Abstract
Objective
To quantify reporting errors, measure incidence of postpartum haemorrhage (PPH) and define risk factors for PPH (≥500 ml) and progression to severe PPH (≥1500 ml).
Design
Prospective observational study.
Setting
Two UK maternity services.
Population
Women giving birth between 1 August 2008 and 31 July 2009 (n = 10 213).
Methods
Weighted sampling with sequential adjustment by multivariate analysis.
Main outcome measures
Incidence and risk factors for PPH and progression to severe PPH.
Results
Errors in transcribing blood volume were frequent (14%) with evidence of threshold preference and avoidance. The incidences of PPH ≥500, ≥1500 and ≥2500 ml were 33.7% (95% CI 31.2–36.2), 3.9% (95% CI 3.3–4.6) and 0.8% (95% CI 0.6–1.0). New independent risk factors predicting PPH ≥ 500 ml included Black African ethnicity (adjusted odds ratio [aOR] 1.77, 95% CI 1.31–2.39) and assisted conception (aOR 2.93, 95% CI 1.30–6.59). Modelling demonstrated how prepregnancy- and pregnancy-acquired factors may be mediated through intrapartum events, including caesarean section, elective (aOR 24.4, 95% CI 5.53–108.00) or emergency (aOR 40.5, 95% CI 16.30–101.00), and retained placenta (aOR 21.3, 95% CI 8.31–54.7). New risk factors were identified for progression to severe PPH, including index of multiple deprivation (education, skills and training) (aOR 1.75, 95% CI 1.11–2.74), multiparity without caesarean section (aOR 1.65, 95% CI 1.20–2.28) and administration of steroids for fetal reasons (aOR 2.00, 95% CI 1.24–3.22).
Conclusions
Sequential, interacting, traditional and new risk factors explain the highest rates of PPH and severe PPH reported to date.
Keywords: Blood loss, observational study, pregnancy, progression, risk factors, severe adverse maternal morbidity
Introduction
Postpartum haemorrhage (PPH), defined as blood loss ≥500 ml, is a major cause of maternal mortality and morbidity worldwide.1 For every death, 20 women live with the consequences of associated morbidities,2 with the greatest burden in low-income countries.3 PPH is a common emergency, and readily treatable when appropriate resources are available.4
Severe PPH (variously defined from 1000 ml upwards) has been used as a measure of severe morbidity and is an appropriate adjunct to mortality reports.4–6 In Europe, one in eight maternal deaths are linked to PPH.7 In the UK, despite the widespread availability of effective treatments and guidelines, deaths from PPH still occur (9/107 direct deaths in 2006–2008, 0.39/100 000 maternities; 95% CI 0.20–0.75).5 Additionally, for each death, 15 women undergo hysterectomy.8
Despite surgical, medical and training innovations, PPH rates remain high in several high-income countries including the UK9–11 with an incidence of 13% recently reported, and evidence that both PPH12 and severe PPH13 are increasing. The causes are likely to be multifactorial with shifting demography and health status widely cited, e.g. age, obesity, comorbidity, multiple pregnancy and ethnicity,14–19 in addition to rising caesarean section rates.10,17,20 These suppositions require formal evaluation.
The quantification of blood loss remains problematic. Although recognised as unreliable,21,22 the usual method is visual assessment following minimal training.23 Accurate estimation is critical because volume thresholds are used to initiate treatment and resuscitation protocols. Despite this, rigorous evaluation of those errors, which may reduce the accuracy of estimated blood loss (EBL), has seldom been attempted.24–26
This prospective observational study aimed to: (i) quantify common EBL reporting errors; (ii) measure PPH incidence; (iii) identify chronologically ordered risk factors (pre-existing or acquired) for PPH and progression to severe PPH.
Methods
This is the quantitative component of the mixed methodology STOP (Surveillance and Treatment of Postpartum haemorrhage) study. PPH management and qualitative results will be reported separately.
A prospective observational study was undertaken in two maternity services incorporating an inner London tertiary referral teaching hospital and a district general hospital in South East England.
Patients and data collection
The population studied comprised all women giving birth between 1 August 2008 and 31 July 2009 (n = 10 213).
In both centres, maternity data were primarily documented in paper records that remained with the woman throughout her pregnancy and early puerperium. Summary data, transcribed from the notes, were entered onto electronic patient databases immediately following birth. This procedure is widespread in UK maternity units.
For the study, blood loss and minimal demographic/delivery data were imported within 1 week of birth from the hospital clinical electronic databases (Healthware™ and EuroKing™) to a secure, bespoke data management system (MedSciNetAB). Preservation of anonymity, data handling and storage were in compliance with the UK Data Protection Act 1988.
Weighted sample
Detailed review of all maternity records was impractical and limited by resource and time constraints. Therefore a weighted sample design (disproportionate stratified sampling), commonly employed in national statistics, accountancy and business surveys, was adopted27,28 (see Supporting information, Appendix S1 Supplementary Methods).
Data extraction and analysis
Two researchers reviewed all clinical data from the original handheld records to more accurately evaluate blood loss and identify transcription errors. Additional information was obtained from other electronic sources (blood transfusion, routine haematology and ultrasound). Variation between researchers of the total volume documented was always <5%; and was always resolved by discussion.
Data analysis was performed using Stata, version 11.2 (Stata Corp, College Station, TX, USA). Summaries, estimates and comparisons were calculated using proportional weighting to adjust for the sampling plan.
Definitions
Study definitions, including the categorisation of PPH by EBL are listed (see Supporting information, Table S1).
Estimation of errors in reported clinical data
Discrepancies were determined using three approaches: (i) evaluating the frequency and magnitude of transcription errors for EBL between paper and electronic records; these were compared to calculated errors for maternal age, maternal date of birth, mode of delivery, baby date of birth, time of birth, sex and birthweight; (ii) cross-checking the observed discrepancies; this was undertaken by two researchers and decision was deferred to a third when required, and to the study Chief Investigator (CI) if there was persistent disagreement; (iii) independently re-examining every tenth set of notes and related electronic records within the weighted sample.
Precise EBL was not recorded in 61/101 waterbirths (including eight homebirths) but following review of these notes, 57 were categorised within 0–499 ml and four within 500–999 ml.
Assessment of incidence of PPH
The incidence of PPH and all other analyses was calculated after adjustment of the EBL categories following inspection of the handheld records.
Determination of potential risk factors for PPH to be assessed
A detailed list of potential risk factors was compiled with the intention of determining which were associated with increased blood loss or increased risk of PPH. This included previously identified and potential risk factors assessed in three sequential groups: (a) pre-pregnancy, (b) during pregnancy, (c) labour and birth. These were further subdivided into pre-defined subgroups arranged, as far as possible, in the order they generally occur (Appendix S1: Supplementary Methods).
The ultimate causes, identified from group (a) may be of public health importance, while intermediate and immediate causes from groups (b) and (c) may have more clinical relevance. The strength of this approach is that appropriately adjusted estimates for both earlier and later predictors are obtained. While earlier factors can possibly be confounders for later events, the reverse does not apply. For example, age influences multiple pregnancy, which is itself believed to be a risk factor for caesarean section and blood loss. Age is therefore a potential confounder of the effect of multiple pregnancy, and multiple pregnancy is a potential confounder of the effect of caesarean section, but not vice versa.
Models were designed to investigate three clinically important aspects of blood loss: (i) in all women, absolute blood loss (ml) (linear regression) and (ii) PPH ≥ 500 ml (logistic regression); (iii) in women with PPH ≥ 500 ml, the risk of progression onto severe PPH ≥ 1500 ml (logistic regression). These models address the three questions: ‘How much blood is this woman likely to lose?’, ‘Is this woman at risk of PPH (≥500 ml)?’ and ‘Having lost 500 ml, what is the likelihood of this woman experiencing severe PPH (≥1500 ml) requiring major intervention?’ (more fully described in Appendix S1: Supplementary Methods).
