Abstract
Background
Suboptimal vitamin D status is prevalent in HIV-infected patients and associated with increased risk of disease severity and morbidity. We aimed to determine 12-mo safety and efficacy of daily 7000IU vitamin D3 (vitD3) vs placebo to sustain increased serum 25-hydroxyvitamin D (25(OH)D) and improve immune status in HIV-infected subjects.
Methods
This was a double-blind trial of perinatally- (PHIV) or behaviorally-acquired (BHIV) HIV-infected subjects (5.0–24.9y). Safety, 25(OH)D-related parameters, and immune status were assessed at baseline, 3, 6, and 12 months.
Results
Fifty-eight subjects enrolled (67% male, 85% African-American, 64% BHIV) and 50 completed with no safety concerns. In unadjusted analyses, there were no differences between randomization groups at baseline; at 3, 6, and 12 months, 25(OH)D was higher with supplementation than baseline and higher than with placebo (P<0.05). In adjusted mixed models, in the supplementation group, the fixed effect of 25(OH)D was higher (P<0.001). Percentage of naïve T helper cells (Th naïve%) were significantly (P<0.01) and T helper cells (CD4%) marginally (P<0.10) increased with supplementation in those taking highly active antiretroviral therapy (HAART), and RNA viral load was reduced (P ≤ 0.05). In exploratory linear models, change in 25(OH)D predicted RNA viral load at 3 and 12 months and CD4% at 3 months (P<0.05).
Conclusions
Daily 7000IU vitD3 for 12 months was safe in HIV-infected subjects and effective in increasing 25(OH)D. Supplementation improved in some clinically important HIV immune markers in subjects on HAART. Adjunct therapy with high-dose, daily vitD3 for HIV-infected subjects and for those on/off HAART requires further investigation.
Keywords: Vitamin D3 supplementation, pediatric HIV, cholecalciferol, nutrition
INTRODUCTION
Suboptimal vitamin D (vitD) status is prevalent in HIV-infected patients and associated with increased risk of disease severity and morbidity.1–4 Low serum 25-hydroxyvitamin D (25(OH)D) and disease severity associations are noted in antiretroviral-treated and treatment naïve patients, with little information in those with perinatally- (PHIV) or behaviorally-acquired HIV infection (BHIV).1;2 VitD and its metabolites are required for essential functions including immune modulation through adaptive and innate mechanisms and anti-inflammatory actions.5;6 Higher 25(OH)D status is associated with higher CD4+ cell counts7–9 and lower RNA viral load.10;11 We reported cholecalciferol (vitD3) dose (4000 and 7000IU/d) selection trials in HIV-infected US youth12 and in Botswanan youth and adults (Steenhoff AP, 2014, personal communication). In both settings, short-term safety was established and improvements seen in several HIV immune status indicators. Efficacy, as 25(OH)D ≥32ng/mL in ≥80% of US participants, required 7000IU/d.12 In Botswana, both dose groups achieved the efficacy criteria.
Few randomized placebo controlled trials of vitD3 supplementation have been conducted in HIV-infected children and young adults.13;14 There are two high-dose trials: Arpadi et al15 in PHIV children, aged 6–16y, with 100,000IU vitD3 bimonthly for 12 mo; and Havens et al16 in young adults, aged 18–24y, with 50,000IU vitD3 monthly for 3 mo. Post-treatment significant increases in 25(OH)D were found in these intermittent dose studies, but no effect on CD4% or RNA viral load was reported.15
Interest in non-skeletal benefits of 25(OH)D and the low cost of vitD3 supplementation support further investigation as adjunct therapy for HIV. The primary aim was to determine the long-term safety and efficacy of daily high-dose vitD3 (7000IU/d), compared with placebo, to sustain an increase in 25(OH)D over 12 mo in 5.0–24.9y participants with PHIV and BHIV. The secondary aim was to determine if supplementation modulates HIV immune status. To our knowledge, this is the first randomized, double-blind, placebo-controlled trial in HIV-infected children and young adults with daily high-dose vitD3 supplementation.
SUBJECTS AND METHODS
Subjects were from eight regional centers (July, 2011 to June, 2013). Inclusion criteria were: PHIV, 5.0–24.9y or BHIV, 15.0–24.9y; usual state of good health two weeks before enrollment. Exclusion criteria included: other adverse growth, dietary intake, or nutritional status conditions; pregnancy or lactation; vitD3 supplementation use. Subjects willing to discontinue supplementation underwent a minimum 2-mo washout period before treatment.17
Parents/guardians or subjects (≥18y or emancipated minor) gave informed consent, and children assented. Study examinations were performed at the Children’s Hospital of Philadelphia (CHOP), in mornings, with blood drawn fasting. The CHOP Institutional Review Board approved the protocol. A monitoring committee reviewed interval data and established subject withdrawal rules. The trial was registered with clinicaltrials.gov as NCT01475890.
Intervention Adherence and Safety Monitoring
Subjects were stratified by PHIV/BHIV and randomized in parallel (1:1 ratio) to receive either 7000IU/d vitD3 or placebo (Life Extension, Ft. Lauderdale, FL). Those unable to swallow capsules took 0.49 mL/d of 400IU vitD3 or placebo drops (J.R. Carlson Laboratories, Inc., Arlington Heights, IL). VitD3 products were independently assessed for potency. Enrollment was balanced by season and reflects the age/sex distribution of PHIV/BHIV groups. A 3.5-mo supply was provided at baseline and 3-mo visits, and a 7-mo supply at the 6-mo visit. Subjects returned supplement/placebo containers at each visit, residual capsules/volumes were recorded. Cumulative 12-mo adherence was determined (percent taken). Adverse events were collected at each visit by structured interviews. The vitD3 withdrawal rule was: three consecutive 25(OH)D values <11ng/mL necessitated withdrawal from study and referral for treatment.
Safety and Efficacy Outcomes
The primary safety hypothesis was that 7000IU/d vitD3 supplementation for 12 mo was safe, defined by low incidence (<5%) of the serious adverse event of simultaneously elevated serum calcium and 25(OH)D >160ng/mL. The primary efficacy hypothesis was that 7000IU/d vitD3 for 12 mo would result in increased 25(OH)D, compared with placebo. 25(OH)D status for non-bone health outcomes was defined based on contemporaneous literature: sufficient, ≥32ng/mL; insufficient, 20–31.9ng/mL; deficient, <20ng/mL; and severely deficient, <11ng/mL.18–20 Secondary outcomes included HIV immune status, vitD-related and metabolic variables.
Laboratory Assessments
Serum 25(OH)D was determined using liquid chromatography tandem mass spectrometry (CHOP Laboratory), serum 1,25-dihydroxyvitamin D (1,25(OH)D) and intact parathyroid hormone (PTH) by radioimmunoassay (Heartland Assays, Ames, IA), and vitD binding protein (VDBP) by enzyme linked immunosorbent assay (R&D Systems, Minneapolis, MN). Bioavailable 25(OH)D not bound to VDBP or albumin was calculated.21
At all visits, safety assessments for serum calcium, phosphorus, and gamma-glutamyl transferase (GGT) (CHOP Laboratory). A comprehensive metabolic panel and lipid panel were assessed. Insulin was measured (ARUP Laboratories, Salt Lake City, UT) at baseline and 6 mo, and serum neopterin (EIA, MP Biomedicals, Solon, OH) at baseline and 3 mo. Urine was collected at each visit for calcium and creatinine (CHOP Laboratory).
