Abstract
Objective
To determine whether the utilization of healthcare resources is reduced after chronic migraine patients are treated for 6 months with onabotulinumtoxinA.
Background
OnabotulinumtoxinA is indicated for headache prophylaxis in patients with chronic migraine, but its effect on healthcare resource use is unknown.
Methods
We analyzed data from an open-label study of 230 chronic migraine patients refractory to ≥2 oral prophylactics who presented to a headache specialty clinic and who were treated with two cycles of onabotulinumtoxinA. Frequency and cost of migraine-related healthcare resource use, including visits to emergency departments, urgent care, or hospitalization, were compared for the 6 months before and after initial treatment. Costs were based on publicly available sources.
Results
Compared with the 6 months predating initial treatment, patients had 55% fewer emergency department visits (174 vs 385), 59% fewer urgent care visits (61 vs 150), and 57% fewer hospitalizations (19 vs 45) during the 6-month treatment period (P < .01 for all). Analysis of treatment-related costs yielded an average reduction of $1219.33/patient, off-setting 49.7% of the total estimated cost for 6 months of treatment with onabotulinumtoxinA.
Conclusions
Although we are unable to distinguish onabotulinumtoxinA's treatment effect from other potential confounding variables, our analysis showed that severely afflicted, treatment-refractory patients with chronic migraine experienced a significant cost-offset through reduced migraine-related emergency department visits, urgent care visits, and hospitalizations in the 6 months following treatment initiation of onabotulinumtoxinA. Future analyses will assess the longer-term effect of onabotulinumtoxinA treatment and the potential contribution of regression to the mean.
Keywords: chronic migraine, onabotulinumtoxinA, healthcare resource use, prophylaxis, cost-offset
Chronic migraine (CM), characterized by ≥15 headache days/month for 3 months, is a disabling condition associated with a high economic and societal burden. It is estimated to impact approximately 1.4-2.2% of the adult population globally and approximately 1% of those in the United States, imparting significant impact on quality of life and disability.1,2 Due to increased acute medication use, physician visits, hospitalizations, and emergency department (ED) visits, individuals with CM have been shown to incur significantly higher healthcare costs than individuals with episodic migraine.3,4
Two parallel, large-scale, placebo-controlled trials were conducted to evaluate onabotulinumtoxinA therapy for CM: the Phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) trials. The PREEMPT trials represent the largest studies in CM to date (n = 1384), and their results indicate that onabotulinumtoxinA treatment is both safe and effective.5–9 While the PREEMPT trials clearly established the efficacy of onabotulinumtoxinA in improving both clinical and quality-of-life measures, less clear are the impact of onabotulinumtoxinA on real-world outcomes such as migraine-related healthcare resource use (HRU) and, more specifically, net cost (vs cost-savings) associated with the use of onabotulinumtoxinA to treat CM.
We sought to evaluate migraine-related healthcare resource utilization and direct medical costs prior to and following initiation of treatment with open-label onabotulinumtoxinA for treatment-refractory CM patients.
