Abstract
Context
Coronary computed tomographic (CT) angiography is a noninvasive anatomic test for diagnosis of coronary stenosis that does not determine whether a stenosis causes ischemia. In contrast, fractional flow reserve (FFR) is a physiologic measure of coronary stenosis expressing the amount of coronary flow still attainable despite the presence of a stenosis, but it requires an invasive procedure. Noninvasive FFR computed from CT (FFRCT) is a novel method for determining the physiologic significance of coronary artery disease (CAD), but its ability to identify ischemia has not been adequately examined to date.
Objective
To assess the diagnostic performance of FFRCT plus CT for diagnosis of hemodynamically significant coronary stenosis.
Design, Setting, and Patients
Multicenter diagnostic performance study involving 252 stable patients with suspected or known CAD from 17 centers in 5 countries who underwent CT, invasive coronary angiography (ICA), FFR, and FFRCT between October 2010 and October 2011. Computed tomography, ICA, FFR, and FFRCT were interpreted in blinded fashion by independent core laboratories. Accuracy of FFRCT plus CT for diagnosis of ischemia was compared with an invasive FFR reference standard. Ischemia was defined by an FFR or FFRCT of 0.80 or less, while anatomically obstructive CAD was defined by a stenosis of 50% or larger on CT and ICA.
Main Outcome Measures
The primary study outcome assessed whether FFRCT plus CT could improve the per-patient diagnostic accuracy such that the lower boundary of the 1-sided 95% confidence interval of this estimate exceeded 70%.
Results
Among study participants, 137 (54.4%) had an abnormal FFR determined by ICA. On a per-patient basis, diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of FFRCT plus CT were 73% (95% CI, 67%–78%), 90% (95% CI, 84%–95%), 54% (95% CI, 46%–83%), 67% (95% CI, 60%–74%), and 84% (95% CI, 74%–90%), respectively. Compared with obstructive CAD diagnosed by CT alone (area under the receiver operating characteristic curve [AUC], 0.68; 95% CI, 0.62–0.74), FFRCT was associated with improved discrimination (AUC, 0.81; 95% CI, 0.75–0.86; P<.001).
Conclusion
Although the study did not achieve its prespecified primary outcome goal for the level of per-patient diagnostic accuracy, use of noninvasive FFRCT plus CT among stable patients with suspected or known CAD was associated with improved diagnostic accuracy and discrimination vs CT alone for the diagnosis of hemodynamically significant CAD when FFR determined at the time of ICA was the reference standard.
Coronary computed tomographic (CT) angiography is a noninvasive test that enables direct visualization of coronary artery disease (CAD) and correlates favorably with invasive coronary angiography (ICA) for measures of stenosis severity.1 However, CT cannot determine the hemodynamic significance of CAD, and even among CT-identified obstructive stenoses confirmed by ICA, fewer than half are ischemia-causing.2,3 These findings underscore an unreliable relationship of stenosis severity to ischemia and have raised concerns that use of CT may precipitate unnecessary ICA and coronary revascularization for patients who do not have ischemia.4,5
These concerns stem from recent randomized trials that have identified no survival benefit for patients who undergo angiographically based coronary revascularization.6,7 As an adjunct to ICA, fractional flow reserve (FFR) has served as a useful tool to determine the likelihood that a coronary stenosis hinders the delivery of oxygen to the heart muscle or causes myocardial ischemia. As the currently accepted reference standard for determining lesion-specific ischemia, FFR is an invasive procedure performed at the time of ICA and represents the ratio of the mean coronary pressure distal to a coronary stenosis to the mean aortic pressure during maximal coronary blood flow.8 This ratio expresses the coronary flow still attainable despite the presence of a coronary stenosis. The addition of physiologic measures of coronary flow by FFR to anatomic-based assessment of stenosis severity by ICA to guide decisions of coronary revascularization improves event-free survival in a manner that is long-lived and cost-effective.9–11 To date, however, this integrated anatomic-physiologic approach has not been available through noninvasive methods.
Noninvasive calculation of FFR from CT (FFRCT) is a novel method that applies computational fluid dynamics to determine the physiologic significance of CAD.12 Fractional flow reserve from CT enables calculation of rest and hyperemic pressure fields in coronary arteries without additional imaging, modification of CT acquisition protocols, or administration of medications.13 In this multicenter international study, we evaluated the performance of noninvasive FFRCT compared with an invasive FFR reference standard for diagnosis of ischemia.
METHODS
Study Design
The rationale and design of the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study has been previously described.14 Briefly, De-FACTO was designed to evaluate the accuracy of FFRCT to diagnose hemodynamically significant CAD, as defined by an invasive FFR reference standard, with a targeted population of patients with suspected native CAD who were referred for clinically indicated nonemergent ICA within 60 days of performance of CT. Patients with prior coronary artery bypass graft (CABG) surgery and suspected instent restenosis on the basis of CT were excluded. The DeFACTO study was conducted at 17 centers in 5 countries (Belgium [n = 1], Canada [n = 1], Latvia [n = 1], South Korea [n = 2], and United States [n = 12]). The De-FACTO study protocol was designed by the steering committee and approved by the institutional review board at each site. All patients provided written informed consent.
