Abstract
Background
Pharmacological evidence suggests that inhalational general anesthetics induce neurodegeneration in vitro and in vivo through overactivation of inositol trisphosphate receptor (InsP3R) Ca2+ release channels, but it is not clear whether these effects are due to direct modulation of channel activity by the anesthetics.
Methods
Using single-channel patch clamp electrophysiology, we examined the gating of rat recombinant type 3 inositol InsP3R (InsP3R-3) Ca2+-release channels in isolated nuclei (N = 3 to 15) from chicken lymphocytes modulated by isoflurane at clinically relevant concentrations in the absence and presence of physiological levels of the agonist InsP3. We also examined the effects of isoflurane on InsP3R-mediated Ca2+ release from the endoplasmic reticulum, and changes in intracellular Ca2+ concentration ([Ca2+]i).
Results
Clinically relevant concentrations (~ 1 Minimal Alveolar Concentration) of the commonly used general anesthetic, isoflurane, activated InsP3R-3 channels with open probability similar to channels activated by 1 μM InsP3 (Po≈0.2). This isoflurane modulation of InsP3R-3 Po depended biphasically on [Ca2+]i. Combination of isoflurane with subsaturating levels of InsP3 in patch pipettes resulted in at least two-fold augmentations of InsP3R-3 channel Po compared to InsP3 alone. These effects were not noted in the presence of saturating [InsP3]. Application of isoflurane to DT40 cells resulted in a 30% amplification of InsP3R-mediated [Ca2+]i oscillations, whereas InsP3-induced rise in [Ca2+]i and cleaved caspase-3 activity were enhanced by nearly 2.5 fold.
Conclusion
These results suggest that the InsP3R may be a direct molecular target of isoflurane and a player in the mechanisms of anesthetic-mediated pharmacological or neurotoxic effects.
Introduction
The inositol trisphosphate receptor (InsP3R) is an intracellular Ca2+ release channel found mostly on the membrane of the endoplasmic reticulum (ER). Activation of InsP3R by InsP3 causes Ca2+ release from the ER lumen into the cytoplasm, where it acts as a second messenger to regulate many physiological processes such as cell survival and neurogenesis. 1–4 InsP3R over-activation also regulates some pathological processes 1;5, especially apoptosis and neurodegeneration. 6–8 InsP3R channel activity can be regulated by a diverse array of interacting proteins, 4 including neurodegenerative-associated proteins such as Huntingtin-associated protein 1 and Alzheimer’s disease mutant presenilin 1 and presenilin 2. 9;10
More than 260 million patients worldwide receive surgeries under general anesthesia each year. The diversity of the molecular mechanisms of general anesthetics is still not clear. Activation of InsP3Rs may play important roles in anesthetic-mediated regulation of intracellular Ca2+ homeostasis and some physiological and pathological processes. 11–14 General anesthetics, especially isoflurane, may precondition cells and provide cytoprotection by moderately activating InsP3Rs and Ca2+ release from the ER. 12 Isoflurane may also cause apoptosis and neurodegeneration by causing abnormal Ca2+ release from the ER via overactivation of InsP3R. 3;11;13;15–17 Sensitized InsP3R activity in Alzheimer’s and Huntington diseases appears to render neurons vulnerable to isoflurane-induced Ca2+ release from the ER and subsequent apoptosis. 15;17;18 Isoflurane increases levels of β-site amyloid β precursor protein-cleaving enzyme 16 and aggregation of mutated Huntingtin proteins 17 via activation of InsP3R. General anesthetics may cause neuronal apoptosis by disruption of intracellular Ca2+ homeostasis. 18–21 Despite these strong associations of anesthetic exposure and InsP3R activation in normal and pathological conditions, there is a lack of evidence to support direct activation of the InsP3R by isoflurane.
In this study, we report for the first time that clinically relevant concentrations of isoflurane directly modulate the activity of the InsP3R channel and sensitize the channel to basal levels of InsP3, resulting in InsP3R mediated Ca2+ release from the ER and amplification of InsP3-induced [Ca2+]i signals, and induction cell apoptosis. These results suggest that the InsP3R may be one of the molecular mechanisms of anesthetic-mediated pharmacological and toxic effects in neurodegeneration.
Materials and Methods
Cell Culture
DT40 chicken lymphocytes
DT40 cells lacking the genes for all 3 isoforms of InsP3Rs (DT40-KO, RIKEN Cell Bank No. RCB 1467, Ibaraki, Japan) and DT40-KO cells stably over-expressing the rat type 3 InsP3R (DT40-R3) 22 were used in this study. While the work was conducted in a chicken lymphocyte cell line, the channel studied is the rat recombinant InsP3R channel. We elected to use InsP3R-3 instead of other InsP3R subtypes because InsP3R-3 has been shown to have relatively higher opening probability activated by InsP3R agonists and a useful in vitro model to examine the InsP3R activation by its agonists. 22 Given the high level of primary sequence homology among mammalian InsP3Rs, and the substantial similarities in the major features of the regulation of endogenous and recombinant InsP3R channels by cytoplasmic InsP3 and Ca2+ (biphasic activation and inhibition by Ca2+; and monotonic, saturable activation by InsP3), 4 we expect that our findings that isoflurane affects Ca2+ and InsP3 regulation of InsP3R channels have relevance for similar regulations of neuronal InsP3R channels, and thereby have relevance for Ca2+ homeostasis in neurons. Cells were maintained in suspension culture in Roswell Park Memorial Institute medium 1640 media containing 10% fetal calf serum, 1% chicken serum, penicillin (100 units/ml), streptomycin (100 μg/ml), and glutamine (2 mM) at 37°C in a 95% air and 5% CO2 humidified incubator.
