Abstract
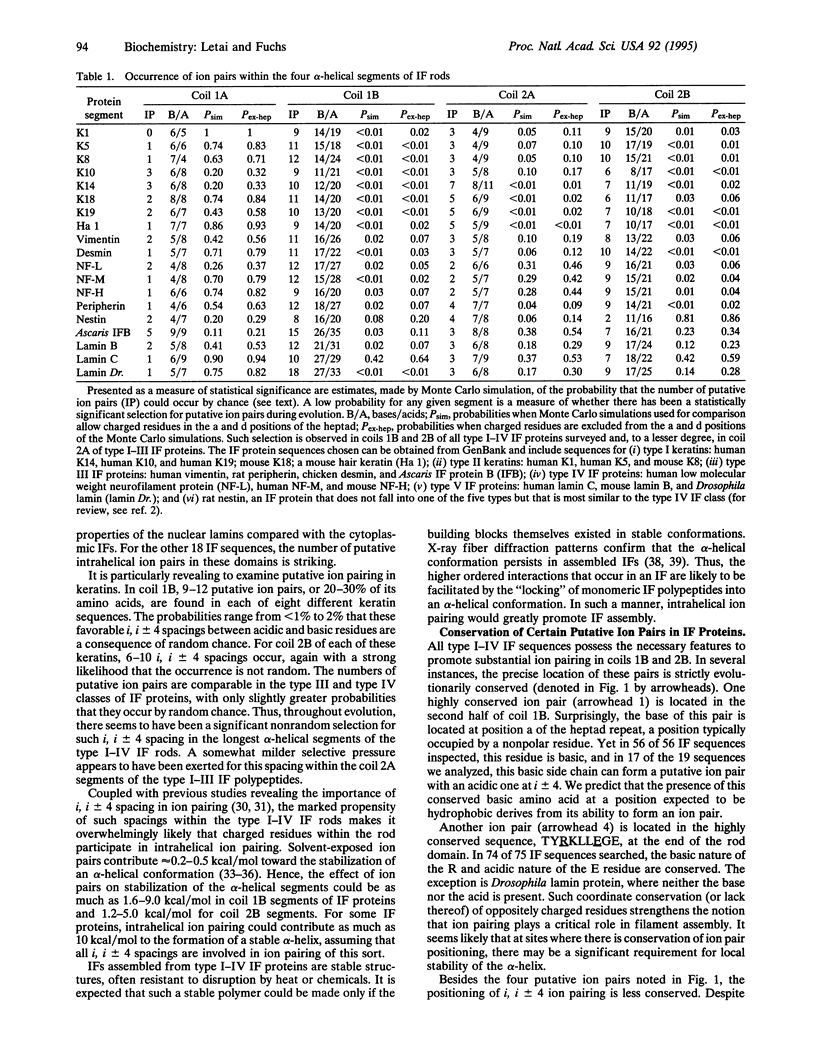
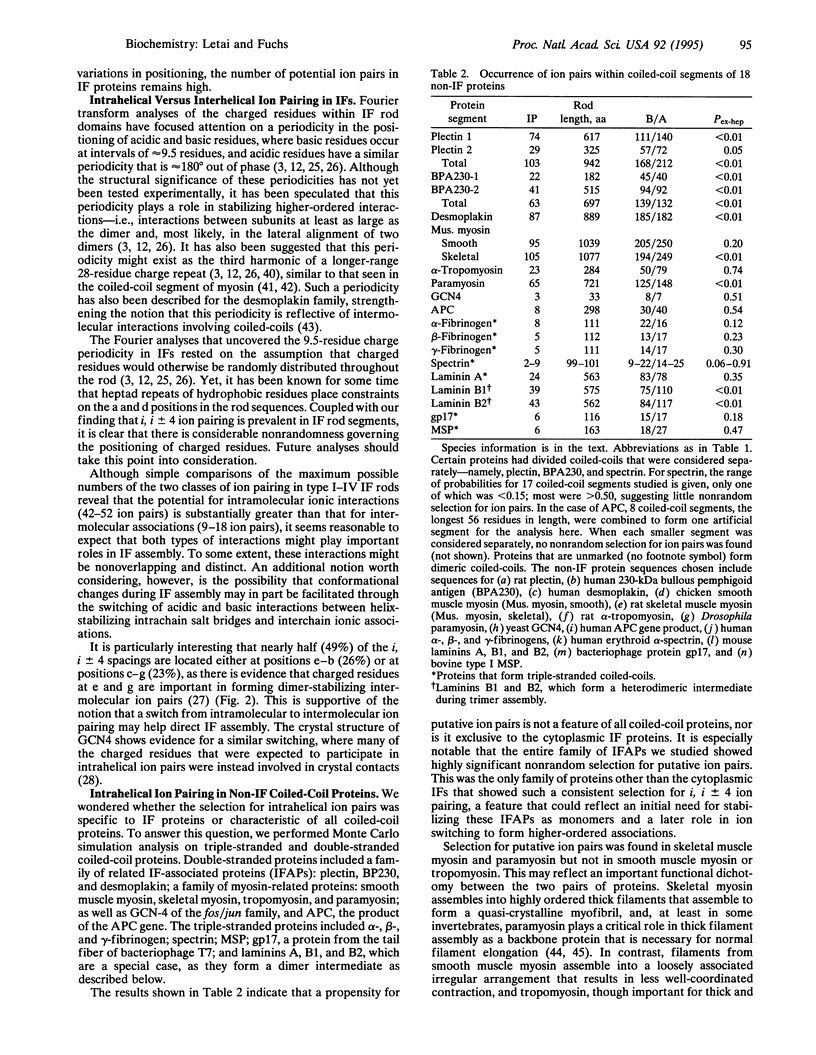
Nuclear and cytoskeletal networks of 10-nm intermediate filaments (IFs) are probably ubiquitous in multicellular eukaryotes. They likely play a role in maintaining the mechanical integrity of a cell. With the exception of the nuclear lamins, IF proteins can form IFs in vitro in the absence of cofactors or associated proteins. Below we present data suggesting that the large alpha-helical "rod" domains of IF proteins are stabilized by large numbers (up to 50) of intra-helical ion pairs formed by residues of opposite charge situated four residues apart. These many ion pairs, sometimes involving up to 30% of the residues within a coiled-coil IF segment, can potentially contribute as much as 10-25 kcal/mol (1 kcal = 4.18 kJ) to the stability of a single alpha-helical rod. Such stabilization is likely to play a major role in the chemical and physical stability of IF networks in vitro and in vivo. An investigation of other coiled-coil proteins shows that selection for intrahelical ion pairing is not simply a property intrinsic to coiled-coil proteins. Rather, there is a correlation between the degree to which there is selection for intrahelical ion pairs and the extent to which a coiled-coil protein participates in highly ordered multimolecular interactions--e.g., as in IFs and myosin thick filaments. The propensity of putative ion pairs in some IF proteins--e.g., epidermal keratins--suggests that an underlying structural stability at the level of the monomer may play an important role in the extraordinary stability of dimers and higher ordered structures in cytoplasmic IFs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986 Oct 9;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Albers K., Fuchs E. The expression of mutant epidermal keratin cDNAs transfected in simple epithelial and squamous cell carcinoma lines. J Cell Biol. 1987 Aug;105(2):791–806. doi: 10.1083/jcb.105.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J. F., Parry D. A. Structural features in the heptad substructure and longer range repeats of two-stranded alpha-fibrous proteins. Int J Biol Macromol. 1990 Oct;12(5):328–334. doi: 10.1016/0141-8130(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Chan Y. M., Albers K., Fuchs E. Deletions in epidermal keratins leading to alterations in filament organization in vivo and in intermediate filament assembly in vitro. J Cell Biol. 1990 Dec;111(6 Pt 2):3049–3064. doi: 10.1083/jcb.111.6.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Fuchs E. Elucidating the early stages of keratin filament assembly. J Cell Biol. 1990 Jul;111(1):153–169. doi: 10.1083/jcb.111.