Immunologic and Virologic Assessments
HIV-1 RNA plasma quantitative assay for viral load (copies/mL) was performed (CHOP Laboratory), and HIV-specific multicolor flow cytometry immunophenotyping assessed at each visit with a Becton Dickinson LSR II flow cytometer and BD FACSDiva software (Becton Dickinson, San Diego, CA).22 White blood cell count, absolute lymphocyte count, and immunophenotyping of peripheral lymphocytes were performed as previously described.23 The expression of natural cytotoxicity receptors, NKp46, NKp44 and NKp30, on natural killer (NK) cells was measured.12
Anthropometry, Skin Reflectance and Dietary Intake Assessments
Anthropometric measurements were obtained following standard techniques.24 Age- and sex-specific z-scores for height, weight and BMI (kg/m2) were calculated;25 19.9y was used for subjects 20.0–24.9y. Baseline pubertal status was determined by self-assessment questionnaire26 in subjects <18y (≥18y assumed fully mature). Skin reflectance of inner arm determined the melanin (pigmentation) and erythema (vascularization) indices (DSM II Colorimeter, Cortex Technology, Hadsund, Denmark).
At baseline, three 24-hour dietary recalls were obtained and averaged (Nutrition Data System, University of Minnesota Nutrition Coordinating Center, Minneapolis, MN). VitD and calcium intakes were compared with Recommended Dietary Allowance (RDA).27
Health History
Medical record reviews documented HIV disease status (CDC classification),12;24 age at diagnosis and initiation of highly active antiretroviral therapy (HAART). HAART was categorized as protease inhibitor- (PI), non-nucleoside reverse transcriptase inhibitor- (NNRTI), and nucleoside reverse transcriptase inhibitor-based (NRTI) regimens, and whether tenofovir or efavirenz were part of regimens. Medication and supplement use were from medical records and socio-demographic information by questionnaire.
Sample Size and Power
The sample size was based upon the assumption that change in 25(OH)D from baseline to 12 mo for placebo group will be zero, while change in supplementation group will be 14.9ng/mL, standard deviation (SD), 13.5ng/mL.28 A sample size of 17 subjects per arm was required to demonstrate an increase in 25(OH)D from baseline 17.1ng/mL to a target goal of ≥32ng/mL with supplementation, with 80% power and α=0.02. The 17.1ng/mL value was determined from our study in Philadelphia children.7
Statistical Analyses
Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) or STATA 12.0 (STATA, College Station, TX) and using intention-to-treat models, except for exploratory analyses for magnitude of effect of secondary outcomes. Variables were tested for normality, and either transformed, and/or results were confirmed using nonparametric tests. RNA (copies/mL) was the only variable that was so skewed that it required transformation and is presented as log10. Several immune outcomes had results confirmed using transformed data. Continuous variables are presented as mean±SD. Least square means (LSMean) from regression or mixed models are LSMean±SE. Comparisons between randomization groups were made at baseline using Student’s t-tests (continuous) and Pearson’s chi-square tests or Fisher’s exact tests (categorical variables).
For longitudinal efficacy data, difference between randomization groups at each visit time was tested using an unpaired Student’s t-test and change from baseline within each randomization group by paired t-test. Persistence of response over the three study visits was assessed using mixed models (SAS PROC MIXED), i.e., multilevel regression models, and generalized autoregressive structure, adjusting for baseline values and controlling for visit. Results from mixed models (LSMeans±SE) are the fixed effects of randomization group; tests of significance were made with values log10-transformed. The mixed model allows for inclusion of all subjects, including drop-outs, those with missing values, and those withdrawn for persistently low 25(OH)D in the regressions. HIV immune status was analyzed similarly. For those with missing RNA viral load at baseline, a value of 2.2 log10 copies/mL was used (mean for all log10 values, including for those with undetectable levels as 1.6/√2).
Exploratory analyses using multiple regression models with randomization groups combined were used to assess associations among baseline 25(OH)D, change (Δ) in 25(OH)D, and immune status outcomes. Other potential predictors tested included age, sex, African-American race, season, enrollment date, HAART use at baseline, and whether HAART status changed during the study. Statistical significance level was set at P=0.05 for all tests.
RESULTS
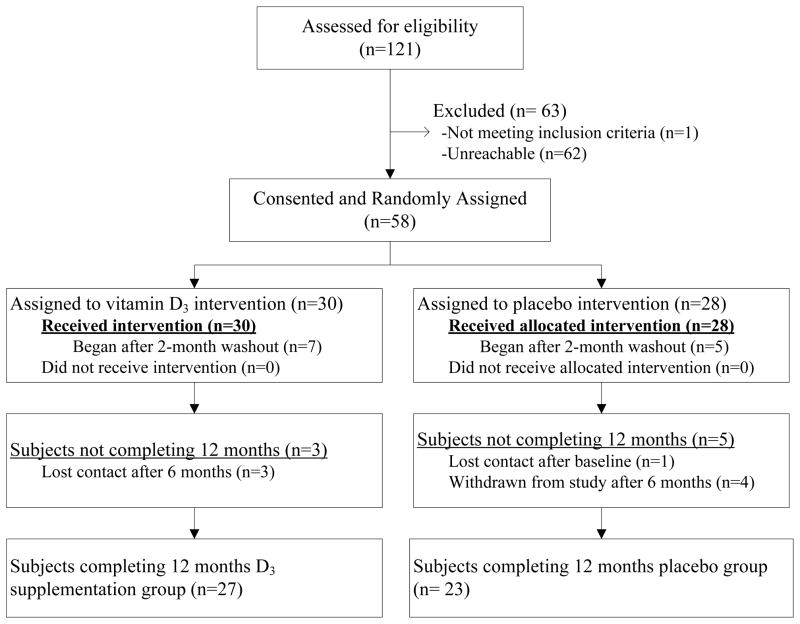
Fifty-eight subjects were recruited (Figure 1), 28 randomized to placebo and 30 to vitD3 supplementation. One after baseline (supplementation group) and three subjects after 6 mo (one placebo, two supplementation) were lost to follow-up. Four subjects (placebo group) were withdrawn after 6 mo and referred for treatment.
FIGURE 1.
Flow diagram for subjects randomized, drop-outs, and completing the placebo-controlled trial of daily 7000IU vitamin D3 supplementation in HIV-infected children and young adults.
Subjects were enrolled over all seasons, were 67% male, 84% African-American, 16% Hispanic, and aged 20.7±3.7y (range 9.6–24.9) (Table 1). Growth status was adequate, one-third were PHIV, and two-thirds BHIV. At baseline, 95% were suboptimal for 25(OH)D, 64% deficient, and 26% severely deficient. Dietary vitD intake was low, with intake for half of subjects <30% RDA. Randomization groups did not differ in demographic disposition, growth or maturity status, vitD and metabolic status, safety measures, HIV disease status, PHIV/BHIV, years since HIV diagnosis, or HAART status.
TABLE 1.