Electrophysiology
There is no way to directly observe single-channel activity of the InsP3R in the native ER membrane since performing patch-clamp electrophysiology on the ER directly has not been achieved. Since the outer nuclear membrane is continuous with the ER membrane topologically, and since there is no evidence to suggest that the biochemical properties of the outer nuclear membrane are distinct from those of the rest of the ER, nuclear patch clamping of the ER membrane is a tool to record ER-localized ion channel activities, including the InsP3R. 4 We have examined the effects of isoflurane on InsP3Rs located on the outer nuclear membrane. Preparation of isolated nuclei from DT40 cells was performed as described. 10;22–26 Cells were washed twice with PBS and suspended in a nuclear isolation solution containing 150 mM KCl, 250 mM sucrose, 1.5 mM 2-mercapoethanol, 10 mM Tris·HCl, 0.05 mM PMSF, and protease inhibitor mixture (Complete; Roche Molecular Biochemicals, Indianapolis, IN), adjusted to pH 7.3 with KOH. Isolated nuclei were placed in a recording chamber containing a standard bath solution: 140 mM KCl, 10 mM Hepes, and 0.5 mM BAPTA, ≈200 nM free-Ca2+, adjusted to pH 7.3 with KOH. During the nuclear isolation protocol and the introduction of nuclei onto the stage of the patch clamp microscope, any endogenous IP3 is washed away. Selected intact nuclei were patch-clamped and single channel activities were recorded in the on-nucleus configuration. Recording pipettes with resistances of 8–10 MΩ were used. The pipette solution contained 140 mM KCl, 0.5 mM ATP, 10 mM Hepes (pH 7.3), with varying concentrations of isoflurane, InsP3 and Ca2+. The free Ca2+ concentration was varied by addition of an appropriate Ca2+ chelator, as previously described. 27 Single-channel currents were amplified using an Axopatch-200B amplifier (Molecular Devices, Downingtown, PA), filtered at 1 kHz, and digitized at 5 kHz with an ITC-16 interface (Instrutech, Port Washington, NY) and Pulse+ Pulse Fit software (HEKA Electronik, Farmingdale, NY). All recordings were performed at room temperature with the pipette electrode at −40 mV relative to the reference bath electrode. Single channel-analyses were performed on recordings exhibiting only a single channel using QuB (University of Buffalo, Baffalo, New York) and Igor Pro (WaveMetrics, Lake Oswego, OR) software. Figures were generated using Igor Pro software and Adobe Illustrator (Adobe System Incorporated, San Jose, CA).
Measurement of cytoplasmic Ca2+ concentration ([Ca2+]i)
DT40-KO or DT40-R3 cells were seeded onto glass coverslips coated with 0.01% poly-L-ornithine for 1 hr before measurements. Cells were loaded with 2.5 μM Fura-2AM (Molecular Probes, Grand Island, NY) for 30 min at room temperature in Hanks’ balanced salt solution (HBSS, Sigma, Pittsburgh, PA) containing 1.8 mM CaCl2 and 0.8 mM MgCl2, pH 7.4. Coverslips were then placed in a sealed perfusion chamber (Warner Instruments, Hamden, CT) and continuously perfused at room temperature with HBSS. Fura-2AM was alternately excited at 340 and 380 nm, and emitted fluorescence (510 nm) was collected and recorded using a CCD-based imaging system running IPLab v3.7 software (Biovision Technologies, Exton, PA). The data are presented as the ratio of fluorescence intensities recorded at 340 nm and 380 nm excitations (F340/F380) during baseline and isoflurane application. F340/F380 ratios were recorded from > 30 cells in at least three separate experiments. For analyses of [Ca2+]i oscillations, ratio images were collected from > 60 cells over 20 min in at least four separate experiments. The percentage of cells with obvious [Ca2+]i responses (a single transient rise or sustained oscillations), peak [Ca2+]i, area under the [Ca2+]i versus time curve, and oscillation frequency were determined and analyzed as described previously. 28 HBSS samples were collected from inflow and outflow tubes of the recording chamber to determine the anesthetic concentration to which the cells were exposed. High performance liquid chromatography measurements showed that isoflurane was consistently maintained at 0.4 mM (not shown). This concentration corresponds to a minimum alveolar concentration of ~1.
Detection of caspase-3 activity
Proteolytic activation of caspase-3 was measured in lysates from DT40 wild type (DT40-wt), DT40-KO and DT40-R3 cells following exposure to isoflurane using previously described protocols.13 Caspase-3 activity was measured using Ac-DEVD-AFC hydrolysis kit (Caspase-3, Calbiochem, Billerica, MA). Briefly, DT40-KO or DT40-R3 cells grown in six well plates were exposed to isoflurane (2.4%) for 24 h which resulted in 0.8 mM isoflurane in the culture medium29 and reliably produced isoflurane toxicity in this anesthesia exposure cell model,13 harvested via trypsinization, and washed with phosphate buffered saline. The cell pellet was gently resuspended in CelLytic™ M lysis buffer with protease inhibitor cocktail (Sigma). Lysate was centrifuged and the resultant supernatant was used for the assay. Ac-DEVD-AFC, the caspase substrate, was added at a final concentration of 50 μM and the samples were incubated for 45 min at 37°C. Ac-DEVD-AFC hydrolysis was monitored by fluorescence emission of the released AFC (excitation, 400 nm; emission, 500 nm) using a multi-wavelength excitation dual wavelength-emission flurorimeter (Delta RAM; photon Technology International, Edison, NJ). Under these excitation and emission conditions, Ac-DEVD-AFC hydrolysis produced a yellow-green fluorescence as suggested by the manufacturer (Calbiochem).
Analysis and Statistics
Data and statistically analyses were done with IGOR Pro software (Wavemetrics). All data are presented as mean ± SEM. Sample size for the electrophysiological and Ca2+ imaging experiments were used based on previous experiences 27;30. Experimenters were not blinded to conditions. To avoid error introduced by subjectivity, all electrophysiological experiments in which nuclear membrane patches were successfully isolated were recorded and analyzed.
Statistical comparisons between data points were done using two-tailed tests. All statistically significant differences between data points stated were established using unpaired t-tests (with Bonferroni correction for multiple comparisons within a family of inferences). All lack of statistically significant differences between data points stated were established by ANOVA. Unless a more stringent P-value is stated, P < 0.05 was used for rejecting the null hypothesis.
