Abstract
Background
Ischemic mitral regurgitation (MR) is a frequent complication of myocardial infarction (MI) associated with left ventricular (LV) dilatation and dysfunction that doubles mortality. At the molecular level, moderate ischemic MR is characterized by a biphasic response with initial compensatory rise in pro-hypertrophic and anti-apoptotic signals followed by their exhaustion. We have shown that early MR repair 30 days after MI is associated with LV reverse remodeling. It is not known if MR repair performed after the exhaustion of compensatory mechanisms is also beneficial. We hypothesised that late repair will not result in LV reverse remodeling.
Methods and Results
Twelve sheep underwent distal left anterior descending coronary artery ligation to create apical MI, and implantation of a LV-to-left atrium shunt to create standardized moderate volume overload. At 90 days, animals were randomized to shunt closure (late repair) vs sham (no repair). LV remodeling was assessed by 3D echocardiography, dP/dt, preload recruitable stroke work (PRSW) and myocardial biopsies. At 90 days, animals had moderate volume overload, LV dilatation and reduced ejection fraction (all p<0.01 vs baseline, p=NS between groups). Shunt closure at 90 days corrected the volume overload (regurgitant fraction 6±5% vs 27±16% for late repair vs sham, p<0.01), but was not associated with changes in LV volumes (end-diastolic volume 106±15 vs 110±22 ml; end-systolic volume 35±6 vs 36±6 ml), or increases in PRSW (41±7 vs 39±13 ml·mmHg) or dP/dt (803±210 vs 732±194 mmHg/sec) at 135 days (all p=NS). Activated Akt, central in the hypertrophic process, and STAT3, critical node in the hypertrophic stimulus by cytokines, were equally depressed in both groups.
Conclusion
Late correction of moderate volume overload after MI did not improve LV volume or contractility. Up-regulation of pro-hypertrophic intra-cellular pathways was not observed. This contrasts with previously reported study in which early repair (30 days) reversed LV remodeling. This suggests a “window of opportunity” to repair ischemic MR, after which no beneficial effect on LV is observed despite successful repair.
Keywords: Mitral regurgitation, ischemic heart disease, remodeling
Background
Ischemic mitral regurgitation (MR) is caused by altered left ventricular geometry and function1–3, and doubles heart failure and mortality after myocardial infarction (MI)4. Still common despite improvement in revascularization and medical treatment after MI, its treatment remains frustrating and controversial5–10.
Expansion of infarcted tissue begins acutely after myocardial infarction (MI), but a more gradual remodeling also involves noninfarcted areas11. Initially compensatory, this process can become maladaptive, as the ventricle enlarges and contracts poorly12, 13, with reduced survival. While severe non-ischemic MR has been shown to promote LV remodeling and increase mortality14–16, even mild or moderate ischemic MR has been associated with poorer prognosis4. We have previously demonstrated17 that moderate MR, simulated by an LV-to-LA shunt, added to a small antero-apical MI (causing no intrinsic MR) causes greater ventricular remodeling than a comparable infarction alone, with an earlier transition to a failure phenotype. Whole-heart changes parallel cellular and molecular abnormalities in the non-infarcted myocardium reflecting the complex remodeling process. These molecular events progress differently after MI and moderate volume overload than with comparable infarction alone, with an initial rise in pro-hypertrophic and anti-apoptotic signals (specifically, the Akt-PI3K and gp130-JAK/STAT3 pathways) followed by their exhaustion. In the same model, early repair (after 1 month)18 of the MR-equivalent LV-to-LA shunt prevents down-regulation of these compensatory pathways and induces LV reverse remodeling manifested by normalization of both functional and anatomical parameters, also consistent with other ischemic MR models and therapeutic approaches10, 19, 20. However it is not known if the molecular alterations and LV remodeling can be reversed by a late intervention done when the pro-hypertrophic and anti-apoptotic signals are already down-regulated.
Based on this observed biphasic molecular response in ischemic MR, we specifically hypothesized that late MR repair would not result in LV reverse remodeling. This was tested using our previously described animal model of ischemic MR combining an apical MI and a LV-to-LA shunt, with shunt ligation at 3 months post MI to simulate MR repair.