Background, Individual, and Disease Characteristics of Subjects with HIV by Randomization Group at Baseline
| All (n = 58) | Placebo (n = 28) | VitD3 Supplementation (n = 30) | |
|---|---|---|---|
| Background characteristics | |||
| Age (y) | 20.7 ± 3.7* | 20.0 ± 4.1 | 21.3 ± 3.3 |
| Sex, male, n (%) | 39 (67) | 19 (68) | 20 (67) |
| Racial identification, n (%) | |||
| African-American | 49 (84) | 23 (82) | 26 (87) |
| White | 2 (3) | 2 (7) | 0 (0) |
| Other and mixed | 7 (12) | 3 (11) | 4 (13) |
| Hispanic ethnicity, n (%) | 9 (16) | 5 (17) | 4 (13) |
| Skin reflectance indices† | |||
| Melanin index | 74.8 ± 18.5 | 70.8 ± 18.8 | 78.8 ± 17.7 |
| Erythema index | 18.1 ± 2.6 | 17.6 ± 2.9 | 18.7 ± 2.3 |
| Season at enrollment, n (%) | |||
| Summer/Fall | 20 (34) | 8 (28) | 12 (40) |
| Winter/Spring | 38 (66) | 20 (72) | 18 (60) |
| Individual characteristics | |||
| Height-for-age (z score) | −0.29 ± 1.03 | −0.21 ± 0.91 | −0.36 ± 1.14 |
| Weight-for-age (z score) | 0.11 ± 1.27 | 0.25 ± 1.25 | −0.12 ± 1.28 |
| BMI (kg/m2) | 24.2 ± 6.6 | 24.9 ± 8.0 | 23.5 ± 5.0 |
| BMI-for-age (z score) | 0.14 ± 1.26 | 0.25 ± 1.25 | 0.04 ± 1.29 |
| Tanner stage 5, n (%)‡ | 49 (85) | 22 (79) | 27 (90) |
| Vitamin D status | |||
| 25(OH)D (ng/mL) | 17.6 ± 8.7 | 17.0 ± 9.2 | 18.2 ± 8.4 |
| 25(OH)D classification, n (%) | |||
| < 11 ng/mL | 15 (26) | 9 (32) | 6 (20) |
| 11–19.9 ng/mL | 22 (38) | 8 (29) | 14 (47) |
| 20–31.9 ng/mL | 18 (31) | 10 (36) | 8 (27) |
| ≥32 ng/mL | 3 (5) | 1 (4) | 2 (7) |
| Dietary vitamin D intake (% RDA)§ | 30 ± 33 | 29 ± 26 | 30 ± 20 |
| Dietary calcium intake (% RDA)§ | 78 ± 35 | 71 ± 29 | 85 ± 39 |
| Safety measures | |||
| Calcium (mg/dL) | 9.4 ± 0.4 | 9.4 ± 0.4 | 9.5 ± 0.4 |
| Phosphorus (mg/dL) | 4.0 ± 0.6 | 4.1 ± 0.6 | 4.0 ± 0.6 |
| Urinary calcium/creatinine (mg/dL) | 0.05 ± 0.05 | 0.06 ± 0.06 | 0.05 ± 0.04 |
| Alanine aminotransferase (U/L) | 30 ± 11 | 31 ± 10 | 28 ± 12 |
| Gamma-glutamyl transferase (U/L) | 34 ± 19 | 35 ± 23 | 34 ± 15 |
| Metabolic status | |||
| Glucose (mg/dL) | 81 ± 10 | 81 ± 9 | 81 ± 12 |
| Insulin (μIU/mL) | 14.4 ± 11.4 | 15.2 ± 11.8 | 13.7 ± 11.3 |
| Triglycerides (mg/dL) | 88 ± 56 | 93 ± 79 | 84 ± 29 |
| LDL cholesterol (mg/dL) | 96 ± 28 | 93 ± 30 | 99 ± 28 |
| Disease characteristics | |||
| Acquisition route, n (%) | |||
| Perinatal | 21 (36) | 11 (39) | 10 (33) |
| Behavioral | 37 (64) | 17 (61) | 20 (67) |
| Age at diagnosis (y) | 12.5 ± 8.6 | 11.6 ± 8.8 | 13.3 ± 8.5 |
| Years since diagnosis (y) | 8.2 ± 7.1 | 8.4 ± 6.6 | 8.0 ± 7.5 |
| Age at HAART (y)¶ | 11.7 ± 9.5 | 9.8 ± 9.4 | 13.5 ± 9.4 |
| Years on HAART (y)¶ | 8.4 ± 7.4 | 9.3 ± 7.3 | 7.6 ± 7.5 |
| On HAART (yes), n (%) | 44 (76) | 21 (75) | 23 (77) |
| PI, n (%)|| | 21 (48) | 7 (33) | 14 (61) |
| NNRTI, n (%)|| | 24 (55) | 14 (67) | 10 (43) |
| Efavirenz, n (%) | 21 (48) | 11 (52) | 10 (43) |
| NRTI, n (%)|| | 44 (100) | 21 (100) | 23 (100) |
| Tenofovir, n (%) | 34 (77) | 16 (76) | 18 (78) |
| RNA detectable (yes), n (%)** | 24 (44) | 11 (41) | 13 (46) |
| RNA viral load (log10 copies/mL)†† | 3.17 ± 0.96 | 3.29 ± 0.92 | 3.06 ± 1.02 |
| T helper cells % (CD4%) | 31.8 ± 12.1 | 32.9 ± 14.4 | 30.8 ± 9.5 |
| Immunity category at baseline, n (%) | |||
| CD4+ count ≥ 500 | 36 (62) | 17 (61) | 19 (63) |
| CD4+ count 200–499 | 19 (33) | 9 (32) | 10 (33) |
| CD4+ count < 200 | 3 (5) | 2 (7) | 1 (3) |
| Serum neopterin (ng/mL) | 3.3 ± 2.3 | 3.2 ± 2.1 | 3.4 ± 2.6 |
Abbreviations: BMI, body mass index; HAART, highly active antiretroviral therapy; LDL cholesterol, low-density lipoprotein cholesterol; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; VitD3, vitamin D3; 25(OH)D, 25-hydroxyvitamin D.
Data are mean ± SD or number (%).
There were no significant differences between the placebo and vitamin D3 supplementation groups for any of the baseline, individual, vitamin D status, safety, metabolic status, dietary intake, or disease characteristics. All blood status values are from analyses on serum. The table includes the one subject in the placebo group who had no follow-up, i.e., dropped out after the baseline visit; analyses excluding the subject with no follow-up did not change the pattern of the results or significance.
For skin reflectance measures, n = 50 (25 placebo, 25 supplementation).
Tanner stage 5 for breast (female) or genital (male) development for adolescents < 19y or presumed maturity for subjects 19y and older.
Intake classified as percentage of the RDA for age and sex established in 2010 IOM report.37
For age at HAART initiation and years on HAART, n = 44 subjects who were on HAART at baseline (21 placebo, 23 supplementation).
The denominators for the specific drug classes are the numbers of subjects who are on HAART, n = 44 on HAART at baseline (21 placebo, 23 supplementation); subjects could be taking more than one drug from a class.
For RNA detectable %, n = 55 (27 placebo, 28 supplementation).
For detectable RNA (log10 copies/mL), n = 24 (11 placebo, 13 supplementation).
Safety and Metabolic Outcomes
No subject experienced the study-defined serious safety event; in fact, none had 25(OH)D >80ng/mL at any time. Randomization groups did not differ in any safety measure or reported adverse events. There was no change from baseline for any safety measure except serum calcium which increased from 9.5±0.4 to 9.6±0.4mg/dL at 12 mo (P<0.05) in the supplementation group (not shown). No changes were noted in metabolic outcomes, growth, or BMI status with either placebo or supplementation, nor between groups
Efficacy Outcomes
Supplementation was effective over 12 mo in increasing 25(OH)D (Table 2). More subjects in the supplementation compared with placebo group exceeded the 25(OH)D cut-off value for sufficiency (≥32ng/mL) at 3 mo (40% vs 4%, P<0.01) and 12 mo (33% vs 9%, P<0.05), the deficiency cut-off (≥20ng/mL) at 3 mo (83% vs 33%, P<0.001), and the severe deficiency cut-off value (≥11ng/mL) out to 12 mo (93% vs 61%, P<0.02). In unadjusted analyses, 25(OH)D with supplementation was higher than baseline at 3, 6 and 12 mo; 25(OH)D for the placebo group remained unchanged. In mixed models, controlling for study visit time and adjusting for baseline value, 25(OH)D response to supplementation across the 12 mo was significantly different from placebo (+12.1±2.8ng/mL). VDBP did not differ between groups at baseline (122±91 and 150±97 μg/mL, placebo and supplementation, respectively). Free and bioavailable 25(OH)D showed responses to 12 mo and overall; 1,25(OH)D showed response to 6 mo and overall with supplementation. There was no change in PTH. Melanin index skin reflectance was negatively associated with VDBP (r=−0.45, P<0.001), and erythema index was positively associated with bioavailable 25(OH)D (r=0.28, P=0.049). Adherence to supplement/placebo was 92±13% over 3 mo and 92±8% over 12 mo, with no differences between groups.
