Abstract
Objective
To determine the effects of maternal undernutrition (MUN) on the reproductive axis of aging offspring.
Design
Animal (rat) study.
Setting
Research Laboratory.
Animals
Female Sprague-Dawley rats.
Intervention(s)
Food restriction during the second half of pregnancy in rats.
Main Outcome Measures
Circulating gonadotropins, Anti-Mullerian Hormone (AMH), ovarian morphology, estrous cyclicity and gene expression studies in the hypothalamus and ovary in 1 day old (P1) and aging adult offspring.
Results
Offspring of MUN dams had low birth weight (LBW) and by adult age developed obesity. 80% of adult LBW offspring had disruption of estrous cycle by 8 months of age with the majority of animals in persistent estrous. Ovarian morphology was consistent with acyclicity with ovaries exhibiting large cystic structures and reduced corpora lutea. There was an elevation in circulating testosterone (T), increased ovarian expression of enzymes involved in androgen synthesis, an increase in plasma Leuteinizing (LH/)/Follicle Stimulating hormone (FSH) levels, reduced estradiol (E2) levels and no changes in AMH in adult LBW offspring compared to control offspring. Hypothalamic expression of leptin receptor (OBRb), estrogen receptor-α (ER-α) and Gonadotropin Releasing hormone (GnRH) protein were altered in an age-dependent manner with increased ObRb, ER-α expression in P1 LBW hypothalami and a reversal of this expression pattern in adult LBW hypothalami.
Conclusion
Our data indicates that the maternal nutritional environment programs reproductive potential of the offspring through alteration of the hypothalamic-pituitary-gonadal axis. The premature reproductive senescence in LBW offspring could be secondary to development of obesity and hyperleptinemia in these animals in adult life.
Keywords: Reproductive aging, maternal undernutrition, hypothalamus, ovary, leptin, obesity, low birth weight, fetal programming
Introduction
The incidence of obesity and type 2 diabetes has dramatically increased not only in adults but also in children and adolescents (1,2,3). This increase has significant consequences as obesity is known to impair reproductive function (4). During the last decade the onset of puberty has been noted to advance and this is likely secondary to increasing incidence of childhood obesity and early accumulation of body fat which is thought to sequester sufficient estradiol in body fat to initiate secondary sexual development (5). Thus in obese adolescent girls, excess body fat may cause early onset of reproductive function followed by later reproductive impairment. The underlying mechanism of obesity related reproductive dysfunction may involve adipose tissue derived adipokines, namely leptin and adiponectin, which are known to influence reproductive maturation and function (6,7,8). The ability of leptin to restore fertility to mice that are genetically deficient in leptin and to accelerate the onset of puberty in normal mice (9,10,11) suggests that leptin may be a key signal triggering the onset of sexual maturation. Adiponectin is known to improve insulin sensitivity; its overexpression impairs reproductive function (12).
Obesity is also associated with insulin resistance and hyperinsulinemia (13). Recent evidence shows that hyperinsulinemia plays a critical role in the reproductive dysfunction in patients with polycystic ovary syndrome (PCOS) (13). Insulin has direct stimulatory effects at the ovarian level to simulate androgen synthesis and secretion by increasing P450c17 enzyme activity (14) and stimulating the expression of 3β-Hydroxy steroid dehydrogenase (HSD) in human luteinized granulosa cells (15). In addition, insulin increases pituitary gonadotrope sensitivity to GnRH (16), and inhibits hepatic sex hormone biding globulin synthesis (17), thereby increasing the levels of free circulating steroids.
An adverse intrauterine environment such as caloric or protein restriction or reduction of uterine blood flow induce fetal growth restriction which predisposes to the development of metabolic syndrome later in life (18). Severe starvation is known to impair reproductive function in adult animals (19,20,21) and stress hormones secreted in excess during starvation alter the activity of hypothalamic-pituitary-gonadal (HPG) axis (22). In addition, maternal under- or overnutrition will also influence offspring reproductive function in a species dependant manner (23). In humans this dysfunction manifests itself early in the adolescence period with advancement of pubertal onset (24), and is considered a prelude to development of hyperinsulinemia (25), elevated plasma dehydroepiandrosterone sulfate, reduced plasma concentration of sex hormone binding globulin (26), reduced ovulation rate (27) and polycystic ovary syndrome (25). There are scant studies on birth weight and fertility in adulthood in humans although one study reported that high or low birth weight was associated with prolonged time to conceive (28).
