The rapid transfer of nearly 4000 HIV-infected patients from a hospital-based clinic to community-based clinics had an estimated success of 82%. Close collaboration between transferring and receiving clinics is necessary to ensure that the health gains from PEPFAR funding are maintained.
Keywords: PEPFAR, transfer of HIV care, South Africa, linkage to care, community-based clinics
Abstract
Background
President's Emergency Plan for AIDS Relief (PEPFAR) funding changes have resulted in human immunodeficiency virus (HIV) clinic closures. We evaluated linkage to care following a large-scale patient transfer from a PEPFAR-funded, hospital-based HIV clinic to government-funded, community-based clinics in Durban.
Methods
All adults were transferred between March and June 2012. Subjects were surveyed 5–10 months post-transfer to assess self-reported linkage to the target clinic. We validated self-reports by auditing records at 8 clinics. Overall success of transfer was estimated using linkage to care data for both reached and unreached subjects, adjusted for validation results.
Results
Of the 3913 transferred patients, 756 (19%) were assigned to validation clinics; 659 (87%) of those patients were reached. Among those reached, 468 (71%) had a validated clinic record visit. Of the 46 who self-reported attending a different validation clinic than originally assigned, 39 (85%) had a validated visit. Of the 97 patients not reached, 59 (61%) had a validated visit at their assigned clinic. Based on the validation rates for reached and unreached patients, the estimated success of transfer for the cohort overall was 82%.
Conclusions
Most patients reported successful transfer to a community-based clinic, though a quarter attended a different clinic than assigned. Validation of attendance highlights that nearly 20% of patients may not have linked to care and may have experienced a treatment interruption. Optimizing transfers of HIV care to community sites requires collaboration with receiving clinics to ensure successful linkage to care.
South Africa, in partnership with the US President's Emergency Plan for AIDS Relief (PEPFAR), began to roll out antiretroviral therapy (ART) in 2004 and now has the largest ART program in the world, with over 2.4 million people on treatment [1, 2]. However, as the program shifts toward “country ownership,” PEPFAR support to South Africa will decrease by almost 50% between 2012 and 2017, with a concomitant shift in focus from providing direct medical care to offering technical support [3–8]. The initial phase of PEPFAR was implemented primarily through nongovernmental organizations, many of which were hospital-based and doctor-managed facilities [9, 10]. Recent South African guidelines have focused on nurse-initiated ART [11]; with the decrease in direct medical care funding provided by PEPFAR, patient care is now shifting to government-funded, nurse-managed primary healthcare clinics in the community [12].
Limited data suggest similar clinical outcomes for those treated within nurse-managed primary healthcare clinics compared to hospital-based programs [13–15]. However, these studies reflect outcomes of selected, clinically stable patients referred to community programs [16–18]. Further, in extant studies, nurses at accepting clinics received additional training through a clinical trial [13], the sickest patients were still cared for by doctors [19], or a substantial fraction of patients refused the referral [14, 17]. Although studies from Malawi and Swaziland reveal less lost to follow-up from primary healthcare centers compared to hospital-based human immunodeficiency virus (HIV) programs, some also report higher mortality [14, 20–22].
The recent closure of a PEPFAR-funded, hospital-based HIV clinic in Durban, South Africa, necessitated the rapid transfer of patients to government-funded, community-based clinics. To date, no study has evaluated a mandatory transfer of all patients, regardless of clinical state, to primary healthcare centers. Given that the highest rates of lost to follow-up generally occur in the immediate transition between the last hospital-based visit and the first clinic visit [18], this clinic closure provided an opportunity to perform real-time assessment of linkage to clinic-based care. As funding for direct patient care declines, more hospital-based and nongovernmental programs throughout sub-Saharan Africa may close and transition patients to public sector community clinics. We sought to assess initial linkage to transfer clinics following a large-scale transfer of HIV-infected patients from hospital-based to primary healthcare clinics in South Africa.