Study Population
Enrolled patients were adults with suspected CAD who underwent clinically indicated ICA after CT with no intervening coronary event. Patients were not eligible if they had a history of CABG surgery; prior percutaneous coronary intervention (PCI) with suspected instent restenosis; contraindication to adenosine; suspicion of or recent acute coronary syndrome; complex congenital heart disease; prior pacemaker or defibrillator; prosthetic heart valve; significant arrhythmia; serum creatinine level greater than 1.5 mg/dL; allergy to iodinated contrast; pregnant state; body mass index greater than 35 (calculated as weight in kilograms divided by height in meters squared); evidence of active clinical instability or life-threatening disease; or inability to adhere to study procedures.
Image Acquisition and Analysis for CT
Computed tomographic angiography was performed on 64– or higher detector row scanners with prospective or retrospective electrocardiographic gating in accordance with Society of Cardiovascular Computed Tomography guidelines.15, 16 Computed tomographic angiograms were transferred to a central core laboratory (Harbor UCLA Medical Center, Los Angeles, California) for blinded interpretation using an 18-segment coronary model. Investigators evaluated CTs for maximal patient-, vessel-, and segment-based diameter stenosis, which was categorized as 0%, 1% to 29%, 30% to 49%, or 50% or larger. Lesions of 50% or larger were categorized as subtotally (≥90%) or totally (100%) occluded.
Per-patient and per-vessel CAD stenosis were the maximal stenoses identified in all segments or in all segments within a vessel distribution, respectively. Vessel distributions were categorized for the left anterior descending (distribution including the first and second diagonal branches), left circumflex (distribution including the ramus intermediate, first and second obtuse marginal branches, and left posterolateral branch), and right coronary artery (distribution including the right posterolateral branch and posterior descending artery). Computed tomographic angiograms were judged as excellent, good, adequate, or nondiagnostic, as previously described.17
Image Acquisition and Analysis for ICA
Selective ICA was performed by standard protocol, with a minimum of 2 projections obtained per vessel distribution and with angles of projection optimized based on the cardiac position.18 Invasive coronary angiograms were transferred to a central angiographic core laboratory (University of British Columbia, Vancouver, Canada) for blinded quantitative coronary angiography of all vessels using commercially available software (Discovery Quinton).
Fractional Flow Reserve
Fractional flow reserve was performed at the time of ICA (PressureWire Certus, St Jude Medical Systems; ComboWire, Volcano Corp). Investigators performed FFR in vessels deemed clinically indicated for evaluation and demonstrating an ICA stenosis between 30% and 90%. Vessels deemed not clinically indicated for FFR were not interrogated. After administration of nitroglycerin, a pressure-monitoring guide wire was advanced distal to a stenosis. Hyperemia was induced by administration of intravenous adenosine at a rate of 140 μg/kg per minute. Fractional flow reserve was calculated by dividing the mean distal coronary pressure by the mean aortic pressure during hyperemia. Fractional flow reserve was considered diagnostic of ischemia at a threshold of 0.80 or less.9
Blinded Integration of FFR and CT
Direct comparison of FFRCT with FFR necessitated FFRCT calculation at the precise location of the wire transducer at the time of FFR. To maintain blinding of the FFRCT core laboratory to CT, ICA, and FFR findings, an integration core laboratory (Minneapolis Heart Institute, Minneapolis, Minnesota) was used. The integration core laboratory identified the location on CT that corresponded to the point where the FFR was measured. The location was communicated to the FFRCT core laboratory by an arrow on a 3-dimensional volume-rendered CT image of the coronary arteries.
Computation of FFRCT
Computation of FFRCT was performed in blinded fashion by the FFRCT core laboratory (HeartFlow Inc, Redwood City, California). Calculations of FFRCT were performed by computational fluid dynamic modeling after semiautomated segmentation of coronary arteries and left ventricular mass. This process required approximately 6 hours per case. Three-dimensional blood flow simulations of the coronary arteries were performed, with blood modeled as a Newtonian fluid using incompressible Navier-Stokes equations, and solved subject to appropriate initial and boundary conditions using a finite element method on a parallel supercomputer.13
Since coronary flow and pressure were unknown a priori, a method to couple lumped parameter models of the microcirculation to the outflow boundaries of the 3-dimensional model was used. Coronary blood flow was simulated under conditions modeling adenosine-mediated coronary hyperemia. The FFRCT ratio was obtained by dividing the mean pressure distal to the coronary stenosis by the mean aortic pressure. Similar to invasive FFR, default FFRCT values of 0.50 and 0.90 were assigned to subtotally and totally occluded arteries or nonstenotic coronary arteries, respectively.
Statistical Analyses
The primary study end point was the diagnostic accuracy of FFRCT plus CT for diagnosis of per-patient ischemia compared with an FFR reference standard. The study was powered based on the protocol-specified primary analysis, in which diagnostic accuracy of FFRCT dichotomized at the 0.80 threshold was to be significantly greater than 70% using a 1-sided test at the .05 level of significance. Assuming a 0.35 proportion of patients with ischemia by FFR, a total of 219 evaluable patients were required to achieve 85% power. A total of 252 patients were enrolled, which provided greater than 90% power to answer the primary study hypothesis. Primary analyses were conducted for FFRCT on an intention-to-diagnose sample, defined as all patients with interpretable CTs (as determined by the CT core laboratory prior to and independent of the FFRCT core laboratory) and with invasive FFR, which served as the reference standard.