Most studies in rodents have pointed to maternal undernutrition causing a delay in onset of puberty (29,30), although one group reported advancement of pubertal onset (19). Reduced reproductive success of female offspring of mice with food restriction during pregnancy has been reported (31). Previous studies have attributed the reproductive dysfunction in low birth weight (LBW) offspring to hyperleptinemia, and leptin resistance associated with obesity in these offspring in adulthood (6). Our group has developed an animal model for gestational programming in which rat dams are food restricted by 50% in the second half of gestation. Offspring of these dams are growth restricted at birth and develop obesity, glucose intolerance, and lipid abnormalities in adulthood (32,33). These offspring at birth have reduced circulating leptin and hypothalamic leptin signaling, and paradoxical upregulation of adipogenic signaling pathway (34,35). At 3 weeks of life the LBW offspring develop hyperleptinemia and an increase in adipose tissue levels of leptin (33). In the course of phenotypic characterization of these LBW offspring as adults we found that that the female offspring failed to exhibit the typical estrous cyclicity of control offspring. This study characterizes the defects in the reproductive axis of LBW female offspring. Since leptin has a key influence on the reproductive axis and LBW offspring develop hyperleptinemia prior to onset of reproductive dysfunction, we hypothesized that the reproductive dysfunction of adult LBW offspring is secondary to the hyperleptinemia associated with obesity.
Material and Methods
Animals
The study was approved by the Animal Use and Care Committee at Los Angeles Biomedical Research Institute at the Harbor-UCLA Medical Center. We used a well-characterized animal model of fetal programming developed by our group (32) in which first-time-pregnant Sprague-Dawley rats (Charles River Laboratories, Hollister, CA) were housed under constant temperature and humidity with a 12h/12h light-dark cycle. On day 10 of gestation, rats were provided either an ad libitum diet (Control group) of standard laboratory chow (Lab Diet 5001, Brentwood, MO: protein 23%, fat 4.5%, metabolizable energy 3030 kcal/kg), or 50% food restricted diet (MUN group) determined by quantification of normal intake in the controls. At day 1 after birth, 4 females and 4 males per litter were cross-fostered to ad libitum fed control dams. At posnatal age 21 all offspring were weaned to an ad libitum diet and housed in individual cages. The data reported is derived from offspring from 6 different litters.
Estrous cycle Monitoring
Starting at 8 months of age LBW and control offspring were assessed for their reproductive cycles. Vaginal cytology was determined by daily examination of vaginal smears and also by measurement of the electrical impedance of vaginal epithelium which is known to vary during the different stages of the estrous cycle using the EC40 estrous cycle monitor (Fine Sciences Tools, Foster City, CA) (36). Daily smears and impedance were determined daily over a 2 month period. There was 100% agreement between the smear cytology and impedance methods in determining the phase of the cycle.
Tissue Harvest
At P1 and 10 months of life female offspring were sacrificed by decapitation for the neonates and by pentobarbital oversdose (200mg/kg, i.p) for adult animals. The hypothalmi and half of ovaries from both P1 and 10 month old offspring were snap frozen for future extraction of RNA and protein. Some of the ovaries were fixed in 4% paraformaldehyde for histology analysis.
Histology
Ovaries were fixed in Bouin’s fixative (Sigma), washed in PBS and paraffin embedded and 10 micron sectioned for staining with hematoxylin and eosin. Every 10th section of the ovary was examined under light microscopic examination at 20x magnification to determine the relative quantity of corpora lutea, small, medium and large follicles. Three fields were examined per slide in a blinded fashion by the same examiner (E.R).
Plasma Hormones
Plasma hormone levels were determined by commercial rat specific RIA or ELISA kits as follows: estradiol (DSL-4400, Diagnostic Systems laboratories, Salem, NH), LH(076-65102, MP Biomedical LLC, Belgium), FSH (29-AELUTHUoE01, Alpco Diagnostics, Windham, NH), and Anti-Mullerian hormone (CK-E11351, Eastbiopharm, Zhejiang, China). For P1 samples plasma from 4 animals were pooled for each reading.
Real Time RT-PCR
Total RNA was extracted from P1 and 10 months old rat ovary tissues of control and MUN groups using Trizol (Invitrogen, Grand Island, NY). The quantity and quality of the isolated RNAs was determined using ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE) and 2μg of total RNA was reverse transcribed using random primer with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s guidelines. Quantitative RT-PCR was carried out using SYBR green PCR master mix and reactions were incubated for 10 min at 95°C followed by 40 cycles of 15 seconds at 95°C and 1 min at 60°C and level of mRNAs expression was determined using Applied Biosystems StepOnePlus Real-Time PCR Detection System with 18S used for normalization. All reactions were run in triplicate and relative expression was analyzed with the comparative cycle threshold method (2−ΔΔCT) according to the manufacturer (Applied Biosystems).