MATERIALS AND METHODS
Between January 2007 and April 2011, we evaluated all patients presenting to a university-based subspecialty headache clinic for potential treatment with onabotulinumtoxinA. We obtained a thorough, headache-directed history from each patient, and among the historical variables we recorded were age; gender; race/ethnicity; age at time of migraine onset; duration of daily or near daily headaches, when applicable; baseline “headache frequency/severity profile” (described elsewhere6); number and specific names of prophylactic therapies previously tried (adequately) and failed; presence vs absence of active overuse of abortive medication (according to International Classification of Headache Disorders, second edition [ICHD-2] criteria10); and frequencies of hospitalization, ED, or urgent care center utilization for acute migraine treatment within the 6 months prior to our initial evaluation. Baseline migraine-related disability was measured by the Migraine Disability Assessment (MIDAS) scale, a seven-item measure of migraine-related disability in the previous 3 months that includes five scored items assessing the number of days that migraine prevented or limited activities, including work, education, household work, and family, social, and leisure activities.11 An overall score is computed as a sum of the number of days for these five items. MIDAS scores are classified into four severity grades: scores of 0-5 represent Grade I (minimal or infrequent disability); 6-10 are Grade II (mild or infrequent disability); 11-20 are Grade III (moderate disability); 21-40 are Grade IVa (severe disability); and 41-270 are Grade IVb (very severe disability).12
Patients were considered to be eligible for treatment with onabotulinumtoxinA if they met ICHD-2 criteria for CM, reported failure to respond to adequate trials of at least two prophylactic agents (of which one was required to be divalproex sodium, topiramate, or zonisamide), had no history of prior treatment with a neurotoxin for any indication, and were likely to be available for long-term follow-up. Previous trials of prophylactic agents that involved inadequate maximum dose and/or inadequate duration of treatment were not counted as failed attempts. Patients overusing abortive medication were not excluded, and at the discretion of the treating physician, patients were allowed to remain on oral prophylactic therapy if that therapy had been initiated 3 months or more prior to the baseline pretreatment data collection month.
During a 30-day baseline pretreatment period, we asked all patients who consented to onabotulinumtoxinA therapy to keep a paper diary. In that diary, they recorded days of headache experienced, maximum intensity of headache on those days, any symptomatic medication administered, and any migraine-related use of an urgent care center or ED or any migraine-related hospitalization.
At the conclusion of this baseline period, all patients whose diaries were consistent with a diagnosis of CM were treated with open-label onabotulinumtoxinA. One investigator (JFR) who previously had served as a principal investigator in PREEMPT was the treating physician for the entire group, and our injection technique and dosing paradigm were identical to those used in that larger, placebo-controlled, double-blind study.13 All patients received a minimum dose of 155 units of onabotulinumtoxinA administered as 31 fixed-site, fixed-dose injections across seven specific head and neck muscle areas (to the procerus and bilaterally to the corrugator, frontalis, temporalis, occipitalis, cervical paraspinal, and trapezius muscles), with the option, at the physician's discretion, of injecting up to an additional 40 units into the temporalis, occipitalis, and trapezius muscles if those muscles were disproportionally involved in the patient's headache attacks or were particularly tender to palpation on exam. Following initial treatment, we asked all patients to maintain a paper diary identical to that utilized during the baseline period.
OnabotulinumtoxinA treatment was repeated at 3 months, and at 6 months, all patients returned for follow-up and potentially continued treatment with onabotulinumtoxinA.
Healthcare resource utilization in the 6 months prior to initiation of treatment with onabotulinumtoxinA was compared with the subsequent 6 months by calculating a mean per-patient reduction as well as the incident event rate ratio for each type of HRU. Cost of HRU was assessed by applying national average cost estimates, adjusting for inflation to 2013 using the medical services component of the Bureau of Labor Statistics Consumer Price Index, where applicable (Table 1). The cost of an ED visit for migraine was estimated at $473.82 using the Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost & Utilization Project (HCUP) State Emergency Department Databases. This represents a weighted average of the mean ED visit costs for patients with a primary diagnosis code of 346.0-346.9. The HCUP online query tool (HCUPnet, US Dept for Health and Human Services, AHRQ, Rockville, MD, USA) was used to search the National Inpatient Statistics databases for inpatient hospital costs. The cost of an inpatient hospital stay for CM was estimated at $6155.42, a weighted average of the mean hospital costs for inpatient stays with a primary diagnosis code of 346.7-346.73. To estimate the cost of an urgent care visit, we began with the 2013 Medicare Physician Fee Schedule (MPFS) reimbursement, without geographic adjustment, for Current Procedural Terminology (CPT) 99214 (office or other outpatient visit for an established patient requiring a detailed history and examination of at least 25 minutes). This figure was then divided by 0.82 because according to the latest Medicare Payment Advisory Commission Report to the Congress, Medicare reimbursements for physician and other health services were 82% of commercial reimbursements in 2011. The resulting cost of an urgent care visit was $130.28.14
Table 1.
