Abstract
Radiation exposure due to radiological terrorism and military circumstances are a continuing threat for the civilian population. In an uncontrolled radiation event, it is likely that there will be other types of injury involved, including trauma. While radiation combined injury is recognized as an area of great significance, overall there is a paucity of information regarding the mechanisms underlying the interactions between irradiation and other forms of injury, or what countermeasures might be effective in ameliorating such changes. The objective of this study was to determine if difluoromethylornithine (DFMO) could reduce the adverse effects of single or combined injury if administered beginning 24 h after exposure. Eight-week-old C57BL/J6 young-adult male mice received whole-body cesium-137 (137Cs) irradiation with 4 Gy. Immediately after irradiation, unilateral traumatic brain injury was induced using a controlled cortical impact system. Forty-four days postirradiation, animals were tested for hippocampus-dependent cognitive performance in the Morris water maze. After cognitive testing, animals were euthanized and their brains snap frozen for immunohisto-chemical assessment of neuroinflammation (activated microglia) and neurogenesis in the hippocampal dentate gyrus. Our data show that single and combined injuries induced variable degrees of hippocampus-dependent cognitive dysfunction, and when given 24 h post trauma, DFMO treatment ameliorated those effects. Cellular changes including neuro-genesis and numbers of activated microglia were generally not associated with the cognitive changes. Further analyses also revealed that DFMO increased hippocampal protein levels of the antioxidants thioredoxin 1 and peroxiredoxin 3 compared to vehicle treated animals. While the mechanisms responsible for the improvement in cognition after DFMO treatment are not yet clear, these results constitute a basis for further development of DFMO as a countermeasure for ameliorating the of risks for cognitive dysfunction in individuals subjected to trauma and radiation combined injury.
INTRODUCTION
Given the growing worldwide threat of radiological/ nuclear terrorism, the White House Office of Science and Technology Policy’s Radiological/Nuclear Threat Countermeasures Working Group concluded that radiation combined injury (RCI) should be a high priority research area (1, 2). Casualty estimates based on previous atomic bomb detonations predict that 65–70% of victims exposed to radiation will likely have additional trauma, whereas only 15–20% will be affected by radiation alone (3). The radiation accident at Chernobyl resulted in 60–70% of the radiation-exposed population sustaining combined injuries (3, 4). While RCI is recognized as an area of great significance, overall there is a paucity of information regarding the magnitudes and mechanisms underlying the interactions between radiation exposure and other forms of injury and how such injuries are handled.
Uncontrolled radiation exposure in an urban or battlefield situation will likely involve a wide range of delivered doses and subsequent tissue/body effects. In addition, radiation effects will likely be exacerbated by other types of injury (e.g., trauma, burns, infection) that may occur either at the time of radiation exposure or at some time thereafter. Although exposure of the brain to low-dose radiation may not directly affect the brain structure or function, it may make the tissue more sensitive to a secondary injury. For instance, a traumatic insult could exacerbate latent radiation injury, resulting in cellular/tissue injury that could impact function or cognitive performance.
The pathophysiology of RCI is complicated and has not been well characterized, nor have effective measures to combat such injuries been established. Thus, the National Institute of Allergy and Infectious Diseases (NIAID) concluded that the search for medical countermeasures for RCI should begin with compounds that are recognized as the standard of care for the individual component injuries (2). Difluoromethylornithine (DFMO) was synthesized almost 30 years ago as an irreversible inhibitor of ornithine decarboxylase (ODC). It was shown that DFMO decreased edema after cortical impact (5) and ischemic injury volume after experimental cerebral ischemia (3). Our earlier work showed that DFMO treatment reduced radiation-induced necrosis, vascular permeability and associated vasogenic edema after focal radiation-induced injury (6–8). We recently demonstrated that when administered immediately after injury and for 41 consecutive days, DFMO effectively reversed cognitive impairments after trauma alone and particularly after RCI (9). To determine if DFMO could ameliorate the adverse effects of RCI when drug delivery was delayed, we started the DFMO treatment 24 h after a single or combined injury and continued the DFMO treatment for 41 consecutive days.
MATERIALS AND METHODS
Animals
A total of 120 eight-week-old C57BL/6J male mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were housed and cared for in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and Institutional Animal Care and Use Committees (IACUCs). Animals were randomly assigned to four treatment cohorts: 0 Gy/ surgery (surgery, n = 30), 4.0 Gy/surgery (radiation, n = 30); 0 Gy/ trauma (trauma, n = 30); 4.0 Gy/trauma (RCI, n = 30). Fifteen animals per treatment group received vehicle, while the other 15 mice were given DFMO. The day that water maze testing was to begin, 5 mice per treatment group were euthanized for biochemical analysis. Animals were group housed (5 mice/cage) throughout the study.
Irradiation
Whole-body irradiation was performed using a 137Cs irradiator (Gamma cell 3000; MDS Nordion Inc., Ottawa, ON, Canada) as previously described (9). Animals were irradiated individually in a specially designed restrainer that fit into the irradiator and allowed minimal movement. Dosimetry was performed using film exposure within the cesium irradiator and employing the same geometry used for the animal treatments. The film readings were calibrated against a range of doses obtained using a linear accelerator. For this study we used a 4.0 Gy dose, which does not induce significant changes in the gut or bone marrow in mice. The total irradiation time was approximately 1 min.
DFMO Treatment
DFMO was generously provided by Dr. Victor A. Levin. DFMO is stable in drinking water for prolonged periods of time (months) if there is no growth or contamination by microorganisms.2 DFMO (1%) was dissolved in drinking water and the solution was changed weekly. DFMO was made available to the mice starting 24 h after their recovery from surgery and for a total of 41 days (Fig. 1). DFMO treatment was stopped the day prior to the initiation of the cognitive testing.