Methods
Ischemic mitral regurgitation model
A previously described ischemic MR model was created in twelve adult Dorsett hybrid sheep (weight>45 kg)17, 18. This model, combining an apical infarction by distal LAD ligation (not causing MR by itself) and an LV to LA shunt (8-cm long, 8-mm diameter reinforced Teflon graft with a cross-sectional area of 50 mm2 implanted into the LV base and left atrial appendage as shown in Figure 1), has been shown to produce standardized and consistent MI size and moderate volume overload. 90 days after the initial surgery, a second thoracotomy was repeated and sheep were randomized to shunt closure (late repair group) or sham surgery (no repair group). The animals were observed for an average of 6 more weeks and sacrificed on day 135 (Figure 2). The 90 days late repair timing was chosen on the basis of previous studies in the same model showing exhaustion of pro-hypertrophic compensatory pathways at this time17, 18. Animal studies conformed to National Institutes of Health guidelines (National Research Council, Washington, DC, 1996) and were Institutional Review Board-approved.
Figure 1.
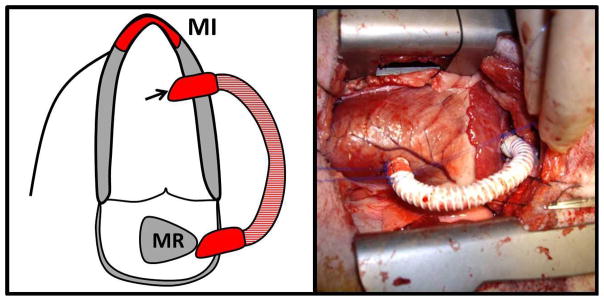
Schematic representation (left) and perioperative view (right) of the left ventricle to left atrial appendage shunt.
Figure 2.
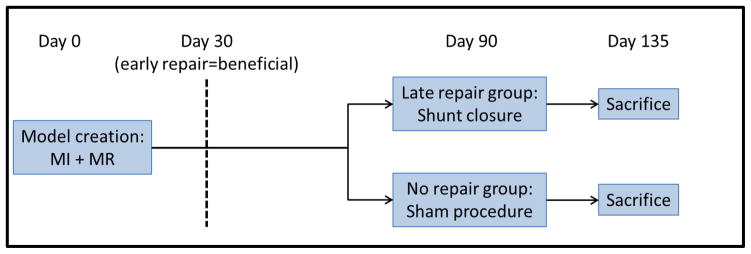
Protocol summary with model creation at day 0, randomization to late repair or sham procedure at day 90 and sacrifice at day 135. It has been previously shown that an early repair at 30 days is beneficial to stop and reverse the LV remodeling cascade.
Echocardiography analysis
Standard 2D and full volume 3D datasets were obtained epicardially at each time point (baseline, 90 days and 135 days) using high frequency (S5, X3) probes and a Philips iE33 scanner (Andover, MA). Shunt patency or closure was confirmed by color flow Doppler. 3D analysis was performed blinded to group and time point using the custom software Omni4D (MD Handschumacher) as previously described18. LV end-diastolic and end-systolic volumes (EDV and ESV) were measured and LV ejection fraction (LVEF) computed. Regurgitant volume was calculated as total 3D stroke volume (EDV – ESV) minus forward aortic stroke volume calculated by pulsed wave Doppler and the continuity equation. Regurgitant volume and fraction therefore included the regurgitant flow through the LV-LA shunt, but also accounted for any additional functional MR that could have occurred secondary to LV dilatation in the context of combined MI and volume overload.
LV pressure-volume analysis
At baseline and day 135, a high fidelity conductance catheter (Scisense, Ontario, Canada) was inserted trans-apically to record LV maximal dP/dt and pressure-volume relationship at different preload conditions by simultaneously partially occluding the inferior vena cava, with subsequent computation of preload-recruitable stroke work (PRSW)18, 21, a preload independent measure of LV systolic contraction.
