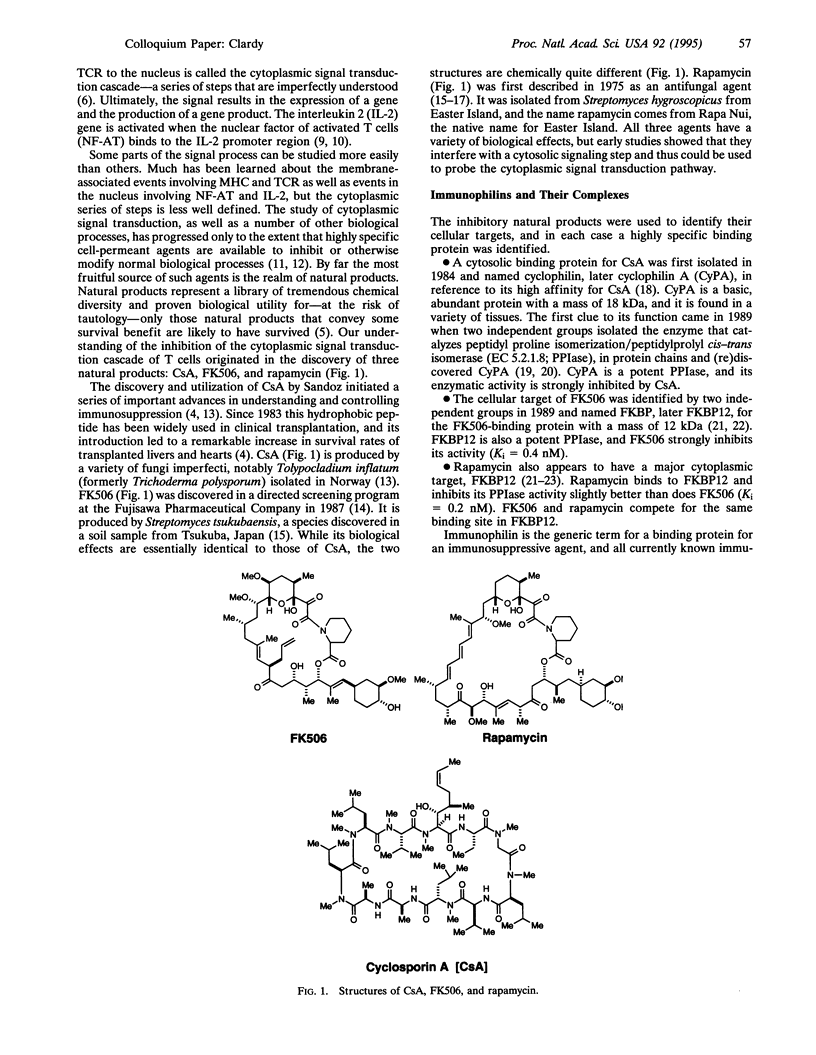
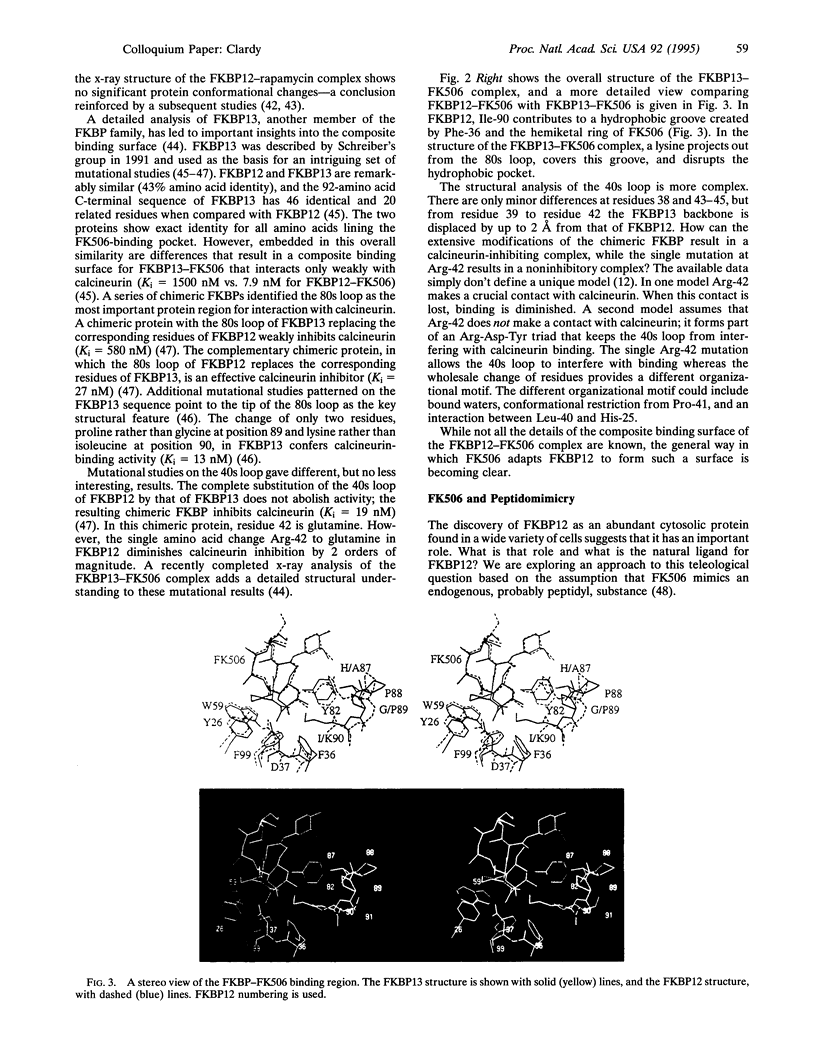
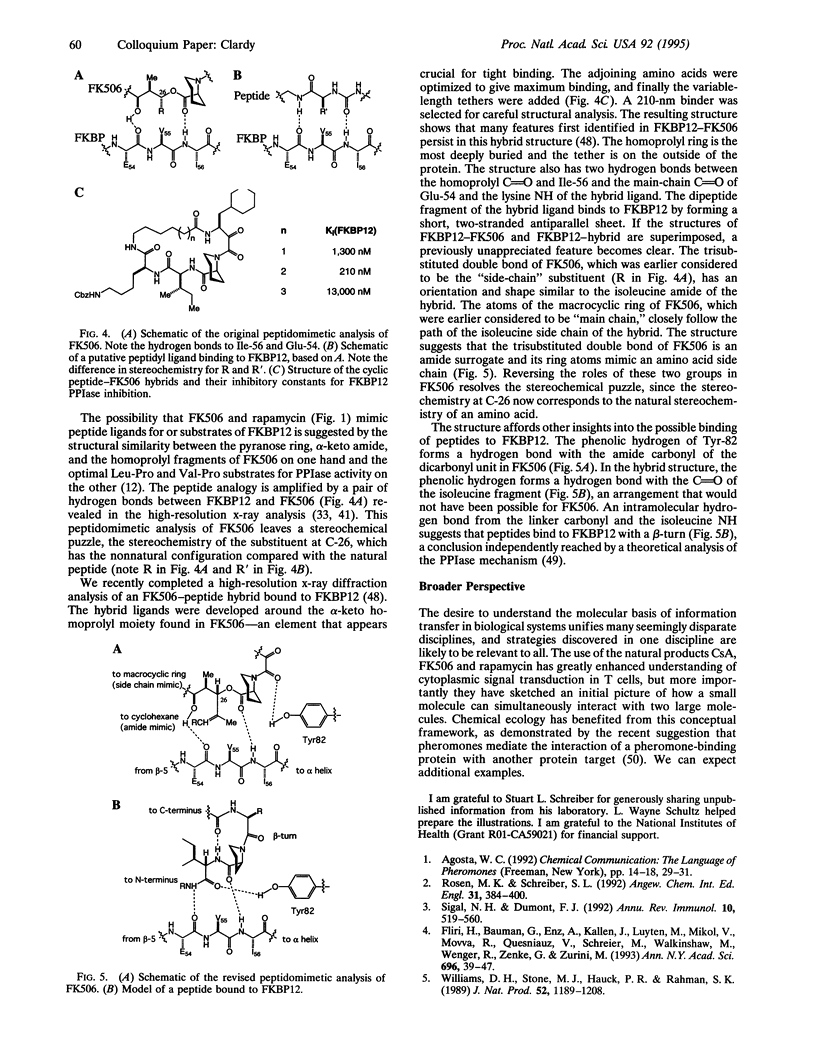
Abstract
Several disciplines, including chemical ecology, seek to understand the molecular basis of information transfer in biological systems, and general molecular strategies are beginning to emerge. Often these strategies are discovered by a careful analysis of natural products and their biological effects. Cyclosporin A, FK506, and rapamycin are produced by soil microorganisms and are being used or considered as clinical immunosuppressive agents. They interrupt the cytoplasmic portion of T-cell signaling by forming a complex with a binding protein--FKBP12 in the case of FK506 and rapamycin and cyclophilin A (CyPA) in the case of cyclosporin A (CsA). This complex in turn inhibits a protein target, and the best understood target is calcineurin, which is inhibited by FK506-FKBP12 and CyPA-CsA. Mutational and structural studies help define how FK506-FKBP12 interacts with calcineurin, and the results of these studies are summarized. The existence of strong FK506-FKBP12 binding suggests that FK506 is mimicking some natural ligand for FKBP12. Synthetic and structural studies to probe this mimicry are also described.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. W., Rotonda J., McKeever B. M., Chan H. K., Marcy A. I., Wiederrecht G., Hermes J. D., Springer J. P. FK-506-binding protein: three-dimensional structure of the complex with the antagonist L-685,818. J Biol Chem. 1993 May 25;268(15):11335–11339. [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994 Jun 30;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G. Analysis of MHC-presented peptides: applications in autoimmunity and vaccine development. Immunol Today. 1994 Apr;15(4):155–160. doi: 10.1016/0167-5699(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Clardy J. Structural studies of complexed FK-506 binding protein. Ann N Y Acad Sci. 1993 Jun 23;685:37–45. doi: 10.1111/j.1749-6632.1993.tb35848.x. [DOI] [PubMed] [Google Scholar]