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. F., Berliner G. C., Casey D. L., Ortiz I. Purified thick filaments from the nematode Caenorhabditis elegans: evidence for multiple proteins associated with core structures. J Cell Biol. 1988 Jun;106(6):1985–1995. doi: 10.1083/jcb.106.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Weber K. Antiparallel orientation of the two double-stranded coiled-coils in the tetrameric protofilament unit of intermediate filaments. J Mol Biol. 1985 Mar 5;182(1):173–177. doi: 10.1016/0022-2836(85)90035-x. [DOI] [PubMed] [Google Scholar]
- Geisler N., Schünemann J., Weber K. Chemical cross-linking indicates a staggered and antiparallel protofilament of desmin intermediate filaments and characterizes one higher-level complex between protofilaments. Eur J Biochem. 1992 Jun 15;206(3):841–852. doi: 10.1111/j.1432-1033.1992.tb16992.x. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. doi: 10.1002/j.1460-2075.1982.tb01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. J., Virata M. L., Elgart G. W., Stanley J. R., Parry D. A. Comparative structural analysis of desmoplakin, bullous pemphigoid antigen and plectin: members of a new gene family involved in organization of intermediate filaments. Int J Biol Macromol. 1992 Jun;14(3):145–153. doi: 10.1016/s0141-8130(05)80004-2. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M., Weber K. The coiled coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratins: use of site-specific mutagenesis and recombinant protein expression. J Cell Biol. 1990 Apr;110(4):1199–1210. doi: 10.1083/jcb.110.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins S., Wong P. C., Müller S., Goldie K., Cleveland D. W., Aebi U. The rod domain of NF-L determines neurofilament architecture, whereas the end domains specify filament assembly and network formation. J Cell Biol. 1993 Dec;123(6 Pt 1):1517–1533. doi: 10.1083/jcb.123.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghues-Despointes B. M., Scholtz J. M., Baldwin R. L. Helical peptides with three pairs of Asp-Arg and Glu-Arg residues in different orientations and spacings. Protein Sci. 1993 Jan;2(1):80–85. doi: 10.1002/pro.5560020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A., Coulombe P. A., Fuchs E. Do the ends justify the mean? Proline mutations at the ends of the keratin coiled-coil rod segment are more disruptive than internal mutations. J Cell Biol. 1992 Mar;116(5):1181–1195. doi: 10.1083/jcb.116.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu P. C., Gans P. J., Kallenbach N. R. Energetic contribution of solvent-exposed ion pairs to alpha-helix structure. J Mol Biol. 1992 Jan 5;223(1):343–350. doi: 10.1016/0022-2836(92)90735-3. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. M., Jr, Epstein H. F. Paramyosin is necessary for determination of nematode thick filament length in vivo. Cell. 1980 Dec;22(3):747–755. doi: 10.1016/0092-8674(80)90551-6. [DOI] [PubMed] [Google Scholar]