Sources of Unit Prices
| Healthcare Resource | Unit Price | Source |
|---|---|---|
| Emergency department visit | $473.82/Visit | State Emergency Department. Databases (2013). Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost & Utilization Project. Weighted average of mean hospital costs associated with International Classification of Diseases, Ninth Revision (ICD9) 346.x. |
| Inpatient hospitalization | $6155.42/Hospitalization | National Inpatient Statistics (2009). Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost & Utilization Project. Weighted average of mean hospital costs associated with ICD9 346.7-346.73. Inflated to 2013 US$ using the medical care consumer price index. |
| Urgent care visit | $130.28/Visit | 2013 Medicare Physician Fee Schedule (MPFS) reimbursement, without geographic adjustment, for Current Procedural Terminology (CPT) 99214 (office or other outpatient visit for an established patient requiring a detailed history and examination of at least 25 minutes). Divided by 0.82 to estimate commercial reimbursements from Medicare reimbursements (per Medicare Payment Advisory Commission Report). |
| OnabotulinumtoxinA drug | $5.25/Unit | Redbook wholesale acquisition cost. |
| OnabotulinumtoxinA administration | $175.51/Administration | 2013 MPFS reimbursement, without geographic adjustment, for CPT 64615 (chemodenervation of muscle[s]; muscle[s] innervated by facial, trigeminal, cervical spinal and accessory nerves, bilateral [eg, for chronic migraine]). Divided by 0.82 to estimate commercial reimbursements from Medicare reimbursements (per Medicare Payment Advisory Commission Report). |
To estimate the cost of onabotulinumtoxinA therapy, we considered both drug cost and cost of administration. The drug cost of onabotulinumtoxinA was estimated using the 2013 wholesale acquisition cost, $5.25/unit.15 To account for wastage, the cost of two full 100-unit vials was considered, resulting in a drug cost of $1050/treatment session. The cost of onabotulinumtoxinA administration was estimated using the same method used to estimate urgent care costs. The 2013 MPFS reimbursement, without geographic adjustment, associated with the CPT code 64615 (chemodenervation of muscle[s]; muscle[s] innervated by facial, trigeminal, cervical spinal and accessory nerves, bilateral [eg, for CM]) was divided by 0.82, resulting in a cost of administration of $175.51/treatment session. Therefore, the estimated cost for two cycles of onabotulinumtoxinA therapy (including administration fee and two 100-unit vials, to account for wastage) was $2451.02. All cost analyses were conducted using Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Statistical Analysis
Descriptive statistics are presented as means or medians either with ranges or with interquartile ranges (IQR), where available. We examined the mean change in each type of HRU before and after treatment by using a paired t-test. The assumptions underlying this parametric test were evaluated using histograms and descriptive statistics. When data were highly skewed, the nonparametric equivalent (Wilcoxon test) was used. The difference in the proportion of patients reporting an ED visit, urgent care visit, or hospitalization before treatment vs after treatment was assessed by McNemar's chi-square test. To maintain an overall type one error rate of 0.05, we interpreted a P value of less than or equal to .017 as statistically significant for each of the three primary outcomes.
We performed this study under the auspices of the University of Alabama at Birmingham's Institutional Review Board where the study was conducted and where JFR was an investigator during the period in which the study occurred. JFR had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Results
There were 237 consecutive patients with CM evaluated for study inclusion, of whom 230 (97%) were treated; reasons for exclusion were patient refusal (n = 5) and prior treatment with onabotulinumtoxinA (n = 2). Of the treated patients, 203 (88.3%) were female, and the mean age was 42.3 years (range: 16-79 years). Median MIDAS score at baseline was 70 (IQR: 37.5-113), and 82.6% (n = 190) had severe or very severe disability. There were 126 participants (54.8%) who had daily or near daily headaches at baseline, and 93 (73.8%) of these had experienced daily headaches for greater than 6 months (IQR 16-60). The median number of prophylactic therapies adequately tried and failed was 3 (IQR 2-5). There were 187 participants who (81.3%) had previously tried topiramate. A total of 84 (36.5%) were overusing abortive medication at baseline, and along with other patient characteristics, the type and distribution of medications overused are listed in Table 2. Of the treatment group, 112 (48.7%) were taking an oral prophylactic agent at the time of initial treatment with onabotulinumtoxinA and remained on that agent at least until the second set of injections.