Justification of study duration, population and sample size
All births over a complete year were studied to eliminate seasonal fluctuations. Population diversity was increased by inclusion of two centres. As this observational study did not assess the influence of a single risk factor or intervention, a conventional power calculation was not undertaken.
Comparison with previous and contemporary evidence
To examine time trends, comparison was undertaken with historical data from a prospective population-based case–control study of severe maternal morbidity, involving the same NHS Trusts (1997/98),16 using present study PPH definitions. Comparison of PPH ≥ 2500 ml was made using the Scottish national morbidity audit for the same time frame as the current study.13
Results
Population and demography
Following selection by weighting, 1897 case notes were examined; two women had no documentation of blood loss. Allowing for weighting, the sample represented 9939 women of whom 9937 (>99.9%) had sufficient data (Figure 1, STROBE diagram).
Figure 1.
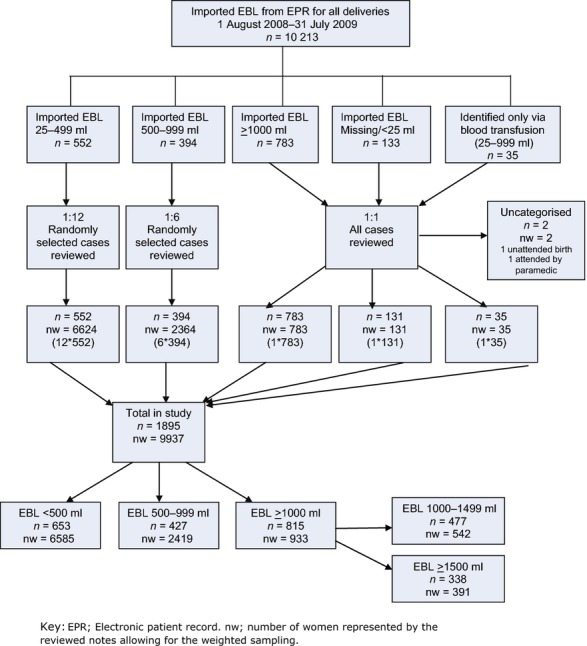
STROBE diagram. Identification and classification of cases according to imported electronic patient records, recategorisation by blood loss documented in handheld maternity notes and sample weighting.
In the whole group, 60% were ≥30 and 26% were ≥35 years old. Almost 60% were White, with Black African the largest minority ethnic group (17%). Over 37% were overweight or obese, 39% lived in areas of highest deprivation and 12% were cigarette smokers. Table S2 (see Supporting information) shows population demography and outcomes in women with and without PPH.
Estimated blood loss error in electronic clinical records
Comparison with paper records revealed a 14% error rate in recorded EBL. This compares with error rates of 0.5% maternal age, 0.2% maternal date of birth, 2.0% mode of delivery, 0.0% baby date of birth, 0.4% time of delivery, 0.4% sex and 2.0% birthweight.
Table 1 shows categories of PPH by EBL. Following review of the paper records 207/1895 weighted sample were re-categorised: 1688 (89.1%) were unchanged, 192 (10.2%) were moved to a higher and 15 (0.8%) to a lower EBL category; women with no electronically recorded EBL (131/10213, 1.28%) were assigned to categories 0–499 ml (n = 101), 500–999 ml (n = 19), 1000–1499 ml (n =6) and ≥1500 ml (n = 5).
Table 1.
Estimated rates of blood loss as % in each category in study population (allowing for weighted sampling)
| Category | Blood loss (ml) | All (%) | 95% CI |
|---|---|---|---|
| No PPH | <500 | 66.3 | 63.8–68.8 |
| PPH – All | ≥500 | 33.7 | 31.2–36.2 |
| PPH - Minor | 500–999 | 24.3 | 22.0–26.6 |
| PPH – Moderate | 1000–1499 | 5.5 | 4.8–6.1 |
| PPH – Severe | 1500–1999 | 2.0 | 1.6–2.4 |
| 2000–2499 | 1.1 | 0.74–1.5 | |
| ≥1500 | 3.9 | 3.3–4.6 | |
| ≥2500 | 0.82 | 0.63–1.0 |
There was a preference to record EBLs ending in 0, or as multiples of 5, 10, 50 and 100. In the full cohort (n = 10 213) disproportionate numbers of women had documented blood loss at exact thresholds; 500 ml (8.4%), 1000 ml (2.1%), 1500 ml (0.8%), 2000 ml (1.5%) and 2500 ml (0.2%). At each threshold, volumes just under (within 50 ml) were favoured over those just above.
PPH incidence
Following adjustment for transcription errors, 33.7% had a PPH ≥ 500 ml, 3.9% ≥ 1500 ml and 0.82% ≥ 2500 ml (Table 1).
Determination of risk factors for PPH and progression to severe PPH
Unadjusted results for all 211 variables are given in the Supporting information, Table S3, including tests for changes in blood loss, PPH ≥ 500 ml and progression onto severe PPH (≥1500 ml). Fifty predictors of PPH ≥ 500 ml (ten prepregnancy, 15 during pregnancy and 25 intrapartum) selected on the basis of unadjusted significance were entered into the next stage of the PPH model. A different 50 predictors of progression to PPH ≥ 1500 ml (11 prepregnancy, 15 during pregnancy and 24 intrapartum) were similarly entered into the progression model.
Modelling according to chronological sequence
Table 2 summarises the prediction of PPH. It uses only predictors significant before adjustment. Each column shows the adjusted odds ratios from a single model. Column 1 deals with prepregnancy factors only (appropriate at first antenatal appointment). Column 2 includes risk factors arising during pregnancy (appropriate prelabour). Column 3 includes risk factors linked to labour and birth (appropriate postpartum).
Table 2.