METHODS
Study Site
McCord Hospital is a 142-bed state-aided, semiprivate general hospital serving a predominantly urban population from the greater Durban area. The Sinikithemba HIV clinic at McCord began treating patients with ART in 1999 and became a PEPFAR-funded site in 2004, rapidly expanding its clinical services and becoming an integral part of the South African ART scale-up. Sinikithemba served a predominantly African, Zulu-speaking population and initiated over 10 000 patients on ART. Because McCord Hospital was semiprivate, patients paid a monthly, all-inclusive fee (180 ZAR [approximately 18 USD] based on the approximate exchange rate at the time of transfer) for comprehensive outpatient care per South African guidelines [11]. Patients were seen by both doctors and nurses throughout the course of their ART initiation and long-term care. In addition to being available daily for consultations, doctors took on medically complex cases. The clinic had an electronic medical record and a dedicated monitoring and evaluation team and was considered a Center of Excellence locally. The HIV clinic was scheduled to lose PEPFAR funding and at the end of 2011 created a transition plan to explore alternative funding options. Due to the inability to secure alternative public or private funding, the clinic had a short timeline to plan and execute the transfer of all patients (stable and unstable) to the public sector by the end of June 2012. Between July 2010 and June 2011, 48% of patients enrolled at McCord were still in care at the clinic, 29% changed providers with a formal transfer, 10% died, 9% were lost to follow-up, and 4% stopped treatment.
Sinikithemba Transfer Process
Patients were referred for transfer if they returned to the clinic for clinical appointments, laboratories, or pharmacy refills between March 12 and June 30, 2012, the “transfer period.” Patients seen during this period underwent a group counseling session explaining the imminent clinic closure and transfer procedures. Patients were then individually evaluated by a clinician and a counselor and transferred to one of 171 different clinics in the Durban area (Fig. 1). The list of transfer clinics and the number of patients each could absorb was compiled in collaboration with district managers from the municipal and provincial Departments of Health. Counselors identified target clinics for patients based on their care needs and residential address, with the Department of Health requesting that patients attend clinics within their designated geographic area. Patients taking first-line ART were transferred to primary healthcare clinics; those taking second-line line ART were transferred to community health clinics. Patients with comorbidities requiring medications not on the South African Essential Drug List [23] were referred to hospital-based clinics. Clinicians completed a transfer form with clinical information for patients to bring to their transfer clinic. In addition to demographic data, clinical data such as date of HIV diagnosis, baseline and most recent CD4 count, current ART regimen, and current contact details were entered into a database. Patients were issued 1 month of medication or a buffer supply of several months if their transfer clinic appointment was delayed.
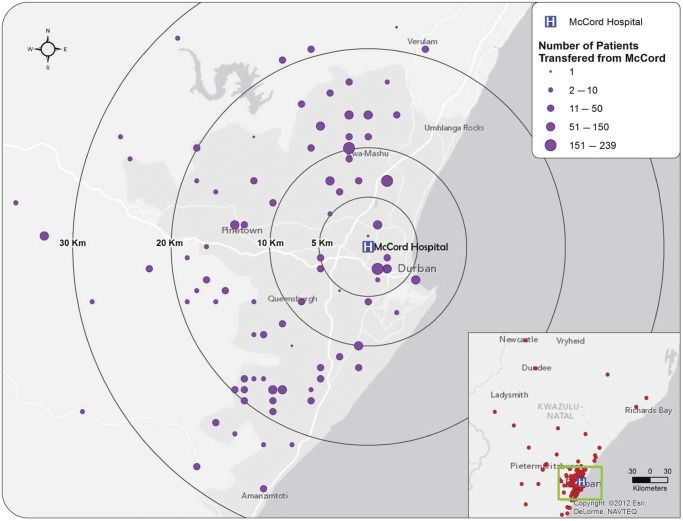
Fig. 1.
Map showing the location of transfer clinics within 40 km of McCord Hospital, Durban, South Africa, and the relative number of people transferred to that clinic (indicated by purple circle size). The inset includes points (red circles) for all transfer clinics and their geographic distribution. Clinics depicted are those with street addresses for which GPS coordinates could be generated. Abbreviation: GPS, global positioning system.
Most patients were assigned a transfer clinic at their first visit to Sinikithemba during the transfer period. However, acutely ill or medically complex patients, those with suspected drug resistance, those who recently initiated ART (in the 3 months prior), and patients with recent ART regimen changes were flagged with a “red dot” sticker on their clinical folder. Red dot, or “high-risk,” patients were retained in care and transferred at a subsequent visit before clinic closure.
Study Population
The study population included all adults (≥18 years) on ART who visited the clinic in the year prior to the transfer period. Pre-ART patients were excluded from the formal transfer process and also from this study. The study protocol was approved by the McCord Hospital Research Ethics Committee (Durban, South Africa) and the Partners Human Research Committee (2012-P-001122/1, Boston, MA).
Data Collection
Research assistants contacted patients by phone 5–10 months after their last Sinikithemba visit to administer a brief questionnaire. Patients were asked about their HIV care, including whether they attended their assigned transfer clinic, reasons for delays or failures to link, whether they experienced treatment interruption, and the date of their last transfer clinic visit. During the phone interview, subjects were asked to give oral consent to have anonymized transfer data used for research purposes. Research assistants attempted to contact patients on 3 different days of the week and at different times. If a patient was not reached after multiple attempts, they were considered unreached. Family members answering a subject's phone who described the subject as deceased were queried regarding the date and cause of death. Responses were entered into a password-protected electronic database.