FIG. 1.

Schematic diagram showing experimental design. Two-month-old C57BL/6J male mice received whole-body irradiation (4 Gy) and immediately after (~15 min) received either focal traumatic brain injury or surgery only. Two weeks later, animals were injected daily for 7 days with BrdU (100 mg/kg). DFMO was made available in the drinking water 24 h after surgery and for a total of 41 days and was stopped the day before cognitive testing in the Morris water maze.
Traumatic Brain Injury
Traumatic brain injury was induced ~15 min after irradiation (Fig. 1). Each mouse was anesthetized with 4% isoflurane, maintained by a non-rebreathing apparatus connected to a nose cone on the stereotaxic head frame (Kopf, Tujunga, CA). Ointment was applied to the eyes to protect vision and heads were shaved with an electric clipper. The skin was scrubbed with betadine solution, and a midline incision was made through the scalp. A circular craniotomy, 3.5 mm in diameter was made in the left parietal skull between the bregma and lambda, 0.5 mm lateral to the midline. The skullcap was carefully removed without disruption of the dura. All mice, regardless of injury type, were subjected to this surgical procedure. Mice that were randomly selected for the trauma only group (no irradiation) or the RCI treatment group were subjected to a controlled cortical impact (10, 11). The lesion was produced with a pneumatic impact device using a 3 mm diameter convex tip, mounted 20° from the vertical to account for the curvature of the mouse skull. The contact velocity was set at 4.5 m/s with a deformation 1.5 mm below the dura and a sustained depression of 150 msec, producing a moderate lesion to the cortex without overtly damaging the hippocampus. After the procedure, the scalp was sutured and each animal received a subcutaneous injection of warm physiologic saline (1 ml) to prevent dehydration. During surgery and subsequent recovery, body temperature was maintained with a circulating water heating pad.
The controlled cortical impact model of traumatic brain injury is widely used because it generates many of the cognitive impairments seen in trauma patients (12). In the open head model, a portion of the skull was removed, and an impacting rod was driven into the dura to produce deformation of the cortex. Increasing the depth and velocity of the impact intensifies cortical cavitation and the effect on behavioral function (13, 14). The impact depth of 1.5 mm was chosen based on previous studies performed in young adult mice where a 0.50 mm deformation produced mild injury, a 1.0 mm deformation produced moderate injury and a 2.0 mm deformation produced severe injury (14).
BrdU Injection
Fourteen days after treatment, all mice received daily injections of BrdU (100 mg/kg) for 7 consecutive days. Four weeks after the first BrdU injection 5 mice per treatment group were euthanized for biochemical analyses. The remaining animals underwent Morris water maze training and testing and were then euthanized by cervical dislocation, after which tissues were collected for analysis of neurogenesis and activated microglia (Fig. 1).
Morris Water Maze
Assessment of hippocampus-dependent cognitive performance was performed 6 weeks after irradiation, trauma or RCI using the Morris water maze test (15). A circular pool (diameter 140 cm) was filled with opaque water (24°C) and mice were trained to locate a platform (luminescence: 200 lux). To determine if treatment affected the ability to swim or learn the water maze task, mice were first trained to locate a clearly marked platform (visible platform, day 1 and 2). Mice were subsequently trained to locate the platform when it was hidden beneath the surface of the opaque water (day 3–5). Hidden platform training (acquisition) required the mice to learn the location of the hidden platform based on extra-maze spatial cues. For both visible and hidden platform paradigms, there were 2 daily sessions that were 2 h apart. Each session consisted of 3 trials (with 10-min intertrial intervals). A trial ended when the mice located the platform. Mice that failed to locate the platform within 60 s were led to the platform by placing a finger in front of their swim path. Mice were taken out of the pool after they were physically on the platform for a minimum of 3 s. During visible platform training, the platform was moved to a different quadrant of the pool for each session. For the hidden platform training, the platform location was kept constant. Mice were placed into the water facing the edge of the pool in one of 9 randomized locations. The start location was changed for each trial. Distance moved and probe trials were recorded with the Noldus Ethovision video tracking system (Ethovision XT, Noldus Information Technology, Wageningen, Netherlands) set at 6 samples per second.
To measure spatial memory retention, probe trials (platform removed) were conducted 1 h after the last trial on each day of hidden platform training (i.e., 3 separate probe trials). For the probe trials, mice were placed into the water in the quadrant opposite from the target quadrant and allowed to search for the platform 60 s. The time spent in the target quadrant, i.e., where the platform was previously located during hidden platform training, was compared to the time spent in the 3 nontarget quadrants. Average velocity and distance to platform were also used as a measure of performance for the hidden sessions.
Histological Procedures
After the last probe trial mice were killed by cervical dislocation and decapitated. Brains were removed quickly (within 60 s), frozen in isopentane and then stored at −80°C until being sectioned on a cryostat. Brain sections were taken across the entire the hippocampus. Tissues from multiple animals were blocked together and cryosectioned (16). Each slide contained a 20 μm thick sample from each of the four experimental conditions: 0 Gy/surgery; 4.0 Gy/surgery; 0 Gy/ trauma; and 4.0 Gy/trauma. All slides were stored at −80°C until processed for histological analysis.