- Marqusee S., Baldwin R. L. Helix stabilization by Glu-...Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Scheraga H. A. The effect of neighboring charges on the helix forming ability of charged amino acids in proteins. Macromolecules. 1975 Jul-Aug;8(4):491–493. doi: 10.1021/ma60046a022. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Coiled coil formation and sequence regularities in the helical regions of alpha-keratin. J Mol Biol. 1978 Sep 5;124(1):297–304. doi: 10.1016/0022-2836(78)90163-8. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982 Sep 16;299(5880):226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Klemm J. D., Kim P. S., Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991 Oct 25;254(5031):539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Lumb K. J., Kim P. S. Peptide 'Velcro': design of a heterodimeric coiled coil. Curr Biol. 1993 Oct 1;3(10):658–667. doi: 10.1016/0960-9822(93)90063-t. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B. Compound helical configurations of polypeptide chains: structure of proteins of the alpha-keratin type. Nature. 1953 Jan 10;171(4341):59–61. doi: 10.1038/171059a0. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Conway J. F., Steinert P. M. Structural studies on lamin. Similarities and differences between lamin and intermediate-filament proteins. Biochem J. 1986 Aug 15;238(1):305–308. doi: 10.1042/bj2380305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. A., Crewther W. G., Fraser R. D., MacRae T. P. Structure of alpha-keratin: structural implication of the amino acid sequences of the type I and type II chain segments. J Mol Biol. 1977 Jun 25;113(2):449–454. doi: 10.1016/0022-2836(77)90153-x. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Steven A. C., Steinert P. M. The coiled-coil molecules of intermediate filaments consist of two parallel chains in exact axial register. Biochem Biophys Res Commun. 1985 Mar 29;127(3):1012–1018. doi: 10.1016/s0006-291x(85)80045-0. [DOI] [PubMed] [Google Scholar]
- Parry D. A. Structure of rabbit skeletal myosin. Analysis of the amino acid sequences of two fragments from the rod region. J Mol Biol. 1981 Dec 5;153(2):459–464. doi: 10.1016/0022-2836(81)90290-4. [DOI] [PubMed] [Google Scholar]
- Potschka M., Nave R., Weber K., Geisler N. The two coiled coils in the isolated rod domain of the intermediate filament protein desmin are staggered. A hydrodynamic analysis of tetramers and dimers. Eur J Biochem. 1990 Jul 5;190(3):503–508. doi: 10.1111/j.1432-1033.1990.tb15602.x. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Cohlberg J. A., Schiller D. L., Hatzfeld M., Franke W. W. Heterotypic tetramer (A2D2) complexes of non-epidermal keratins isolated from cytoskeletons of rat hepatocytes and hepatoma cells. J Mol Biol. 1984 Sep 15;178(2):365–388. doi: 10.1016/0022-2836(84)90149-9. [DOI] [PubMed] [Google Scholar]