Table 2.
Patient Characteristics and Medication Use Characteristics
| Characteristic | N = 230 |
|---|---|
| Age, mean (range) | 42 (16-79) |
| Gender | |
| Male, n (%) | 27 (11.7) |
| Female, n (%) | 203 (88.3) |
| Race | |
| White | 198 (86.1) |
| African American | 27 (11.7) |
| Other | 5 (2.1) |
| MIDAS, median (IQR)† | 70 (37.5-113) |
| Grade I (0-5, little disability), n (%) | 1 (0.4) |
| Grade II (6-10, mild disability), n (%) | 4 (1.7) |
| Grade III (11-20, moderate disability), n (%) | 18 (7.8) |
| Grade IV-A (21-40, severe disability), n (%) | 36 (15.7) |
| Grade IV-B (41-270, very severe disability), n (%) | 154 (67.0) |
| Daily or near daily headaches, n (%) | 126 (54.8) |
| Daily or near daily headaches for >6 months, n (%) | 98 (42.6) |
| Duration of daily or near daily headache (months), median (IQR) | 36 (16-60) |
| History of prophylaxis | |
| Number of agents previously tried, median (IQR) | 3 (2-5) |
| History of topiramate, n (%) | 187 (81.3) |
| Medication overuse, n (%) | 84 (36.5) |
| Medication overuse by class | |
| Triptans (sumatriptan, Treximet, Relpax, etc), n (%) | 23 (11.3) |
| OTC analgesics and OTC combination products (acetaminophen, ibuprofen, etc), n (%) | 32 (15.8) |
| Barbiturates and barbiturate-containing combination products (butalbital, Fioricet, etc), n (%) | 17 (8.4) |
| Opioids and opioid-containing combination products (oxycodone, codeine, etc), n (%) | 31 (15.3) |
†Counts do not add to 230 due to missing data.
IQR = interquartile range; MIDAS = MIgraine Disability ASsessment scale; OTC = over the counter.
Within the initial 6-month treatment period, a total of 226 patients (98.3%) received both sets of injections. Four patients (1.7%) refused the second set of injections, but all 230 patients returned for the 6-month follow-up visit.
Table 3 displays the changes in HRU after the addition of onabotulinumtoxinA to the treatment regimen. The proportion of patients with one or more ED visit was 54.3% in the pretreatment period vs 31.7% in the posttreatment (P < .001), proportion with one or more urgent care visit 20.0 vs 9.6% (P = .06), and proportion with one or more hospitalization 15.7 vs 7.4% (P = .01) (Fig. 1). During the 6 months preceding treatment, patients averaged 1.67 ED visits, 0.65 urgent care visits, and 0.20 hospitalizations (Fig. 2). Following initiation of onabotulinumtoxinA treatment, our patient sample demonstrated an absolute mean reduction of 0.92 ED visits, 0.39 urgent care visits, and 0.11 hospitalizations. Compared with rates before treatment, the incidence of ED visits was reduced by 55% (95% confidence interval [CI] 44-63%); the incidence of urgent care visits was reduced by 59% (95% CI 41-72%); and the incidence of hospitalizations for migraine was reduced by 57% (95% CI 26-75%). Application of conservative national estimates for related costs yielded a mean reduction of $1219.33/patient, with an added cost of $2451.02 for two cycles of onabotulinumtoxinA therapy. Thus, this reduction in HRU offset 49.7% of the estimated cost for 6 months of treatment with onabotulinumtoxinA. It should be noted however that we did not stratify by prior healthcare utilization; therefore, these reductions may not reflect savings for all CM patients, especially lower healthcare resource utilizers.
Table 3.