Neurogenesis
To evaluate the effect of DFMO on the survival of newly born cells in the dentate subgranular zone (SGZ) a double labeling protocol was used to identify newly born (BrdU+) neurons (NeuN+). For BrdU staining, sections were fixed for 8 min in 2% paraformaldehyde, then rinsed in Tris buffered saline (TBS) (pH 7.4). Endogenous peroxidase activity was quenched by 30 min incubation in freshly prepared 3% H2O2 solution. After 2 × 5 min washes in TBS the tissue was treated with 2N hydrochloric acid for 30 min at 37°C to denature DNA. The slides were immersed in 0.1 M Na2B4O7 to neutralize the acid, followed by eight rinses in TBS for 5 min each to return the pH to approximately 7.4. Nonspecific antigen binding was blocked with TBS containing TSA blocking reagent (PerkinElmer Life Sciences, Emeryville, CA) at ambient temperature for 30 min. Newly born cells were stained using rat anti-BrdU (1:50, Accurate Chemical & Scientific Corporation, Westbury, NY) incubated overnight at 4°C. The primary antibody was then detected by 2 h incubation with an anti-rat Red X (Jackson ImmunoResearch Laboratories Inc., Carlsbad, CA). Neuronal staining was performed using an antibody for the neuron-specific nuclear protein NeuN (1:500; Millipore, Billerica, MA). The primary antibody was then detected by 2 h incubation with an anti-mouse Alexa Fluor® 488 (Molecular Probes®, Eugene, OR). BrdU and NeuN positive cells were double labeled in the same section.
Total and Newly Born Activated Microglia
To determine if DFMO treatment reduced inflammatory responses occurring after injury, the number of activated microglia, either total or newly born, was determined by counting cells labeled for CD68 alone (total) or double-labeled with CD68 and BrdU (newly born). Briefly, sections were fixed for 10 min in 4% paraformaldehyde and after three washes with TBS and quenching of endogenous peroxidase activity in 1% H2O2 solution, they were incubated in TSA blocking buffer containing 3% normal rabbit serum for 30 min to block nonspecific antigen binding. The sections were then incubated with rat anti-mouse CD68 antibody (1:1,000, Abcam®, Cambridge, MA) overnight at 4°C followed by incubation with rabbit anti-rat IgG (1:200, Vector Laboratories, Burlingame, CA) for 2 h at room temperature. Staining signals were further amplified with an avidin/biotin amplification system (Vector Laboratories) followed by Cy3 tyramide amplification (PerkinElmer Life Sciences). To label newly born activated microglia (CD68+/BrdU+), sections were further washed with TBS-tween and treated for DNA denaturation as described above. Rat anti-BrdU primary antibody (1:50, Accurate Chemical & Scientific Corporation) was applied for overnight at 4°C and detected with anti-rat FITC (Jackson ImmunoResearch Laboratories Inc.) secondary antibody. Only those cells for which the BrdU nucleus was unambiguously associated with the marker for activated microglia (CD68) were scored as positive for newly born activated microglia. The results were expressed as numbers of cells/mm2.
Microscopic Analysis
Images were reconstructed as described previously (9). Briefly, mosaics were collected with a Zeiss Axio Imager ApoTome microscope using a 20× objective (Carl Zeiss, Hertfordshire, UK). The parameters were kept constant across sections. Regions of interest were selected using AxioVision imaging software (Carl Zeiss) and the numbers of positive cells were counted in a blinded fashion. Every sixth section (120 μm interval), covering the entire hippocampus was processed for immunohistochemical analysis. Cells were counted (without knowledge of treatments) under high power (40×) using an ApoTome Zeiss microscope (Carl Zeiss) and expressed as the number of newborn cells per mm2 of hippocampal dentate gyrus.
Western Blot Analyses
To determine if the major antioxidant enzymes were altered by DFMO treatment, hippocampi were collected from 20 mice each from the vehicle treatment and DFMO treatment groups on the day water maze testing began. Brains (n = 5) from each of the experimental conditions (i.e., 0 Gy/surgery; 4.0 Gy/surgery; 0 Gy/trauma; 4.0 Gy/ trauma) were dissected on ice, flash frozen in liquid nitrogen and stored at −80°C.
For Western blot analyses, hippocampal tissues from each animal were homogenized individually (weight to volume ratio 1:20) in T-PER buffer (Thermo Scientific, Rockford, IL), containing Complete® protease inhibitor and phosphatase inhibitor (Roche, Switzerland). Homogenized samples were centrifuged at 10,000g at 4°C for 5 min, and the supernatants were stored in 20 μl aliquots at −80°C. Protein concentration of each sample was determined with a NanoVue™ spectrophotometer (GE Healthcare, Pittsburgh, PA), and 50 μg of total proteins from each sample were used for the analysis. Protein levels of antioxidant enzymes, CuZn superoxide dismutase (CuZnSOD), Mn superoxide dismutase (MnSOD), thioredoxin 1 (Trx1), peroxiredoxin 1 (Prdx1) and peroxiredoxin 3 (Prdx3) were determined. Primary antibodies used in this study have been previously described (17, 18). IRDye-conjugated secondary antibodies were used for signal detection and quantification of all Western blot samples, and protein bands were visualized using an Odyssey® Infrared Imaging System (Licor, Nebraska). Blots were reprobed with an antibody against β-actin (A3854, 1:5,000, Sigma-Aldrich LLC, St. Louis, MO) for normalization. To adjust for the gel-to-gel variations in Western blot signals, a common hippocampal sample was loaded in all gels for standardization.
Data Analysis
Statistical analyses for the behavioral data were generated using R statistical programming language (GNU General Public License). There were two sets of analyses performed on the distance moved data. The first of these assessed the effects of treatment and time on the abilities of the mice to learn during the visible and hidden platform water maze studies and involved using mixed model analyses of variance (ANOVAs). Next, the corresponding pair-wise comparisons of condition-specific estimates of the day × treatment estimated slopes were calculated. The second round of analysis compared group performance on the same day. In that analysis, radiation and treatment were between group factors and day was treated as categorical to facilitate specific comparisons among groups and to eliminate the assumption of a linear trend in performance over time. To correct for the observed heteroskedasticity, a logarithmic transformation was used. Separate analyses were conducted for the visible and hidden platform learning curves. Distance moved refers to the water maze learning curve. For analysis of performance in the water maze probe trials, one-way ANOVAs were used along with Bonferroni’s multiple comparisons test when appropriate.