Analyses were performed on a per-patient as well as per-vessel basis. In the per-patient analysis, vessels with the most adverse clinical status were selected to represent a given patient (minimum FFR, minimum FFRCT, highest CT stenosis category). Fractional flow reserve and FFRCT measurements were recorded on a continuous scale and dichotomized at the 0.80 threshold (values ≤0.80 considered diseased). Stenosis on CT was recorded on an ordinal scale and dichotomized at the 50% threshold, with stenoses of 50% or larger considered obstructive.
In patient-based analysis, diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value were calculated as simple proportions with corresponding 95% confidence intervals. In vessel-based analyses, these values were computed using generalized estimating equations to account for within-patient correlation. Discrimination was quantified using the area under the receiver operating characteristic curve (AUC), and AUCs were compared using the method of DeLong et al19 in the per-patient analysis and percentile bootstrap with 999 resamples in the per-vessel analysis. Pearson correlation coefficients were calculated to determine the relationship between FFRCT and FFR.
One vessel had missing FFRCT and 2 had missing CT data. Missing data were handled by exclusion of these vessels as well as by the worst-case imputation. Because the results based on both of these methods did not differ materially, we present the analyses excluding the single vessel with missing FFRCT. Because FFR and FFRCT were not available for vessels not clinically indicated for FFR, we evaluated performance in keeping with prior multicenter trials, wherein a value of 0.50 was imputed for both FFR and FFRCT in vessels with stenosis on CT of 90% or larger and a value of 0.90 was imputed when maximal stenosis severity by CT was 30% or smaller, to define FFRCT test characteristics that would be expected across the full range of vessels analyzed by CT within the study.
As a secondary analysis, we evaluated the diagnostic performance of FFRCT among patients with intermediate CT stenosis severity wherein the clinical utility of FFRCT would be most commonly expected for use. We restricted this analysis to patients in the clinically equivocal range of CT, with at least 1 stenosis on CT between 30% and 70% and no stenoses larger than 70%.2 All analyses were performed using SAS software, version 9.1 or higher (SAS Institute Inc).
RESULTS
Among 285 patients who underwent CT, ICA, FFR, and FFRCT between October 2010 and October 2011, 31 patients were excluded by the CT core laboratory for nonevaluable CTs and 2 patients were excluded for unresolvable integration of the FFR wire transducer location by ICA to its corresponding location on CT, resulting in 252 patients included in the analyses (Figure 1). Baseline demographic and clinical characteristics of the study population are listed in Table 1. About 77% of patients had experienced angina within the last month. The median duration between CT and ICA plus FFR was 15.5 days (interquartile range, 5–33 days).
Figure 1.
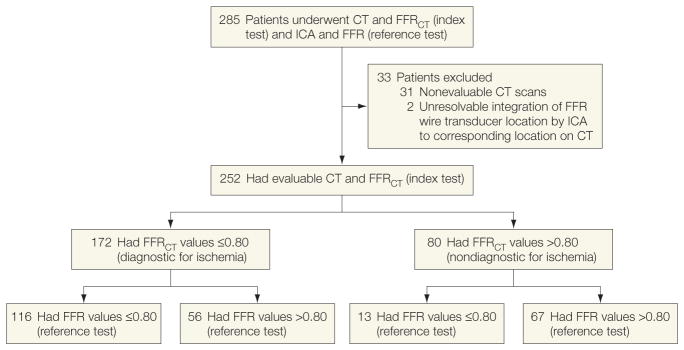
Study Enrollment
CT indicates computed tomographic angiography; FFR, fractional flow reserve; FFRCT, fractional flow reserve calculated from CT; ICA, invasive coronary angiography.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | No. (%)of Patients (N = 252)a |
|---|---|
| Age, mean (SD), y | 62.9 (8.7) |
|
| |
| Male | 178 (70.6) |
|
| |
| Hispanic or Latino | 12 (4.8) |
|
| |
| Race | |
| White | 169 (67.1) |
|
| |
| Asian | 78 (31.0) |
|
| |
| Black or African American | 4 (1.6) |
|
| |
| American Indian or Alaska Native | 1 (0.4) |
|
| |
| Diabetes mellitus | 53 (21.2) |
|
| |
| Hypertension | 179 (71.2) |
|
| |
| Hyperlipidemia | 201 (79.8) |
|
| |
| Family history of coronary artery disease | 50 (19.9) |
|
| |
| Current smoker | 44 (17.5) |
|
| |
| Prior myocardial infarction | 15 (6.0) |
|
| |
| Prior percutaneous coronary intervention | 16 (6.3) |
|
| |
| Angina within the past monthb | 195 (77.2) |
|
| |
| Angina type (worst type)c | |
| Stable | 201 (79.7) |
|
| |
| Worsening | 43 (17.2) |
|
| |
| Silent ischemia | 1 (0.5) |
|
| |
| Other | 7 (2.6) |
Data are reported as No. (%) unless otherwise indicated.
Available in 250 patients.
Available in 192 patients.
The per-patient prevalence of obstructive CAD of 50% or greater by CT and ICA and prevalence of ischemia by FFR and FFRCT are presented in Table 2. Among 615 study vessels, 271 had less than 30% stenosis and 101 had at least 90% stenosis. Four hundred seven vessels were directly interrogated by both FFR and FFRCT (Figure 2). The numbers of patients within the intention-to-diagnose sample with FFRCT and FFR values above and below the 0.80 threshold are listed in Table 3.