TABLE 2.
Efficacy of Daily 7000IU Vitamin D3 Supplementation in Improving Serum 25(OH)D and Related Parameters
| Time of study visit | Fixed effect of randomization* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 mo | 6 mo | 12 mo | ||||||
| n | mean ± SD | n | mean ± SD | n | mean ± SD | n | mean ± SD | LSMean ± SE | |
| 25(OH)D (ng/mL) | |||||||||
| Placebo | 28 | 17.0 ± 9.2 | 27 | 17.7 ± 9.0 | 27 | 18.4 ± 10.4 | 23 | 16.9 ± 9.3 | 17.7 ± 2.0 |
| Supplement | 30 | 18.2 ± 8.4 | 30 | 32.5 ± 13.6§,** | 30 | 29.2 ± 14.4‡,** | 27 | 28.4 ± 19.8§,¶ | 29.8 ± 1.9‡‡ |
| Free 25(OH)D (pg/mL) | |||||||||
| Placebo | 28 | 11.3 ± 7.6 | 27 | 11.4 ± 6.5 | 23 | 10.5 ± 6.2** | 10.2 ± 1.4 | ||
| Supplement | 30 | 10.3 ± 6.4 | 30 | 19.7 ± 12.4‡,** | 27 | 17.0 ± 13.1†,|| | 18.9 ± 1.3‡‡ | ||
| Bioavailable 25(OH)D (ng/mL) | |||||||||
| Placebo | 28 | 4.4 ± 3.1 | 27 | 4.5 ± 2.6 | 23 | 4.2 ± 2.3 | 4.1 ± 0.6 | ||
| Supplement | 30 | 4.1 ± 2.5 | 30 | 7.6 ± 4.7‡,** | 27 | 6.9 ± 5.9†,|| | 7.5 ± 0.6‡‡ | ||
| 1,25(OH)D (pg/mL) | |||||||||
| Placebo | 28 | 30.6 ± 10.6 | 27 | 35.9 ± 27.5 | 27 | 36.2 ± 14.5 | 23 | 33.6 ± 14.0 | 35.4 ± 2.2 |
| Supplement | 30 | 32.7 ± 11.9 | 30 | 43.1 ± 15.7** | 30 | 41.8 ± 13.1|| | 27 | 32.3 ± 10.3 | 38.9 ± 2.1†† |
| PTH (pg/mL) | |||||||||
| Placebo | 28 | 31.5 ± 16.3 | 27 | 37.5 ± 19.0 | 23 | 36.3 ± 17.7 | 37.8 ± 3.0 | ||
| Supplement | 30 | 34.2 ± 25.9 | 30 | 34.3 ± 30.4 | 27 | 42.6 ± 32.1 | 37.0 ± 2.8 | ||
Abbreviations: PTH, intact parathyroid hormone; LSMean, least square mean; 1,25(OH)D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Analytic note: Laboratory values were acquired with good reliability. For serum 25(OH)D, inter- and intra-assay coeffients of variation (CV) were <8%. Inter- and intra-assay CV were 12.6% and 9.8% for 1,25(OH)D and 2.7% and 4.3% for PTH, respectively.
Results are the fixed effect of randomization group from multilevel regression model (SAS PROC MIXED) testing for persistence of response at 3–12 months based on untransformed data, also controlling for time of study visit and adjusting for baseline values; data are LSMean ± SE. The significance of the fixed effect was assessed or confirmed based on log10-transformed data.
Significantly different from placebo mean within time of study visit by Student’s t-test at P < 0.05;
P < 0.01;
P < 0.001.
Significantly different from baseline mean within randomization group by paired t-test at P < 0.05;
P < 0.01;
P < 0.001.
Fixed effect for supplementation significantly different from placebo at P < 0.05;
P< 0.001.
Secondary Outcomes
Several immune indicators improved with supplementation (Table 3). For those with a detectable RNA viral load at any time point, in mixed models adjusting for baseline viral load, the supplementation group viral load was less. Detectable RNA viral load (copies/mL) continued to rise for the placebo group and stayed at a higher level. Exclusion of subjects who were missing and/or undetectable at baseline but had detectable levels at later points did not change the significance of these findings.
TABLE 3.
Effects of Daily 7000IU Vitamin D3 Supplementation on Markers of HIV Immune Status at 3, 6, and 12 Months
| Time of study visit | Fixed effect of randomization* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 mo | 6 mo | 12 mo | ||||||
|
| |||||||||
| n | n (%) | n | n (%) | n | n (%) | n | n (%) | ||
| RNA detectable (yes) | |||||||||
| Placebo | 27 | 11 (41) | 27 | 12 (44) | 27 | 13 (48) | 23 | 13 (57) | |
| Supplement | 28 | 13 (46) | 30 | 12 (40) | 30 | 15 (50) | 27 | 15 (56) | |
|
| |||||||||
| n | mean ± SD | n | mean ± SD | n | mean ± SD | n | mean ± SD | LSMean ± SE | |
|
| |||||||||
| RNA detectable (log10 copies/mL) | |||||||||
| Placebo | 11 | 3.06 ± 1.02 | 12 | 3.57 ± 1.23 | 13 | 3.36 ± 0.94 | 13 | 3.78 ± 1.06† | 3.53 ± 0.15 |
| Supplement | 13 | 3.29 ± 0.92 | 12 | 2.99 ± 0.84 | 15 | 3.03 ± 1.00 | 15 | 3.12 ± 0.81 | 3.06 ± 0.14§ |
| CD3% | |||||||||
| Placebo | 28 | 79.4 ± 8.4 | 27 | 79.6 ± 10.0 | 26 | 81.1 ± 9.5 | 23 | 81.0 ± 10.1 | 81.8 ± 1.0 |
| Supplement | 30 | 78.8 ± 5.8 | 30 | 80.1 ± 6.8 | 30 | 81.4 ± 6.4‡ | 27 | 82.7 ± 6.7‡ | 80.1 ± 0.9 |
| CD4% | |||||||||
| Placebo | 28 | 32.9 ± 14.4 | 27 | 31.2 ± 14.1† | 26 | 31.9 ± 13.7 | 23 | 33.1 ± 14.8 | 30.6 ± 1.0 |
| Supplement | 30 | 30.8 ± 9.5 | 30 | 31.8 ± 9.2 | 30 | 31.9 ± 8.0 | 27 | 32.3 ± 11.1 | 33.1 ± 0.9|| |
| CD8% | |||||||||
| Placebo | 28 | 43.1 ± 15.1 | 27 | 45.0 ± 15.2 | 26 | 45.9 ± 14.6 | 23 | 45.0 ± 14.4 | 45.6 ± 1.2 |
| Supplement | 30 | 44.8 ± 9.0 | 30 | 45.6 ± 10.3 | 30 | 46.5 ± 10.3 | 27 | 46.7 ± 10.3 | 46.2 ± 1.1 |
| CD19% | |||||||||
| Placebo | 28 | 10.6 ± 5.2 | 27 | 8.7 ± 4.7 | 27 | 8.9 ± 6.2 | 23 | 8.1 ± 5.4† | 8.3 ± 0.6 |
| Supplement | 30 | 9.5 ± 3.5 | 30 | 8.3 ± 4.0 | 30 | 7.8 ± 3.9† | 27 | 7.1 ± 4.0† | 7.9 ± 0.6 |
| Th memory% | |||||||||
| Placebo | 28 | 51.1 ± 13.7 | 27 | 49.7 ± 16.2 | 26 | 50.8 ± 16.4 | 23 | 52.0 ± 19.0 | 49.2 ± 1.2 |
| Supplement | 30 | 47.4 ± 11.4 | 30 | 44.6 ± 9.2† | 30 | 45.4 ± 10.9 | 27 | 46.8 ± 9.8 | 47.2 ± 1.1 |
| Th naïve% | |||||||||
| Placebo | 28 | 44.4 ± 12.3 | 27 | 42.6 ± 12.1 | 26 | 43.6 ± 11.4 | 23 | 42.5 ± 15.5 | 42.9 ± 1.0 |