Western Blot Analysis
Protein was extracted in RIPA lysis buffer and protein levels were determined by BCA solution (Pierce, Rockford, IL). For P1 animals protein extracted from 4 animals derived from different litters were pooled for each reading. 50ug of protein was mixed with SDS buffer and separated on a 10% polyacrylamide gel and transferred electrophoretically to pure nitrocellulose membrane (BIO-RAD, Hercules, CA). Non specific binding was blocked with 5% non-fat dry milk in Tris-buffered saline (TBS) with 0.1% Tween 20. The membrane was then incubated with the appropriate antibody from Santa Cruz Laboratories (Santa Cruz, CA) (GnRh (10/6.7KD, SC-32292), Leptin receptor ObRb (120KD, SC-1832), ER-α (66KD, SC-787) in 5% milk in TBS overnight at 4 C. Anti-rabbit or anti-mouse IgG secondary antibody labeled with horse radish peroxidase (BIO-RAD (Hercules, CA) 1;2000 in 5% milk was then added on the membrane and incubated at room temperature. SuperSignal West Pico Chemiluminescent Substrate (Piecre, Rockford, IL) was then used to detect the targeted protein. The band density was analyzed by Alpha DigiDoc Gel documentation and Image analysis system (Alpho Innotech Corporation, San Leandro, CA). Data was normalized to β-actin and expressed as fold change.
Statistics
Data was analyzed using the NCSS statistical software (NCSS LLC, Kaysville, UT) by Student’s t-test. Normal distribution was determined by histogram plots, Kurtosis and Omnibus. Significance was established at P<0.05.
Results
MUN dams gave birth to pups that had lower body weights (LBW) and decreased plasma leptin levels at 1 day of life (P1). When nursed by ad libitum fed dams, LBW offspring demonstrated rapid catch-up growth at 3 week, resulting in increased weight, percent body fat, and plasma leptin levels in the presence of insulin resistance in adults (37,38).
Table 1 shows the plasma hormone levels. In the P1 offspring, there was a significant increase in plasma LH, a decrease in estradiol and no change in FSH levels in LBW offspring as compared with controls. In the adult female offspring during diestrus, there was persistent increase in LH and lower estradiol levels as compared to controls in the same phase of the cycle. Furthermore, LBW adult females showed increased FSH and testosterone levels though no difference in Anti-Mullerian hormone levels as compared to adult control females.
Table 1.
Plasma hormone levels in 1 Day (P1) offspring and 10 month old adult offspring.
| P1 Offspring | Control | LBW |
|---|---|---|
| Estradiol (pg/ml) | 17.3 ± 2.1 | 11.3 ± 1.8* |
| FSH (ng/ml) | 6.0 ± 0.5 | 6.7 ± 0.7 |
| LH (ng/ml) | 0.27 ± 0.02 | 0.58 ± 0.08* |
| AMH (ng/ml) | 2.33 ± 0.157 | 2.57 ± 0.14 |
| 10 Month Offspring | Control | LBW |
| Estradiol (pg/ml) | 58.3 ± 8.9 | 19.9 ± 4.9* |
| Testosterone (ng/ml) | 0.30 ± 0.07 | 0.57 ± 0.08* |
| FSH (ng/ml) | 4.6 ± 0.4 | 7.8 ± 0.9* |
| LH (ng/ml) | 0.23 ± 0.02 | 0.47 ± 0.08 |
| AMH (ng/ml) | 2.73 ± 0.08 | 2.92 ± 0.07 |
Values are mean ± SE N=12 for all hormones in P1 offspring except AMH (N=8 Controls; N=5 LBW). N=10 for all hormones in 10 month old offspring except AMH (N=18 Controls; N=19 LBW).
Estrous cyclicity was significantly disrupted in the adult LBW offspring as shown in Fig. 1. As demonstrated in this figure, 100% of the control offspring exhibited normal estrous cyclicity as compared to 20% of the LBW offspring. Specifically 80% of the LBW offspring were in persistent estrous, which is the hallmark of reproductive aging in rodents, and 20% in persistent diestrus as compared to none of the controls, indicating that by 8 months of age the majority of LBW offspring had entered the first phase of reproductive senescence in contrast to none of the controls.
Figure 1. Reproductive Cycle of 10 Month old Females.
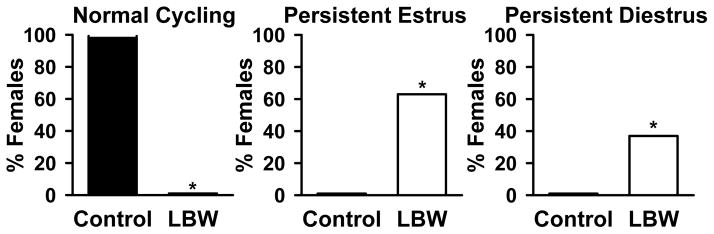
Percentage of females showing normal and abnormal (persistent estrus and persistent diestrus) reproductive cycle from control (■) and LBW (□) groups. *P<0.001 vs. control; N=24 animals from 6 litters.