Table 2.
Patient and Vessel Characteristics by ICA, FFR, CT, and FFRCT
| Characteristics | No. (%)of Vesselsa |
|---|---|
| ICA and FFR characteristics (n = 408 vessels from 252 patients) | |
| Obstructive CAD (≥50% stenosis)b | 190 (46.5) |
|
| |
| Average diameter stenosis, mean (SD), %b | 46.8 (15.7) |
|
| |
| Lesion location | |
| LAD | 223 (54.6) |
|
| |
| LCx | 95 (23.3) |
|
| |
| RCA | 90 (22.1) |
|
| |
| FFR ≤0.80b | 151 (37.1) |
|
| |
| CT and FFRCT characteristics (n = 406 vessels from 252 patients) | |
| Obstructive CAD (≥50% stenosis)c | 216 (53.2) |
|
| |
| >90% Stenosis | 79 (19.5) |
|
| |
| Coronary calcium score, mean (SD), Agatston unitsd | 381.5 (401.0) |
|
| |
| Scan quality | |
| Excellent | 309 (76.2) |
|
| |
| Good | 86 (21.0) |
|
| |
| Satisfactory | 11 (2.8) |
|
| |
| Poor | 0 |
|
| |
| FFRCT ≤0.80b | 216 (53.3) |
Abbreviations: CAD, coronary artery disease; CT, computed tomographic angiography; FFR, fractional flow reserve; ICA, invasive coronary angiography; LAD, left anterior descending; LCx, left circumflex; RCA, right coronary artery.
Data are reported as No. (%) unless otherwise indicated.
By quantitative coronary angiography in vessels directly interrogated by FFR and FFRCT (n=407).
Computed tomographic interpretation was missing in 2 patients.
Available in 218 patients.
Figure 2.
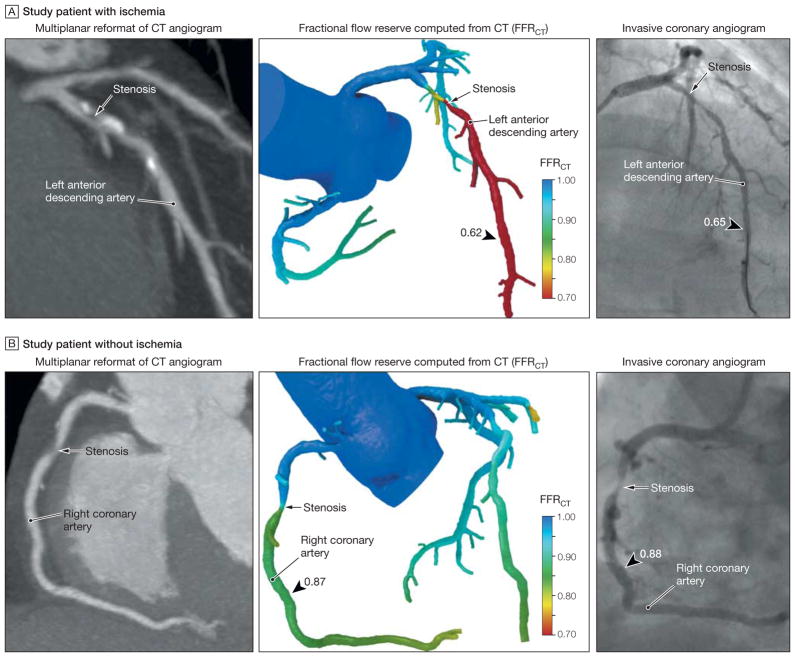
Representative Examples of 2 Patients From the DeFACTO Study
Although both patients have obstructive coronary artery disease by computed tomographic angiography (CT), one patient (A) has ischemia and the other patient (B) does not have ischemia. A, Multiplanar reformat of a CT angiogram demonstrating obstructive stenosis of the proximal portion of the left anterior descending artery (LAD) and a computed fractional flow reserve (FFRCT) value of 0.62, indicating vessel ischemia. Invasive coronary angiogram demonstrates obstructive stenosis of the proximal portion of the LAD and measured fractional flow reserve (FFR) values of 0.65, indicating vessel ischemia. B, CT angiogram demonstrating obstructive stenosis of the mid portion of the right coronary artery (RCA) and an FFRCT value of 0.87, indicating no vessel ischemia. Invasive coronary angiogram demonstrates obstructive stenosis of the mid portion of the RCA and a measured FFR value of 0.88, indicating no vessel ischemia.
Table 3.
No. of Patients With FFRCT and FFR Above and Below the 0.80 Threshold in the Intention-to-Diagnose Sample
| Per-Vessel Performance
|
Per-Patient Performance
|
|||
|---|---|---|---|---|
| FFRCT ≤0.80 | FFRCT >0.80 | FFRCT ≤0.80 | FFRCT >0.80 | |
| FFR ≤0.80 | 121 | 30 | 116 | 13 |
|
| ||||
| FFR >0.80 | 96 | 160 | 56 | 67 |
Abbreviations: FFR, fractional flow reserve; FFRCT, fractional flow reserve calculated from computed tomography.