Tissue samples and molecular analysis
Myocardial needle biopsy was performed at baseline before model creation, and both peri-infarct and remote myocardium were biopsied at day 90 and 135. We measured levels of pAKT (protein kinase B), central in the hypertrophic process, and pSTAT3, a critical node in the hypertrophic stimulus by cytokines. These are both at their respective levels (cytosol and membrane) important crossroads in prohypertrophic signaling18. Western blot analysis was performed on cell lysates from biopsies at baseline, day 90 and 135. pSTAT3 and anti-phosphoAkt (Santa Cruz Biotechnology, Santa Cruz, Calif) were detected with peroxidase-conjugated anti-mouse IgG and chemiluminescence with α-actin as the housekeeping control. Integrated blot pixel density was assessed using standard software (ImageJ; NIH, Bethesda, Md).
Statistics
Continuous variables are expressed as mean±standard deviation. Differences within and between groups at different time points was assessed with repeated measures analysis of variance (ANOVA). Rank based ANOVA were performed when normality was excluded by the Shapiro-Wilk test. p<0.05 was considered significant. Based on previous data with the same model, it was calculated that a sample size of 6 per group would provide at least 80% power (alpha level: 0.05) to detect an effect comparable to early MR repair between groups (25 ml*mmHg in PRSW and 40 ml in ESV). Statistical analysis was performed with Stata/IC 11.2 (StataCorp LP, TX). The authors had full access to and take full responsibility for the integrity of the data.
RESULTS
Left ventricular volumes and function
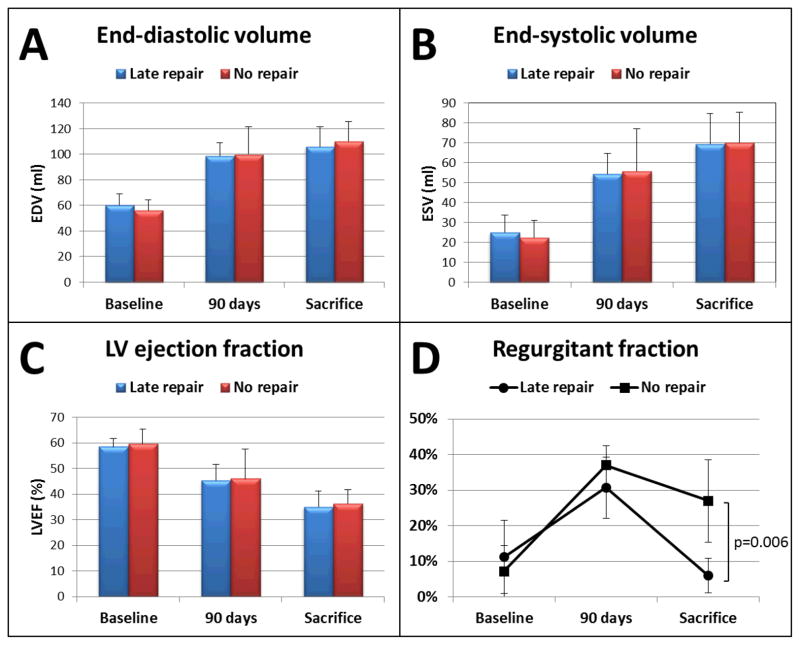
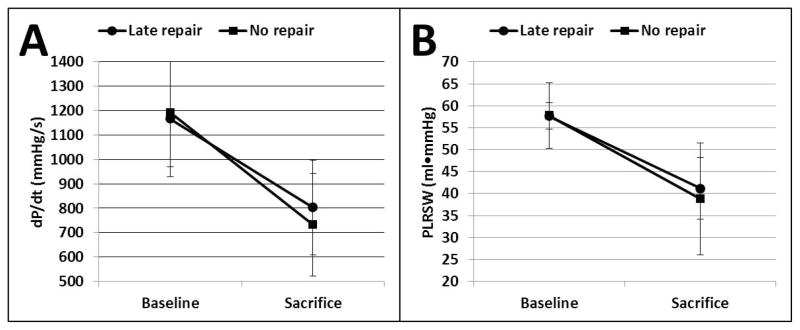
A total of 16 sheep underwent the initial procedure of MI+MR. 4 sheep died suddenly within the first 48 hours, presumably from malignant arrhythmia. A total of 12 sheep completed the protocol successfully, without death occurring after the randomization of MR repair vs sham procedure. As expected, the initial procedure of MI+MR resulted in progressive increase in LV EDV and ESV (both p<0.01 vs baseline), decrease in LVEF (p<0.01 vs baseline), and moderate volume overload (regurgitant volume 15±4 ml; regurgitation fraction 34 ± 8%) at 90 days, with no significant difference between groups (Figure 3). Shunt ligation at 90 days in the late repair group successfully corrected the volume overload, while the no repair group had persistent regurgitation until sacrifice (regurgitant fraction 6 ± 5% vs 27 ± 16% for late repair vs no repair, p=0.006; regurgitant volume 2 ± 2 ml vs 12 ± 7 ml for late repair vs no repair, p=0.007). However, this late correction did not prevent progression of LV remodeling at 135 days and both EDV (106 ± 15 vs 110 ± 22 ml for late repair vs no repair, p=0.72) and ESV (69 ± 15 ml vs 70 ± 16 ml for late repair vs no repair, p=0.95) were further increased at sacrifice with reduced LVEF (35 ± 6 vs 36 ± 6 % for late repair vs no repair, p=0.70), with no significant difference between groups (Figure 3). LV hemodynamics showed the same pattern: PRSW was significantly and equally reduced at sacrifice in both groups (Figure 4, 41 ± 7 vs 39 ± 13 ml·mmHg, p=0.70). Maximal dP/dt also declined over time comparably in both groups at sacrifice (803 ± 210 vs 732 ± 194 mmHg/sec, p=0.56).