Results
Activation of InsP3R by isoflurane
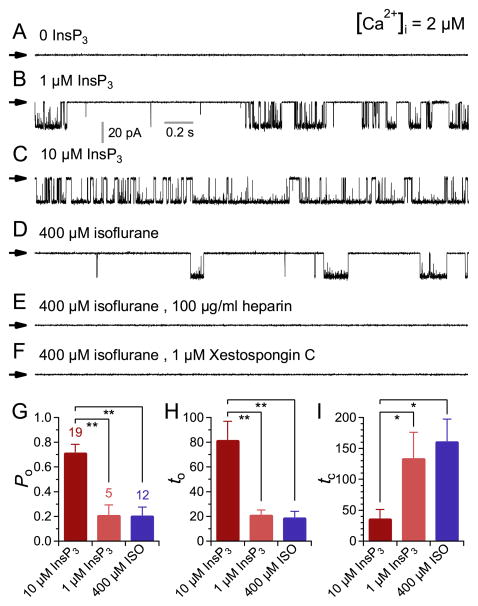
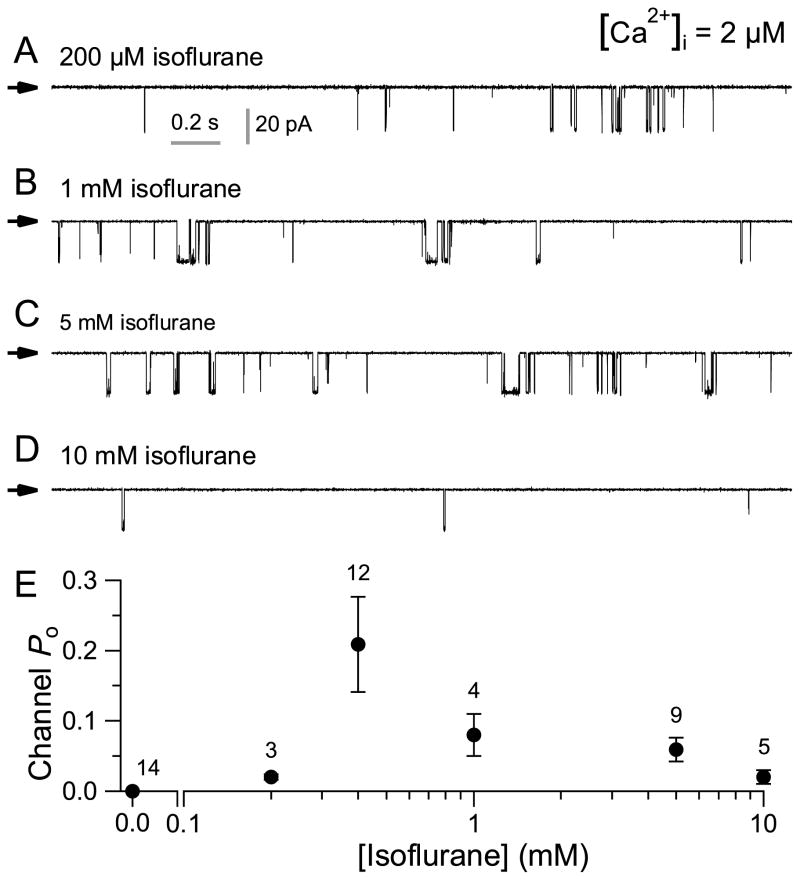
Previous studies indicated that InsP3Rs are involved in isoflurane-mediated increases in [Ca2+]i in PC12 cells, DT40 chicken B lymphocytes, Huntington’s striatal neuronal cell lines and primary cortical neurons. 11;13;16;31 To test whether isoflurane might directly modulate the activity of the InsP3R, we measured single InsP3R channel currents in native ER membranes by nuclear patch clamp electrophysiology. 4;10;23;24;26;28 Homo-tetrameric InsP3R-3 channel activities were recorded by patch-clamping outer membranes of nuclei isolated from DT40-KO cells stably expressing the rat type 3 InsP3R. 10;22;32 This isoform has ligand regulation and permeation properties similar to other InsP3R isoforms, but with robust gating that provides sensitive detection of modulation of channel activity in patch clamp electrophysiology. InsP3R-3 channel activity was observed with 2 μM Ca2+ and either 1 or 10 μM InsP3 in the pipette solution (fig. 1A–C) in ≥ 90% of patches (table 1; 18/20 and 31/34 for 1 and 10 μM InsP3, respectively). Surprisingly, with 400 μM isoflurane in the pipette solution, InsP3R channel activity could be elicited in the absence of InsP3 (fig. 1D), albeit with low open probability (Po) and in < 20% of patches (table 1; 15/85). The channels activated by isoflurane were identified as InsP3R by their sensitivity to the InsP3R antagonists heparin (100 μg/ml) or xestospongin C (1 μM) (fig. 1E and F; table 1). The mean open probability, open duration (to) and closed time (tc) of the InsP3R-3 channels activated by 400 μM isoflurane were similar to those of channels activated by subsaturating 1 μM InsP3 (fig. 1G–I), suggesting that isoflurane activates InsP3R-3 channels with similar kinetics to the endogenous agonist InsP3 but with lower efficacy. Interestingly, the concentration dependence of the modification of InsP3R-3 channel activity by isoflurane was biphasic: at low [isoflurane] (< 400 μM), increases in [isoflurane] increased channel Po, but channel Po decreased as [isoflurane] was increased beyond 400 μM. (fig. 2). Thus, InsP3R-3 channels are activated within a narrow range of [isoflurane]. This is very different from InsP3 activation of the channel, in which channel Po increases with [InsP3] until the saturating [InsP3] is reached. Beyond that, further increase in [InsP3] does not enhance channel activity any more. 32
Figure 1. Isoflurane activates single inositol trisphosphate receptor (InsP3R) channel gating.
(A–F) Typical single-channel current traces in membrane patches from nuclei isolated from DT40-KO cells stably expressing rate type 3 InsP3R. Concentrations of InsP3, isoflurane and InsP3R inhibitors used are as indicated. In this and all subsequent figures, currents shown were recorded at room temperature with applied potential (Vapp) = −40 mV, and arrows indicate the closed channel current level. Concentration of free Ca2+ ([Ca2+]i) = 2 μM in all the experiments unless stated otherwise. (G–I) Bar graphs showing InsP3R gating characteristics: mean open probability (Po), open duration (to) and closed time (tc), respectively, in various inositol trisphosphate (InsP3) or isoflurane (ISO) concentrations. Error bars indicate standard error of the mean, and number of experiments analyzed is tabulated. ** and * indicate P (t-test with Bonferroni correction) < 0.005 and 0.05, respectively, for the quantities connected by the brackets.
Table 1.
Channel Detection Rate (Pd) of Isoflurane-activated Type 3 InsP3R
| Isoflurane (0.4 mM) | InsP3 (μM) | Heparin (100 μg/ml) | Xestospongin C (μM) | Channel Detection Rate (Pd) | Patches (n) | |
|---|---|---|---|---|---|---|
| Type-3 InsP3R | − | 0 | − | − | 0/14(0) | 14 |
| + | 0 | − | − | 15/85(0.18) | 85 | |
| − | 1 | − | − | 18/20(0.9) | 20 | |
| − | 10 | − | − | 31/34(0.92) | 34 | |
| + | − | + | + | 0/40(0) | 40 | |
| + | − | − | + | 0/40(0) | 40 | |
|
| ||||||
| InsP3R Knockout | − | 10 | − | − | 0/10(0) | 10 |
| + | 0 | − | − | 0/40(0) | 40 | |
InsP3R – inositol 1,4,5-trisphosphate receptors.