- Sali D., Bycroft M., Fersht A. R. Surface electrostatic interactions contribute little of stability of barnase. J Mol Biol. 1991 Aug 5;220(3):779–788. doi: 10.1016/0022-2836(91)90117-o. [DOI] [PubMed] [Google Scholar]
- Serrano L., Horovitz A., Avron B., Bycroft M., Fersht A. R. Estimating the contribution of engineered surface electrostatic interactions to protein stability by using double-mutant cycles. Biochemistry. 1990 Oct 9;29(40):9343–9352. doi: 10.1021/bi00492a006. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Marekov L. N., Fraser R. D., Parry D. A. Keratin intermediate filament structure. Crosslinking studies yield quantitative information on molecular dimensions and mechanism of assembly. J Mol Biol. 1993 Mar 20;230(2):436–452. doi: 10.1006/jmbi.1993.1161. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. Organization of coiled-coil molecules in native mouse keratin 1/keratin 10 intermediate filaments: evidence for alternating rows of antiparallel in-register and antiparallel staggered molecules. J Struct Biol. 1991 Oct;107(2):157–174. doi: 10.1016/1047-8477(91)90019-s. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A. The conserved H1 domain of the type II keratin 1 chain plays an essential role in the alignment of nearest neighbor molecules in mouse and human keratin 1/keratin 10 intermediate filaments at the two- to four-molecule level of structure. J Biol Chem. 1993 Feb 5;268(4):2878–2887. [PubMed] [Google Scholar]
- Stellwagen E., Park S. H., Shalongo W., Jain A. The contribution of residue ion pairs to the helical stability of a model peptide. Biopolymers. 1992 Sep;32(9):1193–1200. doi: 10.1002/bip.360320909. [DOI] [PubMed] [Google Scholar]
- Stewart M. Intermediate filament structure and assembly. Curr Opin Cell Biol. 1993 Feb;5(1):3–11. doi: 10.1016/s0955-0674(05)80002-x. [DOI] [PubMed] [Google Scholar]
- Stewart M., Quinlan R. A., Moir R. D. Molecular interactions in paracrystals of a fragment corresponding to the alpha-helical coiled-coil rod portion of glial fibrillary acidic protein: evidence for an antiparallel packing of molecules and polymorphism related to intermediate filament structure. J Cell Biol. 1989 Jul;109(1):225–234. doi: 10.1083/jcb.109.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. C., Cleveland D. W. Characterization of dominant and recessive assembly-defective mutations in mouse neurofilament NF-M. J Cell Biol. 1990 Nov;111(5 Pt 1):1987–2003. doi: 10.1083/jcb.111.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]