Summary of Total Resource Utilization Counts and Cost Over the Observational Period
| Healthcare Resource | Pretreatment† | Posttreatment | Change | P value |
|---|---|---|---|---|
| Emergency department visits | ||||
| Proportion of patients with one or more | 54.3% | 31.7% | −22.6% | <.001 |
| Total count | 385 | 174 | −211 | — |
| Mean (range) number of visits | 1.67 (0-18) | 0.76 (0-17) | −0.92 ± 2.04‡ | <.001 |
| Estimated cost/patient | $791.28 | $360.10 | −$431.18§ | — |
| Urgent care visits | ||||
| Proportion of patients with one or more | 20.2% | 9.6% | −10.6% | .061 |
| Total count | 150 | 61 | −89 | — |
| Mean (range) number of visits | 0.65 (0-12) | 0.27 (0-8) | −0.39 ± 1.46‡ | <.001 |
| Estimated cost/patient | $84.68 | $35.18 | −$49.50§ | — |
| Hospitalizations | ||||
| Proportion of patients with one or more | 15.7% | 7.4% | 8.3% | .013 |
| Total count | 45 | 19 | −26 | — |
| Mean (range) number of visits | 0.20 (0-5) | 0.08 (0-2) | −0.11 ± 0.55‡ | .002 |
| Estimated cost/patient | $1231.08 | $492.43 | −$738.65§ | — |
| OnabotulinumtoxinA | ||||
| Estimated cost/patient | $0 | $2451.02 | +$2451.02 | — |
| Net cost/patient | ||||
| Net cost | — | — | +$1219.33 | — |
| % Cost offset | — | — | 49.7% | — |
Pretreatment values were obtained through patient recall of the previous 6 months. Healthcare resource utilization in the 6 months prior to initiation of treatment with onabotulinumtoxinA was compared with the subsequent 6 months by calculating a mean per-patient reduction as well as the incident event rate ratio for each type of healthcare resource utilization.
Mean reduction in visits rounded to the nearest hundredth ± standard deviation.
Calculated by the mean reduction in visits multiplied by the unit cost of each healthcare resource.
— = not applicable.
Figure 1.
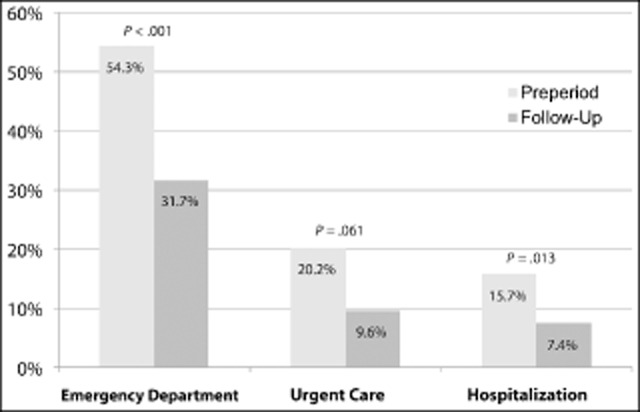
Reductions in the utilization of migraine-related healthcare resource use over 6 months. Pretreatment/preperiod values were obtained through patient recall of the previous 6 months.
Figure 2.
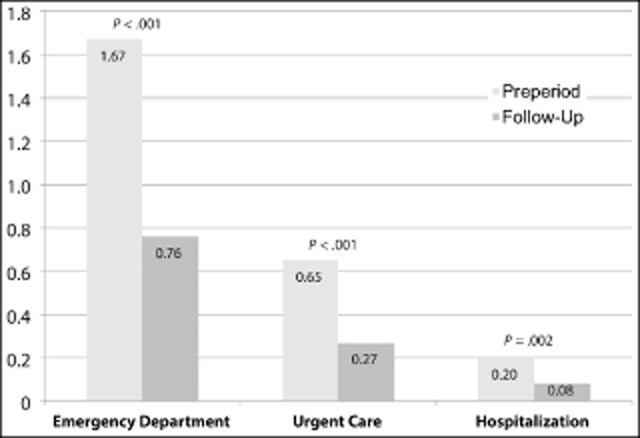
Mean migraine-related healthcare resource use over 6 months.Pretreatment/preperiod values were obtained through patient recall of the previous 6 months.