Figure 2. Dependence of inositol trisphosphate receptor (InsP3R) channel activity on isoflurane concentration.
(A–D) Typical single-channel current traces of type 3 (InsP3R-3) channel activity in various isoflurane concentrations as indicated. Concentration of free Ca2+ ([Ca2+]i) = 2 μM. See figure 1D for single InsP3R channel current trace in 400 μM isoflurane. (E) [isoflurane] dependence of InsP3R-3 channel open probability Po. Error bars indicate standard error of the mean, and number of experiments analyzed is tabulated.
[Ca2+]i dependence of isoflurane-induced InsP3R channel activity
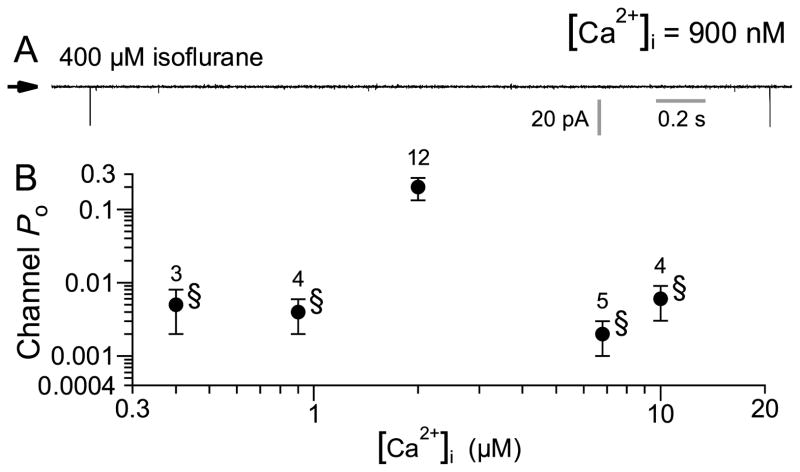
Gating of InsP3Rs is under complex allosteric regulation by InsP3 and [Ca2+]i. In general, InsP3R channel activity is biphasically regulated by [Ca2+]i with maximum channel Po observed over a broad range of [Ca2+]i in the presence of saturating (10 μM) InsP3. In the presence of subsaturating [InsP3], sensitivity of the channel to inhibition by high [Ca2+] is enhanced, resulting in a narrower Po versus [Ca2+]i dependence. 4;32 Thus, we investigated the [InsP3] and [Ca2+]i dependencies of isoflurane-activated InsP3R channel activity. At low (0.4 or 0.9 μM) or high (6 and 10 μM) [Ca2+]i, 400μM isoflurane-activated channel Po was substantially lower (Po < 0.006) than that observed in 2 μM Ca2+i, (Po = 0.2; fig. 3), resulting in a narrow biphasic [Ca2+]i dependence.
Figure 3. Ca2+ dependence of isoflurane-activated type 3 inositol trisphosphate receptor (InsP3R-3) channel.
(A) Typical single channel current traces of InsP3R channels activated by 400 μM isoflurane in free Ca2+ concentration ([Ca2+]i) of 900 nM. (B) [Ca2+]i dependence of InsP3R-3 channel open probability (Po) in 400 μM isoflurane. Error bars indicate standard error of the mean, and number of experiments analyzed is tabulated. Note the logarithmic channel Po axis. Low channel Po (<0.01, marked with §) were observed in all [Ca2+]i examined other than 2 μM. There is no statistically significant difference among these low channels Po (P > 0.05, ANOVA test). These channel Po were so low that the number of active channel(s) (N) in the membrane patch cannot be accurately determined. Thus, the data plotted are effectively the NPo value, which may be an over-estimate of the actual Po value.
Isoflurane modulates InsP3R channel sensitivity to InsP3
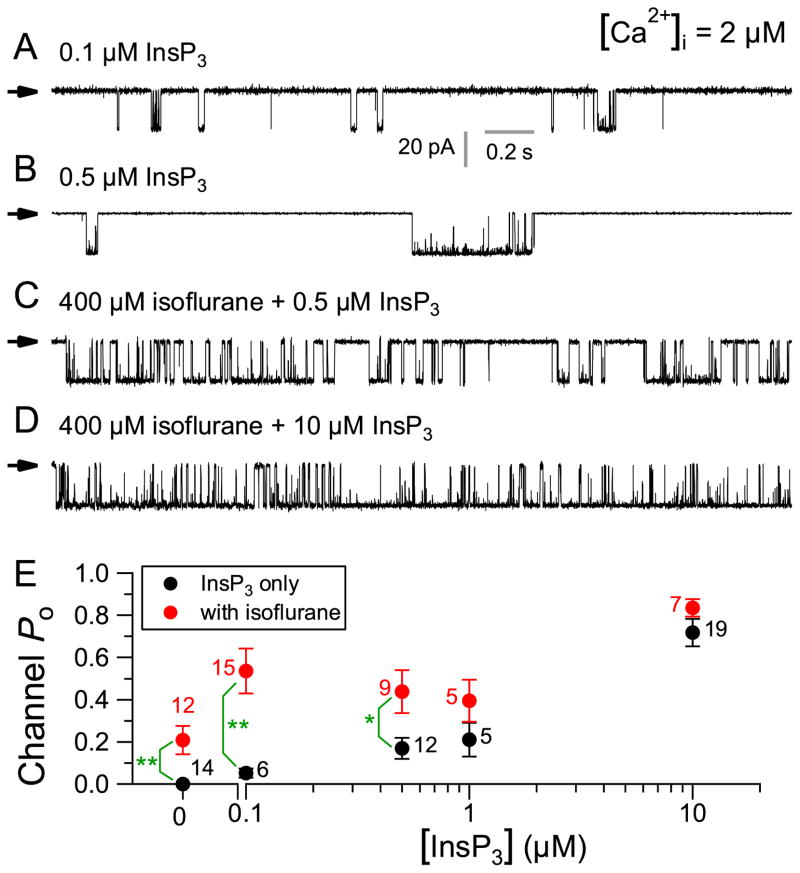
Although isoflurane activated InsP3R-3 channel activity in the absence of InsP3 in the pipette solution, the sensitivity of isoflurane activation of the channel to the competitive inhibitor heparin suggests that isoflurane may activate the InsP3R-3 by sensitizing the channel to low [InsP3] generated locally in the patched nuclear membrane. To test this, we recorded InsP3R channel activity in DT40-R3 cells with 400 μM isoflurane, 2 μM Ca2+ and a range of [InsP3] in the pipette solution. As expected, InsP3 enhanced channel Po in a dose-dependent manner (fig. 4A–D). At very low (0.1 and 0.5 μM) [InsP3], 400 μM isoflurane potentiated channel activity to a level greater than either agonist alone (fig. 4E). In contrast, at higher (1 μM) [InsP3], 400 μM isoflurane no longer potentiated channel activity significantly (fig. 4E). In saturating (10 μM) [InsP3], 400 μM isoflurane did not enhance channel activity measurably (fig. 4E). These results suggest that isoflurane increases the functional sensitivity of the InsP3R-3 to InsP3 only at low, subsaturating [InsP3] (<0.1 μM).
