz A, Jimenez C, Pick U (1995) Isolation and characterization of a protein associated with carotene globules in the alga Dunaliella bardawil. Plant Physiol 108: 1657–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer G. (2009) The green algal eyespot apparatus: a primordial visual system and more? Curr Genet 55: 19–43 [DOI] [PubMed] [Google Scholar]
- Kroll D, Meierhoff K, Bechtold N, Kinoshita M, Westphal S, Vothknecht UC, Soll J, Westhoff P (2001) VIPP1, a nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. Proc Natl Acad Sci USA 98: 4238–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, et al. (2012) The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res 40: D1202–D1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Lechner M, Findeiss S, Steiner L, Marz M, Stadler PF, Prohaska SJ (2011) Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics 12: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Lazarowitz SG (2010) Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc Natl Acad Sci USA 107: 2491–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Godzik A (2006) Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–1659 [DOI] [PubMed] [Google Scholar]
- Lin IP, Jiang PL, Chen CS, Tzen JTC (2012) A unique caleosin serving as the major integral protein in oil bodies isolated from Chlorella sp. cells cultured with limited nitrogen. Plant Physiol Biochem 61: 80–87 [DOI] [PubMed] [Google Scholar]
- Lippold F, vom Dorp K, Abraham M, Hölzl G, Wewer V, Yilmaz JL, Lager I, Montandon C, Besagni C, Kessler F, et al. (2012) Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis. Plant Cell 24: 2001–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SM, Theg SM (2012) Role of vesicle-inducing protein in plastids 1 in cpTat transport at the thylakoid. Plant J 71: 656–668 [DOI] [PubMed] [Google Scholar]
- Lohse M, Nagel A, Herter T, May P, Schroda M, Zrenner R, Tohge T, Fernie AR, Stitt M, Usadel B (2014) Mercator: a fast and simple web server for genome scale functional annotation of plant sequence data. Plant Cell Environ 37: 1250–1258 [DOI] [PubMed] [Google Scholar]
- Lundquist PK, Davis JI, van Wijk KJ (2012a) ABC1K atypical kinases in plants: filling the organellar kinase void. Trends Plant Sci 17: 546–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist PK, Poliakov A, Bhuiyan NH, Zybailov B, Sun Q, van Wijk KJ (2012b) The functional network of the Arabidopsis plastoglobule proteome based on quantitative proteomics and genome-wide coexpression analysis. Plant Physiol 158: 1172–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist PK, Poliakov A, Giacomelli L, Friso G, Appel M, McQuinn RP, Krasnoff SB, Rowland E, Ponnala L, Sun Q, et al. (2013) Loss of plastoglobule kinases ABC1K1 and ABC1K3 causes conditional degreening, modified prenyl-lipids, and recruitment of the jasmonic acid pathway. Plant Cell 25: 1818–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara A, Dalcorso G, Leister D, Jahns P, Baldan B, Furini A (2013) AtSIA1 and AtOSA1: two Abc1 proteins involved in oxidative stress responses and iron distribution within chloroplasts. New Phytol 4: 12533. [DOI] [PubMed] [Google Scholar]
- Martinis J, Glauser G, Valimareanu S, Kessler F (2013) A chloroplast ABC1-like kinase regulates vitamin E metabolism in Arabidopsis. Plant Physiol 162: 652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattjus P. (2009) Glycolipid transfer proteins and membrane interaction. Biochim Biophys Acta 1788: 267–272 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Benning C (2010) RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot Cell 9: 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ. (2012) The dynamic roles of intracellular lipid droplets: from archaea to mammals. Protoplasma 249: 541–585 [DOI] [PubMed] [Google Scholar]
- Nacir H, Bréhélin C (2013) When proteomics reveals unsuspected roles: the plastoglobule example. Front Plant Sci 4: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HM, Baudet M, Cuiné S, Adriano JM, Barthe D, Billon E, Bruley C, Beisson F, Peltier G, Ferro M, et al. (2011) Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: with focus on proteins involved in lipid metabolism. Proteomics 11: 4266–4273 [DOI] [PubMed] [Google Scholar]