| Supplement | 30 | 45.1 ± 13.0 | 30 | 47.0 ± 10.7† | 29 | 47.8 ± 11.3 | 27 | 46.7 ± 10.0 | 47.1 ± 0.9¶ |
| NK% | |||||||||
| Placebo | 28 | 8.4 ± 5.8 | 27 | 10.0 ± 8.3 | 26 | 9.8 ± 7.7 | 23 | 9.3 ± 8.0 | 10.1 ± 0.8 |
| Supplement | 30 | 8.8 ± 4.6 | 30 | 9.2 ± 4.9 | 30 | 8.5 ± 3.8 | 27 | 9.0 ± 5.4 | 8.5 ± 0.7 |
| NKp30% | |||||||||
| Placebo | 28 | 44.8 ± 23.9 | 27 | 40.6 ± 22.2 | 27 | 42.2 ± 22.7 | 23 | 43.9 ± 22.1 | 40.8 ± 2.1 |
| Supplement | 30 | 41.4 ± 25.8 | 30 | 40.2 ± 20.9 | 30 | 39.5 ± 19.7 | 27 | 40.1 ± 26.5 | 41.3 ± 2.0 |
| NKp44% | |||||||||
| Placebo | 28 | 1.7 ± 2.0 | 27 | 0.8 ± 1.3† | 27 | 1.0 ± 0.9† | 23 | 1.6 ± 2.7 | 1.1 ± 0.2 |
| Supplement | 30 | 1.6 ± 2.0 | 30 | 1.1 ± 1.2 | 30 | 1.8 ± 3.2 | 27 | 1.2 ± 1.8 | 1.4 ± 0.2§ |
| NKp46% | |||||||||
| Placebo | 28 | 52.8 ± 23.8 | 27 | 55.9 ± 23.3 | 27 | 48.8 ± 23.5 | 23 | 50.7 ± 25.5 | 50.4 ± 2.9 |
| Supplement | 30 | 48.5 ± 25.1 | 30 | 49.2 ± 21.1 | 30 | 47.5 ± 19.6 | 27 | 47.1 ± 24.8 | 49.0 ± 2.8 |
| HLA-DR% | |||||||||
| Placebo | 28 | 25.4 ± 17.6 | 27 | 27.4 ± 17.9 | 26 | 25.1 ± 16.7 | 23 | 22.0 ± 15.7 | 24.9 ± 1.9 |
| Supplement | 30 | 24.2 ± 14.3 | 30 | 22.5 ± 14.1 | 30 | 22.0 ± 14.2 | 27 | 23.5 ± 14.5 | 23.0 ± 1.7 |
Definitions: CD3%, T cells %, CD45+/CD3+; CD4%, T helper cells %, CD45+/CD3+/CD4+; CD8%, T cytotoxic cells %, CD45+/CD3+/CD8+; CD19%, B cells %, CD45+/CD3−/CD19+; HLA-DR%, activated cytotoxic T cells %, CD3+/CD8+/CD38+; LSMean, least square mean; NK%, NK (natural killer) cells %, CD45+/CD3−/CD16+CD56+; NKp30%, NKp30+ NK cells % (natural cytotoxicity receptor), CD45+/CD3−/CD16+CD56+/NKp30+; NKp44%, NKp44+ NK cells % (natural cytotoxicity receptor), CD45+/CD3−/CD16+CD56+/NKp44+; NKp46%, NKp46+ NK cells % (natural cytotoxicity receptor), CD45+/CD3−/CD16+CD56+/NKp46+; Th memory%, memory T helper cells %, CD3+/CD4+/CD45RA−/CD45RO+; Th naïve%, naïve T helper cells %, CD3+/CD4+/CD45RA+/CD62L+.
Results are the fixed effect of randomization group from multilevel regression model (SAS PROC MIXED) testing for persistence of response at 3–12 months based on untransformed data, also controlling for time of study visit and adjusting for baseline values; data are LSMean ± SE. The significance of the fixed effect was assessed or confirmed based on log10-transformed data.
Significantly different from baseline within randomization group by paired t-test at P < 0.05;
P < 0.01.
Fixed effect for supplementation significantly different from placebo at P < 0.05;
P < 0.01.
Fixed effect for supplementation significantly different from placebo at P < 0.10.
For CD3%, CD4%, Th memory% and Th naïve%, differences over time are in the direction of indicating clinical improvement for the supplementation group compared with placebo, with marginal or significant changes in CD4% and Th naïve%, respectively. Neopterin was unchanged from baseline to 3 mo for both groups (not shown). There were no significant differences for other immunologic markers.
Further analyses were conducted to determine if HAART was associated with changes in 25(OH)D and immune indicators. In these models, changes in HAART status (on/off) over the course of the study were covaried. For 25(OH)D, there was a significant 25(OH)D and HAART interaction such that in post hoc comparisons vitD3 supplementation appeared effective only in the presence of HAART. For those in the supplementation group on HAART at baseline, the fixed effect was 33.3±2.1ng/mL, compared with 17.2±4.1ng/mL for those off HAART (P<0.01). There were no differences in 25(OH)D for the placebo group by HAART status. For Th naïve%, the pattern was similar, with supplementation improving status only with HAART. The response on HAART for Th naïve% with supplementation (47.4±1.0%) compared with placebo (43.2±1.1%) was significant (P<0.01).
Vitamin D Status Predicting HIV Immune Status
Table 4 presents the results of exploratory regression models including all subjects with baseline 25(OH)D and change (increase or decrease) in 25(OH)D (Δ25(OH)D) predicting immune markers at 3 and 12 mo, after adjusting for covariates. Δ25(OH)D significantly negatively predicted RNA viral load at 3 and 12 mo; increased 25(OH)D predicted a significant decrease in viral load over time. For those with detectable viral load at baseline, this remained significant at 3 mo. Δ25(OH)D significantly positively predicted CD4% at 3 mo, with baseline 25(OH)D showing a marginal effect. Both baseline and Δ25(OH)D significantly negatively predict NK% at 3 mo and baseline 25(OH)D significantly negatively predicted NK% at 12 mo. Baseline 25(OH)D also negatively predicted HLA-DR%, a marker of immune activation, at 3 and 12 mo with Δ25(OH)D having a marginal negative effect. At 3 mo only, Δ25(OH)D significantly positively predicted Th naïve%. In summary, RNA viral load was decreased with increased 25(OH)D short- and long-term, and CD4% and Th naïve% were increased and NK% decreased short-term. Conversely, decreased 25(OH)D was associated with increased RNA viral load and NK%, and decreased CD4% and Th naïve%. Subjects with higher 25(OH)D at baseline had greater reductions in NK% and HLA-DR% short- and long-term. There were no significant seasonal effects on Δ25(OH)D or immune outcomes.
TABLE 4.