- Du G., Ng C. S., Prestwich G. D. Odorant binding by a pheromone binding protein: active site mapping by photoaffinity labeling. Biochemistry. 1994 Apr 26;33(16):4812–4819. doi: 10.1021/bi00182a009. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Fischer S., Michnick S., Karplus M. A mechanism for rotamase catalysis by the FK506 binding protein (FKBP). Biochemistry. 1993 Dec 21;32(50):13830–13837. doi: 10.1021/bi00213a011. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Albers M. W., Lane W. S., Bierer B. E., Schreiber S. L., Burakoff S. J. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepre C. A., Thomson J. A., Moore J. M. Solution structure of FK506 bound to FKBP-12. FEBS Lett. 1992 May 4;302(1):89–96. doi: 10.1016/0014-5793(92)80292-o. [DOI] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Wandless T. J., Luan S., Alberg D. G., Belshaw P. J., Cohen P., MacKintosh C., Klee C. B., Schreiber S. L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992 Apr 28;31(16):3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Meadows R. P., Nettesheim D. G., Xu R. X., Olejniczak E. T., Petros A. M., Holzman T. F., Severin J., Gubbins E., Smith H., Fesik S. W. Three-dimensional structure of the FK506 binding protein/ascomycin complex in solution by heteronuclear three- and four-dimensional NMR. Biochemistry. 1993 Jan 26;32(3):754–765. doi: 10.1021/bi00054a004. [DOI] [PubMed] [Google Scholar]
- Michnick S. W., Rosen M. K., Wandless T. J., Karplus M., Schreiber S. L. Solution structure of FKBP, a rotamase enzyme and receptor for FK506 and rapamycin. Science. 1991 May 10;252(5007):836–839. doi: 10.1126/science.1709301. [DOI] [PubMed] [Google Scholar]
- Moore J. M., Peattie D. A., Fitzgibbon M. J., Thomson J. A. Solution structure of the major binding protein for the immunosuppressant FK506. Nature. 1991 May 16;351(6323):248–250. doi: 10.1038/351248a0. [DOI] [PubMed] [Google Scholar]
- Petros A. M., Gampe R. T., Jr, Gemmecker G., Neri P., Holzman T. F., Edalji R., Hochlowski J., Jackson M., McAlpine J., Luly J. R. NMR studies of an FK-506 analogue, [U-13C]ascomycin, bound to FKBP: conformation and regions of ascomycin involved in binding. J Med Chem. 1991 Sep;34(9):2925–2928. doi: 10.1021/jm00113a037. [DOI] [PubMed] [Google Scholar]
- Rahfeld J. U., Schierhorn A., Mann K., Fischer G. A novel peptidyl-prolyl cis/trans isomerase from Escherichia coli. FEBS Lett. 1994 Apr 18;343(1):65–69. doi: 10.1016/0014-5793(94)80608-x. [DOI] [PubMed] [Google Scholar]