Figure 4. Effects of 400 μM isoflurane on the activity of inositol trisphosphate receptor (InsP3R) channel in various [InsP3].
(A–D) Typical single-channel current traces of InsP3R channels in various [InsP3] in the absence and presence of 400 μM isoflurane, as indicated. See figure 1A–C for InsP3R channel current traces in 0, 1, and 10 μM InsP3 with no isoflurane, and figure 1D for InsP3R channel current trace in 400 μM isoflurane only. (E) Statistically similar InsP3R channel activities (P > 0.05, ANOVA test) were observed in 100 nM ≤ [InsP3] ≤ 1 μM in the absence of isoflurane. With 400 μM isoflurane, statistically similar InsP3R channel activities (P > 0.05, ANOVA test) were also observed in 0 ≤ [InsP3] ≤ 1 μM. Importantly, 400 μM isoflurane significantly increased InsP3R channel activity at low [InsP3] (0, 100, and 500 nM). Error bars indicate standard error of the mean, and number of experiments analyzed is tabulated. ** and * indicate P (t-test) < 0.005 and 0.05, respectively, for the quantities connected by the brackets. InsP3R channel Po in saturating 10 μM InsP3 were significantly higher (P < 0.05, t-test with Bonferronic correction) than those in subsaturating [InsP3], in the presence or absence of 400 μM isoflurane. However, InsP3R channel activity in saturating (10 μM) InsP3 was not enhanced by 400 μM isoflurane (P > 0.05, t-test).
Isoflurane modulates InsP3R-mediated [Ca2+]i signaling and apoptosis
Our single channel recordings indicate that isoflurane at clinically relevant concentrations potentiates the activity of the InsP3R-3 in low levels of [InsP3] that can exist in un-stimulated cells in basal conditions. To determine whether this effect influences intracellular Ca2+ signaling, we measured [Ca2+]i in individual DT40-KO or DT40-R3 cells kept in complete growth medium. Application of 400 μM isoflurane resulted in a significant transient increases in [Ca2+]i in the DT40-R3 cells that were absent in DT40-KO cells lacking InsP3R expression (fig. 5A–C).
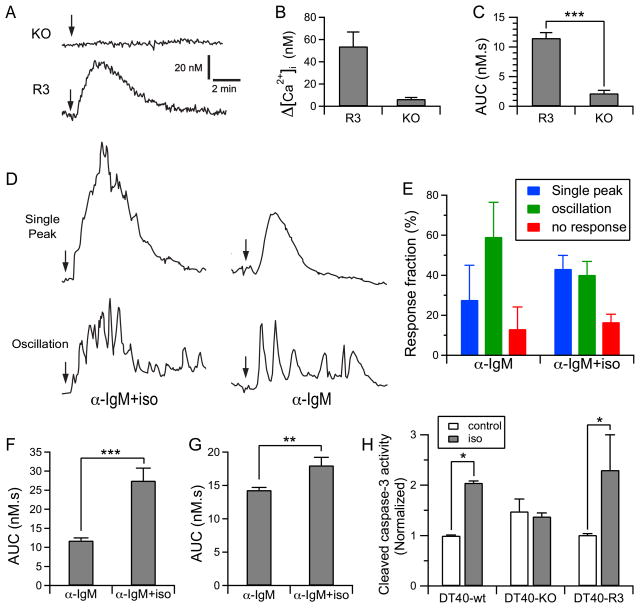
Figure 5. Isoflurane amplifies cytosolic Ca2+ signals in DT40 cells by sensitizing inositol trisphosphate receptor (InsP3R) activation and induces cell apoptosis.
(A) Representative single cell Ca2+ responses to 400 μM isoflurane (Iso) in DT-40 cells with triple knock out of InsP3R (DT40-KO) or DT40-KO transfected with rat recombinant type 3 InsP3R (DT40-R3) cells. Arrows mark the addition of iso to the complete growth medium. (B) Peak amplitudes and (C) area under curve (AUC) for DT40-KO and DT40-R3 cells responding to isoflurane. (D) Representative single cell Ca2+ responses and oscillations induced by addition (marked by arrows) of 50 ng/ml anti-IgM antibody (α-IgM) or 50 ng/ml α-IgM plus isoflurane in Dulbecco’s Modified Eagle Medium. (E) Fraction of cells with various kind of response to stimulation by α-IgM or α-IgM+iso in the medium. (F) AUC of cells with single Ca2+ peak response triggered by α-IgM or α-IgM+iso. (G) AUC of cells with Ca2+ oscillations in response to stimulation by α-IgM or α-IgM+iso. (H) Isoflurane significantly elevated caspase-3 activity only in DT40 wild type (DT40-wt) cells or in DT40 expressing only InsP3R (DT40-R3), but not in DT40 cells with triple knock out of InsP3R (DT40-KO). All calcium measurement data are summary of at least 141 cells (N≥141, fig. 5 B and C) or 212 cells (N≥212, fig. 5 E, F, and G) from 4 separate experiments. The N values for caspase-3 activity are the average from three separate experiments (N = 3). Error bars indicate standard error of the mean, and number of experiments analyzed is tabulated. *, **, and *** indicate significant difference (P < 0.05, P < 0.01 and P < 0.001, respectively, t test).* indicate significant difference (P < 0.05, t-test) between quantities connected by the brackets in the bar graphs.