The primary outcome of interest was linkage to care at the referral clinic, defined as a self-reported clinic visit within 90 days of transfer. This window reflects the period of time during which patients would have needed to link to care to avoid a treatment interruption. Secondary outcomes included: (1) a validated transfer clinic visit, as documented by medical record review and (2) death, as ascertained through family members answering patients' phones and cross-matching with the South African death registry using national ID numbers [24].
Validation of Self-Report
We randomly selected a sample of clinics to validate patients' self-reported first transfer clinic visit. To facilitate access to clinics by the research team, we selected the 80 clinics closest to the hospital based on global positioning system (GPS) coordinates and divided the clinics into deciles based on the cumulative proportion of the cohort transferred there. One clinic was sampled without replacement from each decile, with the goal of validating at least 10% of patient visits.
Validation of self-reported visits to the sampled transfer clinics was conducted by identifying a clinic record of patient visits in ART registers and medical records. Patients were searched for by name (English and Zulu), surname, date of birth, and South African ID number. All patients assigned to a validation clinic, regardless of whether they were reached for the phone questionnaire, were sought, as were patients who were assigned elsewhere but self-reported attending one of the validation clinics. If a patient was neither assigned to nor reported attending a validation clinic, their records were not intentionally sought at the validation clinic. Because not all clinics specifically marked the records of McCord transfers, the number of patients found at the validation clinics is a conservative figure. We estimated the overall success of transfer for the entire cohort using the rates of linkage to care for subjects reached and not reached for the survey, adjusted for results of the validation visits:
where R, percent of patients reached; FAC, percent of reached patients found at their assigned clinic; E, percent among those reached who were not found at assigned clinic and reported going elsewhere; FSR, percent found among patients who self-reported going to a nonassigned validation clinic; UAC, percent of unreached patients found at their assigned clinic.
We calculated 95% confidence intervals for the transfer rate estimate using a bootstrapping procedure with 1000 repetitions. Univariate risk ratios with 95% confidence intervals were used to examine correlates of not being found at the validation clinic to which the patient was assigned. To assess the independent impact of red dot status on the likelihood of not being found at their assigned transfer clinic, we used a Poisson regression model, controlling for other factors potentially important to the outcome. The model included red dot (high-risk) status, gender, age, most recent CD4 count prior to transfer, distance of transfer clinic from McCord, assignment to a community health clinic, and whether the patient was reached for the survey. Associations were examined at a P < .05 significance level with 95% confidence intervals. We did not exclude any variables based on their P-values. Statistical analyses were performed using Stata statistical software, 2008 (Stata Corporation, College Station, TX).
RESULTS
Cohort Characteristics
In the year prior to the transfer period, 4678 patients visited the clinic (Fig. 2A) and were considered eligible for transfer. There were 765 (16%) patients who did not visit during the March to June 2012 transfer period: 482 (63%) changed service providers prior to the transfer period, 179 (23%) were considered lost to follow-up by the clinic for not having had a visit for 6 months or more, and 104 (14%) died. Thus, 3913 (84%) eligible patients had at least 1 visit during the transfer period and are considered the transfer cohort. Of these, 3383 (86%) were reached for the telephone survey, 500 (13%) were unreached, 3 (0.08%) refused the survey, and 27 (0.7%) were known deaths. Fifty-nine percent of the transfer cohort was female, with a mean age of 40 years (standard deviation [SD] 9.5) and a most recent median CD4 count prior to transfer of 375/µL (interquartile range [IQR] 250–530/µL; Table 1). Two-thirds of patients (2537) were transferred to a primary healthcare clinic. There were 254 (6%) high-risk (red dot) patients who were asked to return for a subsequent visit during the transfer period. The baseline characteristics of the reached and unreached groups did not differ substantially (Table 1).
Fig. 2.