Two enrolled patients experienced coronary dissection during FFR wire crossing that required PCI, and 1 patient experienced a retroperitoneal bleed requiring blood transfusion and corrective surgery. No untoward events were identified following CT, with no episodes of serious contrast reactions or contrast-induced nephropathy noted. The median radiation dose of CT was 6.4 mSv (inter-quartile range, 4.4–15.0 mSv).
Diagnostic Accuracy of FFRCT for Diagnosis of Ischemia
Per-patient performance of FFRCT plus CT is listed in Table 4. Diagnostic accuracy for FFRCT plus CT was 73% (95% CI, 67%–78%), which did not meet the prespecified primary end point of greater than 70% of the lower bound of the 95% confidence interval. By comparison, diagnostic accuracy of CT alone was 64% (95% CI 58%–70%). When comparing FFRCT alone with CT stenosis of 50% or greater alone, FFRCT demonstrated superior discrimination (AUC, 0.81 [95% CI, 0.75–0.86] vs 0.68 [95% CI, 0.62–0.74]; difference, 0.13[95% CI, 0.06 – 0.20]; P < .001) (Figure 3A).
Table 4.
Per-Patient Diagnostic Performance of FFRCT ≤0.80 and CT ≥50% vs FFR ≤0.80 in the Intention-to-Diagnose Sample
| FFRCT ≤0.80
|
CT ≥50%
|
|||
|---|---|---|---|---|
| Estimate, % (95% CI) | No. of Patients in Group | Estimate, % (95% CI) | No. of Patients in Group | |
| Accuracy | 73 (67–78) | 252 | 64 (58–70) | 252 |
|
| ||||
| Sensitivity | 90 (84–95) | 129 | 84 (77–90) | 129 |
|
| ||||
| Specificity | 54 (46–83) | 123 | 42 (34–51) | 123 |
|
| ||||
| PPV | 67 (60–74) | 172 | 61 (53–67) | 180 |
|
| ||||
| NPV | 84 (74–90) | 80 | 72 (61–81) | 72 |
Abbreviations: CT, computed tomographic angiography; FFRCT, fractional flow reserve calculated from CT; NPV, negative predictive value; PPV, positive predictive value.
Figure 3.
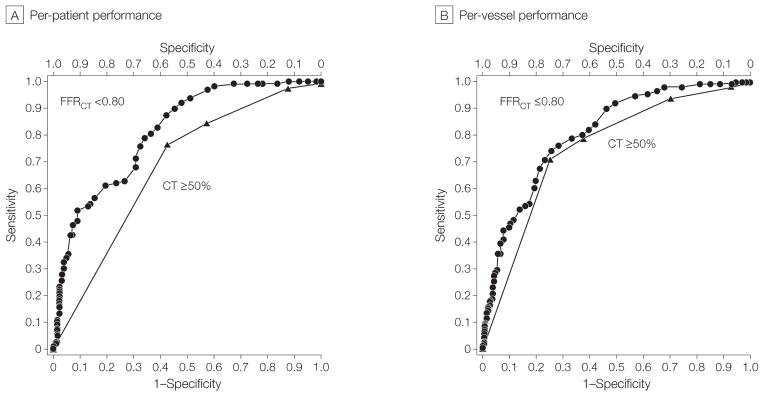
Areas Under the Receiver Operating Characteristic Curve of Per-Patient and Per-Vessel Performance of FFRCT ≤0.80 and CT Stenosis ≥50% Compared With Invasive FFR for Diagnosis of Ischemia
FFR indicates fractional flow reserve; CT, computed tomographic angiography; FFRCT, fractional flow reserve calculated from CT.
For vessels directly interrogated by FFR, FFRCT also demonstrated higher discriminatory power than CT stenosis alone (Figure 3B). For these vessels, the diagnostic sensitivity and specificity of FFRCT alone were 80% (95% CI, 73%–86%) and 61% (95% CI, 54%–67%), respectively. When FFR and FFRCT values were imputed in cases of greater than 90% or less than 30% stenosis, the diagnostic sensitivity and specificity of FFRCT alone were 83% (95% CI, 76%–88%) and 78% (95% CI, 73%–82%), respectively. Direct per-vessel correlation of FFRCT to FFR was good (Pearson correlation coefficient, 0.63; 95% CI, 0.56–0.68), with under-estimation of FFRCT compared with FFR (mean difference, 0.058; 95% CI, 0.05–0.07).
Diagnostic Performance of FFRCT for Patients With Intermediate Stenosis Severity by CT
In patient-based analysis restricted to those with an intermediate stenosis ranging from 30% to 70%, diagnostic accuracy, sensitivity, positive predictive value, and negative predictive value were higher for FFRCT than for CT, with similar specificity (Table 5).
Table 5.
Per-Patient Diagnostic Performance of FFRCT ≤0.80 and CT ≥50% vs FFR ≤0.80 Among Patients With Intermediate CT Stenosis Severity (30%–70%)
| FFRCT ≤0.80
|
CT >50%
|
|||
|---|---|---|---|---|
| Estimate, % (95% CI) | No. of Patients in Group | Estimate, % (95% CI) | No. of Patients in Group | |
| Accuracy | 71 (61–80) | 83 | 57 (46–67) | 83 |
|
| ||||
| Sensitivity | 82 (63–92) | 27 | 37 (22–56) | 27 |
|
| ||||
| Specificity | 66 (53–77) | 56 | 66 (53–77) | 56 |
|
| ||||
| PPV | 54 (39–68) | 41 | 34 (20–53) | 29 |
|
| ||||
| NPV | 88 (75–95) | 42 | 68 (55–79) | 54 |
Abbreviations: CT, computed tomographic angiography; FFRCT, fractional flow reserve calculated from CT; NPV, negative predictive value; PPV, positive predictive value.