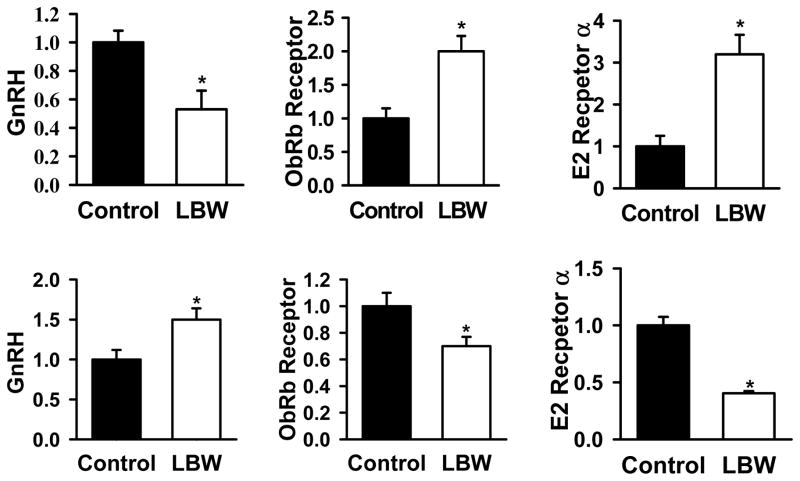
Hypothalamic protein expression of GnRH, ER-α and leptin receptor (ObRb) in P1 and adult offspring are shown in Fig. 2. As demonstrated in the top panel there was a significant decrease in GnRH protein, an increase in leptin and estrogen receptor protein levels in P1 LBW offspring as compared to controls. By 10 months of age, there was an increase in GnRH, a decrease in leptin and estrogen receptor protein expression in the LBW offspring as compared with controls.
Figure 2. Hypothalamic Protein Expression.
Hypothalamic protein expression of GnRH, leptin receptor (ObRb) and estrogen receptor-α (ER-α) in 1 day old (upper pane) and in 10 month old (lower panel) control (■) and LBW (□) females. Values are expressed as fold change (mean ± SE) relative to control set at 1. *P<0.01 vs. control; N=6 per group (For P1 each N was derived from pooling of protein from 4 animals).
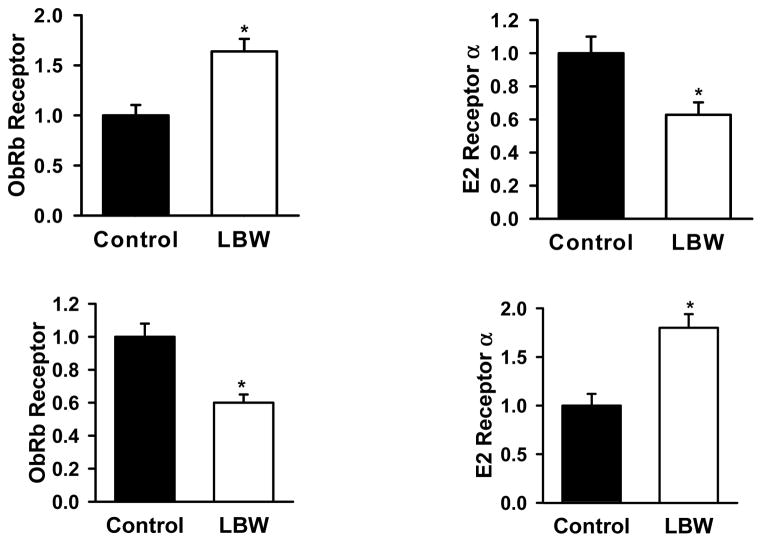
Ovarian expression of leptin and estrogen receptor in P1 and adult offspring are demonstrated in Fig. 3. As shown in the top panel there was an increase in the ovarian expression of leptin receptor and a decrease in estrogen receptor in P1 LBW offspring. In the adult offspring the expression of leptin receptor protein was significantly lower whereas the estrogen receptor expression was higher in the LBW ovaries as compared to controls.
Figure 3. Ovarian Protein Expression.
Ovarian protein expression of leptin receptor (ObRb) and estrogen receptor (E2a) in 1 day old (upper panel) and in 10 month old (lower panel) control (■) and LBW (□) females. Values are expressed as fold change (mean ± SE) relative to control set at 1. *P<0.01 vs. control; N=6 per group.
The mRNA expression of ovarian steroidogenic enzymes and gonadotropin receptors in the ovaries are shown in Supplemental Fig. 1. In P1 offspring there was a decrease in the expression of HSD3B1 and HSD3B2 expression in LBW ovaries as compared with the controls, though no differences in the mRNA expression of CYP11A1, HSD-17β1, HSD-17β2, CYP19A1, LH and FSH receptors. In the adult offspring, there was a decrease in the expression of CYP11A1, HSD-3β1, CYP19A1, LH receptors and no changes in HSD-3β2, HSD-17β1, HSD-17β2, and FSH receptor mRNA levels in the LBW offspring as compared to controls.