Statistical analyses for the immunohistochemistry cell counts were performed using GraphPad Prism software (La Jolla, CA). Two-way ANOVAs were used to test whether there were main effects of DFMO and treatment (surgery, irradiation, trauma or RCI) or an interaction between DFMO and treatment. When the interaction between DFMO and treatment was significant (P < 0.05), a step-down procedure for multiple comparisons was followed with two separate one-way ANOVAs. When the overall one-way ANOVA was significant (P < 0.05), individual between-groups comparisons were performed with the Newman Keuls post hoc test. Data for the ipsilateral and contralateral hemispheres were analyzed separately. Western blot data were analyzed using a two-way ANOVA with Holm’s correction. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Cognitive Studies
Distance moved
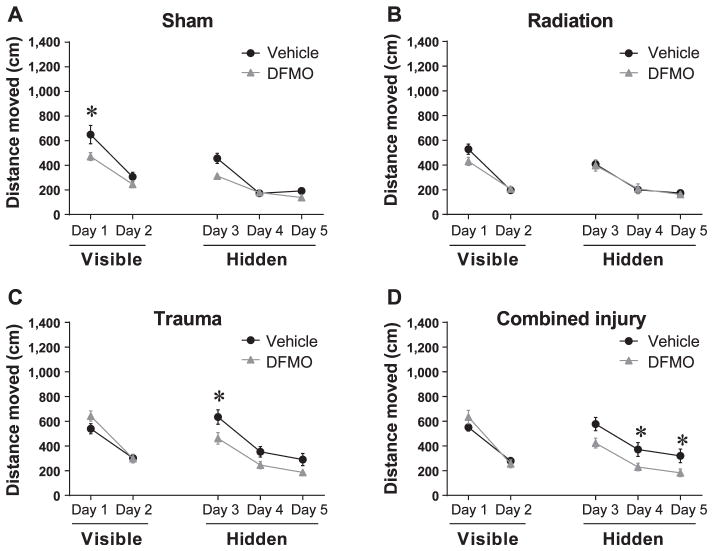
The Morris water maze was used to determine if DFMO treatment reverses cognitive deficits after single or combined injury. During the visible platform sessions, which measured the animal’s ability to learn the task, there was an effect of treatment on distance moved to target (F(3, 76) = 6.36, P < 0.01). Multiple comparison analyses showed that on day 1, mice that received surgery only and treated with DFMO swam significantly shorter distances to locate the visible platform compared to mice that received surgery and vehicle only [DFMO vs. vehicle treated; t = −2.05, P < 0.05 (Fig. 2A)]. There was also a trend toward significance on day 2 [DFMO vs. vehicle treated; t = −1.65, P = 0.09 (Fig. 2A)].
FIG. 2.
Distance moved to the target platform during visible and hidden training sessions. Panel A: During the visible platform training DFMO treated, sham-irradiated mice that received surgery only swam significantly shorter distances to locate the platform compared to vehicle treated, sham-irradiated mice on day 1 (*P < 0.05). Panel B: There were no significant differences between irradiated mice that were DFMO treated and those that were vehicle treated. Panel C: During the hidden platform training (day 3–5), vehicle treated mice that received trauma swam significantly distances compared to DFMO treated mice on day 3 (*P < 0.05). Panel D: Day 4 and 5 DFMO treated RCI mice swam significantly shorter distances to locate the hidden platform compared to vehicle treated RCI mice (*P < 0.05; day 4–5). Each datum point represents the mean of 9–10 mice; error bars are standard error of the mean (SEM).
For the analysis of the hidden platform sessions, which measures acquisition of spatial learning, overall there was an effect of DFMO (F(1, 76) = 18.56, P < 0.001). Multiple comparison analyses showed that mice receiving trauma and vehicle treatment moved significantly longer distances to locate the platform compared to DFMO treatment animals on day 3 [day 3, DFMO + trauma vs. vehicle + trauma; t = −2.27, P < 0.05 (Fig. 2C)]. Multiple comparison analyses also showed that after RCI, mice treated with DFMO swam significantly shorter distances to locate the hidden platform compared to RCI mice treated with vehicle on day 4 and 5 [day 4: DFMO + RCI vs. vehicle + RCI = −2.28, P < 0.05; day 5: DFMO + RCI vs. vehicle + RCI = −2.91, P < 0.01 (Fig. 2D)], and a trend toward significance on day 3 [DFMO vs. vehicle; t = −1.71, P = 0.08 (Fig. 2D)]. While all treatment groups exhibited improved learning, only mice that received RCI plus DFMO consistently showed improved ability to locate the hidden platform compared to mice that received RCI plus vehicle treatment.