COMMENT
In this multicenter international study of stable patients with suspected or known CAD, we observed that FFRCT—a novel method that applies computational fluid dynamics to derive physiologic data from CT—demonstrated improved diagnostic accuracy vs CT alone for diagnosis of ischemia, although this study did not satisfy its prespecified primary end point of diagnostic accuracy of greater than 70% of the lower bound of the 1-sided 95% confidence interval. Taken together, these study results suggest the potential of FFRCT as a promising non-invasive method for identification of individuals with ischemia. The present study findings can be considered proof of concept of the feasibility of this novel technology and, to our knowledge, represent the first large-scale prospective demonstration of the use of computational models to calculate rest and hyperemic coronary pressure fields from typically acquired CT images.
At the patient level, FFRCT, when added to CT, improved diagnostic accuracy vs CT alone, driven by improvements in sensitivity as well as specificity. These results suggest that FFRCT can impart considerable discriminatory power to identify and exclude ischemia in patients with suspected CAD. These findings are supported by the receiver operating characteristics curves—which are generally considered to be independent of disease prevalence—wherein enhanced diagnostic performance of FFRCT vs CT alone was reflected by greater discrimination on both a per-patient and per-vessel basis. Importantly, the sensitivity and negative predictive value of FFRCT were high, indicating a low rate of false-negative studies.
These diagnostic features of FFRCT may encourage a greater sense of diagnostic certainty that patients who undergo CT who have ischemia are not overlooked, such that clinicians may be confident in not proceeding to invasive angiography in patients with stenoses on CT when FFRCT results are normal. Nevertheless, despite its superiority to CT alone, the diagnostic specificity and positive predictive value of FFRCT for ischemia detection remained low, suggesting that while false-positive studies would be reduced with this approach, a substantial rate would remain. In this regard, universal application of FFRCT to guide invasive assessment may result in referral of a nonnegligible number of patients without ischemia, and future studies will be needed to determine the potential clinicoeconomic effectiveness of FFRCT for this particular indication.
Compared with CT alone, we also observed improvements in diagnostic accuracy of FFRCT for ischemia in patients with lesions of intermediate stenosis severity, who represent a particularly challenging clinical subset among whom angiographic severity is an often ambiguous metric for ischemia diagnosis.20 Notably, however, the improvements in diagnostic accuracy afforded by FFRCT among patients with lesions of intermediate stenosis severity were for measures of sensitivity rather than specificity, the latter of which was identical for FFRCT and CT. Similar to per-patient findings, these performance characteristics suggest a low false-negative rate if assessments by FFRCT were used to identify ischemia-causing intermediate lesions, with negligible effects on reductions of false-positive results. In this regard, the use of FFRCT may significantly advance clinical assessment of patients without conventional measures of anatomic high-grade coronary stenosis, largely by proper identification of a significantly greater proportion of patients with manifest ischemia rather than as a safeguard to further invasive evaluation.
In recent years, CT has emerged as a noninvasive imaging test that permits direct visualization of coronary stenoses with high performance compared with invasive angiography.1 However, overestimation of stenosis severity by CT has been observed, and even among stenoses considered obstructive by CT analysis that are subsequently confirmed by ICA, only a minority cause ischemia.2 This discordance is not restricted to CT but has been observed for ICA-determined stenosis as well and underscores the complex relationship between stenosis severity and ischemia. One contemporary example of this anatomic-physiologic incongruity was observed in the nuclear substudy of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, wherein among patients with severe angiographic stenosis, only 32% exhibited moderate or severe ischemia and 40% manifested none or mild ischemia by single-photon emission CT.21 This marked disparity suggests that even in the setting of severe coronary stenosis, other factors are operative in the regulation of ischemia.
By invasive methods, addition of physiologic assessment of CAD by FFR to ICA augments assessment of coronary stenoses in a clinically and economically efficient manner.9 In the multicenter Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) study, combined assessment of stenosis and ischemia by ICA and FFR, respectively, for decisions about revascularization resulted in improved event-free survival compared with revascularization based on anatomic stenosis alone. However, this approach is invasive and associated with potential complications related to coronary vessel instrumentation. In the present study, we observed 2 coronary dissections and 1 significant retroperitoneal hemorrhage in these clinically indicated invasive studies. In contrast, calculation of FFRCT can be performed noninvasively without these risks. Indeed, FFRCT can be calculated from typically acquired CTs without additional imaging, added radiation, modification of image acquisition protocols, or administration of medications. In the present study, performance of FFRCT was reflective of “real-world” practice, wherein vessels interrogated by site investigators were those that were deemed clinically necessary for FFR evaluation, irrespective of size and across a wide range of CT image quality. Given these study characteristics, the high number of participating centers, and the international scope, the present findings should be considered robust and widely generalizable.