Risk pathways for PPH ≥ 500 ml according to chronological variables grouped as prepregnancy, during pregnancy, labour and birth
| Risk factors included in final model | Pre-Pregnancy Variable subgroups 1–4 (1895 women included) OR (95% CI), p | During Pregnancy Variable subgroups 1–10 (1868 women included; 27 excluded due to 1 missing data and 26 perfectly predicted) OR (95% CI), p | Labour and Birth Variable subgroups 1–16 (1724 women included; 171 excluded due to 135 missing data, 36 perfectly predicted) OR (95% CI), p |
|---|---|---|---|
| (1) Sociodemographic | |||
| Age, for each 10 years | 1.45 (1.19–1.76), 0.000 | 1.44 (1.18–1.75), 0.000 | 0.93 (0.73–1.19), 0.57 |
| Black African | 1.77 (1.31–2.39), 0.000 | 1.77 (1.30–2.41), 0.000 | 1.94 (1.35–2.79), 0.000 |
| (2) Local Deprivation: index of multiple deprivation, most deprived UK quintile (%) | |||
| Barriers – housing and services | 1.06 (0.87–1.33), 0.61 | 1.05 (0.83–1.32), 0.69 | 1.04 (0.79–1.36), 0.80 |
| Education, skills and training | 0.93 (0.64–1.36), 0.72 | 0.93 (0.64–1.36), 0.71 | 1.03 (0.57–1.62), 0.89 |
| (3) General and medical risk factors | |||
| Current smoker | 0.76 (0.54–1.09), 0.14 | 0.77 (0.53–1.10), 0.15 | 0.82 (0.53–1.28), 0.38 |
| BMI (kg/m2) per unit | 1.03 (1.01–1.05), 0.006 | 1.03 (1.00–1.05), 0.016 | 1.01 (0.99–1.04), 0.32 |
| Assisted conception | 2.93 (1.30–6.59), 0.010 | 2.28 (0.99–5.29), 0.054 | 2.10 (0.83–5.33), 0.19 |
| (4) Previous obstetric history | |||
| Previous PPH | 2.34 (1.33–4.12), 0.003 | 2.45 (1.38–4.35), 0.002 | 2.75 (1.40–5.44), 0.003 |
| Multiparous previous caesarean | 1.32 (0.93–1.87), 0.19 | 1.30 (0.91–1.86), 0.14 | 0.96 (0.61–1.51), 0.86 |
| Multiparous no previous caesarean | 0.33 (0.26–0.42), 0.000 | 0.33 (0.25–0.42), 0.000 | 0.79 (0.56–1.11), 0.18 |
| (5) Current pregnancy | |||
| Multiple pregnancy | 2.27 (1.04–4.96), 0.039 | 2.02 (0.82–5.00), 0.13 | |
| Admissions >24 weeks | 0.82 (0.57–1.18), 0.28 | 0.82 (0.53–1.29), 0.39 | |
| (6) Antenatal day unit (ADU) attendances | |||
| Any ADU attendance | 1.06 (0.84–1.34), 0.62 | 0.95 (0.72–1.26), 0.74 | |
| Pre-eclampsia screen | 1.06 (0.65–1.75), 0.81 | 1.04 (0.57–1.91), 0.89 | |
| Generally unwell | 1.22 (0.68–2.18), 0.50 | 1.33 (0.69–2.60), 0.40 | |
| (7) Placenta praevia: All 26 women with major or anterior placenta praevia PPH > 500 ml | |||
| (8) Antepartum haemorrhage (APH) and urinary tract infection | |||
| APH | 1.11 (0.62–1.99), 0.74 | 1.27 (0.65–2.51), 0.48 | |
| ‘Warning APH’ | 8.95 (1.02–78.7), 0.048 | 1.92 (0.19–19.3), 0.58 | |
| (9) Pre-eclampsia (PET) and anaemia | |||
| Gestational hypertension | 1.83 (0.83–4.03), 0.13 | 2.22 (0.87–5.63), 0.093 | |
| Pre-eclampsia | 3.16 (1.12–8.93), 0.030 | 3.21 (0.94–10.90), 0.062 | |
| (10) Medications in pregnancy pre-birth | |||
| Antibiotics | 1.35 (1.01–1.80), 0.043 | 1.14 (0.77–1.66), 0.52 | |
| Antihypertensives (including for PET) | 0.75 (0.44–1.29), 0.30 | 0.66 (0.33–1.32), 0.24 | |
| Diabetic Rx | 1.89 (0.79–4.56), 0.15 | 1.20 (0.43–3.37), 0.73 | |
| Steroids for fetal reasons | 0.90 (0.57–1.43), 0.65 | 1.23 (0.69–2.18), 0.49 | |
| (11) Gestation at birth | |||
| Gestation at delivery (weeks) | 0.98 (0.90–1.08), 0.70 | ||
| (12) Birthweight | |||
| Maximum birthweight (kg) | 2.19 (1.62–2.99), 0.000 | ||
| (13) Onset of labour | |||
| No labour onset | 1.51 (0.47–4.90), 0.49 | ||
| Induction | 0.75 (0.39–1.46), 0.40 | ||
| Augmentation | 0.83 (0.42–1.64), 0.59 | ||
| ROM > 2 hours before onset | 0.95 (0.64–1.41), 0.79 | ||
| ROM > 6 hours before onset | 1.35 (0.90–2.02), 0.14 | ||
| ROM not recorded | 1.03 (0.60–1.77), 0.91 | ||
| (14) Intrapartum: all ten women with evidence of chorioamnionitis PPH > 500 ml | |||
| Prostin | 1.04 (0.53–2.02), 0.91 | ||
| Syntocinon® | 1.44 (0.95–2.16), 0.085 | ||
| Spinal anaesthesia | 0.87 (0.51–1.49), 0.60 | ||
| Epidural analgesia | 1.08 (0.71–1.65), 0.71 | ||
| Raised temperature (per degree >37.0°C) | 2.62 (1.24–5.52), 0.011 | ||
| Temperature not recorded | 0.75 (0.50–1.11), 0.15 | ||
| (15) Birth | |||
| Instrumental vaginal | 3.50 (2.21–5.24), 0.000 | ||
| Elective caesarean | 24.4 (5.53–108.00), 0.000 | ||
| Emergency caesarean section | 40.5 (16.30–101.00), 0.000 | ||
| (16) Third stage | |||
| Physiological | 1.48 (0.80–2.77), 0.22 | ||
| Syntometrine® intramuscular | 0.55 (0.33–0.91), 0.019 | ||
| Syntocinon® intravenous bolus | 0.58 (0.27–1.25), 0.17 | ||
| Syntocinon® 40/50 IU infusion commenced | 0.61 (0.38 –0.99), 0.045 | ||
| Retained placenta | 21.3 (8.31–54.70), 0.000 | ||
| Suture interval after vaginal birth (hours) | 2.03 (1.65–2.50), 0.000 | ||
| Suture interval not recorded | 2.2 (1.32–3.69), 0.003 | ||
Full regression model; result of three multiple regression models selecting the principal significant variables. In each model, an additional group of predictors is added. Results adjusted for other members of the same group and for previous groups only.
Only 15 factors remained significant postpartum. Black African ethnicity, previous PPH, placenta praevia (anterior and major), maximum birthweight, temperature per degree >37°C, chorioamnionitis, instrumental delivery, elective caesarean, emergency caesarean, retained placenta, interval to suturing (time taken and unrecorded) increased risk. Intramuscular Syntometrine® and Syntocinon® 40/50 IU infusion were protective.
Eight variables significant in columns 1 or 2 are not significant in column 3 (age, body mass index [BMI], assisted conception, multiple pregnancy, ‘warning’ antepartum haemorrhage, pre-eclampsia, antibiotics and multiparity without previous caesarean). Their effects may be mediated through the 15 significant factors (see Methods).
Table 3 deals similarly with risk factors for progression to severe PPH (≥1500 ml) and Figure 2A,B show all significant variables diagramatically.
Table 3.