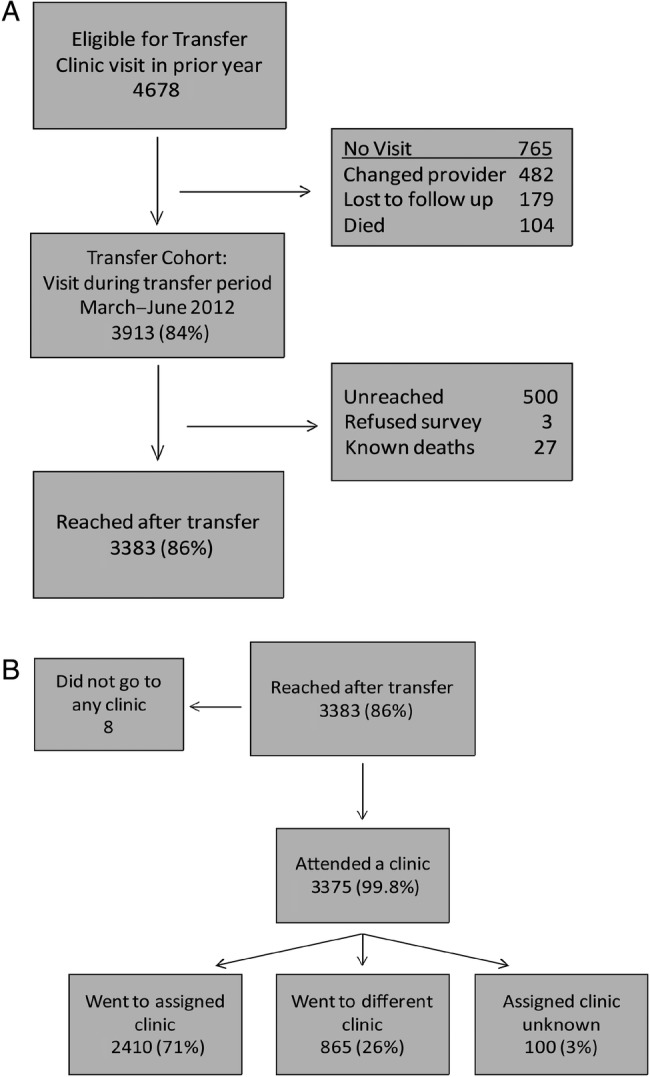
Schematic of patient transfers and clinic attendance. (A) Patients eligible for transfer who had a visit and were reached after transfer. (B) Self-reported outcomes for reached patients.
Table 1.
Characteristics of Patients Who Visited McCord Hospital during the Transfer Period, Comparing Those Reached and Unreached by Phone Following Transfer
| Total N = 3913 |
Reached N = 3383 |
Not Reached N = 530 |
|
|---|---|---|---|
| Female, N (%) | 2324 (59) | 2022 (60) | 302 (57) |
| Age, Mean (SD) | 40 (9.5) | 40 (9.5) | 40 (9.9) |
| Most recent CD4 count, Median/µL (IQR) | 375 (250–530) | 376 (251–531) | 366 (246–528) |
| Red dot, N (%)a | 254 (6) | 212 (6) | 42 (8) |
| Transferred to primary healthcare clinic, N (%) | 2537 (65) | 2225 (66) | 312 (59) |
| Transferred to community health clinic, N (%) | 770 (20) | 653 (19) | 117 (22) |
| Transferred to hospital-based clinic, N (%) | 524 (13) | 446 (13) | 78 (15) |
| Transferred to private doctor, N (%) | 82 (2) | 59 (2) | 23 (4) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; SD, standard deviation.
a Red dot patients were those deemed “high-risk” for transfer: acutely ill or medically complex patients, those in whom there were concerns about drug resistance, recent ART initiates (prior 3 months), and patients with recent ART regimen changes.
Self-reported Linkage to Care
Of the 3383 patients reached for the survey, 3375 (99.8%) reported attending a transfer clinic; 2410 (71%) said they went to the clinic assigned to them by McCord and 865 (26%) reported going to a clinic other than the one assigned (Fig. 2B). The most common reasons for attending alternative clinics were: being told by the receiving clinic to go elsewhere (23%), stigma concerns (16%), and inconvenient location (14%).
Validation of Self-reported Transfer Clinic Visits
The 10 validation clinics each had 27–275 patients assigned to them for transfer. Two clinics were excluded from the validation analysis. One clinic did not record identifiers such as name, date of birth, or South African identification number in their daily visit log; they used only site-specific folder numbers, which could not be linked to the study database variables. The other clinic included names but no date of birth or South African identification number in their clinic register, therefore, identities could not be confirmed.