Figure 3.
Left ventricular volume and function in both groups overtime. A: Left ventricular end-diastolic volume. B: Left ventricular end-systolic volume. C: Left ventricular ejection fraction. D: Regurgitant fraction. Despite successful shunt closure and marked reduction in volume overload at 90 days, no difference is seen at 135 days vs sheep without repair: left ventricular volumes are equally increased, as the LVEF decreases.
Figure 4.
Left ventricular dP/dt (A) and Preload recruitable stroke work (B) at baseline and sacrifice, showing a similar pattern compared with the echocardiographic analysis: late volume overload correction at 90 days did no prevent loss of left ventricular function at 135 days.
Molecular analysis
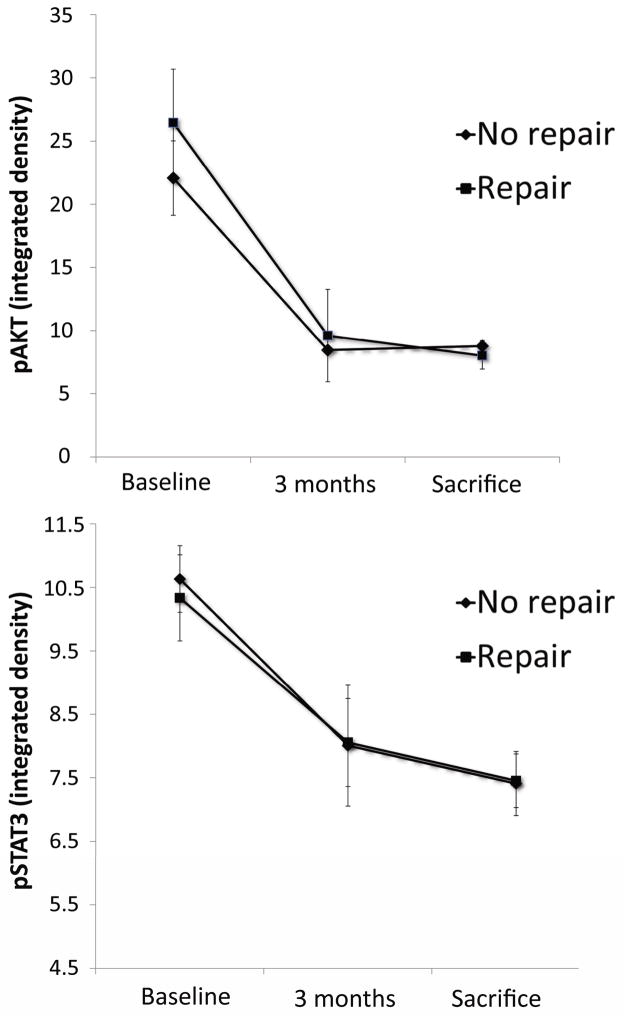
Western blot integrated density of pro-hypertrophic markers pAKT and pSTAT3 at three time points are displayed in Figure 5. As expected, both groups had similarly decreased signals for these markers at 90 days. However, late repair did not result in recovery and both groups were equally depressed at day 135 (p=0.16).