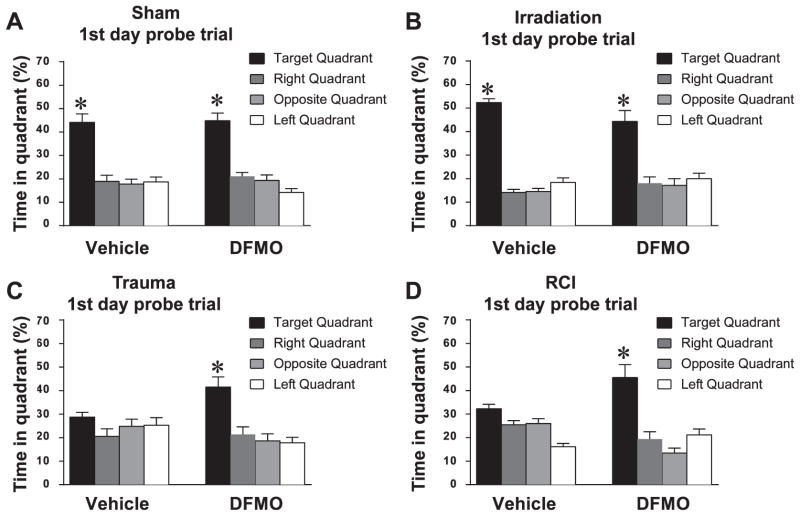
Probe trial
During the first probe trial, vehicle treated mice that received surgery only or radiation only demonstrated memory retention by spending more time in the target quadrant than any other quadrant. In contrast, vehicle treated animals that received trauma, or RCI, did not show spatial memory retention [surgery: target vs. any other quadrant, P < 0.05 (Fig. 3A); radiation: target vs. any other quadrant, P < 0.05 (Fig. 3B); trauma: target vs. any other quadrant, P > 0.05 (Fig. 3C); RCI: target vs. any other quadrant, P > 0.05 (Fig. 3D)]. During subsequent probe trials 2 and 3, all of the treatment groups showed memory retention, spending more time searching in the target quadrant than in any other quadrant (not shown).
FIG. 3.
Spatial memory retention during the probe trail. Panels A and B: Mice that received surgery or radiation only showed a significant preference for the target quadrant. Panels C and D: Vehicle treated mice that received trauma or RCI showed an impairment of hippocampal-dependent spatial memory during the first probe trials. All of the DFMO treated mice, showed memory retention in the water maze by spending most of their time in the target quadrant that contained the hidden platform. For all 4 treatments, when time spent in the target quadrant was compared to all other quadrants, there was a significant (*P < 0.05) preference for the target quadrant. Each bar represents the mean of 8–10 mice; error bars are standard error of the mean (SEM).
In DFMO treated mice, spatial memory retention was seen in the first probe trial after all treatments, with mice spending more time in the target quadrant than in any other quadrant [surgery: target vs. any other quadrant, P < 0.05 (Fig. 3a); radiation: target vs. any other quadrant, P < 0.05, (Fig. 3b); trauma: target versus any other quadrant, P < 0.05 (Fig. 3C); RCI: target vs. any other quadrant, P < 0.05 (Fig. 3b)]. The results from probe trials 2 and 3 were the same as trial 1 with all animals showing retention of spatial memory.
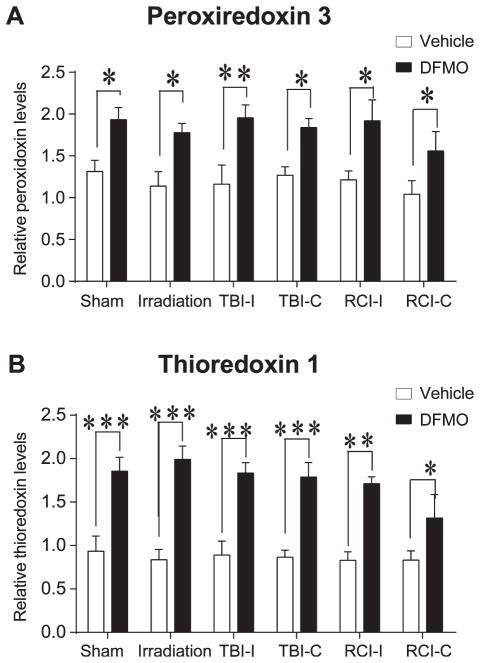
Western Blot Analysis of Antioxidants
Western blot analyses were performed to examine the effects of DFMO treatment on the expression of antioxidants associated with cellular redox regulation. Protein levels of CuZnSOD, MnSOD, peroxiredoxin 1, peroxir-edoxin 3 and thioredoxin 1 were measured from hippo-campal tissue. Compared to vehicle treated mice, there was a significant main effect of DFMO treatment on the levels of peroxiredoxin 3 (F(1, 48) = 110.2, P < 0.01) and thioredoxin 1 (F(1, 48) = 14.74, P < 0.001) present within the hippocampus. Holm-Sidak’s multiple comparison analysis revealed that DFMO significantly increased hippocampal thioredoxin 1 and peroxiredoxin 3 across all treatment groups [thioredoxin 1: surgery DFMO vs. vehicle, P < 0.001; radiation DFMO vs. vehicle, P < 0.001; trauma ipsilateral DFMO vs. vehicle, P < 0.001; trauma contralateral DFMO vs. vehicle, P < 0.001; RCI ipsilateral DFMO vs. vehicle, P < 0.01; RCI contralateral DFMO vs. vehicle, P < 0.05 (Fig. 4A)] [peroxiredoxin 3: surgery DFMO vs. vehicle, P < 0.05; radiation DFMO vs. vehicle, P < 0.05; trauma ipsilateral DFMO vs. vehicle, P < 0.01; trauma contralateral DFMO vs. vehicle, P < 0.05; RCI ipsilateral DFMO vs. vehicle, P < 0.05; RCI contralateral DFMO vs. vehicle, P < 0.05 (Fig. 4B)]. Our analyses did not detect a main effect of DFMO treatment on the levels of CuZnSOD, MnSOD, peroxir-edoxin 1 precluding post hoc comparison. This suggested that at least for these enzymes analyzed here, there were no apparent significant changes when mice were treated with DFMO.
FIG. 4.
Protein levels of antioxidant enzymes in the hippocampal formation Western blot analyses of antioxidant enzymes revealed that DFMO significantly increased mitochondrial peroxiredoxin (Prdx3) (panel A) and cytosolic thioredoxin (Trx1) (panel B) across all treatment groups. All levels were normalized to that of β-actin. (*P < 0.05; **P < 0.01; ***P < 0.001)
Immunohistochemistry
BrdU
The presence of BrdU+ cells 4 weeks after BrdU injection represents the long-term survival of newly generated cells, independent of phenotype. Our initial analytical approach was to assess these end points in both hemispheres independently. In both hemispheres of the animals that received surgery only, DFMO treatment resulted in nonsignificant reductions in the numbers of BrdU+ cells (Table 1).