- Rüegger A., Kuhn M., Lichti H., Loosli H. R., Huguenin R., Quiquerez C., von Wartburg A. Cyclosporin A, ein immunsuppressiv wirksamer Peptidmetabolit aus Trichoderma polysporum (Link ex Pers.) Rifai. Helv Chim Acta. 1976;59(4):1075–1092. doi: 10.1002/hlca.19760590412. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Sehgal S. N., Baker H., Vézina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1975 Oct;28(10):727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F. J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994 Mar 17;368(6468):215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Barthelmess I. B., Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989 Dec 21;342(6252):953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- Van Duyne G. D., Standaert R. F., Karplus P. A., Schreiber S. L., Clardy J. Atomic structure of FKBP-FK506, an immunophilin-immunosuppressant complex. Science. 1991 May 10;252(5007):839–842. doi: 10.1126/science.1709302. [DOI] [PubMed] [Google Scholar]
- Van Duyne G. D., Standaert R. F., Karplus P. A., Schreiber S. L., Clardy J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J Mol Biol. 1993 Jan 5;229(1):105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- Vézina C., Kudelski A., Sehgal S. N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975 Oct;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Weber C., Wider G., von Freyberg B., Traber R., Braun W., Widmer H., Wüthrich K. The NMR structure of cyclosporin A bound to cyclophilin in aqueous solution. Biochemistry. 1991 Jul 2;30(26):6563–6574. doi: 10.1021/bi00240a029. [DOI] [PubMed] [Google Scholar]
- Wiederrecht G., Brizuela L., Elliston K., Sigal N. H., Siekierka J. J. FKB1 encodes a nonessential FK 506-binding protein in Saccharomyces cerevisiae and contains regions suggesting homology to the cyclophilins. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1029–1033. doi: 10.1073/pnas.88.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. H., Stone M. J., Hauck P. R., Rahman S. K. Why are secondary metabolites (natural products) biosynthesized? J Nat Prod. 1989 Nov-Dec;52(6):1189–1208. doi: 10.1021/np50066a001. [DOI] [PubMed] [Google Scholar]