To further study the potentiating effects of isoflurane on intracellular Ca2+ signaling in weakly stimulated cells, the cells were perfused with complete growth medium containing a low concentration (50 ng/ml) of anti-IgM antibody to weakly stimulate the B-cell receptor to generate low, but higher than basal, levels of InsP3. 33 82 ± 4% of DT40-R3 cells responded to anti-IgM antibody with either a single transient elevation [Ca2+]i or with [Ca2+]i oscillations (fig. 5D and E). Whereas the addition of 400 μM isoflurane did not change the percentage of cells responding to anti-IgM antibody (fig. 5D and E), it enhanced the percentage of cells that responded with a single prolonged [Ca2+]i peak (fig. 5E), consistent with a stronger InsP3R response. Because of the variable responses of cells (oscillations vs. single-peak responses), we quantified the [Ca2+]i responses in all cells by the areas under the [Ca2+]i-versus-time curves (AUC). The presence of isoflurane significantly increased the AUC for cells responding with a single-peak transient (fig. 5F; P < 0.001) or with [Ca2+]i oscillations (fig. 5G; P < 0.01). These results suggest that isoflurane, at a clinically used concentration, potentiates low level InsP3-mediated [Ca2+]i signaling.
Prolonged exposure to isoflurane is associated with widespread cell death in diverse in vivo and in vitro systems. 13;16 Because activation of InsP3R-mediated Ca2+ signaling has been linked to apoptosis, 34;35 and considering the effects of isoflurane on InsP3R gating observed here, we assessed the role of this effect in isoflurane-mediated apoptosis. Caspases are a family of cysteine proteases that play crucial roles in apoptosis. Activation of caspase-3 is a central event in the progression of programmed cell death. We therefore monitored caspase-3 cleavage as a measure of apoptosis. In agreement with our previous observations of cells expressing endogenous InsP3R, 13;16 isoflurane triggered apoptosis in the DT40-R3 cells, but not in the InsP3R deficient cells (fig. 5H). These observations support a role for exaggerated activation of InsP3R in isoflurane-induced apoptosis, consistent with previous observations. 11;13;16
Discussion
We have, for the first time, demonstrated that isoflurane at clinically relevant concentrations modulates the activity of the InsP3R Ca2+ release channel at the single channel level. The modulating effects of isoflurane on the InsP3R regulate Ca2+ release from the ER that results in exaggerated [Ca2+]i signaling. We have also demonstrated, for the first time at the molecular level, that isoflurane causes Ca2+ release from the ER via this activation of InsP3R, and can therefore affect intracellular Ca2+ homeostasis, regulation of cytosolic Ca2+ oscillations and cell survival. These results provide novel insights into possible molecular mechanisms of anesthesia-mediated effects on neurodegeneration and cognitive function.
Inhalational anesthetic modulate InsP3R activation
It was previously demonstrated that both isoflurane and halothane may elevate [Ca2+]i primarily by inducing Ca2+ release from the intracellular Ca2+ stores in neurons. 36 Our results provide the first evidence that an inhalational anesthetic can modulate activation of InsP3R channels. Interestingly, isoflurane activates InsP3R channels with a biphasic dose response with optimal concentrations around 0.4 – 1mM, close to clinically used concentrations for general anesthesia. This activation of InsP3R channels by isoflurane showed a strong Ca2+ concentration dependence, with the optimum [Ca2+]i of 2 μM, qualitatively similar to the [Ca2+] dependence of InsP3-activated channel activity. The InsP3 competitive antagonist, heparin, blocked the ability of isoflurane to activate InsP3Rs. Whether isoflurane requires InsP3 for it to activate the channel, or isoflurane activation is direct but also sensitive to heparin inhibition is unclear. The former may be likely since isoflurane (0.4 mM) potentiated activation of the InsP3R by low concentrations of InsP3, but failed to further enhance InsP3R-3 activity at saturating [InsP3]. Nevertheless, further studies are needed to investigate possible biochemical interactions between isoflurane and the InsP3R. Together, these results establish the InsP3R as a novel target of isoflurane and perhaps other inhalational or intravenous general anesthetics.
Isoflurane enhances Ca2+ signals and cell apoptosis via activation of InsP3R
The biphasic effects of [Ca2+]i on InsP3R activation play important roles on intracellular Ca2+ oscillations, waves, and spreading of global Ca2+ signals. 37 Our finding that isoflurane activates InsP3R-3 channels raised the possibility that it could enhance [Ca2+]i signals. Application of 400 μM isoflurane resulted in a transient increase in [Ca2+]i in DT-40 cells expressing InsP3R-3 but not in DT-40 cells lacking the channels. In addition, [Ca2+]i signals generated by IgM stimulation of DT40-R3 cells, both single-peaks and oscillations, were more prominent in the presence of isoflurane. (fig. 5F and G).
We previously showed in in vitro and in vivo models that exposure to isoflurane for prolonged durations significantly induced apoptosis that required activation of InsP3Rs.11;13 Our results here suggest that this is medicated at least in part by isoflurane activation of InsP3R channel gating. The effects of isoflurane on changes in [Ca2+]i are in good agreement with the conclusions reached from the single channel recordings of the effects of isoflurane on InsP3R activity observed in the current study. It has long been known that anesthetics including halothane activate the other major ER Ca2+ release channels, the RyR channel complex,38 and this is thought to be the basis for malignant hyperthermia. 39 Like InsP3Rs, RyRs are expressed throughout the nervous system and play important roles in both normal cell functions 40 and in various neurodegenerative diseases. 2;41–43 Our previous study indicated a role of RyR activation in isoflurane-induced apoptosis in neuronal tissue cultures. 29 Both InsP3R and RyR contribute to regulation of intracellular Ca2+ homeostasis and may interact with each other through Ca2+-induced Ca2+ release in a common pathway in normal neuronal function and neurodegeneration. Excessive Ca2+ release from the ER via these release channels could cause Ca2+ overload in mitochondria and depletion of ER Ca2+, both of which can contribute to apoptosis. 6;34;44 In addition, mitochondrial Ca2+ overload causes cytochrome C release, activating caspase-3, which can cleave the InsP3R, resulting in a constituent Ca2+ leak from the ER. 45 Thus, excessive or prolonged activation of InsP3R by isoflurane may set in motion a cascade of events resulting in apoptosis in different tissue culture cells including neurons, 3;11;13;15–17 and in developing brains.16 Our data suggest that the InsP3R may represent an ideal target for prevention of the harmful side effects of inhalational anesthetics. Inhibition of excessive activation of the InsP3R may ameliorate or prevent anesthesia-mediated neurodegeneration as demonstrated in animal models. 16 This is especially relevant in pediatric 46;47 and aged patients, 48 who appear to be the most vulnerable to the harmful side effects of anesthetics. Unfortunately, there is no good pharmacology for the InsP3R. Although heparin is an InsP3R antagonist, its poor penetration across cell membranes limits its use for protection against anesthetic neurotoxicity in animals or patients. It should be noted that anesthetics have also been shown to be protective against various stresses, also by activation of InsP3Rs in different tissue culture models. 3;12;14;49;50 As demonstrated here (fig. 6), mild or moderate activation of InsP3R and moderate Ca2+ release from the ER by isoflurane provides cytoprotection, possibly via physiological Ca2+ uptake into mitochondria and stimulation of ATP production, 25 or by activation of AKT and MAPK/ERK cytoprotective pathways. 12;14;50 Nevertheless, it is prudent to minimize the use of general anesthetics as much as possible, so that their beneficial effects can be utilized and the harmful effects be minimized.