Risk pathways for progression of PPH from ≥500 ml to severe PPH ≥ 1500 ml according to chronological variables grouped as prepregnancy, during pregnancy, labour and birth
| Risk factors included in final model (1230 women included; 70 excluded due to missing data in addition to all women with EBL<500 ml) | Prepregnancy Variable subgroups 1–4 OR (95% CI), P | During pregnancy Variable subgroups 1–10 OR (95% CI), P | Labour and birth Variable subgroups 1–16 OR (95% CI), P |
|---|---|---|---|
| (1) Sociodemographic | |||
| Age, for each 10 years | 0.85 (0.67–1.07), 0.16 | 0.90 (0.68–1.09), 0.20 | 0.99 (0.77–1.29), 0.96 |
| Black African | 0.86 (0.62–1.19), 0.36 | 0.84 (0.61–1.17), 0.31 | 0.91 (0.64–1.30), 0.61 |
| (2) Local Deprivation: index of multiple deprivation, most deprived UK quintile (%) | |||
| Barriers to housing and services | 0.79 (0.60–1.03), 0.076 | 0.78 (0.59–1.02), 0.069 | 0.75 (0.56–1.01), 0.055 |
| Education, skills and training | 1.75 (1.11–2.74), 0.015 | 1.84 (1.16–2.92), 0.009 | 1.82 (1.10–3.00), 0.019 |
| (3) General and medical risk factors | |||
| Current smoker | 0.59 (0.36–0.97), 0.039 | 0.56 (0.33–0.93), 0.026 | 0.67 (0.38–1.17), 0.16 |
| BMI (kg/m2) per unit | 1.03 (1.00–1.05), 0.022 | 1.03 (1.00–1.05), 0.023 | 1.04 (1.01–1.06), 0.008 |
| Assisted conception | 1.41 (0.79–2.50), 0.25 | 1.02 (0.53–1.93), 0.096 | 1.18 (0.60–2.35), 0.64 |
| (4) Previous obstetric history | |||
| Previous PPH | 1.79 (1.06–3.02), 0.030 | 1.93 (1.13–3.31), 0.016 | 2.39 (1.33–4.28), 0.003 |
| Multiparous previous caesarean | 0.70 (0.49–1.01), 0.055 | 0.63 (0.43–0.92), 0.018 | 0.84 (0.54–1.30), 0.43 |
| Multiparous no previous caesarean | 1.65 (1.20–2.28), 0.002 | 1.55 (1.12–2.16), 0.009 | 1.17 (0.80–1.74), 0.42 |
| (5) Current pregnancy | |||
| Multiple pregnancy | 2.00 (1.05–3.82), 0.035 | 2.60 (1.27–5.38), 0.009 | |
| Admissions >24 weeks | 0.67 (0.44–1.02), 0.062 | 0.83 (0.53–1.32), 0.43 | |
| (6) Antenatal day unit (ADU) attendances | |||
| Any ADU attendance | 1.17 (0.89–1.56), 0.26 | 1.05 (0.77–1.43), 0.75 | |
| Pre-eclampsia screen | 0.93 (0.55–1.57), 0.80 | 1.15 (0.66–2.01), 0.62 | |
| Generally unwell | 1.49 (0.82–2.70), 0.19 | 1.69 (0.90–3.20), 0.11 | |
| (7) Placenta praevia | |||
| Anterior | 3.37 (0.86–13.30), 0.082 | 5.55 (1.29–23.9), 0.022 | |
| Major | 0.72 (0.17–3.05), 0.660 | 0.97 (0.22–4.25), 0.97 | |
| (8) Antepartum haemorrhage (APH) and urinary tract infection | |||
| APH | 1.26 (0.67–2.37), 0.48 | 1.25 (0.62–2.52), 0.53 | |
| ‘Warning APH’ | 1.70 (0.56–5.20), 0.35 | 1.99 (0.58–6.81), 0.27 | |
| (9) Pre-eclampsia (PET) and anaemia | |||
| Gestational hypertension | 1.00 (0.47–2.16), 0.99 | 0.98 (0.43–2.22), 0.97 | |
| Pre-eclampsia (PET) | 1.03 (0.43–2.50), 0.95 | 0.87 (0.32–2.13), 0.69 | |
| (10) Medications in pregnancy prebirth | |||
| Antibiotics | 1.02 (0.74–1.40), 0.91 | 0.95 (0.65–1.39), 0.79 | |
| Antihypertensives (including for PET) | 0.99 (0.56–1.78), 0.92 | 0.91 (0.49–1.70), 0.77 | |
| Diabetic Rx | 0.98 (0.44–2.18), 0.96 | 1.23 (0.52–2.91), 0.64 | |
| Steroids for fetal reasons | 2.00 (1.24–3.22), 0.004 | 2.00 (1.17–3.41), 0.011 | |
| (11) Gestation at birth | |||
| Gestation at delivery (weeks) | 0.95 (0.86–1.04), 0.25 | ||
| (12) Birthweight | |||
| Maximum birthweight (kg) | 1.17 (0.87–1.59), 0.30 | ||
| (13) Onset of labour | |||
| No labour onset | 1.28 (0.54–3.03), 0.58 | ||
| Induction | 1.07 (0.56–2.04), 0.83 | ||
| Augmentation | 1.37 (0.73–2.58), 0.33 | ||
| ROM >2 hours before onset | 1.01 (0.60–1.70), 0.96 | ||
| ROM >6 hours before onset | 1.16 (0.73–1.85), 0.52 | ||
| ROM unknown | 0.95 (0.52–1.73), 0.86 | ||
| (14) Intrapartum | |||
| Prostin | 1.12 (0.60–2.11), 0.73 | ||
| Syntocinon® | 0.75 (0.49–1.13), 0.17 | ||
| Spinal anaesthesia | 0.73 (0.45–1.18), 0.20 | ||
| Epidural analgesia | 1.20 (0.78–1.85), 0.41 | ||
| Raised temperature (per degree >37.0°C) | 1.21 (0.75–1.94), 0.44 | ||
| Temperature not recorded | 1.40 (0.86–2.27), 0.17 | ||
| Chorioamnionitis | 2.70 (0.70–10.5), 0.15 | ||
| (15) Birth | |||
| Instrumental vaginal | 0.79 (0.49–1.29), 0.36 | ||
| Elective caesarean | 0.14 (0.04–0.46), 0.001 | ||
| Emergency caesarean section | 0.34 (0.15–0.80), 0.013 | ||
| (16) Third stage | |||
| Physiological | 3.74 (1.72–8.10), 0.001 | ||
| Syntometrine® intramuscular | 1.12 (0.66–1.91), 0.68 | ||
| Syntocinon® intravenous bolus | 1.35 (0.63–2.87), 0.44 | ||
| Syntocinon® 40/50 IU infusion commenced | 0.97 (0.65–1.44), 0.87 | ||
| Retained placenta | 1.40 (0.77–2.54), 0.27 | ||
| Suture interval after vaginal birth (hours) | 1.16 (0.99–1.35), 0.058 | ||
| Suture interval not recorded | 0.44 (0.25–0.79), 0.006 | ||
Full regression model: result of three multiple regression models selecting the principal significant variables. In each model, a new additional group of predictors is used. Results are adjusted for other members of the same group and for previous groups only. Women with EBL <500 ml are excluded.
Figure 2.
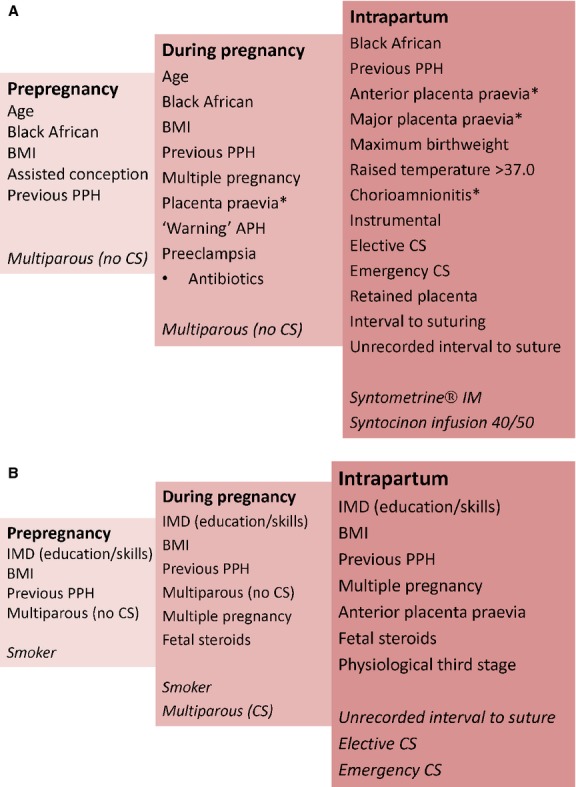
Diagram of multiple logistic and chronological regression analysis showing (A) predictors of PPH ≥ 500 ml (protective factors in italics; asterisks indicate perfect predictors, i.e. all women had PPH) and (B) predictors of PPH ≥ 500 ml onto severe PPH ≥ 1500 ml (protective factors in italics).
Incidence of severe PPH in historical comparison
Numbers of births increased from 8329 to 10 213 (19.7% rise) between 1997/9816 and 2008/09. Over this 11-year period, there was a 3.4 (95% CI 2.7–4.3) risk ratio for PPH ≥ 1500 ml (n = 93 versus n = 391) and an 8.3 (95% CI 4.0–17.1) risk ratio for PPH ≥ 2500 ml (n = 8 versus n = 81).
Incidence of severe PPH in contemporary cohort
The incidence of PPH ≥ 2500 ml was 0.82% (95% CI 0.63–1.0, n = 81/9937) compared with 0.56% (95% CI 0.49–0.62, n = 306/54910) in Scotland during the same period.13
Discussion
Main findings
The incidences of PPH and severe PPH in the present study are, to our knowledge, the highest reported from any high-income or low-income country.1,3,9–12 The novel application of weighted sampling highlighted errors between clinical notes and electronic summary data. Established and novel risk factors for both PPH and progression to severe PPH have been quantified. Rigorous and chronological assessment of contributory factors illuminate the complex multifactorial origin of recent rises in PPH.