TABLE 1.
Total Number of BrdU Positive and BrdU/NeuN Positive Cells per mm2 in the Contralateral and Ipsilateral Dentate Subgranular Zone
| Treatment (0 Gy)
|
Treatment (4 Gy)
|
||||
|---|---|---|---|---|---|
| Sham | Trauma | Irradiation | RCI | ||
| BrdU | |||||
| Vehicle | Contralateral | 112.3 ± 12.6 | *41.7 ± 12.2 | *68.9 ± 11.7 | *61.4 ± 3.9 |
| DFMO | Contralateral | 89.1 ± 8.4 | 77.0 ± 6.3 | 62.1 ± 7.8 | 64.5 ± 5.7 |
| Vehicle | Ipsilateral | 101.5 ± 10.8 | 58.8 ± 15.6 | 51.8 ± 6.7 | 78.9 ± 11.8 |
| DFMO | Ipsilateral | 83.4 ± 12.5 | 110.2 ± 8.0 | 56.8 ± 8.1 | 106.5 ± 20.7 |
| NeuN/BrdU | |||||
| Vehicle | Contralateral | 86.2 ± 9.4 | *33.6 ± 11.1 | *53.8 ± 10.2 | *47.4 ± 3.23 |
| DFMO | Contralateral | 63.9 ± 5.8 | 56.5 ± 4.1 | 48.8 ± 6.2 | 50.6 ± 5.1 |
| Vehicle | Ipsilateral | 80.5 ± 9.0 | 49.3 ± 14.2 | 40.0 ± 7.0 | 67.8 ± 10.9 |
| DFMO | Ipsilateral | 65.1 ± 10.7 | 79.9 ± 8.8 | 44.3 ± 8.1 | 94.0 ± 13.5 |
Indicates significant values compared to sham-irradiated mice.
Neurogenesis
As for newly born neurons (BrdU+/NeuN+), the changes observed with respect to hemispheric differences and changes induced by injury and modulated by DFMO were generally the same as those observed for BrdU alone (Table 1).
Activated Total and Newly Born Microglia
For total numbers of activated microglia (CD68+) there was no interaction between DFMO and injury (F(3, 35) = 0.58, P = 0.62; two-way ANOVA) and there were no significant differences between treatment groups with or without DFMO (not shown). For newly born activated microglia (CD68+/BrdU+), there was a trend towards an interaction between DFMO and injury (F(3, 35) = 2.54, P = 0.07; two-way ANOVA), but there were no significant differences in cell number as a function of insult or DFMO treatment (Table 2).
TABLE 2.
Total Numbers of Newly Born Microglia (BrdU+/CD68+) Within the Contralateral and Ipsilateral Dentate Subgranular Zone
| Treatment (0 Gy)
|
Treatment (4 Gy)
|
||||
|---|---|---|---|---|---|
| Sham | Trauma | Irradiation | RCI | ||
| CD68/BrdU | |||||
| Vehicle | Contralateral | 36.7 ± 8.0 | 60.5 ± 5.3 | 35.3 ± 6.5 | 47.9 ± 5.9 |
| DFMO | Contralateral | 25.4 ± 4.0 | 24.3 ± 5.3 | 24.7 ± 3.0 | 30.7 ± 2.7 |
| Vehicle | Ipsilateral | 37.6 ± 6.7 | 68.4 ± 21.2 | 54.3 ± 3.5 | 63.3 ± 5.5 |
| DFMO | Ipsilateral | 24.4 ± 5.2 | 52.9 ± 13.3 | 27.8 ± 6.1 | 57.8 ± 8.07 |
DISCUSSION
Our data show that when treatment with DFMO is started 24 h after injury, the effects of trauma and RCI on hippocampus-dependent learning and memory are ameliorated. In addition, DFMO treatment upregulates the expression of antioxidant enzymes in the hippocampus that may protect neurons from excitotoxicity. This is a very important finding, given the brain’s inherent susceptibility to oxidative injury due to relatively low levels of endogenous antioxidants (19, 20). It has been suggested that this sensitivity plays a role in a number of neurodegenerative conditions, aging and in ischemic, traumatic and excitotoxic damage (21–24).
In the current study, all animals that received DFMO, independent of the type of injury, showed significant increases in Trx1 and Prdx3 compared to vehicle treated controls (Fig. 4A, B). The thioredoxin system protects cells against H2O2-induced cell death and its inhibition promotes oxidative stress. Trx1 has also been shown to have neuroprotective effects on both brain ischemia and excitotoxic hippocampal injury in transgenic mice overexpressing human Trx1 (25). Prdx3 is located exclusively in the matrix of mitochondria (26, 27). Studies have shown that overexpression of Prdx3 in cell lines lead to a reduction in cellular reactive oxygen species (ROS) and that a knockdown elevates cellular ROS suggesting that Prdx3 plays an important role in quenching H2O2 in mitochondria (28, 29). Prdx3 not only scavenges H2O2 in cooperation with thiol, but also peroxynitrate (ONOO−) by itself (30). The redox regulating activity of Trx1 and Prdx3 may contribute to the protective/mitigative effects of DFMO after traumatic brain injury or combined radiation-traumatic brain injury by diminishing oxidative stress.