Serum 25(OH)D Status (ng/mL) at Baseline and Change to 3 and 12 Months, Predicting HIV Immune Markers for Total Sample
| 3 Mo Outcomes | 12 Mo Outcomes | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Variable | Predictor | Coefficient* | SE | P-value | Coefficient | SE | P-value |
| RNA (log10)† (all) |
25(OH)D baseline | 0.00 | 0.01 | NS | −0.01 | 0.01 | NS |
| Δ 25(OH)D | −0.02 | 0.01 | 0.013 | −0.02 | 0.01 | 0.045 | |
| RNA (log10)‡ (detectable) |
25(OH)D baseline | 0.01 | 0.02 | NS | −0.01 | 0.05 | NS |
| Δ 25(OH)D | −0.05 | 0.02 | 0.014 | −0.06 | 0.05 | NS | |
| CD4%§ | 25(OH)D baseline | 0.18 | 0.10 | 0.077 | 0.16 | 0.12 | NS |
| Δ 25(OH)D | 0.13 | 0.06 | 0.037 | 0.07 | 0.06 | NS | |
| NK%§ | 25(OH)D baseline | −0.13 | 0.07 | 0.047 | −0.19 | 0.09 | 0.037 |
| Δ 25(OH)D | −0.08 | 0.04 | 0.047 | −0.04 | 0.05 | NS | |
| HLA-DR%§ | 25(OH)D baseline | −0.38 | 0.19 | 0.050 | −0.44 | 0.21 | 0.044 |
| Δ 25(OH)D | −0.22 | 0.12 | 0.075 | −0.19 | 0.11 | 0.082 | |
| Th naïve%¶ | 25(OH)D baseline | 0.03 | 0.14 | NS | −0.02 | 0.14 | NS |
| Δ 25(OH)D | 0.19 | 0.08 | 0.026 | 0.03 | 0.07 | NS | |
Abbreviations: HAART, highly active antiretroviral therapy; 25(OH)D, 25-hydroxyvitamin D; Δ 25(OH)D, change in 25-hydroxyvitamin D; NS, not significant.
Definitions: CD4%, T helper cells %, CD45+/CD3+/CD4+; HLA-DR%, activated cytotoxic T cells %, CD3+/CD8+/CD38+; NK%, NK (natural killer) cells, CD45+/CD3−/CD16+CD56+; Th naïve%, naïve T helper cells %, CD3+/CD4+/CD45RA+/CD62L+.
Covariates included in all models were immune status at baseline, age, African-American, baseline HAART use, and change in HAART use during the study. For RNA (log10) (all), levels that were not detectable were set to the limit of detection (LOD) as LOD/√2. All overall models were significant at P < 0.001, with the exception of RNA (log10) (detectable only) at 12 mo which was not significant.
Sample sizes for baseline and 12 months, respectively: n = 54 and 47, RNA (log10) (all);
n = 23 and 19, RNA (log10) (detectable at baseline only);
n = 57 and 50, CD4%, NK%, HLA-DR%;
n = 56 and 50, Th naïve%.
DISCUSSION
This 12-mo randomized, double-blind, placebo-controlled trial in predominantly African-American HIV-infected subjects with PHIV and BHIV demonstrated the safety and efficacy of 7000IU/d vitD3 supplementation. Supplementation improved vitD status and several markers of HIV immune status, including increased CD4% and decreased RNA viral load. These latter improvements appear small but are statistically significant and may be clinically meaningful.29 HAART status predicted the change with supplementation in 25(OH)D and HIV immune markers. Our sample was 95% insufficient at baseline, 64% deficient, and 26% severely deficient. Others have reported similar 25(OH)D status in HIV-infected children and adults,7;15;16;30–36 and those with poor status are at risk for greater disease severity.1;2;8;10 African-Americans are disproportionately affected by HIV infection and are at high risk for 25(OH)D deficiency.37 Low 25(OH)D status in our inner-city sample may be due to a combination of inadequate sunlight, low dietary/supplemental vitD intake, skin pigmentation, specific drug therapy, malabsorption, or unknown HIV-associated factors. Participants are similar to the HIV-infected population currently in care in the US, with 45% asymptomatic for HIV and 56% with undetectable RNA viral load. This was the first randomized, clinical trial evaluating the long-term safety and efficacy of daily high-dose vitD3 in HIV-infected children and young adults. A daily dose regimen may have a clinical advantage by maintaining stable increased 25(OH)D.
Treatment was safe. No subject experienced a study-defined serious safety event, and none had 25(OH)D >80ng/mL. These results are consistent with trials in healthy adults and African-Americans using 4000IU/d to 10,000IU/d without adverse events20;38–40 and in HIV-infected subjects.12;41 This extends our 3-mo findings that both 4000IU/d and 7000IU/d vitD3 are safe and well-tolerated in HIV-infected participants.12
Supplementation with 7000IU/d maintained a +12.1±2.8ng/mL increase in 25(OH)D, compared with placebo. Similar 25(OH)D responses were found in the three placebo-controlled trials of HIV-infected children and young adults that used very high doses in non-daily dose regimens, i.e., 100,000IU every 3 mo for 12 mo,33 100,000IU every 2 mo for 12 mo,15 and 50,000IU/mo for 3 mo.16 We found a higher 25(OH)D response than in a placebo-controlled trial of HIV-infected adults administered a dose of 4000IU/d for 3 mo.41 The 7000IU/d regimen improved efficacy and did not result in 25(OH)D peaks and troughs seen with interval dosing.15
In the present study, improved 25(OH)D with supplementation was not associated with a decline in PTH. Decreases in PTH with vitD3 supplementation in HIV-infected subjects have been reported,33;42 but not consistently,42;43 and may be associated with specific anti-retroviral therapy (i.e., tenofovir).43;44 In order to assess the full magnitude of potential efficacy, more sensitive and specific adherence measures to both supplement and HIV treatment are needed.
There is a need to determine the dose regimen to optimize 25(OH)D for subjects with increased risk for cardiovascular and immune-mediated diseases, including HIV.45 Several studies have reported high-dose (≥4000IU/d) safety and efficacy in healthy adults and concluded that vitD3 as high as 10,000IU/d was safe.38–40 The optimal 25(OH)D needed to affect disease outcomes, i.e., ≥20ng/mL, ≥30ng/mL, ≥32ng/mL, or other concentration, is unknown, and trials are needed in different disease and patient groups. The concentration needed will likely differ by disease diagnoses or population, such as African-Americans. In a 3-mo, placebo-controlled trial of over 300 healthy African-American adults, Ng et al20 estimated that 1640IU/d was needed to raise 25(OH)D to ≥20ng/mL in ≥97.5% of subjects; 4000IU/d was needed to achieve ≥33ng/mL for ≥80%. The response of healthy and HIV-infected African-Americans to vitD3 supplementation is also influenced by VDBP genetics. Individuals of African descent have lower VDBP which may result in a greater proportion of 25(OH)D as free or bioavailable for cellular function.46–48 Targeted trials in diverse population and health settings are needed to develop therapeutic regimens.
We demonstrate that subjects receiving 7000IU/d vitD3 had some improvement in HIV immune status, with increased CD4% and Th naïve%, and in those with baseline detectable RNA viral load, decreased viral load by 12 mo. Furthermore, after controlling for age, African-American race, and HAART status, a positive change in 25(OH)D significantly predicted higher CD4% and Th naïve% and lower NK% at 3 mo and lower RNA viral load at 3 and 12 mo. Better 25(OH)D status at baseline was associated with lower NK% and activated cytotoxic T cells (HLA-DR%). This confirms the dosing study findings in predominantly African-American HIV-infected children and young adults from Philadelphia,12 where CD4% increased and RNA viral load, NK% and HLA-DR% decreased after 3-mo supplementation with either 4000 or 7000IU/d. Similarly, in our Botswana study, 3-mo supplementation with 7000IU/d was associated with higher CD4% and lower RNA viral load (Steenhoff AP, 2014, personal communication).
VitD affects immune cells that help control HIV infection, including macrophages49 and T lymphocytes,30 and is known to stimulate monocyte differentiation.50–52 Studies have shown an association between higher 1,25(OH)D and increased CD4+ cell counts,8;53–55 expansion of activated CD4+ and regulatory T cells,56 and decreased RNA viral load.10;11 VitD3 trials have not always demonstrated improvement in HIV disease progression, either increased CD4% and/or reduced RNA viral load.15;32 In these studies, supplementation was provided as non-daily doses, not exceeding the equivalent of 1700IU/d. Perhaps higher and daily doses are necessary to provide sustained improvement in vitD status and impact HIV disease progression.