Strengths and limitations
Strengths include prospective design and contemporaneous, robust data collection, mitigating ascertainment bias. Weighted sample design maintained statistical power and overcame the limitations of case–control studies. Risk assessment using chronological categories was preferable to stepwise regression (Appendix S1: Supplementary Methods). Generalisability may be limited by the higher deprivation (39% lowest quintile) and larger proportion of women >30 years in the current cohort compared with contemporaneous maternity data for England and Wales (60% versus 47%),29 although obesity rates were similar to recent national figures (15.2% versus 15.6%).30 Comparison with UK maternal ethnic distribution was not feasible because these data are not in the public domain.31 Historical comparison could have been influenced by different methodologies, changes in local service provision and shifting population. Despite controlling for known confounding variables, associations cannot necessarily be assumed to be causal. Gynaecological history, intended place of birth and degree of perineal trauma were not included.
Interpretation
Reporting errors
This is the first study, to our knowledge, to identify major errors in blood loss reporting in electronic maternity records. As these summary data form the sole source of information regarding hospital admissions, treatment and management, errors have implications for individual healthcare and policy. Commonest errors were incorrect addition and failure to include documented blood loss in totals, both in paper notes and electronic records. The only relevant study, from 1994, considered electronic data accurate, despite error rates of 5–19%.32 Threshold avoidance and preference biases, although identified for blood pressure33 and birthweight,34 have not been previously reported for blood loss. Underestimation is widespread for all birth modes,35 partly caused by visual assessment23,36 although one study reports overestimation following caesarean section.37 Our data confirm that under-reporting remains unresolved.
Incidence
These PPH rates are higher than previous reports at every threshold. Recent reports of rising rates,10,17,24,38 up to 13% in high-income countries12 may be underestimates, because most studies use routinely collected, retrospective and ‘coded’ electronic data.10,12,17,24,38–40 The incidence of severe PPH ≥ 2500 ml is slightly higher than the contemporaneous Scottish audit,13 suggesting that they are real underlying trends.
Risk factors for PPH, progression to severe PPH and risk pathway modelling according to chronological sequence
Our approach differs from previous studies by highlighting the need to consider underlying interlinked contributing factors, which lead to PPH and contribute to the progression onto severe PPH.
Prepregnancy factors for PPH include age, ethnicity, BMI, previous PPH and assisted conception. The association with age is variably reported11,41–44 although older women have more medical45 and obstetric46 comorbidities and poorer uterine contractility.47 No previous study has specifically identified Black African ethnicity as an independent predictor,16–19,48 possibly because of lack of adjustment for potential confounding variables.49–52 The independent relationship between BMI and PPH concurs with prospective cohort studies,14,53 although retrospective and routine data reports are equivocal.54–56 The 4% increase per BMI unit becomes substantial in higher obesity categories. The new association with assisted conception could reflect multiple pregnancy or abnormal placentation.57 The impact of previous PPH24,38 was confirmed, and quantified, unlike previous caesarean section.58 Established risk factors for severe PPH in the general population included age, BMI, multiple pregnancy and previous caesarean.14–16,54,55,59,60 However, our data did not highlight any association with age, previous caesarean and severe PPH. Although grand multiparity has been associated with PPH,24 our data reveal multiparity as protective.38 The unexpected findings that multiparity without caesarean section and index of multiple deprivation (education, skills and training) were risk factors for progression to severe PPH require validation. Despite known associations with placental abruption,61 the finding that smoking protects against PPH progression may be associated with poor uteroplacental blood flow.62
Confirmed pregnancy-acquired risk factors for PPH include multiple pregnancy,9 placenta praevia,17 pre-eclampsia63 and macrosomia.64,65 The novel association with prelabour antibiotic use could reflect chorioamnionitis. Similarly, multiple pregnancy17 and anterior placenta praevia were confirmed as predictors of progression to severe PPH ≥ 1500 ml.16,17,66,67 The novel association with administration of steroids for fetal reasons could be explained by multiple pregnancy and threatened preterm birth although gestation of delivery showed no effect. Over 62% of women with haemoglobin <8.5 g/l had PPH, 26% of whom progressed to severe PPH, concurring with NICE guidelines identifying this as a threshold for concern.68 Associations with third trimester anaemia using higher thresholds were not confirmed.69,70
Confirmed intrapartum risk factors for PPH were temperature,69 chorioamnionitis,64 instrumental and caesarean births71,72 and retained placenta.65 We found no association with induction and augmentation, agreeing with an earlier report73 but at variance with others.12,47 Although previously reported, the influence of caesarean59 and retained placenta69,74 (adjusted odds ratio [aOR] 21.3) were notable. Severe PPH is related to emergency caesarean75,76 and the Royal College of Obstetricians and Gynaecologists state that it is less likely following elective caesarean.76,77 We observed that both are strongly associated with PPH (aORs 24.4 and 40.5) but apparently protect against progression (aORs 0.14 and 0.34); however, this is probably the result of prompt surgical and anaesthetic interventions. Prophylactic Syntometrine® and high-dose Syntocinon® infusion were protective against PPH, reinforcing concerns78 about current recommendations for intramuscular Syntocinon®.79 Although not associated with PPH, physiological third stage was a risk factor for progression, possibly related to delays in recognition or treatment, although others report <0.5% maternal postnatal transfers.80 Time to complete genital tract repair was confirmed as a risk for PPH.81
Conclusion
Identifying risk pathways is important as predisposing risk can underlie factors that appear, accumulate and dominate later events, including subsequent pregnancies.
These findings have implications for ‘red flags’, training and emergency management. Currently, clinical tools are only designed for PPH ≥ 500 ml.70 Clinicians must remain vigilant, identify and respond to women's accumulating risks, recognise abnormal bleeding, summon assistance and ensure prompt treatment and transfer. Staff must eschew threshold preference and avoidance when assessing blood loss, and keep scrupulous records of cumulative loss. Prompt examination for genital tract trauma and expedient suturing must be ensured. Although current practice requires duplication of data entry,82 health professionals should ensure accurate and complete transcription from paper to electronic records.
Policy and research should tackle the potentially modifiable risk factors. Public health interventions addressing the ageing reproductive population and obesity should be encouraged. Commissioners must consider instrumental and primary caesarean rates,38 which may depend on informed decisions about staffing models83,84 and facilities regarding planned place of birth.85 PPH is identified as a key metric for quality of care86–88 yet this study emphasises abundant flaws in measurement and reporting. Standard procedures, including auditing the incidence of PPH and accuracy of recorded EBL, must be improved, otherwise reliance on EBL may be inappropriate.
Research should focus on the transferability of trauma care innovations89,90 and implementation of clinical improvements, such as cumulative blood loss recording, training and reminders for staff.23,91,92 Weighted sampling is an underused methodology that reduces data entry burden and could be extrapolated to research, audit and monitor other morbidities. Progression predictors and attenuators might inform the design of an emergency strategy to ameliorate severe PPH which otherwise looks set to continue rising.
Disclosure of interests
All authors have completed the Unified Competing Interest form available on request from the corresponding author and declare the following: AB, HB, PTS and SB had financial support from Guy's and St Thomas' Charity for the submitted work; AB, LP and PTS received separate financial support from Tommy's Charity (Registered charity no. 1060508 and SC039280); AB also received support from KHP BRC; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Contributions to authorship
The authors were involved as follows: AB, SB conception; AB, SB, PTS, GT, MW design; AB, HB, GT, MW data acquisition; AB, SB, PTS analysis and interpretation; AB, SB, PTS, JS, LP and RMT input into drafting article; AB, PTS, HB, GT, MW, LP, JS, RMT, SB were responsible for revision and final approval of manuscript.
Details of ethics approval
The South East Multicentre Research Ethics Committee (07/H1102/79) approved the study.