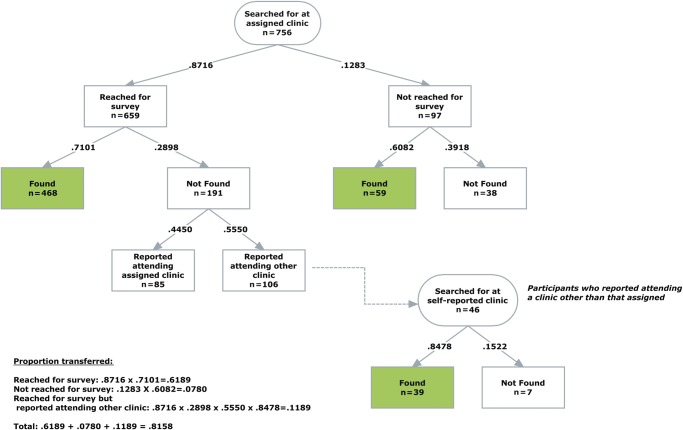
Of the 756 transfer patients assigned to the 8 clinics used for validation, the mean age was 40 years (SD 9.3), 61% were female, the median CD4 count prior to transfer was 370/µL (IQR 262–530/µL), and 52 (7%) had red dot (high-risk) status. These characteristics did not differ significantly between the validation cohort and the rest of the transfer cohort (all P > .05). 659 (87%) were reached for the survey. Among respondents, 468 (71%) were found at their assigned clinic (Fig. 3). An additional 46 people self-reported attending a validation clinic they were not originally assigned to and, of these, 39 (85%) had a validated visit. Of the 97 unreached patients, 59 (61%) were identified at their assigned clinic. Based on the validation rates for reached patients reporting attending assigned clinic, reached patients who self-reported attending a different clinic and unreached patients, the estimated success of transfer for the cohort overall was 82% (95% confidence interval [CI]: 79%–85%).
Fig. 3.
Number and proportion of patients reached and unreached by phone following transfer, including only the 756 patients assigned to the validation clinics. Patients are further delineated by whether they were found in the clinic record at the validation clinic, were not found in the record, or reported going elsewhere.
Correlates of Failure to be Found at Validation Clinic
For every 1 kilometer that the transfer clinic was from McCord, patients were 7% more likely not to be found (adjusted relative risk [aRR] 1.07, CI: 1.02–1.11; Table 2). Those assigned to a community health clinic, as opposed to a primary healthcare clinic or hospital, were 70% more likely not to be found (aRR 1.70, CI: 1.20–2.42). Red dot or high-risk status was not associated with failure to be found at the assigned validation clinic (aRR 1.03, CI: 0.60–1.78).
Table 2.
Correlates of Not Being Found at Assigned Transfer Clinics Visited for Validation
| Factors | Unadjusted relative risk (95% CI) | Adjusted relative risk (95% CI) |
|---|---|---|
| Female | 1.03 (0.78–1.34) | 1.02 (0.78–1.33) |
| Age group (under 30) | 1.30 (0.89–1.89) | 1.27 (0.87–1.86) |
| Most recent CD4 count (per quartile) | 1.05 (0.94–1.18) | 1.06 (0.94–1.19) |
| Distance from McCord to transfer clinica | 1.08 (1.04–1.12) | 1.07 (1.02–1.11) |
| Red dot status | 0.97 (0.57–1.64) | 1.03 (0.60–1.78) |
| Assigned to community health clinicb | 1.92 (1.37–2.70) | 1.70 (1.20–2.42) |
| Reached for survey | 1.35 (0.95–1.91) | 1.29 (0.91–1.84) |
Relative risk: risk for failure to be found at validation clinic.
Abbreviations: aRR, adjusted relative risk for failure to be found at validation clinic, adjusted for all characteristics shown; ART, antiretroviral therapy; CI, confidence interval.
a For every 1 km that the transfer clinic was from McCord, patients were 10% less likely to be found.
b Patients on second-line ART were assigned to community health clinics.
DISCUSSION
We evaluated the rapid transfer of nearly 4000 patients from a PEPFAR-funded, hospital-based HIV clinic to government-funded community clinics. We reached 86% of patients with clinic visits during the transfer period; of those, over 99% self-reported visiting a clinic within 6 months of transfer. We further evaluated these self-reported data through an audit of records at 8 transfer clinics, which validated visits for 85% of reached patients and 61% of unreached patients. Based on the validation rates applied to the entire cohort, an overall estimated 82% were successfully transferred, suggesting that 18% of patients did not link to care after transfer to community-based clinics. We did not find an association between red dot (high risk) patients and failure to successfully link to care.
Given the rapid and widespread nature of the transfer, that an estimated 82% of nearly 4000 patients linked to care may be considered a relative success and may reflect several strengths within the process. This transfer was initiated from a well-resourced clinic with: (1) close coordination between clinic leadership and the Department of Health managers, (2) consideration of patient preferences and specific clinical indications for different levels of clinical care, and (3) highly motivated patients who received group and, if needed, individual counseling related to ART adherence. Less well-resourced clinics may not be able to achieve the success seen in this study. However, promoting a smooth transition between doctor- and nurse-managed clinics is especially important in light of qualitative evidence suggesting that transferring patients are more skeptical of the care they receive in nurse-managed clinics [25].