The primary goal of noninvasive imaging of CAD has been to develop a single test that identifies high-grade stenosis as well as determines the hemodynamic significance of these lesions. At present, professional societies endorse the use of noninvasive stress imaging for evaluation of symptomatic patients with suspected CAD, given the large-scale observational evidence that ischemia may guide decisions of revascularization in a salutary fashion.22–24 These stress imaging studies identify regional differences in coronary flow reserve or wall motion abnormalities as a surrogate for ischemia yet do not directly visualize coronary stenoses or assess the hemodynamic significance of individual coronary lesions. Furthermore, this testing misclassifies a significant proportion of patients as low risk and has significant false-positive and false-negative rates such that the proportion of patients undergoing ICA after testing in whom no obstructive CAD is identified remains substantial.25,26
Accordingly, some have advocated for hybrid imaging with physiologic and anatomic evaluation of CAD by stress testing and CT, respectively.27 However, this approach requires 2 tests and is associated with higher costs and greater per-patient radiation burden. The addition of FFRCT to CT may allow for combined anatomic-physiologic assessment of CAD from performance of a single imaging test in a manner that may promote salutary outcomes. Future studies to assess the clinical effectiveness and cost-effectiveness of such an approach now appear warranted.
Importantly, the prespecified primary end point for FFRCT was selected on the basis of an array of prior studies that have demonstrated 70% to be generally at the mid point of reported diagnostic accuracies for stress imaging, depending on test type, patient population, and disease prevalence.28 In this study, FFRCT demonstrated a per-patient diagnostic accuracy of 73%, with confidence intervals that suggest a diagnostic accuracy as low as 67% and as high as 78%. These findings establish a performance of FFRCT that is within the range of conventional stress imaging testing.
Furthermore, considerable added value of FFRCT exists when an anatomic imaging test such as CT is used as an alternative test to stress imaging for CAD evaluation. The significant gain in discriminatory power of FFRCT relative to anatomic CT stenosis alone supports its potential application to identify individuals with ischemic stenoses, and the combined anatomic and physiologic information imparted by these noninvasive methods may allow for more refined patient-physician discussions regarding treatment options in a manner not possible with either stress imaging or CT testing alone. Given this possibility, studies to address the clinical impact of FFRCT added to CT compared with traditional stress imaging algorithms are currently being designed.
This study has several limitations. The prescribed exclusion criteria disqualified individuals with prior CABG or PCI with suspected instent restenosis from study participation. Furthermore, not every vessel in study participants was interrogated after ethical review due to concerns regarding FFR in very low-risk or very severe coronary stenoses. Yet in this study, all vessels directly interrogated by FFR and FFRCT were ones that were deemed clinically indicated for evaluation. Also, it remains unknown whether revascularization of the ischemic lesions identified by FFRCT would achieve therapeutic reduction in ischemia from revascularization. The computational fluid dynamic–based algorithms that enable calculation of FFRCT also allow for “virtual” revascularization, and the ability of FFRCT to predict the extent of ischemia resolution after revascularization is currently under investigation. Finally, to study patients for whom and vessels for which FFRCT would most likely be used in clinical practice, we examined a subset of patients with intermediate anatomic stenosis severity. This population is the most challenging in which any imaging mode can discriminate ischemia. Despite this restriction, FFRCT compared favorably with CT stenosis alone.
CONCLUSION
Although the study did not achieve its prespecified primary outcome goal for the level of per-patient diagnostic accuracy, use of noninvasive FFRCT plus CT among stable patients with suspected or known CAD was associated with improved diagnostic accuracy and discrimination compared with CT alone for the diagnosis of hemodynamically significant CAD when FFR at the time of ICA served as the referent standard.
Acknowledgments
Funding/Support: This study was funded by HeartFlow Inc.
Role of the Sponsor: HeartFlow Inc had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs Min and Pencina had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Min, Leipsic, Koo, Jaffer, Shaw, Mauri.
Acquisition of data: Leipsic, Koo, van Mieghem, Erglis, Budoff, Cole, Leon, Mancini, Park, Schwartz, Mauri.
Analysis and interpretation of data: Leipsic, Pencina, Berman, Koo, van Mieghem, Lin, Dunning, Apruzzese, Leon, Malpeso, Mancini, Schwartz, Shaw, Mauri.
Drafting of the manuscript: Min, Leipsic, Koo, Erglis, Lin, Apruzzese, Leon, Schwartz, Shaw, Mauri.
Critical revision of the manuscript for important intellectual content: Leipsic, Pencina, Berman, van Mieghem, Lin, Dunning, Budoff, Cole, Jaffer, Leon, Malpeso, Mancini, Park, Schwartz, Shaw, Mauri.
Statistical analysis: Pencina, Berman, Koo, Lin, Dunning, Apruzzese, Shaw, Mauri.
Obtained funding: Mauri.
Administrative, technical, or material support: van Mieghem, Budoff, Cole, Malpeso, Mancini, Park.
Study supervision: Min, Leipsic, van Mieghem, Erglis, Budoff, Jaffer, Mancini, Schwartz, Shaw, Mauri.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Min reports research support from GE Healthcare and Philips Medical. Dr Leipsic reports research support from GE Healthcare and serving as a consultant to Edwards Life-sciences. Dr Pencina reports consulting fees from Heart-Flow to his institution. Dr Berman reports research support from Siemens Medical Systems and Lantheus Medical Imaging and stock options in Spectrum Dynamics. Dr Budoff reports research support from GE Healthcare and grants to his institution from Roche and Pfizer. Dr Cole reports grant for research support from HeartFlow. Dr Jaffer reports serving as consultant to Boston Scientific, Siemens, and Merck and receiving nonfinancial research support from Abbott Vascular. Dr Mancini reports a grant to his institution from HeartFlow. Dr Mauri reports serving as a consultant to Medtronic and Cordis and receiving research support from Medtronic, Cordis, Abbott, Boston Scientific, Eli Lilly, Daiichi Sankyo, Bristol-Myers Squibb, and sanofiaventis; she also reports grants and consulting fees paid to her institution from HeartFlow. No other disclosures were reported.