Funding
The study was funded by Guy's and St Thomas' Charity (Registered charity no.251983) who had no role in study design, data collection, analysis and interpretation, writing of the report or decision to submit the article for publication. The researchers are independent from the funders. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. King's College London is the guarantor for the study.
Acknowledgments
We thank the Study Advisory Group; Professor Charles Wolfe, Ms Laura Pettigrew, Ms Miranda Clarke, Ms Sue Eardley, Ms Andrea Holder and Ms Sarah Gregson; also Mrs Leanne Pratt who contributed to study completion; Professor Martin Bland for statistical and critical review of the manuscript; and Freya Briley for transcribing dice throws.
Data sharing
The authors will be pleased to consider requests for data sharing following publication of the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Supplementary Methods.
Definitions.
Characteristics of the study population by PPH (estimated blood loss >500 ml) or no PPH, following correction of errors, categorisation and weighting.
Changes in estimated blood loss (according to groups and sub-groups of risk factors as appropriate to pre-pregnancy, during pregnancy, labour and birth), principal predictors of postpartum haemorrhage (PPH) >500 ml and conditional predictors of progression to severe PPH >1500 ml; unadjusted associations
References
- 1.Hogan MC, Foreman KJ, Naghavi M, Ahn SJ, Wan M, Makela SM, et al. Maternal mortality in 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375:1609–23. doi: 10.1016/S0140-6736(10)60518-1. [DOI] [PubMed] [Google Scholar]
- 2.United Nations Children's Defence Fund. Progress for Children: A report card on maternal mortality. Vol. 7. New York: UNICEF; 2008. [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gulmezolglu AM, Van Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.Brace V, Penney G, Hall M. Quantifying severe maternal morbidity: a Scottish population study. BJOG. 2004;111:481–4. doi: 10.1111/j.1471-0528.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 5.Centre for Maternal and Child Enquiries (CMACE) Saving Mothers' Lives: reviewing maternal deaths to make motherhood safer: 2006–08. The Eighth Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl. 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 6.Mantel GD, Buchman E, Rees H, Pattinson RC. Severe acute maternal morbidity: a pilot study of a definition for a near-miss. BJOG. 1999;105:985–90. doi: 10.1111/j.1471-0528.1998.tb10262.x. [DOI] [PubMed] [Google Scholar]
- 7.Zeitlin J, Mohangoo A. European Perinatal Health Report. EURO-PERISTAT project in collaboration with SCPE, EUROCAT & EURONEOSTAT; 2008. [ www.europeristat.com ] Accessed 1 March 2013. [Google Scholar]
- 8.Knight M. Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage. BJOG. 2007;114:1380–7. doi: 10.1111/j.1471-0528.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum haemorrhage: United States, 1994-2006. AJOG. 2010;202:e1–6. doi: 10.1016/j.ajog.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle M-H, Ford J, et al. Trends in postpartum haemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy and Childbirth. 2009;9:55. doi: 10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron CA, Roberts CL, Olive EC, Ford JB, Fischer WE. Trends in postpartum haemorrhage. Aust N Z J Public Health. 2006;30:151–6. doi: 10.1111/j.1467-842x.2006.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 12.Lutomski JE, Byrne BM, Devane D, Green RA. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11-year population-based cohort study. BJOG. 2012;119:306–14. doi: 10.1111/j.1471-0528.2011.03198.x. [DOI] [PubMed] [Google Scholar]
- 13.Health Improvement Scotland. Scottish Confidential Audit of Severe Maternal Morbidity. 7th Report.2011. [Google Scholar]
- 14.Blomberg M. Maternal obesity and risk of postpartum haemorrhage. Obstet Gynecol. 2011;118:561–8. doi: 10.1097/AOG.0b013e31822a6c59. [DOI] [PubMed] [Google Scholar]
- 15.Arrowsmith S, Wray S, Quenby S. Maternal obesity and labour complications following induction of labour in prolonged pregnancy. BJOG. 2011;118:578–88. doi: 10.1111/j.1471-0528.2010.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: a case-control study. BMJ. 2001;322:1089–93. doi: 10.1136/bmj.322.7294.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer MS, Dahhou M, Vallerand D, Liston R, Joseph KS. Risk factors for postpartum haemorrhage: can we explain the recent temporal increase? J Obstet Gynaecol Can. 2011;33:810–9. doi: 10.1016/S1701-2163(16)34984-2. [DOI] [PubMed] [Google Scholar]
- 18.Bryant A, Mhyre JM, Leffert LR, Hoban RA, Yakoob MY, Bateman BT. The association of maternal race and ethnicity and the risk of postpartum hemorrhage. Anesth Analg. 2012;115:1127–36. doi: 10.1213/ANE.0b013e3182691e62. [DOI] [PubMed] [Google Scholar]
- 19.Magann EF, Evans S, Hutchinson M, Collins R, Howard B, Morrison JC. Postpartum hemorrhage after vaginal birth: an analysis of risk factors. Southern Med J. 2005;98:419–22. doi: 10.1097/01.SMJ.0000152760.34443.86. [DOI] [PubMed] [Google Scholar]
- 20.Rossen J, Okland I, Nilsen OB, Eggebo TM. Is there an increase in postpartum hemorrhage, and is severe haemorrhage associated with more frequent use of obstetric interventions? Acta Obstet Gynecol Scand. 2010;89:1248–55. doi: 10.3109/00016349.2010.514324. [DOI] [PubMed] [Google Scholar]
- 21.McConnell JS, Fox TJ, Jossen JP, Subramanian A. “About a cupful” - a prospective study into accuracy of volume estimation by medical and nursing staff. Accid Emerg Nurs. 2007;5:101–5. doi: 10.1016/j.aaen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Schorn MN. Measurement of blood loss: review of the literature. J Midwifery Womens Health. 2010;5:20–7. doi: 10.1016/j.jmwh.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Bose P, Regan F, Paterson-Brown S. Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. BJOG. 2006;113:919–24. doi: 10.1111/j.1471-0528.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 24.Driessen M, Bouvier-Colle M-H, Dupont C, Khoshnood B, Rudigoz RC, Deneux-Tharaux C, et al. Postpartum haemorrhage resulting from uterine atony after vaginal delivery: factors associated with severity. Obstet Gynecol. 2011;117:21–31. doi: 10.1097/AOG.0b013e318202c845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homer CSE, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. A novel classification system to audit severe maternal morbidity. Midwifery. 2010;26:532–6. doi: 10.1016/j.midw.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Joseph KS, Rouleau J, Kramer MS, Young DC, Liston RM, Baskett TF. Investigation of an increase in postpartum haemorrhage in Canada. BJOG. 2007;114:751–9. doi: 10.1111/j.1471-0528.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 27.Korn EL, Graubard BI. Analysis of Health Surveys. New York: Wiley; 1999. [Google Scholar]
- 28.Malgarini M. Efficient sample design and weighting methodologies. Joint European Commission-OECD workshops on international development of business and consumer tendency surveys, Task Force on Harmonisation of Survey Operation and Technical Design. Brussels: The Organisation for Economic Co-operation and Development (OECD); 2005. [Google Scholar]
- 29.Office of National Statistics. Statistics Bulletin; 2010. Statistics Bulletin: Live births and deaths in England and Wales by characteristics of mother 2009. [ www.ons.gov.uk ]. Accessed 12 December 2012. [Google Scholar]
- 30.Heselhurst N. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 61 323 births, 1989–2007. Int J Obesity. 2010;34:420–8. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- 31.HESonline. The information centre; 2009. Table 7: Method of onset of labour 2008–9. [ www.HESonline.nhs.uk ]. Accessed 28 June 2012. [Google Scholar]
- 32.Cleary R, Beard RW, Coles J, Roberts S, Schumacher D, Wickings HI. The quality of routinely collected maternity data. BJOG. 1994;101:1042–7. doi: 10.1111/j.1471-0528.1994.tb13579.x. [DOI] [PubMed] [Google Scholar]