Unlike prior studies, which have only assessed the transfer of clinically stable patients [16–18], all patients at the Sinikithemba clinic were transferred, regardless of their desire to transfer or their clinical condition. Red dot patients, who might have been excluded from prior studies due to suspected drug resistance, recent ART initiation, or significant comorbidities, had similar validated linkage rates. This suggests that patients previously seen as high-risk for transfer may be transferred safely. Notably, patients whose transfer clinic was further from McCord were less likely to be found, supporting the idea that some patients purposefully travel a great distance to avoid being recognized at a local clinic [26]. Convenience or transport concerns might motivate patients to elect a closer clinic; however, concerns about stigma might impel them to choose a more distant clinic. The latter concern is evidenced by patients, all of whom were assigned to geographically close clinics, reporting “inconvenient location” as a reason for attending a nonassigned clinic. These data suggest that patient transfer preferences should be considered when choosing a transfer location.
A quarter of transferred patients reported attending a different clinic than assigned. Their reasons are consistent with other studies in sub-Saharan Africa, which find that lack of transport to clinic [27–29] and stigma [30–33] greatly alter a patient's ability to engage in care. Given that nearly one-quarter of those attending a different clinic from the one assigned reported being turned away from their assigned clinic, efforts to optimize transfers to community-based sites will require close collaboration with receiving clinics to ensure successful linkage to care. Coordinating with the Department of Health to ensure a comprehensive list of available sites by geographic area may prevent confusion about the appropriateness of a chosen clinic.
As PEPFAR funding decreases in South Africa, additional large, centralized HIV clinics may be closed and care shifted to South Africa's health system. Despite the notable success of the Sinikithemba transfer process, we estimate 18% of transferred patients may not have linked to care. This represents a substantial proportion of the cohort that may have experienced treatment interruption. Those who were lost from care have a heightened risk of clinical complications and viral rebound, potentially increasing their risk of morbidity and onward HIV transmission. Although South African ownership of direct medical care is an important goal, rapid shifts that result in significant interruptions in care jeopardize the important health gains made [6]. In order to ensure that the significant progress made in HIV treatment is maintained, the South African government and PEPFAR should consider the existing medical infrastructure and the readiness of public clinics to accept a rapid influx of patients [5,9]. Standardized transfer procedures and documentation, including clear expectations about geographic limitations for patient transfers, will facilitate this process. Furthermore, consistent use of a single patient identifier would allow for more effective tracking of patients between sites and would enable the health system to identify patients who have been lost to follow-up [34]. The current study speaks only to an initial transfer clinic visit; the outcomes of transitions in care should be formally monitored to assess not only linkage to care but also retention in care, ART adherence, and ongoing virologic suppression. This type of long-term data gathering is imperative to better inform future clinic closures, ensure that patient care is not compromised, and maximize the continued benefit of PEPFAR aid, even as funding diminishes.
There are several possible explanations for the differential validation rates between the reached and unreached cohorts. Patients were tried 3 times over several weeks on different days and at different times in order to maximize the likelihood of a response. This is consistent with studies that show that up to 83% of patients lost to follow-up were unreachable by phone due to incorrect or incomplete contact information [35–37]. It is also possible that some unreached participants intentionally did not answer or return calls from research assistants because they did not want to admit that they had failed to attend their transfer clinic. By collecting self-reported clinic attendance and validating a portion of the self-reports, we were able to make a more nuanced estimation of the overall linkage to care rate for the cohort.
This study should be viewed in the context of several limitations. Those patients who attended the clinic during the transfer period may represent the most motivated. We do not know the outcomes for those who were eligible for transfer but did not attend during the transfer period; therefore, we may have overestimated the success of the transfer process. About 14% of transferred patients were not reached by phone. Though some were found at their assigned clinic, others may have successfully transferred to another clinic without our knowledge. The primary outcome relied on patients' self-reports of clinic attendance and may thus be subject to social desirability bias. The validation process was used to correct for such bias. Linkage to the transfer clinic was successfully validated for the majority of patients from the transfer cohort. However, due to differences in record-keeping methods, there was variability in how patients were identified in records, and we may not have found all patients with transfer clinic visits. Further, we had to exclude 2 of the validation sites because records were inconsistent and incomplete. One of these clinics had 275 patients assigned to it; this would have increased our validation group by nearly 30% and may have added to the robustness of our findings. We sampled from the 80 clinics closest to McCord, which may have biased the results toward a higher rate of linkage to care. Finally, because Sinikithemba patients paid a monthly fee for care, they may represent a more motivated and financially stable cohort than is typically found in public, hospital-based clinics where care is free of charge. These patients' financial investment in their HIV treatment may have made them more likely to link to a transfer clinic.