Figure 5.
Integrated Density from Western Blot normalised for α-actin. Top: pAKT levels showing comparable suppression in both groups at 90 and 135 days, without improvement in the late MR repair group. Bottom: Similar pattern is observed for pSTAT3. Downregulation of both molecules was however prevented by early repair at 30 days in previous study18.
Discussion
In this study, we show that late correction of a moderate volume overload 3 months post-MI does not reverse or prevent further LV remodeling and dysfunction, with consistent outcomes at the functional and molecular levels. These results contrast with a previously published study18 in which early (1 month) repair was associated with complete normalization of ventricular volume, hemodynamic and molecular alterations.
Despite its clear association with poorer prognosis4, treatment of ischemic MR remains highly controversial. Both clinical5, 22, 23 and experimental6, 10, 18 studies yield conflicting results. Part of this controversy is attributable to the complex nature of LV remodeling associated with ischemic MR: the MI initially causes the MR, but both MI and MR can contribute to cellular and functional changes in the non-infarcted LV17. The relative contribution of MI and MR has been difficult if not impossible to separate in clinical studies. Other confounders relate to MR surgical correction, often performed with simultaneous coronary revascularization and with the frustrating problem of recurrent MR, treating the volume overload often only partially or temporarily7–9. This animal model of apical MI with LV-LA shunt can help resolve these difficulties. By isolating the effect of moderate volume overload on comparable MI size and location, it previously demonstrated the additional contribution of moderate MR to LV remodeling. The second advantage is the adequate and persistent correction of volume overload by shunt ligation, simulating a successful and minimally invasive MR repair. In this controlled environment, the current experimental study provides another possible explanation for discrepancies between clinical studies by suggesting that a successful surgical correction can produce variable results if not performed in a timely manner.
This variable response to correction at different time points needs to be related to the previously observed biphasic molecular response in the myocardium following MI and MR. Cardiac remodeling in ischemic MR is dynamic: increased apoptotic tone (caspase-3) has been shown in remote myocardium, partially counterbalanced by transiently increased pro-hypertrophic and anti-apoptotic pathways: the gp130-STAT3 pathway is related to cytokine-mediated hypertrophy, and proteine kinase B (Akt) is a serine/threonine-specific protein kinase promoting growth factor-mediated cell survival and hypertrophy. Signaling of both pathways is increased after MI, but the presence of moderate volume overload is associated with subsequent down-regulation paralleling the transition to heart failure17, 24. Early MR repair when performed while gp130 and Akt are still overexpressed can interrupt the vicious remodeling cycle and reverse heart failure. The lack of success of the same procedure after down-regulation of these compensatory pathways indicates a point of no return for LV remodeling in ischemic MR, and suggests a limited time window to correct the MR, with less or no beneficial effect if the intervention is not done within this interval. The persistent negative remodeling observed after late MR repair suggests that these profound molecular alterations not only prevent reversal of heart failure, but could also contribute to further LV remodeling even after the correction of the initial volume overload.
Clinical correlation and future studies
Clinical criteria to correct MR surgically are generally based on MR severity, LV dimensions and function. Although clinically important and useful, these parameters might not be sufficient to capture the whole spectrum of LV remodeling or completely predict outcome after surgery for ischemic MR. While the current study suggests that earlier repair is better to prevent LV remodeling, determining the best timing for intervention in a clinical setting will remain a challenge since each patient presents variable MI size, MR severity, and myocardial substrate, and therefore an individual “time window”. Although preoperative echocardiography assessment can help predict procedural success in terms of MR correction25, and that parameters such as LV dimension can be useful to assess the remodeling, it is unclear at this point how well imaging (other than LV dimensions and function) and/or biomarkers can improve the prediction of ventricular response after successful MR repair. Another question will be if and how current and future medical therapies can prolong the time during which beneficial repair can be done. Well-designed clinical and experimental studies are needed to answer these relevant questions properly.