Ovarian morphology was also examined in the adult offspring by histology. Representative H and E stained section is shown in Supplemental Fig. 2. In contrast to LBW ovaries which showed a disruption of normal follicular structures, control ovaries had normal ovarian morphology with follicles of different sizes and copora lutea (Supplemental Fig 2a). The number of ovarian follicles in different phases of the estrous cycle is shown in Supplemental Fig. 2b. In control ovaries small follicles were the most abundant followed by corpora lutea and medium and extra large follicles. In LBW offspring in persistent estrous which constituted the majority of animals, there was a significant reduction in the number of corpora lutea and small follicles and a significant increase in the number of large cystic structures indicative of anovulation.
Discussion
The results of this study show that maternal undernutrition has profound effects on the reproductive potential of the offspring and that growth restricted newborns undergo premature reproductive senescence as adults due to programming effects of maternal under-nutrition on the HPG axis. LBW offspring by 8 months of life had disruption of estrous cyclicity remaining mostly in persistent estrous and some in diestrous state, which is equivalent to peri-menopause in humans. Ovarian morphology was consistent with this acyclicity with ovaries demonstrating large cystic structures and a marked reduction of corpora lutea consistent with anovulation. In LBW offspring in persistent diestrus, which is one of the phases of reproductive aging, (39,40) there was significantly greater numbers of corpora lutea and number of large antral follicles.
Ovarian reserve testing showed no differences in AMH levels in these offspring compared with controls, however plasma FSH were higher in the LBW offspring. Ovarian steroidogenic enzyme expression was altered in LBW offspring in an age-dependant manner, with a relative increase in the expression of enzymes involved in androgen synthesis in adult LBW ovaries. This finding was consistent with elevated circulating levels of testosterone (T) levels in the adult LBW offspring. There were no changes in the expression of gonadotropin receptors in P1 LBW ovaries but in the adult offspring a reduction of ovarian LH receptors was detected. We also determined the ovarian expression of leptin and estrogen receptor-alpha (ER-α) and found that in P1 LBW offspring there was an increase in ObRb receptor and an increase in ER-α protein expression with a complete reversal of this pattern in the adult ovaries. Hypothalamic expression of ObRb, ER-α protein and GnRH protein in LBW offspring were also altered in an age-specific manner with reduced GNRH protein and increased OBRb, ER-α in P1 LBW hypothalami and a reversal of this expression pattern in the adult LBW offspring.
Adipose-tissue derived factors are well known to impact health and disease (7) and fertility (8). Since leptin plays a vital role in nutritional signaling to the brain and in regulation of reproduction (41), we hypothesized that the changes in the HPG axis in LBW offspring are leptin-mediated. Previous studies from our laboratory (33) and others (42) have demonstrated that LBW offspring have low circulating leptin at birth and elevated leptin levels in adult life (33). Leptin exerts its most significant reproductive effects through the hypothalamus (43). Leptin is known to stimulate GnRH secretion in vitro from arcuate nucleus explants (44), and immortalized GnRH secreting neuronal cell line (45). It is also well established that leptin down regulates its own receptor expression in the hypothalmus (46). Evidence for this negative feedback axis between circulating leptin and leptin receptor expression come from our data showing that lower circulating leptin In P1 LBW offspring was associated with elevated hypothalamic ObRb protein, and the reverse pattern in adult LBW offspring. The pattern of changes in hypothalamic ObRb expression LBW offspring were in line with known effects of leptin to stimulate GnRH secretion (47,45), namely in P1 LBW offspring which had lower circulating leptin hypothalamic GnRH was lower whereas in adult LBW offspring with higher circulating leptin, hypothalamic GnRH was higher. Hypothalamic GnRH levels correlated with circulating gondaotropins in the adult LBW offspring. However, in P1 LBW offspring despite lower hypothalmic GnRH, circulating LH levels were higher, indicating a GnRH-independent mechanism for stimulation of LH secretion. The elevated LH levels in LBW offspring detected as early as day one of life and persisting into adult life is reminiscent of women with PCOS with elevated LH levels (48). Prior studies have demonstrated that leptin stimulates LH secretion (49,50), and therefore the hyperleptinemia in adult LBW offspring could account for the elevated LH levels at least in the adult LBW offspring. In line with elevated plasma LH which would be expected to stimulate ovarian androgen synthesis adult LBW offspring also had elevated circulating T levels. These offspring had lower estradiol levels which could be secondary to decreased ovarian expression of the aromatase (CYP19A1) enzyme or direct effect of leptin on ovarian steroidogenesis. The effect of leptin on ovarian steroidogenesis is conflicting with both negative effects (51,52) and positive effects (53) reported depending on the animal model and species. Whether the suppression of CYP19A1 and HSD-3β2 are due to elevated leptin in the LBW offspring cannot be determined from our study. Besides leptin insulin also plays a crucial role in signaling the nutritional status to the hypothalamus (54). Adult LBW offspring have hyperinsulinemia (32) and some of the changes in the HPG axis could have been due to the hyperinsluinemia.