- 33.Shennan A, Halligan A. Blood pressure measurement in pregnancy: room for improvement. Mat Child Health J. 1996;21:55–9. [Google Scholar]
- 34.Edouard L, Senthilselvan A. Observer error and birthweight: digit preference in recording. Public Health. 1997;111:77–9. doi: 10.1016/s0033-3506(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 35.Glover P. Blood loss at delivery: how accurate is your estimation? Aust J Midwifery. 2006;16:21–4. doi: 10.1016/s1031-170x(03)80005-3. [DOI] [PubMed] [Google Scholar]
- 36.Buckland S, Homer CSE. Estimating blood loss after birth: using simulated clinical examples. Women Birth. 2007;20:85–8. doi: 10.1016/j.wombi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Larsson C, Saltvedt S, Wiklund I, Pahlen S, Andolf E. Estimation of blood loss after caesarean section and vaginal delivery has low validity with a tendency to over exaggeration. Acta Obstet Gynecol Scand. 2006;85:1448–52. doi: 10.1080/00016340600985032. [DOI] [PubMed] [Google Scholar]
- 38.Ford JB, Rogers CL, Bell JC, Morris JM. Postpartum haemorrhage occurrence and recurrence: a population-based study. Med J Aust. 2007;187:391–3. doi: 10.5694/j.1326-5377.2007.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 39.Roberts CL, Ford JB, Thompson JF, Morris JM. Population rates of haemorrhage and transfusions among obstetric patients in New South Wales: a short communication. N Z J Obstet Gynaecol. 2009;49:296–8. doi: 10.1111/j.1479-828X.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 40.Winter C, Macfarlane A, Deneux-Tharaux C, Zhang WH, Alexander S, Brocklehurst P, et al. Variations in policies for management of the third stage of labour and the immediate management of postpartum haemorrhage in Europe. BJOG. 2007;114:845–54. doi: 10.1111/j.1471-0528.2007.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montan S. Increased risk in the elderly parturient. Curr Opin Obstet Gynecol. 2007;19:110–2. doi: 10.1097/GCO.0b013e3280825603. [DOI] [PubMed] [Google Scholar]
- 42.Diejomaoh MFE, Al-Shamali IA, Al-Kandari F, Al-Qenae M, Mohd AT. The reproductive performance of women at 40 years and over. Eur J Obstet Gynecol Reprod Biol. 2006;126:33–8. doi: 10.1016/j.ejogrb.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Biro MA, Davey MA, Carolan M, Kealy M. Advanced maternal age and obstetric morbidity for women giving birth in Victoria, Australia: a population-based study. Aust N Z J Obstet Gynaecol. 2012;52:229–34. doi: 10.1111/j.1479-828X.2012.01427.x. [DOI] [PubMed] [Google Scholar]
- 44.Lyndon A, Lee HC, Gilbert WM, Gould JB, Lee KA. Maternal morbidity during childbirth hospitalization in California. J Mat-Fet Neonat Med. 2012;25:2529–35. doi: 10.3109/14767058.2012.710280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhanjal MK. The older mother and medical disorders of pregnancy. In: Bewley SM, Ledger W, Nickolou D, Kehoe S, editors. Reproductive Ageing. London: RCOG Press; 2009. pp. 141–62. [Google Scholar]
- 46.Kenyon A, Bewley S. The effect of age on obstetric (maternal and fetal) outcomes. In: Bewley S, Ledger W, Nickolou D, Kehoe S, editors. Reproductive Ageing. London: RCOG Press; 2009. pp. 125–40. [Google Scholar]
- 47.Smith GCS, Cordeaux Y, White IR, Pasupathy D, Missfelder-Lobos H, Pell JP, et al. The effect of delaying childbirth on primary caesarean section rates. PLoS Med. 2008;5:e144. doi: 10.1371/journal.pmed.0050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabacungan ET, Ngui EM, McGinley EL. Racial/ethnic disparities in maternal morbidities: a statewide study of labor and delivery hospitalizations in Wisconsin. Mat Child Health J. 2012;16:1455–67. doi: 10.1007/s10995-011-0914-6. [DOI] [PubMed] [Google Scholar]
- 49.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 50.Sundararajan V, Reidpath DD, Allotey P. Ethnicity, discrimination and health outcomes: a secondary analysis of hospital data from Victoria, Australia. Diversity in Health and Social Care. 2007;4:21–32. [Google Scholar]
- 51.Calvert C, Thomas SL, Ronsmans C, Wagner KS, Adler AJ, Filippi V. Identifying regional variation in the prevalence of postpartum haemorrhage: a systematic review and meta-analysis. PLoS ONE. 2012;7:e41114. doi: 10.1371/journal.pone.0041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998-2005. Obstet Gynecol. 2010;116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 53.Fyfe EM, Thompson JM, Anderson NH, Groom KM, McCowan LM. Maternal obesity and postpartum haemorrhage after vaginal and caesarean delivery among nulliparous women at term: a retrospective cohort study. BMC Pregnancy and Childbirth. 2012;12:112. doi: 10.1186/1471-2393-12-112. doi: 10.1186/1471-2393-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebire NJ, Jolly M, Harris JP, Regan L, Robinson S. Is maternal underweight really a risk factor for adverse pregnancy outcome? A population-based study in London. BJOG. 2001;108:61–6. doi: 10.1111/j.1471-0528.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- 55.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obesity. 2001;25:1175–82. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 56.Paglia MJ, Grotegut CA, Johnson LN, Thames B, James AH. Body Mass Index and Severe Postpartum Hemorrhage. Gynecol Obstet Invest. 2012;73:70–4. doi: 10.1159/000329335. [DOI] [PubMed] [Google Scholar]
- 57.Kallen B. Maternal morbidity and mortality in in-vitro fertilization. Best Practice Res Clin Obstet Gynaecol. 2008;22:549–58. doi: 10.1016/j.bpobgyn.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 58.Oboro V, Adewunmi A, Olagbuji B, Ezeanochie M, Oyeniran A. Morbidity associated with failed vaginal birth after caesarean section. Acta Obstet Gynecol Scand. 2010;89:1229–32. doi: 10.3109/00016349.2010.499448. [DOI] [PubMed] [Google Scholar]
- 59.Fong A, Leake J, Pan D, Ogunyemi D. Demographic, institutional and obstetrical risk factors for postpartum haemorrhage mortality. J Obstet Gynaecol. 2010;30:470–5. doi: 10.3109/01443615.2010.487576. [DOI] [PubMed] [Google Scholar]
- 60.Coulter-Smith SD, Holohan M, Darling MRN. Previous caesarean section: a risk for major obstetric haemorrhage. J Obstet Gynaecol. 1996;6:349–52. [Google Scholar]
- 61.Tikkanen M, Riihimäki O, Gissler M, Luukkaala T, Metsäranta M, Andersson S, et al. Decreasing incidence of placental abruption in Finland during 1980-2005. Acta Obstet Gynecol Scand. 2012;91:1046–52. doi: 10.1111/j.1600-0412.2012.01457.x. [DOI] [PubMed] [Google Scholar]
- 62.Newnham JP, Patterson L, James I, Reid SE. Effects of maternal cigarette smoking on ultrasonic measurements of fetal growth and on Doppler flow velocity waveforms. Early Hum Dev. 1990;24:23–36. doi: 10.1016/0378-3782(90)90003-2. [DOI] [PubMed] [Google Scholar]
- 63.Eskild A, Vatten LJ. Abnormal bleeding associated with preeclampsia: a population study of 315,085 pregnancies. Acta Obstet Gynecol Scand. 2009;88:154–8. doi: 10.1080/00016340802613242. [DOI] [PubMed] [Google Scholar]
- 64.Malabarey O, Almog B, Borwn R, Abenhaim HA, Shrim A. Postpartum haemorrhage in low risk population. J Perinat Med. 2011;39:495–8. doi: 10.1515/jpm.2011.059. [DOI] [PubMed] [Google Scholar]
- 65.Magann EF, Evans S, Chauhan SP, Lanneau G, Fisk AD, Morrison JC. The length of the third stage of labor and the risk of postpartum haemorrhage. Obstet Gynecol. 2005;105:290–3. doi: 10.1097/01.AOG.0000151993.83276.70. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe T, Matsubara S. No bleeding before, more bleeding later: the relationship between the presence of warning bleeding and the amount of bleeding during caesarean section in placenta praevia. J Obstet Gynaecol. 2010;30:836. doi: 10.3109/01443615.2010.509827. [DOI] [PubMed] [Google Scholar]
- 67.Stones RW, Patterson CM, Saunders NJ. Risk factors for major obstetric haemorrhage. Eur J Obstet Gynecol Reprod Biol. 1993;48:15–8. doi: 10.1016/0028-2243(93)90047-g. [DOI] [PubMed] [Google Scholar]
- 68.National Institute for Health and Clinical Excellence (NICE) Antenatal care: Routine care for healthy pregnant women. 2010. [ www.nice.org.uk ] Accessed 23 January 2014.