Cognitive impairments were quantified using the Morris water maze, a well described method for determining hippocampus-dependent learning and retention of spatial memory (15 and references therein). In the current study, the acquisition of spatial learning was assessed during the hidden platform sessions. Learning was impaired over multiple sessions in vehicle treated mice that received RCI. DFMO ameliorated this learning acquisition deficit. This effect was most apparent on day 4 and 5 when vehicle treated mice that received RCI swam significantly longer distances to locate the hidden platform location (Fig. 2D). After the hidden platform session, spatial memory retention was assessed during the probe trials. Vehicle treated animals that received trauma or RCI did not show spatial memory retention. In contrast, all animals that received DFMO regardless of injury type showed spatial memory retention and spent most of their time searching the target quadrant. Overall these data suggest that a delayed regimen of DFMO treatment can ameliorate the hippocampal-dependent cognitive impairments induced by a single or combined radiation-traumatic brain injury situation.
The hippocampus has been shown to be critically involved in learning and memory, and the results of this current study show that these functions are affected by RCI and are in agreement with our previous studies (9). Hippocampal-dependent learning and memory are strongly influenced by the activity of neural stem cells and their proliferative progeny (31). Increased neurogenesis may result in improved performance in hippocampal-dependent memory tasks (32), while disruption of hippocampal neurogenesis can result in decreased performance in hippocampal-dependent tasks (33). Given our own data, along with those from others, we felt it was possible that changes in neurogenesis might play an important role in the behavioral consequence of RCI. Our current data show that in vehicle treated mice, there was a significant decrease in BrdU+/NeuN+ cells in the contralateral hemisphere in irradiation only, trauma only and RCI groups compared to mice that received surgery only. Previous studies from our laboratory and others have shown that radiation decreases cell proliferation and neurogenesis, suggesting that these decreases seen in the RCI and radiation only groups in the current study are due to the radiation treatment. However, the RCI mice were impaired while the mice receiving radiation only did not display cognitive deficiency. Given these findings, the data suggest that the generation of newly born neurons per se may not be a critical factor in the cognitive performance changes observed here, which supports previous reports on RCI (9, 34).
A number of studies suggest that inflammation may contribute to alterations in neurogenesis and cognitive function after CNS irradiation or trauma (35–38). Microglia are resident cells of the brain involved in the regulatory process critical for maintenance of the neural environment, injury and repair. In the uninjured brain “resting” microglia are ramified, continuously surveying the surrounding neural tissues (39). Under pathological conditions microglia become activated and perform a dual role, scavenging the damaged and dying neurons and initiating a local neuro-inflammatory reaction (40). In the current study, we measured inflammatory changes by quantifying numbers of total activated microglia (CD68 only) and the numbers of newly born activated microglia (BrdU+/CD68+). In mice that were treated with vehicle or DFMO there were no group differences seen in total numbers of activated microglia (CD68 only) (not shown). In addition, when DFMO treatment was delayed 24 h post injury, the drug did not affect the number of newly born neurons on in the ipsilateral hemisphere.
Polyamines putrescine, spermidine and spermine are ubiquitous low-molecular-weight aliphatic cations involved in cell proliferation, differentiation and death (41). Poly-amines play a fundamental role in the developing brain; they are crucial for the regulation of cell division and are present in high concentration. Once neuronal cells have matured and proliferation has ceased, polyamine levels drop. We recognize that there is considerable data available showing that DFMO depletes polyamine levels (42–44). However, it was not the objective of this study to do an in-depth investigation into whether the observed differences in cognition were directly attributed to alerted levels of polyamines. Further studies are warranted to delineate if and how polyamines impact hippocampal antioxidant enzymes and how those changes may influence effects of traumatic brain injury or combined radiation-traumatic brain injury on cognition.
Traumatic brain injury is a likely consequence of a nuclear blast, and is a frequent cause of death and disability when individuals are exposed to explosive forces. Not only are individuals near the site of detonation at risk, but also first-responding personnel (medical/rescue) who tend to the victims, and although the latter may receive lower radiation doses, they are also at risk for other types of injury (e.g., trauma) while performing their duties. This study supports the potential usefulness of DFMO in managing traumatic brain injury alone, and as a countermeasure against RCI. To the best of our knowledge, this is the first report demonstrating a countermeasure administered 24 h post injury against RCI in the brain. Given a broad consideration that DFMO significantly reduced adverse effects for RCI from a biochemical and behavioral perspective, this suggests that this drug may be an effective countermeasure.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant R33 AI080531. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank Julian Habdank-Kolaczkowski for his assistance with the immunohistochemical experiments.
Footnotes
Dr. Victor A. Levin, personal communication.