Discussion
PREEMPT established onabotulinumtoxinA as a safe and efficacious treatment for CM, and it is currently the only therapy approved by the US Food and Drug Administration (FDA) for headache prophylaxis among patients with CM.16 Less rigorous studies involving the use of gabapentin, tizanidine, divalproex sodium, methadone, topiramate, and other medications for the treatment of chronic daily headache, “transformed migraine,” or CM have been performed, but of these, only topiramate possesses an evidence base sufficient to support potential use in patients with CM.17
ED visits for acute headache treatment represent one of the major contributors to direct medical costs related to migraine.18 Our patients reported a significant decline in their frequency of ED utilization, and the associated savings offset a reasonable proportion of the cost of treatment; additional savings in direct costs occurred consequent to reductions in migraine-related utilization of urgent care centers and hospitalizations.
Our patients were similar in their demographic and clinical characteristics to subjects from population-based studies of CM such as the American Migraine Prevalence and Prevention Study and the International Burden of Migraine Study (IBMS).12,19 Before initiating treatment, patients in our study averaged 1.67 ED visits (385 visits for 230 patients), 0.65 urgent care visits (150 visits for 230 patients), and 0.20 hospitalizations (45 hospitalizations for 230 patients) over 6 months. This is somewhat higher than the level of HRU observed in the IBMS, where CM participants reported 0.41 ED visits and 0.09 hospital admissions over the previous 3 months, and these higher rates may reflect an inherent difference in the care-seeking behavior of clinic-based vs general population-based subject groups.
The higher level of HRU observed in our cohort may be attributable to it, comprising a more severely affected subgroup of CM patients who were motivated to seek care in a specialty setting. Our study included only refractory CM patients, with previous failure of at least two oral prophylactic agents. This is potentially a more severely affected group of patients relative to those enrolled in the PREEMPT clinical trials. Thus, our results may have been partly driven by higher healthcare utilizers and may not be representative of the cost-offsets experienced by lower healthcare utilizers. Nevertheless, although it is true that high utilizers have greater room for reduction in utilization, any other real-world sample is also going to be composed of a mix of high utilizers and lower utilizers. Thus, although our estimate of the mean reduction in utilization may not be representative of every patient in our sample or any other sample, it remains representative of the average of any other sample of similar patients in clinical practice. If this population seeking care in a specialty clinic is indeed not representative of the broader CM population, then our estimates of reductions in resource utilization due to initiation of onabotulinumtoxinA would not be generalizable beyond similar patients seeking treatment in similar facilities. Future analyses should stratify by degree of healthcare utilization.
Only four patients (1.7%) refused the second set of injections, suggesting an acceptable patient tolerability. This reinforces the positive safety and tolerability profile demonstrated in the PREEMPT clinical trials.
Our study did not include a control group, which limits our ability to compare treatment options or adjust for reduction in the frequency of headache days or HRU independent of onabotulinumtoxinA therapy. The rate of remission from CM to episodic migraine has been estimated at 26% annually, with lower baseline headache frequency (15-19 vs 25-31 days/month) and the absence of allodynia identified as statistically significant predictors of remission; use of prophylactic therapy was associated with a lower remission rate, suggesting a bias toward people with more persistent CM receiving such treatment.20 As our group of CM patients had experienced daily or near daily headaches for a median of 36 months, with adequate trial and failure of at least two prior prophylactic therapies, we feel that the bias toward spontaneous remission to episodic migraine in this severely impacted cohort likely was limited, but cannot be ruled out in the absence of a control (placebo) group.
The issue of spontaneous remission to episodic migraine aside, it is impossible to distinguish onabotulinumtoxinA's treatment effect from other potential confounding variables present during the observational period (including – but not limited to – changes in abortive therapies, improved patient education, discontinuation of adjunctive oral prophylaxis, and aggressive management of symptomatic medication overuse).