The LBW ovarian morphology with large cystic structures and absence of corpora lutea indicates anovulation further supports a PCOS-like syndrome in the MUN offspring. Reduction of ovulation rates in LBW offspring has been demonstrated in a number of species. Protein restriction during pregnancy in rats resulted in cycle disruption; a reduction is estradiol and progesterone levels and reduced fertility rates (55). Bernal et al (29) also showed reduced number of primordial and secondary follicles in LBW offspring (similar to our LBW offspring in persistent estrous and diestrous) which was proposed to be secondary to increased oxidative stress and decreased ability to repair the oxidative damage (29). The same group reported reduced progesterone levels in these offspring in adult life (30) consistent with oligovulation. Maternal undernutrition also reduced ovulation rates (56), and increased the number of small follicles in the ovary and a reduction in corpora lutea (57) in sheep. Reduced ovulation rate has also been reported in adolescent girls born small for gestational age (27). Potential mechanisms for reduced number of small follicles may be the hyperandrogenic and hypoestrogenic ovarian environment in the MUN offspring; androgens are known to induce follicular atresia (58,59) and estrogen is essential for folliculogenesis to regulate granulosa cell growth and differentiation (59). Alternatively, this effect on ovarian morphology could be secondary to the direct effects of excess leptin on ovarian folliculogenesis (60), or due to leptin’s effect to alter the sensitivity of the ovary to gonadotropins (61).
Despite many similarities in reproductive aging mechanisms between rats and humans, there are few differences worth noting. In postmenopausal women LH and FSH are elevated (62) whereas in rats, LH levels are relatively normal, despite changes in estradiol (62). In addition, follicles have been reported in anetrous rat ovaries unlike postmenopausal human ovaries (62,63). This indicates that the exhaustion of ovarian follicles is not a limiting factor in rodent reproductive aging. Because of these differences in ovarian morphology markers of ovarian reserve in women such as AMH which shows an age-dependent decline (64) are not applicable in rodents (65). This is supported by our data showing no differences in AMH levels in aging LBW offspring compared to controls. In contrast elevated FSH levels are detected in aging female rats which transition from normal estrous cyclicity to an anovulatory state in persistent estrous (66) as in our LBW adult female offspring.
In summary, our data indicates that maternal nutritional environment programs reproductive potential of the offspring through alteration of the HPG axis. The changes in the HPG axis in these offspring are manifested as early as day one of life and are potentially secondary to the known reproductive effects of leptin which are low in the LBW offspring at birth and elevated with onset of obesity in adult life or due to epigenetic mechanisms. The adult LBW offspring exhibit an anovulatory state, and remain in a persistent estrous phase of the cycle which is the reproductive hallmark of rodent aging. This model of reproductive aging should provide fertile ground for design of interventions to block the effects of in utero undernutrition.
Supplementary Material
Acknowledgments
Supported by National Institute of Diabetes and Digestive and Kidney Grants R01DK081756 and National Institute of Child Health and Human Development Grant R01HD054751
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivian EM. Type 2 diabetes in children and adolescents--the next epidemic? Curr Med Res Opin. 2006;22:297–306. doi: 10.1185/030079906X80495. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013;1281:123–140. doi: 10.1111/nyas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mircea CN, Lujan ME, Pierson RA. Metabolic fuel and clinical implications for female reproduction. J Obstet Gynaecol Can. 2007;29:887–902. doi: 10.1016/S1701-2163(16)32661-5. [DOI] [PubMed] [Google Scholar]
- 5.Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet. 2013;106:135–141. doi: 10.1159/000342545. [DOI] [PubMed] [Google Scholar]
- 6.Popovic V, Casanueva FF. Leptin nutrition and reproduction: new insights. Hormones (Athens ) 2002;1:204–217. doi: 10.14310/horm.2002.1169. [DOI] [PubMed] [Google Scholar]
- 7.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2005;130:583–597. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]
- 9.Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138:1190–1193. doi: 10.1210/endo.138.3.5024. [DOI] [PubMed] [Google Scholar]