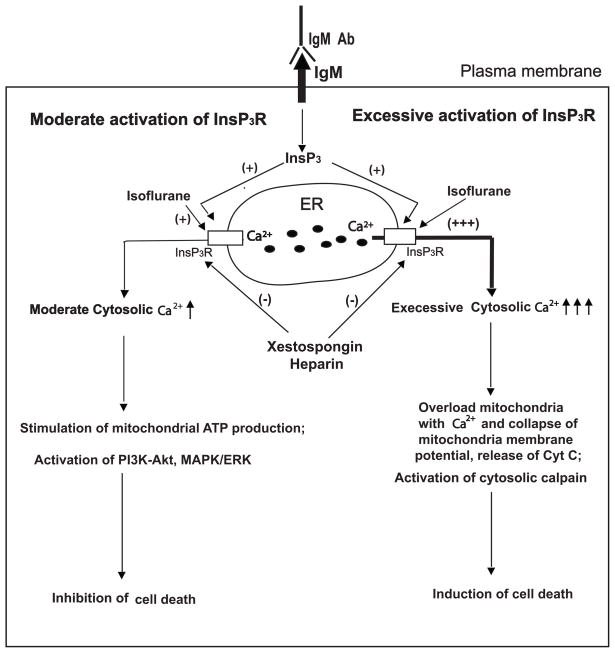
Figure 6. Modulation of inositol trisphosphate (InsP3)receptor (InsP3R) activity by isoflurane and the effects on cell survival.
Isoflurane can activate InsP3R or potentiate the activation of InsP3R by endogenous agonist InsP3 generated by various extracellular stimulation including antibody for IgM (IgM Ab). These effects can be inhibited by InsP3R antagonists xestospongin and heparin. Moderate activation of InsP3R by isoflurane at low concentration for short duration cause moderate Ca2+ release from the endoplasmic reticulum (ER) and elevation of cytosolic Ca2+, which in turn, inhibit cell death by inducing endogenous cytoprotective mechanisms (left side), such as activation of phosphatidylinositol-4,5-bisphosphate 3-kinase(PI3K)- protein kinase B (AKT) and microtubule-associated protein kinase (MAPK)/extracellular-signal-regulated kinases (ERK) pathways. Excessive activation of InsP3R by isoflurane at high concentration for prolonged duration cause excessive Ca2+ release from the endoplasmic reticulum and abnormal elevation of cytosolic Ca2+, resulting in induction of cell death via apoptosis directly (right side), through rerelease of cytochrome C (Cyc C) from mitochondria into cytosolic space. ATP = adenosine triphosphate.
In summary, our results indicate that the commonly used inhalational anesthetic isoflurane modulates gating of the InsP3R Ca2+ release channel, enhancing ER Ca2+ release and contributing to isoflurane-mediated apoptosis. These results suggest that InsP3Rs are molecular targets of general anesthetics and that these receptors may provide the basis for some pharmacologic effects of general anesthetics and therapeutic interventions for anesthesia-mediated cell death by apoptosis.
Acknowledgments
Source of Financial Support: This work was supported by: National Institutes of Health, Baltimore, Maryland, awards GM084979 and GM084979-02S1 to H.W., GM056328 and MH059937 to J.K.F., and K08-GM073224 to H.W.; March of Dimes Birth Defects Foundation Research Grant 12-FY08-167 to H.W., White Plains, New York; and the Research Fund at the Department of Anesthesiology at the University of Pennsylvania, Philadelphia, Pennsylvania.
The authors thank Qingcheng Meng, Ph.D., Department of Anesthesiology, University of Pennsylvania, Philadelphia, Pennsylvania for assistance in measuring isoflurane concentrations used in the electrophysiology and Ca2+ measurement experiments. We appreciate the valuable discussions and support from Roderic Eckenhoff, M.D., Maryellen Eckenhoff, Ph.D., and Lee Fleisher, M.D., Department of Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, Pennsylvania.
Footnotes
Conflict of Interest: The authors declare no competing interests.
Abstracts of this research work were presented at the Annual Meetings for Society for Neuroscience in Chicago of Illinois On October 21, 2009 (Program # 818.11/C4).