An additional limitation of our study involved the reliance on patient recall for HRU in the 6 months preceding initiation of onabotulinumtoxinA, with recall bias lending to the possibility that HRU during the pretreatment period was overestimated. Contravening this is the observation that immediately prior to participating in the study described here, a subset of our patients had participated in an epidemiologic investigation of CM that required prospective recording of HRU. The HRU reported by this subgroup was similar to that retrospectively reported by our remaining subjects. Although we did not perform a formal validation analysis, it would appear that the effect of patient recall bias on HRU consequently was limited.
Cost-offsets demonstrated here are likely to be conservative, as we did not take into account any savings in HRU attributable to decreased use of abortive medication, or prophylactic therapies, elective visits to healthcare providers, diagnostic studies (eg, brain magnetic resonance imaging or computed tomography), or savings in indirect costs (eg, work absenteeism or decreased productivity related to migraine), which have been shown to be substantial for those with CM.21 However, even if these estimates are conservative, they may not be generalizable beyond 6 months after initiation of treatment.
Conclusion
CM is a highly disabling and burdensome disorder.1,4 OnabotulinumtoxinA is the only FDA-approved prophylactic treatment for CM, making onabotulinumtoxinA a logical treatment choice for CM. Although we are unable to distinguish onabotulinumtoxinA's treatment effect from other potential confounding variables, our study showed that in addition to the documented safety and efficacy of onabotulinumtoxinA, severely afflicted, treatment-refractory CM patients who initiate onabotulinumtoxinA therapy experienced a significant cost-offset by a reduction in ED visits, urgent care visits, and hospitalizations for migraine in the 6 months following treatment initiation.
Statement of Authorship
Category 1
-
(a) Conception and Design
John F. Rothrock; Lisa M. Bloudek; Sepideh F. Varon
-
(b) Acquisition of Data
John F. Rothrock; Diane Andress-Rothrock
-
(c) Analysis and Interpretation of Data
John F. Rothrock; Lisa M. Bloudek; Timothy T. Houle; Diane Andress-Rothrock; Sepideh F. Varon
Category 2
-
(a) Drafting the Manuscript
John F. Rothrock; Lisa M. Bloudek; Timothy T. Houle; Diane Andress-Rothrock; Sepideh F. Varon
-
(b) Revising It for Intellectual Content
John F. Rothrock; Lisa M. Bloudek; Timothy T. Houle; Diane Andress-Rothrock; Sepideh F. Varon
Category 3
-
(a) Final Approval of the Completed Manuscript
John F. Rothrock; Lisa M. Bloudek; Timothy T. Houle; Diane Andress-Rothrock; Sepideh F. Varon
Acknowledgments
The authors thank Christopher Hanlon, MD, Department of Anesthesiology, University of Florida, Gainesville, Florida, for contributions to project conception and initial data collection. The authors would also like to acknowledge Silvia Weibelt, RN (The University of Alabama School of Medicine, Birmingham, AL) for her expert assistance in providing care to the patients involved in this research project, Andrew Messali (Allergan, Inc., Irvine, CA) for assisting with obtaining cost estimates, and Imprint Publication Science (New York, NY) and Complete Healthcare Communications (Chadds Ford, PA), for editorial support in the preparation and styling of this paper. This analysis was supported by Allergan, Inc., Irvine, CA, USA. Allergan supported the study analysis, interpretation of the data, and the preparation, review, and approval of the manuscript.
Dr. Rothrock was an employee of the University of Alabama at Birmingham at the time this study was conducted, but is no longer affiliated with the university.