- 10.Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chehab FF, Mounzih K, Lu R, Lim ME. Early onset of reproductive function in normal female mice treated with leptin. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- 12.Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. 2008;22:760–771. doi: 10.1210/me.2007-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmina E. PCOS: metabolic impact and long-term management. Minerva Ginecol. 2012;64:501–505. [PubMed] [Google Scholar]
- 14.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 15.McGee E, Sawetawan C, Bird I, Rainey WE, Carr BR. The effects of insulin on 3 beta-hydroxysteroid dehydrogenase expression in human luteinized granulosa cells. J Soc Gynecol Investig. 1995;2:535–541. doi: 10.1016/1071-5576(94)00061-5. [DOI] [PubMed] [Google Scholar]
- 16.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 17.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 18.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 19.Warren MP. Effects of undernutrition on reproductive function in the human. Endocr Rev. 1983;4:363–377. doi: 10.1210/edrv-4-4-363. [DOI] [PubMed] [Google Scholar]
- 20.Bergendahl M, Perheentupa A, Huhtaniemi I. Starvation-induced suppression of pituitary-testicular function in rats is reversed by pulsatile gonadotropin-releasing hormone substitution. Biol Reprod. 1991;44:413–419. doi: 10.1095/biolreprod44.3.413. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Kaur G. Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: a study of hypothalamo-hypophysial-gonadal axis. PLoS One. 2013;8:e52416. doi: 10.1371/journal.pone.0052416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferin M. Clinical review 105: Stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- 23.Dupont C, Cordier AG, Junien C, Mandon-Pepin B, Levy R, Chavatte-Palmer P. Maternal environment and the reproductive function of the offspring. Theriogenology. 2012;78:1405–1414. doi: 10.1016/j.theriogenology.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Ibanez L, de ZF. Puberty and prenatal growth. Mol Cell Endocrinol. 2006;254–255:22–25. doi: 10.1016/j.mce.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 25.de ZF, Ibanez L. Prenatal growth restraint followed by catch-up of weight: a hyperinsulinemic pathway to polycystic ovary syndrome. Fertil Steril. 2006;86 (Suppl 1):S4–S5. doi: 10.1016/j.fertnstert.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Ibanez L, Lopez-Bermejo A, Diaz M, Suarez L, de ZF. Low-birth weight children develop lower sex hormone binding globulin and higher dehydroepiandrosterone sulfate levels and aggravate their visceral adiposity and hypoadiponectinemia between six and eight years of age. J Clin Endocrinol Metab. 2009;94:3696–3699. doi: 10.1210/jc.2009-0789. [DOI] [PubMed] [Google Scholar]
- 27.Ibanez L, Potau N, Ferrer A, Rodriguez-Hierro F, Marcos MV, de ZF. Reduced ovulation rate in adolescent girls born small for gestational age. J Clin Endocrinol Metab. 2002;87:3391–3393. doi: 10.1210/jcem.87.7.8657. [DOI] [PubMed] [Google Scholar]
- 28.Nohr EA, Vaeth M, Rasmussen S, Ramlau-Hansen CH, Olsen J. Waiting time to pregnancy according to maternal birthweight and prepregnancy BMI. Hum Reprod. 2009;24:226–232. doi: 10.1093/humrep/den357. [DOI] [PubMed] [Google Scholar]
- 29.Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010;5:e15558. doi: 10.1371/journal.pone.0015558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS One. 2009;4:e6744. doi: 10.1371/journal.pone.0006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meikle D, Westberg M. Maternal nutrition and reproduction of daughters in wild house mice (Mus musculus) Reproduction. 2001;122:437–442. doi: 10.1530/rep.0.1220437. [DOI] [PubMed] [Google Scholar]
- 32.Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol. 2007;196:555–557. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 34.Desai M, Guang H, Ferelli M, Kallichanda N, Lane RH. Programmed upregulation of adipogenic transcription factors in intrauterine growth-restricted offspring. Reprod Sci. 2008;15:785–796. doi: 10.1177/1933719108318597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai M, Gayle D, Han G, Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci. 2007;14:329–337. doi: 10.1177/1933719107303983. [DOI] [PubMed] [Google Scholar]
- 36.Ramos SD, Lee JM, Peuler JD. An inexpensive meter to measure differences in electrical resistance in the rat vagina during the ovarian cycle. J Appl Physiol (1985 ) 2001;91:667–670. doi: 10.1152/jappl.2001.91.2.667. [DOI] [PubMed] [Google Scholar]
- 37.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 38.Desai M, Gayle D, Babu J, Ross MG. Permanent reduction in heart and kidney organ growth in offspring of undernourished rat dams. Am J Obstet Gynecol. 2005;193:1224–1232. doi: 10.1016/j.ajog.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 39.Huang HH, Meites J. Reproductive capacity of aging female rats. Neuroendocrinology. 1975;17:289–295. doi: 10.1159/000122367. [DOI] [PubMed] [Google Scholar]