Limitations
While helping to understand the effects and potential implications of volume overload correction on LV remodeling, direct extrapolation to patients with ischemic MR cannot be made at this point. This animal model was designed to separate the results of MI and volume overload on LV remodeling and to study the effects of successful MR repair, but does not fully represent the clinical scenario. However, these results highlight the dynamic nature of LV remodeling in ischemic MR and the importance of timing when considering surgical intervention. Our power was sufficient to rule out changes of the magnitude observed by early repair, but the relative small sample size could not rule out a smaller beneficial effect of late repair compared with no treatment. Although we previously used this model to show the beneficial effects of early repair18, LV volume was measured differently in the present study (real-time 3D echocardiography vs sequential rotational acquisitions). This may possibly account for the larger variability in volume measurement observed in the previous study. Thus, while the differences in evolution following repair in the two studies is striking, direct comparison of LV volume between the studies cannot be made.
Conclusion
In this ischemic MR animal model, late MR repair was not beneficial to prevent additional LV remodeling; in contrast with earlier repair. This suggests a point of no return after which no or limited beneficial effect of MR repair can be achieved despite adequate correction of volume overload. The timing of valve repair in ischemic MR needs to be assessed in future clinical studies, as this could in part resolve controversies and discrepant findings in this area.
Acknowledgments
Funding: AHA-Founders Affiliate post-doctoral fellowship grant 10POST4580055, NIH R01 HL72265 and HL109506, and Israel-USA Binational Science Foundation (BSF) grant no. 2005250. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
Disclosure: none.
References
- 1.Otsuji Y, Handshumacher MD, Schwammethal E, Jiang L, Song J-K, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanisms of functional mitral regurgitation: Direct in vivo demonstration of altered leaflet geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 2.Llaneras MR, Nance ML, Streicher JT, Linden PL, Downing SW, Lima JA, Deac RF, Edmunds J, Henry L. Pathogenesis of ischemic mitral insufficiency. Journal of Thoracic and Cardiovascular Surgery. 1993;105:439–442. [PubMed] [Google Scholar]
- 3.Komeda M, Glasson JR, Bolger AF, Daughters GT, 2nd, Ingels NB, Jr, Miller DC. Papillary muscle-left ventricular “complex”. Journal Of Thoracic And Cardiovascular Surgery. 1997;113:292–300. doi: 10.1016/s0022-5223(97)70326-x. [DOI] [PubMed] [Google Scholar]
- 4.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation : Long-term outcome and prognostic implications with quantitative doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 5.Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. Journal of the American College of Cardiology. 2005;45:381–387. doi: 10.1016/j.jacc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzaki K, Morita M, Hamamoto H, Noma M, Robb JD, Gillespie MJ, Gorman JH, Iii, Gorman RC. Elimination of ischemic mitral regurgitation does not alter long-term left ventricular remodeling in the ovine model. The Annals of Thoracic Surgery. 90:788–794. doi: 10.1016/j.athoracsur.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, Shiota T, Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. Journal Of Thoracic And Cardiovascular Surgery. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Kuwahara E, Otsuji Y, Iguro Y, Ueno T, Zhu F, Mizukami N, Kubota K, Nakashiki K, Yuasa T, Yu B, Uemura T, Takasaki K, Miyata M, Hamasaki S, Kisanuki A, Levine RA, Sakata R, Tei C. Mechanism of recurrent/persistent ischemic/functional mitral regurgitation in the chronic phase after surgical annuloplasty: Importance of augmented posterior leaflet tethering. Circulation. 2006;114:I529–534. doi: 10.1161/CIRCULATIONAHA.105.000729. [DOI] [PubMed] [Google Scholar]