Glossary
- AHRQ
Agency for Healthcare Research and Quality
- CM
chronic migraine
- CPT
Current Procedural Terminology
- ED
emergency department
- HCUP
Healthcare Cost & Utilization Project
- HRU
healthcare resource use
- IBMS
International Burden of Migraine Study
- ICHD-2
International Classification of Headache Disorders, second edition
- IQR
interquartile range
- MIDAS
MIgraine Disability ASsessment scale
- MPFS
Medicare Physician Fee Schedule
- PREEMPT
Phase III REsearch Evaluating Migraine Prophylaxis Therapy trial
References
- 1.Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: Results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52:1456–1470. doi: 10.1111/j.1526-4610.2012.02223.x. [DOI] [PubMed] [Google Scholar]
- 2.Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: A systematic review. Cephalalgia. 2010;30:599–609. doi: 10.1111/j.1468-2982.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 3.Munakata J, Hazard E, Serrano D, et al. Economic burden of transformed migraine: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2009;49:498–508. doi: 10.1111/j.1526-4610.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- 4.Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: Results from the International Burden of Migraine Study (IBMS) Headache. 2011;51:1058–1077. doi: 10.1111/j.1526-4610.2011.01945.x. [DOI] [PubMed] [Google Scholar]
- 5.Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803. doi: 10.1177/0333102410364676. [DOI] [PubMed] [Google Scholar]
- 6.Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 7.Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921–936. doi: 10.1111/j.1526-4610.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 8.Lipton RB, Varon SF, Grosberg B, et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine. Neurology. 2011;77:1465–1472. doi: 10.1212/WNL.0b013e318232ab65. [DOI] [PubMed] [Google Scholar]
- 9.Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–1373. doi: 10.1111/j.1526-4610.2011.01990.x. [DOI] [PubMed] [Google Scholar]
- 10.Silberstein SD, Olesen J, Bousser MG, et al. The International Classification of Headache Disorders, 2nd edition (ICHD-II) – revision of criteria for 8.2 medication-overuse headache. Cephalalgia. 2005;25:460–465. doi: 10.1111/j.1468-2982.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- 11.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56:S20–S28. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 12.Blumenfeld AM, Varon SF, Wilcox TK. Disability, HRQoL and resource use among chronic and episodic migraineurs: Results from the International Burden of Migraine Study (IBMS) Cephalalgia. 2011;31:301–315. doi: 10.1177/0333102410381145. , et al. [DOI] [PubMed] [Google Scholar]
- 13.Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: A safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50:1406–1418. doi: 10.1111/j.1526-4610.2010.01766.x. [DOI] [PubMed] [Google Scholar]
- 14.Medicare Payment Advisory Commission. Report to the Congress: Medicare payment policy. 2013. Available at: http://www.medpac.gov/documents/reports/mar13_entirereport.pdf?sfvrsn=0. Accessed September 16.
- 15.Micromedex®. RED BOOK Online® Version 2.0. Thomson Reuters. 2013. Available at: http://www.redbook.com/redbook/online/. Accessed September 24.
- 16.BOTOX®. Full Prescribing Information. Irvine, CA: Allergan, Inc; 2013. (onabotulinumtoxinA) [Google Scholar]
- 17.Cady RK, Schreiber CP, Porter JA, Blumenfeld AM, Farmer KU. A multi-center double-blind pilot comparison of onabotulinumtoxinA and topiramate for the prophylactic treatment of chronic migraine. Headache. 2011;51:21–32. doi: 10.1111/j.1526-4610.2010.01796.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelley NE, Tepper DE. Rescue therapy for acute migraine, part 1: Triptans, dihydroergotamine, and magnesium. Headache. 2012;52:114–128. doi: 10.1111/j.1526-4610.2011.02062.x. [DOI] [PubMed] [Google Scholar]
- 19.Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428–432. doi: 10.1136/jnnp.2009.192492. [DOI] [PubMed] [Google Scholar]
- 20.Manack A, Buse DC, Serrano D, Turkel CC, Lipton RB. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology. 2011;76:711–718. doi: 10.1212/WNL.0b013e31820d8af2. [DOI] [PubMed] [Google Scholar]
- 21.Stewart WF, Wood GC, Manack A, Varon SF, Buse DC, Lipton RB. Employment and work impact of chronic migraine and episodic migraine. J Occup Environ Med. 2010;52:8–14. doi: 10.1097/JOM.0b013e3181c1dc56. [DOI] [PubMed] [Google Scholar]