This study demonstrates that when PEPFAR support is withdrawn from a high-volume clinic, a substantial proportion of patients can be successfully transferred to community clinics. However, even under circumstances where the transfer process was carefully coordinated, nearly 20% of patients were not found to have had a first visit at a sample of validation clinics. To truly assess the impact of a large-scale transfer of this nature, outcomes beyond the first transfer clinic visit, such as long-term retention in care, adherence to treatment, and virologic suppression, must be evaluated. During this time of transition to South African government-supported HIV care, efforts are urgently needed to strengthen monitoring systems and methods for retaining patients in care to ensure that the remarkable gains supported by PEPFAR are not lost.
Funding
This work was supported by the National Institutes of Health [R01 MH090326-03S1 to I. V. B.; R01 MH090326 to I. V. B.; R01 AI058736 to K. A. F.; R01 MH073445 to R. P.W.; K24 AR057827 to E. L.] and the Harvard University Center for AIDS Research [P30 AI060354 to S. R.].
Acknowledgements
The authors gratefully acknowledge the extensive efforts of Portia Davis and Siphesihle Sithole in contacting the patients transferred from Sinikithemba. We thank Drs Helga Holst and Peninah Thumbi at McCord for providing strong leadership during a time of challenging transition. Finally, the authors acknowledge Penny Dladla, eThekwini District Health Services Manager, and Elizabeth Lutge, Chairperson of the Health Research Committee of the KwaZulu-Natal Provincial Department of Health, for facilitating access to community-based clinics for visit validation.
Conflict of interest statement. The authors have no conflicts of interest to disclose.
References
- 1.UNAIDS. Getting to zero: HIV in eastern and southern Africa. Available at: http://www.sanac.org.za/resources/doc_download/50-unaids-report-2013 . Accessed May 9, 2014.
- 2.United States President's Emergency Plan for AIDS Relief. 10 years of PEPFAR: the power of partnership. Available at: http://southafrica.usembassy.gov/pepfar.html . Accessed May 9, 2014.
- 3.Lawn SD, Myer L, Bekker LG, et al. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: Impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 4.Partnership framework in support of South Africa's National HIV & AIDS and TB Response 2012/13-2016/17 between the Government of the Republic of South Africa and the Government of the United States of America. Available at: http://www.pepfar.gov/documents/organization/196651.pdf . Accessed May 9, 2014.
- 5.Institute of Medicine. Evaluation of PEPFAR. Available at: http://www.iom.edu/~/media/Files/Report%20Files/2013/PEPFAR/PEPFAR_RB.pdf . Accessed May 9, 2014.
- 6.Collins C, Beyrer C. Country ownership and the turning point for HIV/AIDS. Lancet Global Health. 2013;1:e319–20. doi: 10.1016/S2214-109X(13)70092-5. [DOI] [PubMed] [Google Scholar]
- 7.Organisation for Economic Co-operation and Development. The Paris declaration on aid effectiveness. http://www.oecd.org/development/effectiveness/34428351.pdf . Accessed May 13, 2014.
- 8.UNAIDS. Country ownership for a sustainable AIDS response: From principles to practice. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2012/20120717_JC2134_UNAIDS_Country_Ownership_Discussion_Paper.pdf . Accessed May 13, 2014.
- 9.Katz IT, Bassett IV, Wright AA. PEPFAR in transition—implications for HIV care in South Africa. N Engl J Med. 2013;369:1385–7. doi: 10.1056/NEJMp1310982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson E, O'Bra H, Brown JW, et al. Supporting the massive scale-up of antiretroviral therapy: The evolution of PEPFAR-supported treatment facilities in South Africa, 2005–2009. BMC Public Health. 2012;12:173. doi: 10.1186/1471-2458-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SANAC. South Africa National Department of Health clinical guidelines for the management of HIV & AIDS in adults and adolescents. Available at: http://www.who.int/hiv/pub/guidelines/south_africa_art.pdf . Accessed May 9, 2014.