- 9.Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA. Mechanism of recurrent ischemic mitral regurgitation post-annuloplasty: Continued lv remodeling as a moving target. Circulation. 2004;110:85–90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 10.Guy TS, Moainie SL, Gorman JH, Jackson BM, Plappert T, Enomoto Y, St John-Sutton MG, Edmunds LH, Jr, Gorman RC. Prevention of ischemic mitral regurgitation does not influence the outcome of remodeling after posterolateral myocardial infarction. Journal of the American College of Cardiology. 2004;43:377–383. doi: 10.1016/j.jacc.2003.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 12.Picard MH, Wilkins GT, Ray PA, Weyman AE. Progressive changes in ventricular structure and function during the year after acute myocardial infarction. American Heart Journal. 1992;124:24–31. doi: 10.1016/0002-8703(92)90916-j. [DOI] [PubMed] [Google Scholar]
- 13.Davidoff R, Picard MH, Force T, Thomas JD, Guerrero JL, McGlew S, Weyman AE. Spatial and temporal variability in the pattern of recovery of ventricular geometry and function after acute occlusion and reperfusion. American Heart Journal. 1994;127:1231–1241. doi: 10.1016/0002-8703(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 14.Carabello BA, Nakano K, Corin W, Biederman R, Spann JF., Jr Left ventricular function in experimental volume overload hypertrophy. American Journal of Physiology. 1989;256:H974–981. doi: 10.1152/ajpheart.1989.256.4.H974. [DOI] [PubMed] [Google Scholar]
- 15.Spinale FG, Ishihra K, Zile MR, DeFryte G, Crawford FA, Carabello BA. Structural basis for changes in left ventricular function and geometry because of chronic mitral regurgitation and after correction of volume overload. Journal Of Thoracic And Cardiovascular Surgery. 1993;106:1147–1157. [PubMed] [Google Scholar]
- 16.Ling LH, Enriquez-Sarano M, Seward JB, Tajik AJ, Schaff HV, Bailey KR, Frye RL. Clinical outcome of mitral regurgitation due to flail leaflet. New England Journal of Medicine. 1996;335:1417–1423. doi: 10.1056/NEJM199611073351902. [DOI] [PubMed] [Google Scholar]
- 17.Beeri R, Yosefy C, Guerrero JL, Nesta F, Abedat S, Chaput M, del Monte F, Handschumacher MD, Stroud R, Sullivan S, Pugatsch T, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. Mitral regurgitation augments post-myocardial infarction remodeling: Failure of hypertrophic compensation. Journal of the American College of Cardiology. 2008;51:476–486. doi: 10.1016/j.jacc.2007.07.093. [DOI] [PubMed] [Google Scholar]
- 18.Beeri R, Yosefy C, Guerrero JL, Abedat S, Handschumacher MD, Stroud RE, Sullivan S, Chaput M, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. Early repair of moderate ischemic mitral regurgitation reverses left ventricular remodeling: A functional and molecular study. Circulation. 2007;116:I–288–293. doi: 10.1161/CIRCULATIONAHA.106.681114. [DOI] [PubMed] [Google Scholar]
- 19.Hung J, Chaput M, Guerrero JL, Handschumacher MD, Papakostas L, Sullivan S, Solis J, Levine RA. Persistent reduction of ischemic mitral regurgitation by papillary muscle repositioning: Structural stabilization of the papillary muscle-ventricular wall complex. Circulation. 2007;116:I259–263. doi: 10.1161/CIRCULATIONAHA.106.679951. [DOI] [PubMed] [Google Scholar]
- 20.Messas E, Bel A, Szymanski C, Cohen I, Touchot B, Handschumacher MD, Desnos M, Carpentier A, Menasche P, Hagege AA, Levine RA. Relief of mitral leaflet tethering following chronic myocardial infarction by chordal cutting diminishes left ventricular remodeling. Circulation. Cardiovascular imaging. 2010;3:679–686. doi: 10.1161/CIRCIMAGING.109.931840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Rankin JS. Linearity of the frank-starling relationship in the intact heart: The concept of preload recruitable stroke work. Circulation. 1985;71:994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- 22.Prifti E, Bonacchi M, Frati G, Giunti G, Babatasi G, Sani G. Ischemic mitral valve regurgitation grade ii-iii: Correction in patients with impaired left ventricular function undergoing simultaneous coronary revascularization. Journal of Heart Valve Disease. 2001;10:754–762. [PubMed] [Google Scholar]
- 23.Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, Jain AR, Leng Chua Y, Daly R, Senni M, Mokrzycki K, Menicanti L, Oh JK, Michler R, Wróbel K, Lamy A, Velazquez EJ, Lee KL, Jones RH. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial / clinical perspective. Circulation. 2012;125:2639–2648. doi: 10.1161/CIRCULATIONAHA.111.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 25.Magne J, Pibarot P, Dagenais F, Hachicha Z, Dumesnil JG, Senechal M. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation. 2007;115:782–791. doi: 10.1161/CIRCULATIONAHA.106.649236. [DOI] [PubMed] [Google Scholar]