- 69.Arulkumaran S, Mavrides E, Penney GC on behalf of the Guidelines and Audit Committee of the Royal College of Obstetricians and Gynaecologists. Prevention and Management of Postpartum Haemorrhage. London: RCOG Press; 2009. Green Top Guideline 52. [Google Scholar]
- 70.Government of South Australia. Perinatal Practice Guidelines-Postpartum Haemorrhage Government of South Australia. 2012. [ http://www.sahealth.sa.gov.au/wps/wcm/connect/7a6f45804ee56498a97eadd150ce4f37/2013_04_30_postpartum+haemorrhage.pdf?MOD=AJPERES&CACHEID=7a6f45804ee56498a97eadd150ce4f37 ]. Accessed 23 January 2014.
- 71.Sheiner E, Sarid L, Levy A, Seidman DS, Hallak M. Obstetric risk factors and outcome of pregnancies complicated by early postpartum haemorrhage: a population-based study. J Maternal-Fetal & Neonatal Med. 2005;18:149–54. doi: 10.1080/14767050500170088. [DOI] [PubMed] [Google Scholar]
- 72.Unterschneider J, McMenamin M, Cullinane F. Rising rates of caesarean deliveries at full dilatation: a concerning trend. Eur J Obstet Gynecol Reprod Biol. 2011;157:141–4. doi: 10.1016/j.ejogrb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ. 2012;2838:1–13. doi: 10.1136/bmj.e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weeks AD. The retained placenta. Best Pract Res Clin Obstet Gynaecol. 2008;22:1103–17. doi: 10.1016/j.bpobgyn.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Holm C, Langhoff-Roos J, Petersen KB, Norgaard A, Diness BR. Severe postpartum haemorrhage and mode of delivery: a retrospective cohort study. BJOG. 2012;119:596–604. doi: 10.1111/j.1471-0528.2011.03267.x. [DOI] [PubMed] [Google Scholar]
- 76.Al-Zirqi I, Vangen S, Forsen L, Stray-Oedersen B. Effects of onset of labor and mode of delivery on severe postpartum haemorrhage. Am J Obstet Gynecol. 2009;201:e1–9. doi: 10.1016/j.ajog.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Weston N on behalf of RCOG. Severe postpartum haemorrhage less likely with planned caesarean section, finds study. BJOG release. 2012 [ http://www.rcog.org.uk/news/bjog-release-severe-post-partum-haemorrhage-less-likely-planned-caesarean-section-finds-study ] Accessed 23 January 2014. [Google Scholar]
- 78.Rogers C, Villar R, Pisal P, Yearley C. Effects of Syntocinon use in active management of third stage of labour. Br J Midwifery. 2011;19:371–8. [Google Scholar]
- 79.National Institute for Health and Clinical Excellence (NICE) Intrapartum care: care of healthy women and their babies during childbirth. 2007. [ www.nice.org.uk ] Accessed 23 January 2014. [PubMed]
- 80.Rowe RE, Fitzpatrick AR, Hollowell BJ, Kurinczuk AJJ. Transfers of women planning birth in midwifery units:data from the Birthplace prospective cohort study. BJOG. 2012;119:1081–90. doi: 10.1111/j.1471-0528.2012.03414.x. [DOI] [PubMed] [Google Scholar]
- 81.Ozdegirmenci O, Erkaya S, Yalvac S, Dilbaz B, Altinbas S, Haberal A. Does early repair of episiotomy decrease postpartum blood loss: a randomized clinical trial. J Mat-Fet Neonat Med. 2010;23:308–10. doi: 10.1080/14767050903118296. [DOI] [PubMed] [Google Scholar]
- 82.Fawdry R, Bewley S, Cumming G, Perry H. Data re-entry overload: time for paradigm shift in maternity IT? J R Soc Med. 2011;104:405–12. doi: 10.1258/jrsm.2011.110153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Halem M, Sandall J, Devane D, Soltani H, Gates S. Midwife-led versus other models of care for childbearing women. Cochrane Rev. 2008;8:CD004667. doi: 10.1002/14651858.CD004667.pub2. [DOI] [PubMed] [Google Scholar]
- 84.McLachlan HL, Porster DA, Davie MA, Biro MA, Albers L, Flood M, et al. Effects of continuity of care by a primary midwife (caseload midwifery) on caesarean section rates in women of low obstetric risk: the COSMOS randomised controlled trial. BJOG. 2012;119:1483–92. doi: 10.1111/j.1471-0528.2012.03446.x. [DOI] [PubMed] [Google Scholar]
- 85.Birthplace in England Collaborative Group. Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the Birthplace in England national prospective cohort study. BMJ. 2011;343:d7400. doi: 10.1136/bmj.d7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Penney G, Adamson L. Scottish Confidential Audit of Severe Maternal Morbidity. 4th annual report; Edinburgh: SPCERH; 2006. [Google Scholar]
- 87.Woiski MD, Hermens RP, Moddeldorp JM, Kremer JA, Marcus MA, Woulters MG, et al. Haemorrhagia post partum; an implementation study on the evidence-based guideline of the Dutch Society of Obstetrics and Gynaecology (NVOG) and the MOET (Managing Obstetric Emergencies and Trauma-course) instructions; the Fluxim study. BMC Pregnancy & Childbirth. 2010;10:5. doi: 10.1186/1471-2393-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Talungchit P, Laibsuetrakul T, Lindmark G. Development and assessment of indicators for quality of care in severe preeclampsia/eclampsia and postpartum hemorrhage. J Healthcare Qual. 2012;35:22–34. doi: 10.1111/j.1945-1474.2011.00183.x. [DOI] [PubMed] [Google Scholar]
- 89.Byers R. An upshot of war-damage resuscitation. Int Emergency Nursing. 2010;8:221. doi: 10.1016/j.ienj.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Holcomb JB, Zarzabel LA, Michalek JE, Kozar JE, Spinelle PC, Perkins JG, et al. Increased platelet: RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 suppl 3):S318–28. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 91.Draycott T, Owen L, Akande V, Winter C, Reading S, Whitelaw A. Does training in obstetric emergencies improve neonatal outcome? BJOG. 2006;113:177–82. doi: 10.1111/j.1471-0528.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 92.Deneux-Tharaux C, Dupont C, Colin C, Rabilloud M, Touzet S, Lansac J. Multifaceted intervention to decrease the rate of severe postpartum haemorrhage: the PITHAGORE6 cluster-randomised controlled trial. BJOG. 2010;117:1278–87. doi: 10.1111/j.1471-0528.2010.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods.
Definitions.
Characteristics of the study population by PPH (estimated blood loss >500 ml) or no PPH, following correction of errors, categorisation and weighting.
Changes in estimated blood loss (according to groups and sub-groups of risk factors as appropriate to pre-pregnancy, during pregnancy, labour and birth), principal predictors of postpartum haemorrhage (PPH) >500 ml and conditional predictors of progression to severe PPH >1500 ml; unadjusted associations