References
- 1.Stutzmann GE. Calcium dysregulation, IP3 signaling, and Alzheimer’s disease. Neuroscientist. 2005;11:110–5. doi: 10.1177/1073858404270899. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee S, Hasan G. The InsP3 receptor: Its role in neuronal physiology and neurodegeneration. Bioessays. 2005;27:1035–47. doi: 10.1002/bies.20298. [DOI] [PubMed] [Google Scholar]
- 3.Zhao X, Yang Z, Liang G, Wu Z, Peng Y, Joseph DJ, Inan S, Wei H. Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology. 2013;118:537–49. doi: 10.1097/ALN.0b013e3182833fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Criollo A, Vicencio JM, Tasdemir E, Maiuri MC, Lavandero S, Kroemer G. The inositol trisphosphate receptor in the control of autophagy. Autophagy. 2007;3:350–3. doi: 10.4161/auto.4077. [DOI] [PubMed] [Google Scholar]
- 6.Hanson CJ, Bootman MD, Roderick HL. Cell signalling: IP3 receptors channel calcium into cell death. Curr Biol. 2004;14:R933–5. doi: 10.1016/j.cub.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Joseph SK, Hajnoczky G. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis. 2007;12:951–68. doi: 10.1007/s10495-007-0719-7. [DOI] [PubMed] [Google Scholar]
- 8.Decuypere JP, Monaco G, Bultynck G, Missiaen L, De SH, Parys JB. The IP(3) receptor-mitochondria connection in apoptosis and autophagy. Biochimica et Biophysica Acta. 2011;1813:1003–13. doi: 10.1016/j.bbamcr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–39. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung KH, Shineman D, Muller M, Cardenas C, Mei LJ, Yang J, Tomita T, Iwatsubo T, Lee VMY, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP(3) receptor channel gating. Neuron. 2008;58:871–83. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei HF. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–50. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: Role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103:532–9. doi: 10.1097/00000542-200509000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Wei HF, Liang G, Yang H, Wang QJ, Hawkins B, Madesh M, Wang SP, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 14.Bickler PE, Fahlman CS. The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth Analg. 2006;103:419–29. doi: 10.1213/01.ane.0000223671.49376.b2. [DOI] [PubMed] [Google Scholar]
- 15.Liang G, Wang QJ, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei HF. A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg. 2008;106:492–500. doi: 10.1213/ane.0b013e3181605b71. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Liang G, Chen Q, Joseph DJ, Meng Q, Eckenhoff RG, Eckenhoff MF, Wei H. Anesthetic-induced neurodegeneration mediated via inositol 1,4,5-trisphosphate receptors. J Pharmacol Exp Ther. 2010;333:14–22. doi: 10.1124/jpet.109.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Liang G, Yang H, Wang S, Eckenhoff MF, Wei H. The common inhaled anesthetic isoflurane increases aggregation of huntingtin and alters calcium homeostasis in a cell model of Huntington’s disease. Toxicol Appl Pharmacol. 2011;250:291–8. doi: 10.1016/j.taap.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei H. The role of calcium dysregulation in anesthetic-mediated neurotoxicity. Anesth Analg. 2011;113:972–4. doi: 10.1213/ANE.0b013e3182323261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang GH, Dong YL, Zhang B, Ichinose F, Wu X, Culley DJ, Crosby G, Tanzi RE, Xie ZC. Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci. 2008;28:4551–60. doi: 10.1523/JNEUROSCI.5694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao YL, Xiang Q, Shi QY, Li SY, Tan L, Wang JT, Jin XG, Luo AL. GABAergic excitotoxicity injury of the immature hippocampal pyramidal neurons’ exposure to isoflurane. Anesth Analg. 2011;113:1152–60. doi: 10.1213/ANE.0b013e318230b3fd. [DOI] [PubMed] [Google Scholar]
- 21.Sinner B, Friedrich O, Zink W, Zausig Y, Graf BM. The toxic effects of s(+)-ketamine on differentiating neurons in vitro as a consequence of suppressed neuronal Ca2+ oscillations. Anesth Analg. 2011;113:1161–9. doi: 10.1213/ANE.0b013e31822747df. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Wang XL, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci U S A. 2007;104:12565–70. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak DO, Vais H, Cheung KH, Foskett JK. Patch-clamp electrophysiology of intracellular Ca2+ channels. Cold Spring Harb Protoc. 2013;2013:787–97. doi: 10.1101/pdb.top066217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak DO, Vais H, Cheung KH, Foskett JK. Isolating nuclei from cultured cells for patch-clamp electrophysiology of intracellular Ca(2+) channels. Cold Spring Harb Protoc. 2013;2013:880–4. doi: 10.1101/pdb.prot073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–83. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mak DO, Vais H, Cheung KH, Foskett JK. Nuclear patch-clamp electrophysiology of Ca2+ channels. Cold Spring Harb Protoc. 2013;2013:885–91. doi: 10.1101/pdb.prot073064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci U S A. 1998;95:15821–5. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–47. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Vais H, Siebert AP, Ma Z, Fernandez-Mongil M, Foskett JK, Mak DO. Redox-regulated heterogeneous thresholds for ligand recruitment among InsP3R Ca2+-release channels. Biophys J. 2010;99:407–16. doi: 10.1016/j.bpj.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q, Liang G, Yang H, Wang S, Eckenhoff MF, Wei H. The common inhaled anesthetic isoflurane increases aggregation of huntingtin and alters calcium homeostasis in a cell model of Huntington’s disease. Toxicol Appl Pharmacol. 2010;250:291–8. doi: 10.1016/j.taap.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vais H, Foskett JK, Ullah G, Pearson JE, Daniel Mak DO. Permeant calcium ion feed-through regulation of single inositol 1,4,5-trisphosphate receptor channel gating. J Gen Physiol. 2012;140:697–716. doi: 10.1085/jgp.201210804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang R, Cioffi J, Kimberly R, Edberg J, Mayer L. B cell differentiation factor-induced human B cell maturation: Stimulation of intracellular calcium release. Cell Immunol. 1995;164:227–33. doi: 10.1006/cimm.1995.1165. [DOI] [PubMed] [Google Scholar]
- 34.Inan S, Wei H. The cytoprotective effects of dantrolene: A ryanodine receptor antagonist. Anesth Analg. 2010;111:1400–10. doi: 10.1213/ANE.0b013e3181f7181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol Med. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kindler CH, Eilers H, Donohoe P, Ozer S, Bickler PE. Volatile anesthetics increase intracellular calcium in cerebrocortical and hippocampal neurons. Anesthesiology. 1999;90:1137–45. doi: 10.1097/00000542-199904000-00029. [DOI] [PubMed] [Google Scholar]
- 37.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 38.Akata T, Nakashima M, Izumi K. Comparison of volatile anesthetic actions on intracellular calcium stores of vascular smooth muscle: Investigation in isolated systemic resistance arteries. Anesthesiology. 2001;94:840–50. doi: 10.1097/00000542-200105000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Denborough M. Malignant hyperthermia. Lancet. 1998;352:1131–6. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- 40.Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Paschen W, Frandsen A. Endoplasmic reticulum dysfunction - a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem. 2001;79:719–25. doi: 10.1046/j.1471-4159.2001.00623.x. [DOI] [PubMed] [Google Scholar]
- 42.Berridge MJ. Calcium signalling and Alzheimer’s disease. Neurochem Res. 2011;36:1149–56. doi: 10.1007/s11064-010-0371-4. [DOI] [PubMed] [Google Scholar]
- 43.Marambaud P, Dreses-Werringloer U, Vingtdeux V. Calcium signaling in neurodegeneration. Mol Neurodegener. 2009;4:20. doi: 10.1186/1750-1326-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–37. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assefa Z, Bultynck G, Szlufcik K, Nadif KN, Vermassen E, Goris J, Missiaen L, Callewaert G, Parys JB, De Smedt H. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. J Biol Chem. 2004;279:43227–36. doi: 10.1074/jbc.M403872200. [DOI] [PubMed] [Google Scholar]
- 46.Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–12. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 47.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 49.Wei H, Inan S. Dual effects of neuroprotection and neurotoxicity by general anesthetics: Role of intracellular calcium homeostasis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:156–61. doi: 10.1016/j.pnpbp.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–15. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]