References
- 1.Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography a process in evolution. J Am Coll Cardiol. 2010;55(10):957–965. doi: 10.1016/j.jacc.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 2.Meijboom WB, Van Mieghem CA, van Pelt N, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography vs conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52(8):636–643. doi: 10.1016/j.jacc.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Schuijf JD, Bax JJ. CT angiography: an alternative to nuclear perfusion imaging? Heart. 2008;94(3):255–257. doi: 10.1136/hrt.2006.105833. [DOI] [PubMed] [Google Scholar]
- 4.Lauer MS. CT angiography: first things first. Circ Cardiovasc Imaging. 2009;2(1):1–3. doi: 10.1161/CIRCIMAGING.108.841429. [DOI] [PubMed] [Google Scholar]
- 5.Hachamovitch R, Di Carli MF. Methods and limitations of assessing new noninvasive tests, I: anatomy-based validation of noninvasive testing. Circulation. 2008;117(20):2684–2690. doi: 10.1161/CIRCULATIONAHA.107.708586. [DOI] [PubMed] [Google Scholar]
- 6.Boden WE, O’Rourke RA, Teo KK, et al. COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 7.Frye RL, August P, Brooks MM, et al. BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pijls NH, De Bruyne B. Coronary pressure measurement and fractional flow reserve. Heart. 1998;80(6):539–542. doi: 10.1136/hrt.80.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonino PA, De Bruyne B, Pijls NH, et al. FAME Study Investigators. Fractional flow reserve vs angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 10.Pijls NH, Fearon WF, Tonino PA, et al. FAME Study Investigators. Fractional flow reserve vs angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56(3):177–184. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Fearon WF, Bornschein B, Tonino PA, et al. Fractional Flow Reserve Versus Angiography for Multi-vessel Evaluation (FAME) Study Investigators. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation. 2010;122(24):2545–2550. doi: 10.1161/CIRCULATIONAHA.109.925396. [DOI] [PubMed] [Google Scholar]
- 12.Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms: results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Non-invasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58(19):1989–1997. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Vignon-Clementel IE, Coogan JS, Figueroa CA, Jansen KE, Taylor CA. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng. 2010;38(10):3195–3209. doi: 10.1007/s10439-010-0083-6. [DOI] [PubMed] [Google Scholar]
- 14.Min JK, Berman DS, Budoff MJ, et al. Rationale and design of the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography) study. J Cardiovasc Comput Tomogr. 2011;5(5):301–309. doi: 10.1016/j.jcct.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3(3):190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Halliburton SS, Abbara S, Chen MY, et al. Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011;5(4):198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min JK, Koo BK, Erglis A, et al. Effect of image quality on diagnostic accuracy of noninvasive fractional flow reserve: results from the prospective multicenter international DISCOVER-FLOW study. J Cardiovasc Comput Tomogr. 2012;6(3):191–199. doi: 10.1016/j.jcct.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Naidu SS, Rao SV, Blankenship J, et al. Clinical expert consensus statement on best practices in the cardiac catheterization laboratory: Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. doi: 10.1002/ccd.24311. published online March 20, 2012. [DOI] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under 2 or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 20.Fearon WF, Takagi A, Jeremias A, et al. Use of fractional myocardial flow reserve to assess the functional significance of intermediate coronary stenoses. Am J Cardiol. 2000;86(9):1013–1014. doi: 10.1016/s0002-9149(00)01139-5. [DOI] [PubMed] [Google Scholar]
- 21.Shaw LJ, Berman DS, Maron DJ, et al. COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 22.Klocke FJ, Baird MG, Lorell BH, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American Society for Nuclear Cardiology. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) Circulation. 2003;108(11):1404–1418. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 23.Hendel RC, Patel MR, Kramer CM, et al. American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group; American College of Radiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology; North American Society for Cardiac Imaging; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology. ACCF/ACR/SCCT/SCMR /ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48(7):1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107(23):2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 25.Hendel RC, Berman DS, Di Carli MF, et al. American College of Cardiology Foundation Appropriate Use Criteria Task Force; American Society of Nuclear Cardiology; American College of Radiology; American Heart Association; American Society of Echocardiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; Society of Nuclear Medicine. ACCF /ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging. J Am Coll Cardiol. 2009;53(23):2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Berman DS, Kang X, Slomka PJ, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14 (4):521–528. doi: 10.1016/j.nuclcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Gaemperli O, Bengel FM, Kaufmann PA. Cardiac hybrid imaging. Eur Heart J. 2011;32(17):2100–2108. doi: 10.1093/eurheartj/ehr057. [DOI] [PubMed] [Google Scholar]
- 28.Mowatt G, Vale L, Brazzelli M, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Health Technol Assess. 2004;8(30):1–207. doi: 10.3310/hta8300. [DOI] [PubMed] [Google Scholar]