- Nojima D, Yoshino T, Maeda Y, Tanaka M, Nemoto M, Tanaka T (2013) Proteomics analysis of oil body-associated proteins in the oleaginous diatom. J Proteome Res 12: 5293–5301 [DOI] [PubMed] [Google Scholar]
- Otters S, Braun P, Hubner J, Wanner G, Vothknecht UC, Chigri F (2013) The first α-helical domain of the vesicle-inducing protein in plastids 1 promotes oligomerization and lipid binding. Planta 237: 529–540 [DOI] [PubMed] [Google Scholar]
- Rosati C, Aquilani R, Dharmapuri S, Pallara P, Marusic C, Tavazza R, Bouvier F, Camara B, Giuliano G (2000) Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J 24: 413–419 [DOI] [PubMed] [Google Scholar]
- Rouard M, Guignon V, Aluome C, Laporte MA, Droc G, Walde C, Zmasek CM, Périn C, Conte MG (2011) GreenPhylDB v2.0: comparative and functional genomics in plants. Nucleic Acids Res 39: D1095–D1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapire AL, Valpuesta V, Botella MA (2009) Plasma membrane repair in plants. Trends Plant Sci 14: 645–652 [DOI] [PubMed] [Google Scholar]
- Schmidt M, Gessner G, Luff M, Heiland I, Wagner V, Kaminski M, Geimer S, Eitzinger N, Reissenweber T, Voytsekh O, et al. (2006) Proteomic analysis of the eyespot of Chlamydomonas reinhardtii provides novel insights into its components and tactic movements. Plant Cell 18: 1908–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze T, Schreiber S, Iliev D, Boesger J, Trippens J, Kreimer G, Mittag M (2013) The heme-binding protein SOUL3 of Chlamydomonas reinhardtii influences size and position of the eyespot. Mol Plant 6: 931–944 [DOI] [PubMed] [Google Scholar]
- Siddique MA, Grossmann J, Gruissem W, Baginsky S (2006) Proteome analysis of bell pepper (Capsicum annuum L.) chromoplasts. Plant Cell Physiol 47: 1663–1673 [DOI] [PubMed] [Google Scholar]
- Silvestro D, Andersen TG, Schaller H, Jensen PE (2013) Plant sterol metabolism: Δ(7)-sterol-C5-desaturase (STE1/DWARF7), Δ(5,7)-sterol-Δ(7)-reductase (DWARF5) and Δ(24)-sterol-Δ(24)-reductase (DIMINUTO/DWARF1) show multiple subcellular localizations in Arabidopsis thaliana (Heynh) L. PLoS ONE 8: e56429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, McNellis TW (2011) Fibrillin protein function: the tip of the iceberg? Trends Plant Sci 16: 432–441 [DOI] [PubMed] [Google Scholar]
- Tenzer S, Moro A, Kuharev J, Francis AC, Vidalino L, Provenzani A, Macchi P (2013) Proteome-wide characterization of the RNA-binding protein RALY-interactome using the in vivo-biotinylation-pulldown-quant (iBioPQ) approach. J Proteome Res 12: 2869–2884 [DOI] [PubMed] [Google Scholar]
- Vieler A, Brubaker SB, Vick B, Benning C (2012) A lipid droplet protein of Nannochloropsis with functions partially analogous to plant oleosins. Plant Physiol 158: 1562–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vothknecht UC, Otters S, Hennig R, Schneider D (2012) Vipp1: a very important protein in plastids?! J Exp Bot 63: 1699–1712 [DOI] [PubMed] [Google Scholar]
- Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U (2009) Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryot Cell 8: 1856–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal S, Heins L, Soll J, Vothknecht UC (2001) Vipp1 deletion mutant of Synechocystis: a connection between bacterial phage shock and thylakoid biogenesis? Proc Natl Acad Sci USA 98: 4243–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Zhu Z, Fu L, Niu B, Li W (2011) WebMGA: a customizable web server for fast metagenomic sequence analysis. BMC Genomics 12: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287: 303–305 [DOI] [PubMed] [Google Scholar]
- Youssef A, Laizet Y, Block MA, Maréchal E, Alcaraz JP, Larson TR, Pontier D, Gaffé J, Kuntz M (2010) Plant lipid-associated fibrillin proteins condition jasmonate production under photosynthetic stress. Plant J 61: 436–445 [DOI] [PubMed] [Google Scholar]
- Ytterberg AJ, Peltier JB, van Wijk KJ (2006) Protein profiling of plastoglobules in chloroplasts and chromoplasts: a surprising site for differential accumulation of metabolic enzymes. Plant Physiol 140: 984–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Sakamoto W (2013) Possible function of VIPP1 in thylakoids: protection but not formation? Plant Signal Behav 8: e22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.