- 12.Lawn SD, Myer L, Edwards D, et al. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–25. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–98. doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys CP, Wright J, Walley J, et al. Nurse led, primary care based antiretroviral treatment versus hospital care: A controlled prospective study in Swaziland. BMC Health Serv Res. 2010;10:229. doi: 10.1186/1472-6963-10-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanne I, Orrell C, Fox MP, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): A randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long L, Brennan A, Fox MP, et al. Treatment outcomes and cost-effectiveness of shifting management of stable ART patients to nurses in South Africa: An observational cohort. PLoS Med. 2011;8:e1001055. doi: 10.1371/journal.pmed.1001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan AT, Long L, Maskew M, et al. Outcomes of stable HIV-positive patients down-referred from a doctor-managed antiretroviral therapy clinic to a nurse-managed primary health clinic for monitoring and treatment. AIDS. 2011;25:2027–36. doi: 10.1097/QAD.0b013e32834b6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connor C, Osih R, Jaffer A. Loss to follow-up of stable antiretroviral therapy patients in a decentralized down-referral model of care in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2011;58:429–32. doi: 10.1097/QAI.0b013e318230d507. [DOI] [PubMed] [Google Scholar]
- 19.McGuire M, Ben Farhat J, Pedrono G, et al. Task-sharing of HIV care and ART initiation: evaluation of a mixed-care non-physician provider model for ART delivery in rural Malawi. PLoS One. 2013;8:e74090. doi: 10.1371/journal.pone.0074090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bemelmans M, van den Akker T, Ford N, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15:1413–20. doi: 10.1111/j.1365-3156.2010.02649.x. [DOI] [PubMed] [Google Scholar]
- 21.Massaquoi M, Zachariah R, Manzi M, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Trans R Soc Trop Med Hyg. 2009;103:594–600. doi: 10.1016/j.trstmh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Sturm G, Zangerle R for the AHIVCOS Group. Mortality in the combination antiretroviral therapy era: Will switching to another treatment centre become a risk factor?. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Kuala Lumpur, Malaysia. 2013. [Google Scholar]
- 23.South Africa Department of Health. Standard treatment guidelines and essential medicines list for South Africa. Available at: www.kznhealth.gov.za/pharmacy/edladult_2012.pdf . Accessed May 9, 2014.
- 24.Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: Collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukora R, Charalambous S, Dahab M, et al. A study of patient attitudes towards decentralisation of HIV care in an urban clinic in South Africa. BMC Health Serv Res. 2011;11:205. doi: 10.1186/1472-6963-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook PA, Downing J, Wheater CP, et al. Influence of socio-demographic factors on distances travelled to access HIV services: enhanced surveillance of HIV patients in northwest England. BMC Public Health. 2009;9:78. doi: 10.1186/1471-2458-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ware NC, Wyatt MA, Geng EH, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: A qualitative study. PLoS Med. 2013;10:e1001369. doi: 10.1371/journal.pmed.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuller DM, Bangsberg DR, Senkungu J, et al. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: A qualitative study. AIDS Behav. 2010;14:778–84. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tweya H, Feldacker C, Estill J, et al. Are they really lost? “True” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in urban Malawi. PLoS One. 2013;8:e75761. doi: 10.1371/journal.pone.0075761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe WR, Weiser SD, Bangsberg DR, et al. Effects of HIV-related stigma among an early sample of patients receiving antiretroviral therapy in Botswana. AIDS Care. 2006;18:931–3. doi: 10.1080/09540120500333558. [DOI] [PubMed] [Google Scholar]
- 31.Nachega JB, Stein DM, Lehman DA, et al. Adherence to antiretroviral therapy in HIV-infected adults in Soweto, South Africa. AIDS Res Hum Retroviruses. 2004;20:1053–6. doi: 10.1089/aid.2004.20.1053. [DOI] [PubMed] [Google Scholar]
- 32.Talam NC, Gatongi P, Rotich J, Kimaiyo S. Factors affecting antiretroviral drug adherence among HIV/AIDS adult patients attending HIV/AIDS clinic at Moi Teaching and Referral Hospital, Eldoret, Kenya. East Afr J Public Health. 2008;5:74–8. [PubMed] [Google Scholar]
- 33.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: Systematic review and meta-synthesis. J Int AIDS Soc. 2013;16:18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UNAIDS. Developing and using individual identifiers for the provision of health services including HIV: Proceedings from a workshop, 24–26 February 2009, Montreux, Switzerland. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/dataanalysis/20110520_Unique_Identifiers_Meeting_Report_Montreux.pdf . Accessed May 13, 2014.
- 35.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: Systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalal RP, Macphail C, Mqhayi M, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–7. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 37.Maskew M, MacPhail P, Menezes C, Rubel D. Lost to follow up: Contributing factors and challenges in South African patients on antiretroviral therapy. S Afr Med J. 2007;97:853–7. [PubMed] [Google Scholar]