HIV-infected people with high RNA viral load are likely to have increased proportion of activated cytotoxic T cells.57;58 Furthermore, untreated HIV-infected subjects with low CD4+ cell counts (<50/mm3) had significantly higher NK cell counts and cytotoxic activity compared with healthier HIV-infected people.59 The reduction in HLA-DR% and NK% with vitD3 supplementation may reflect improved RNA viral load and CD4%. Further studies are needed to explore this mechanism.
Although the numbers are small, this study included subjects both on HAART and HAART naïve, so that we compared vitD status by HAART status. VitD3 supplementation was effective in increasing 25(OH)D and in improving Th naïve% only with HAART. Some antiretroviral drugs are known to interfere with vitD metabolism.16;43;60–62 While the association between specific classes of HAART and poorer vitD status has been documented in cross-sectional studies, particularly for NNRTI- (efavirenz, nevirapine),63–65 PI-,60 and tenofovir-containing regimens,43;66 little is known regarding the response to vitD3 supplementation for subjects on HAART compared with HAART naïve. In our previous study, either 4000 or 7000IU/d for 3 mo was effective in increasing 25(OH)D in subjects on HAART.12 The mechanisms for differential response to vitD3 supplementation by HAART status are unknown, may include adherence to treatment and require further study. A limitation of this trial is that adherence to HIV treatment (HAART) was not measured and adherence to study treatment was measured only by self-report and return of residuals. With full adherence to HAART and supplementation, our results may have been more significant.
In summary, 12-mo daily, high-dose vitD3 supplementation in children and young adults with HIV was safe and resulted in a significant increase in 25(OH)D in subjects on HAART. This increase was accompanied by small improvement in several markers of HIV immune status. The recent non-vitD3 micronutrient study in asymptomatic, HAART-naïve HIV-infected adults in Botswana also demonstrated safety and reduced risk of immune decline and morbidity with a nutrition intervention.67 Large scale studies are needed to confirm the potential immunologic and virologic benefits of high-dose vitD3 supplementation.
Acknowledgments
Sources of Support:
This work was supported by the NIH/National Center for Complementary and Alternative Medicine, Grant R01AT005531, the National Center for Research Resources, Grant UL1RR024134, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This publication was made possible through core services and support from the University of Pennsylvania Center for AIDS Research (CFAR), an NIH-funded program (P30AI045008). Additional support was from the Jean A. Cortner Endowed Chair, Nutrition Center and the Research Institute at the Children’s Hospital of Philadelphia. Life Extension (Ft. Lauderdale, FL) and J.R. Carlson Laboratories, Inc. (Arlington Heights, IL) donated the vitamin D3 supplements and placebo capsules and drops, respectively.
We are grateful to subjects and families for participation. We thank Savannah Knell, Susan Ellenberg, PhD, Steven Douglas, MD, Eric Riedel, Jennifer Murray, Clinical Translational Research Center, the Special Immunology Family Care Clinic, Adolescent Initiative Program at CHOP, Jonathan Lax Treatment Center, Cooper University Hospital, Alfred I. duPont Hospital for Children, Hospital of the University of Pennsylvania, Temple University Hospital, and Drexel University Hospital.
VAS, JIS, BSZ, RMR designed the research; VAS, JIS, BSZ, KAD, JS, RMR conducted the research; VAS, JIS, MLH, FT, RMR analyzed the data; VAS, JIS, MLH, BSZ, RMR wrote the paper; VAS had the primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Disclaimers: The authors have no funding or conflicts of interest to disclose.
References
- 1.Viard JP, Souberbielle JC, Kirk O, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 2.Vescini F, Cozzi-Lepri A, Borderi M, et al. Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr. 2011;58:163–172. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- 3.Mehta S, Giovannucci E, Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS ONE. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS ONE. 2012;7:e40036. doi: 10.1371/journal.pone.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 6.Ansemant T, Mahy S, Piroth C, et al. Severe hypovitaminosis D correlates with increased inflammatory markers in HIV infected patients. BMC Infect Dis. 2013;13:7. doi: 10.1186/1471-2334-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutstein R, Downes A, Zemel B, Schall J, Stallings V. Vitamin D status in children and young adults with perinatally acquired HIV infection. Clin Nutr. 2011;30:624–628. doi: 10.1016/j.clnu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Haug C, Muller F, Aukrust P, Froland SS. Subnormal serum concentration of 1,25-vitamin D in human immunodeficiency virus infection: correlation with degree of immune deficiency and survival. J Infect Dis. 1994;169:889–893. doi: 10.1093/infdis/169.4.889. [DOI] [PubMed] [Google Scholar]
- 9.Bang UC, Brandt L, Benfield T, Jensen JE. Changes in 1,25-dihydroxyvitamin d and 25-hydroxyvitamin d are associated with maturation of regulatory T lymphocytes in patients with chronic pancreatitis: a randomized controlled trial. Pancreas. 2012;41:1213–1218. doi: 10.1097/MPA.0b013e31824da377. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Gandhi V, Psevdos G, Jr, Espinoza F, Park J, Sharp V. Evaluation of vitamin D levels among HIV-infected patients in New York City. AIDS Res Hum Retroviruses. 2012;28:235–241. doi: 10.1089/AID.2011.0040. [DOI] [PubMed] [Google Scholar]
- 11.Bearden A, Abad C, Gangnon R, Sosman JM, Binkley N, Safdar N. Cross-Sectional Study of Vitamin D Levels, Immunologic and Virologic Outcomes in HIV-Infected Adults. J Clin Endocrinol Metab. 2013;98:1726–1733. doi: 10.1210/jc.2012-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougherty K, Schall J, Zemel B, et al. Safety and efficacy of high dose daily vitamin D3 supplementation in children and young adults infected with HIV. J Pediatr Infect Dis Soc. 2014 doi: 10.1093/jpids/piu012. doi:10,1093/jpids/piu012. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irlam JH, Visser MM, Rollins NN, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database Syst Rev. 2010:CD003650. doi: 10.1002/14651858.CD003650.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Irlam JH, Siegfried N, Visser ME, Rollins NC. Micronutrient supplementation for children with HIV infection. Cochrane Database Syst Rev. 2013;10:CD010666. doi: 10.1002/14651858.CD010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arpadi SM, McMahon D, Abrams EJ, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–e126. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havens PL, Mulligan K, Hazra R, et al. Serum 25-Hydroxyvitamin D Response to Vitamin D3 Supplementation 50,000 IU Monthly in Youth with HIV-1 Infection. J Clin Endocrinol Metab. 2012;97:4004–4013. doi: 10.1210/jc.2012-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney RP. Vitamin D: criteria for safety and efficacy. Nutr Rev. 2008;66:S178–S181. doi: 10.1111/j.1753-4887.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- 19.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 20.Ng K, Scott JB, Drake BF, et al. Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr. 2013 doi: 10.3945/ajcn.113.067777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn PM, Rudy BJ, Douglas SD, et al. Virologic and immunologic outcomes after 24 weeks in HIV type 1-infected adolescents receiving highly active antiretroviral therapy. J Infect Dis. 2004;190:271–279. doi: 10.1086/421521. [DOI] [PubMed] [Google Scholar]
- 23.Starr SE, Sarr M, Campbell DE, Wilson CM, Douglas SD. Increased proliferation within T lymphocyte subsets of HIV-infected adolescents. AIDS Res Hum Retroviruses. 2002;18:1301–1310. doi: 10.1089/088922202320886343. [DOI] [PubMed] [Google Scholar]