References
- 1.Pellmar TC, Rockwell S Radiological/Nuclear Threat Countermeasures Working Group. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–23. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 2.DiCarlo AL, Hatchett RJ, Kaminski JM, Ledney GD, Pellmar TC, Okunieff P, et al. Medical countermeasures for radiation combined injury: radiation with burn, blast, trauma and/or sepsis. Report of an NIAID Workshop, March 26–27, 2007. Radiat Res. 2008;169:712–21. doi: 10.1667/RR1295.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellmar TC, Ledney GD. Combined injury: radiation in combination with trauma, infectious disease, or chemical exposures. Proceedings of Meeting 099, “Radiation Bioeffects and Countermeasures”; Bethesda: NATO Human Factors and Medicine (HFM) Panel Research Task Group (RTG); 2005. pp. 1–9. ( www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA438764) [Google Scholar]
- 4.Johnston AM. Pulmonary effects of combined blast injury and radiation poisoning. J R Army Med Corps. 2004;150 (Suppl 1):22–6. [PubMed] [Google Scholar]
- 5.Baskaya MK, Rao AM, Puckett L, Prasad MR, Dempsey RJ. Effect of difluoromethylornithine treatment on regional ornithine decarboxylase activity and edema formation after experimental brain injury. J Neurotrauma. 1996;13:85–92. doi: 10.1089/neu.1996.13.85. [DOI] [PubMed] [Google Scholar]
- 6.Fike JR, Gobbel GT, Marton LJ, Seilhan TM. Radiation brain injury is reduced by the polyamine inhibitor a-difluoromethylornithine (DFMO) Radiat Res. 1994;138:99–106. [PubMed] [Google Scholar]
- 7.Fike JR, Gobbel GT, Chou D, Wijnhoven BPL, Bellinzona M, Nakagawa M, et al. Cellular proliferation and infiltration following interstitial irradiation of normal dog brain is altered by an inhibitor of polyamine synthesis. Int J Radiat Oncol Biol Phys. 1995;32:1035–45. doi: 10.1016/0360-3016(95)00030-3. [DOI] [PubMed] [Google Scholar]
- 8.Gobbel GT, Marton LJ, Lamborn K, Seilhan TM, Fike JR. Modification of radiation-induced brain injury by a-difluoromethylornithine (DFMO) Radiat Res. 1991;128:306–15. [PubMed] [Google Scholar]
- 9.Rosi S, Ferguson R, Fishman K, Allen A, Raber J, Fike JR. The polyamine inhibitor alpha-difluoromethylornithine modulates hippocampus-dependent function after single and combined injuries. PloS One. 2012;7:e31094. doi: 10.1371/journal.pone.0031094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, et al. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202:189–99. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Rosi S, Belarbi K, Ferguson RA, Fishman K, Obenaus A, Raber J, et al. Trauma-induced alterations in cognition and Arc expression are reduced by previous exposure to 56Fe irradiation. Hippocampus. 2012;22:544–54. doi: 10.1002/hipo.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brody DL, Mac Donald C, Kessens CC, Yuede C, Parsadanian M, Spinner M, et al. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neuro-trauma. 2007;24:657–73. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23:1241–53. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- 14.Yu S, Kaneko Y, Bae E, Stahl CE, Wang Y, van Loveren H, et al. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009;1287:157–63. doi: 10.1016/j.brainres.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 15.Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–31. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim A, Joseph S, Khan A, Epstein CJ, Sobel R, Huang TT. Enhanced expression of mitochondrial superoxide dismutase leads to prolonged in vivo cell cycle progression and up-regulation of mitochondrial thioredoxin. Free Radic Biol Med. 2010;48:1501–12. doi: 10.1016/j.freeradbiomed.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rola R, Zou Y, Huang TT, Fishman K, Baure J, Rosi S, et al. Lack of extracellular superoxide dismutase (EC-SOD) in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med. 2007;42:1131–45. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peuchen S, Bolanos JP, Heales SJ, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–81. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 21.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 22.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–9. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juurlink BH, Paterson PG. Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J Spinal Cord Med. 1998;21:309–34. doi: 10.1080/10790268.1998.11719540. [DOI] [PubMed] [Google Scholar]
- 24.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–31. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, et al. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci U S A. 1999;96:4131–6. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Z, Bhella D, Lindsay JG. Reconstitution of the mitochondrial PrxIII antioxidant defence pathway: general properties and factors affecting PrxIII activity and oligomeric state. J Mol Biol. 2007;372:1022–33. doi: 10.1016/j.jmb.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Go YM, Jones DP. Mitochondrial thioredoxin-2/ peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys. 2007;465:119–26. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Hattori F, Murayama N, Noshita T, Oikawa S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J Neurochem. 2003;86:860–8. doi: 10.1046/j.1471-4159.2003.01918.x. [DOI] [PubMed] [Google Scholar]
- 29.Matsushima S, Ide T, Yamato M, Matsusaka H, Hattori F, Ikeuchi M, et al. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–86. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 30.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–5. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 31.Jenrow KA, Brown SL, Liu J, Kolozsvary A, Lapanowski K, Kim JH. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol. 2010;5:6. doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Rev Neurosci. 2000;1:191–8. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 33.Achanta P, Fuss M, Martinez JL., Jr Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behav Neurosci. 2009;123:1036–45. doi: 10.1037/a0016870. [DOI] [PubMed] [Google Scholar]
- 34.Allen AR, Eilertson K, Chakraborti A, Sharma S, Baure J, Habdank-Kolaczkowski J, et al. Radiation exposure prior to traumatic brain injury induces responses that differ as a function of animal age. Int J Radiat Biol. 2014;90:214–23. doi: 10.3109/09553002.2014.859761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Avila JC, Lam TI, Bingham D, Shi J, Won SJ, Kauppinen TM, et al. Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J Neuroinflammation. 2012;9:31. doi: 10.1186/1742-2094-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–34. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 37.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Experimental Neurol. 2004;188:316–30. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–7. [PubMed] [Google Scholar]
- 39.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 40.Kraft AD, Harry GJ. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Res Public Health. 2011;8:2980–3018. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan DM. Polyamines. An overview. Molecular biotechnology. 1999;11:229–50. doi: 10.1007/BF02788682. [DOI] [PubMed] [Google Scholar]
- 42.Janne J, Alhonen L, Leinonen P. Polyamines: from molecular biology to clinical applications. Ann Med. 1991;23:241–59. doi: 10.3109/07853899109148056. [DOI] [PubMed] [Google Scholar]
- 43.Malaterre J, Strambi C, Aouane A, Strambi A, Rougon G, Cayre M. A novel role for polyamines in adult neurogenesis in rodent brain. Eur J Neurosci. 2004;20:317–30. doi: 10.1111/j.1460-9568.2004.03498.x. [DOI] [PubMed] [Google Scholar]
- 44.Sparapani M, Virgili M, Caprini M, Facchinetti F, Ciani E, Contestabile A. Effects of gestational or neonatal treatment with alpha-difluoromethylornithine on ornithine decarboxylase and polyamines in developing rat brain and on adult rat neurochemistry. Exp Brain Res. 1996;108:433–40. doi: 10.1007/BF00227266. [DOI] [PubMed] [Google Scholar]