- 24.Groleau V, Herold RA, Schall JI, et al. Blood Lead Concentration Is Not Altered by High Dose Vitamin D Supplementation in Children and Young Adults with HIV. J Pediatr Gastroenterol Nutr. 2012;56:316–319. doi: 10.1097/MPG.0b013e3182758c4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. Advance data from vital and health statistics. 314. Hyattsville, MD: National Center for Health Statistics; 2000. CDC Growth Charts: United States; pp. 1–28. [PubMed] [Google Scholar]
- 26.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adol. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 27.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: 2011. [Google Scholar]
- 28.Aloia JF, Patel M, Dimaano R, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–1958. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- 29.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2013. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 30.Stephensen CB, Marquis GS, Kruzich LA, Douglas SD, Aldrovandi GM, Wilson CM. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr. 2006;83:1135–1141. doi: 10.1093/ajcn/83.5.1135. [DOI] [PubMed] [Google Scholar]
- 31.Eckard AR, Judd SE, Ziegler TR, et al. Risk factors for vitamin D deficiency and relationship with cardiac biomarkers, inflammation and immune restoration in HIV-infected youth. Antivir Ther. 2012;17:1069–1078. doi: 10.3851/IMP2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. J Pediatr. 2011;159:951–957. doi: 10.1016/j.jpeds.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Giacomet V, Vigano A, Manfredini V, et al. Cholecalciferol Supplementation in HIV-Infected Youth with Vitamin D Insufficiency: Effects on Vitamin D Status and T-Cell Phenotype: A Randomized Controlled Trial. HIV Clin Trials. 2013;14:51–60. doi: 10.1310/hct1402-51. [DOI] [PubMed] [Google Scholar]
- 34.Meyzer C, Frange P, Chappuy H, et al. Vitamin D Deficiency and Insufficiency in HIV Infected Children and Young Adults. Pediatr Infect Dis J. 2013 doi: 10.1097/INF.0b013e3182a735ed. [DOI] [PubMed] [Google Scholar]
- 35.Arpadi SM, McMahon DJ, Abrams EJ, et al. Effect of supplementation with cholecalciferol and calcium on 2-y bone mass accrual in HIV-infected children and adolescents: a randomized clinical trial. Am J Clin Nutr. 2012;95:678–685. doi: 10.3945/ajcn.111.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steenhoff AP, Redwood A, Pettifor JM, et al. Vitamin D status in HIV-infected patients with and without tuberculosis: a pilot study. J Acquir Immune Defic Syndr. 2012;61:e21–e23. doi: 10.1097/QAI.0b013e3182683cd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics. 2009;123:797–803. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]
- 38.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 39.Vieth R. Vitamin D toxicity, policy and science. J Bone Miner Res. 2007;22:V64–V68. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 40.Vieth R. Implications for 25-hydroxyvitamin D testing of public health policies about the benefits and risks of vitamin D fortification and supplementation. Scand J Clin Lab Invest Suppl. 2012;243:144–153. doi: 10.3109/00365513.2012.682893. [DOI] [PubMed] [Google Scholar]
- 41.Longenecker CT, Hileman CO, Carman TL, et al. Vitamin D supplementation and endothelial function in vitamin D deficient HIV-infected patients: a randomized placebo-controlled trial. Antivir Ther. 2012;17:613–621. doi: 10.3851/IMP1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 43.Havens PL, Stephensen CB, Hazra R, et al. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis. 2012;54:1013–1025. doi: 10.1093/cid/cir968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenvinge MM, Gedela K, Copas AJ, et al. Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. J Acquir Immune Defic Syndr. 2010;54:496–499. doi: 10.1097/qai.0b013e3181caebaa. [DOI] [PubMed] [Google Scholar]
- 45.Tafazoli A, Khalili H. Vitamin D and HIV infection: a review of the clinical evidence. Future Virol. 2013;8:589–606. [Google Scholar]
- 46.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: The free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denburg MR, Kalkwarf HJ, de Boer IH, et al. Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatr Nephrol. 2013;28:1843–1853. doi: 10.1007/s00467-013-2493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girasole G, Wang JM, Pedrazzoni M, et al. Augmentation of monocyte chemotaxis by 1 alpha,25-dihydroxyvitamin D3. Stimulation of defective migration of AIDS patients. J Immunol. 1990;145:2459–2464. [PubMed] [Google Scholar]
- 50.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226–233. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 51.Haug CJ, Muller F, Aukrust P, Froland SS. Different effect of 1,25-dihydroxyvitamin D3 on replication of Mycobacterium avium in monocyte-derived macrophages from human immunodeficiency virus-infected subjects and healthy controls. Immunol Lett. 1998;63:107–112. doi: 10.1016/s0165-2478(98)00065-0. [DOI] [PubMed] [Google Scholar]
- 52.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haug CJ, Muller F, Rollag H, Aukrust P, Degre M, Froland SS. The effect of 1,25-vitamin D3 on maturation of monocytes from HIV-infected patients varies with degree of immunodeficiency. APMIS. 1996;104:539–548. doi: 10.1111/j.1699-0463.1996.tb04909.x. [DOI] [PubMed] [Google Scholar]
- 54.Teichmann J, Stephan E, Discher T, et al. Changes in calciotropic hormones and biochemical markers of bone metabolism in patients with human immunodeficiency virus infection. Metabolism. 2000;49:1134–1139. doi: 10.1053/meta.2000.8609. [DOI] [PubMed] [Google Scholar]
- 55.Teichmann J, Stephan E, Lange U, et al. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J Infect. 2003;46:221–227. doi: 10.1053/jinf.2002.1109. [DOI] [PubMed] [Google Scholar]
- 56.Bang U, Kolte L, Hitz M, et al. Correlation of increases in 1,25-dihydroxyvitamin D during vitamin D therapy with activation of CD4+ T lymphocytes in HIV-1-infected males. HIV Clin Trials. 2012;13:162–170. doi: 10.1310/hct1303-162. [DOI] [PubMed] [Google Scholar]
- 57.Borkowsky W, Stanley K, Douglas SD, et al. Immunologic response to combination nucleoside analogue plus protease inhibitor therapy in stable antiretroviral therapy-experienced human immunodeficiency virus-infected children. J Infect Dis. 2000;182:96–103. doi: 10.1086/315672. [DOI] [PubMed] [Google Scholar]
- 58.Kestens L, Vanham G, Gigase P, et al. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6:793–797. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Ironson G, Balbin E, Solomon G, et al. Relative preservation of natural killer cell cytotoxicity and number in healthy AIDS patients with low CD4 cell counts. AIDS. 2001;15:2065–2073. doi: 10.1097/00002030-200111090-00001. [DOI] [PubMed] [Google Scholar]
- 60.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 61.Drain PK, Kupka R, Mugusi F, Fawzi WW. Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. Am J Clin Nutr. 2007;85:333–345. doi: 10.1093/ajcn/85.2.333. [DOI] [PubMed] [Google Scholar]
- 62.Madeddu G, Spanu A, Solinas P, et al. Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q J Nucl Med Mol Imaging. 2004;48:39–48. [PubMed] [Google Scholar]
- 63.Welz T, Childs K, Ibrahim F, et al. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24:1923–1928. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- 64.Pasquet A, Viget N, Ajana F, et al. Vitamin D deficiency in HIV-infected patients: associated with non-nucleoside reverse transcriptase inhibitor or efavirenz use? AIDS. 2011;25:873–874. doi: 10.1097/QAD.0b013e32834542fa. [DOI] [PubMed] [Google Scholar]
- 65.Conesa-Botella A, Florence E, Lynen L, Colebunders R, Menten J, Moreno-Reyes R. Decrease of vitamin D concentration in patients with HIV infection on a non nucleoside reverse transcriptase inhibitor-containing regimen. AIDS Res Ther. 2010;7:40. doi: 10.1186/1742-6405-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mueller NJ, Fux CA, Ledergerber B, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 67.Baum MK, Campa A, Lai S, et al. Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: a randomized clinical trial. JAMA. 2013;310:2154–2163. doi: 10.1001/jama.2013.280923. [DOI] [PMC free article] [PubMed] [Google Scholar]