- 40.Peng MT, Peng YM. Changes in the uptake of tritiated estradiol in the hypothalamus and adenohypophysis of old female rats. Fertil Steril. 1973;24:534–539. doi: 10.1016/s0015-0282(16)39794-1. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbaum M, Leibel RL. Leptin: a molecule integrating somatic energy stores, energy expenditure and fertility. Trends Endocrinol Metab. 1998;9:117–124. doi: 10.1016/s1043-2760(98)00028-9. [DOI] [PubMed] [Google Scholar]
- 42.Leonhardt M, Lesage J, Croix D, Dutriez-Casteloot I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68:390–400. doi: 10.1095/biolreprod.102.003269. [DOI] [PubMed] [Google Scholar]
- 43.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 44.Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997;138:5055–5058. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- 45.Magni P, Vettor R, Pagano C, Calcagno A, Beretta E, Messi E, et al. Expression of a leptin receptor in immortalized gonadotropin-releasing hormone-secreting neurons. Endocrinology. 1999;140:1581–1585. doi: 10.1210/endo.140.4.6622. [DOI] [PubMed] [Google Scholar]
- 46.Wilsey J, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J Endocrinol. 2004;181:297–306. doi: 10.1677/joe.0.1810297. [DOI] [PubMed] [Google Scholar]
- 47.Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997;138:5055–5058. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- 48.Grulet H, Hecart AC, Delemer B, Gross A, Sulmont V, Leutenegger M, et al. Roles of LH insulin resistance in lean and obese polycystic ovary syndrome. Clin Endocrinol (Oxf) 1993;38:621–626. doi: 10.1111/j.1365-2265.1993.tb02144.x. [DOI] [PubMed] [Google Scholar]
- 49.Carbone S, Szwarcfarb B, Reynoso R, Bollero G, Ponzo O, Rondina D, et al. Leptin stimulates LH secretion in peripubertal male rats through NMDA receptors. Endocr Res. 2005;31:387–396. doi: 10.1080/07435800500458032. [DOI] [PubMed] [Google Scholar]
- 50.Arnaoutoglou C, Keivanidou A, Arnaoutoglou M, Kastrouni E, Samaras V, Kaiki-Astara A, et al. The effect of leptin on the tonic secretion of gonadotropins in the female rats. Neuro Endocrinol Lett. 2008;29:999–1006. [PubMed] [Google Scholar]
- 51.Ghizzoni L, Barreca A, Mastorakos G, Furlini M, Vottero A, Ferrari B, et al. Leptin inhibits steroid biosynthesis by human granulosa-lutein cells. Horm Metab Res. 2001;33:323–328. doi: 10.1055/s-2001-15419. [DOI] [PubMed] [Google Scholar]
- 52.Zachow RJ, Weitsman SR, Magoffin DA. Leptin impairs the synergistic stimulation by transforming growth factor-beta of follicle-stimulating hormone-dependent aromatase activity and messenger ribonucleic acid expression in rat ovarian granulosa cells. Biol Reprod. 1999;61:1104–1109. doi: 10.1095/biolreprod61.4.1104. [DOI] [PubMed] [Google Scholar]
- 53.Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H. Leptin directly stimulates aromatase activity in human luteinized granulosa cells. Mol Hum Reprod. 1999;5:708–713. doi: 10.1093/molehr/5.8.708. [DOI] [PubMed] [Google Scholar]
- 54.Gamba M, Pralong FP. Control of GnRH neuronal activity by metabolic factors: the role of leptin and insulin. Mol Cell Endocrinol. 2006;254–255:133–139. doi: 10.1016/j.mce.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 55.Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol. 2006;572:97–108. doi: 10.1113/jphysiol.2005.103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rae MT, Kyle CE, Miller DW, Hammond AJ, Brooks AN, Rhind SM. The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim Reprod Sci. 2002;72:63–71. doi: 10.1016/s0378-4320(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 57.Kotsampasi B, Chadio S, Papadomichelakis G, Deligeorgis S, Kalogiannis D, Menegatos I, et al. Effects of maternal undernutrition on the hypothalamic-pituitary-gonadal axis function in female sheep offspring. Reprod Domest Anim. 2009;44:677–684. doi: 10.1111/j.1439-0531.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 58.Yu YS, Sui HS, Han ZB, Li W, Luo MJ, Tan JH. Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res. 2004;14:341–346. doi: 10.1038/sj.cr.7290234. [DOI] [PubMed] [Google Scholar]
- 59.Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol. 2006;4:16. doi: 10.1186/1477-7827-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duggal PS, Ryan NK, Van der Hoek KH, Ritter LJ, Armstrong DT, Magoffin DA, et al. Effects of leptin administration and feed restriction on thecal leucocytes in the preovulatory rat ovary and the effects of leptin on meiotic maturation, granulosa cell proliferation, steroid hormone and PGE2 release in cultured rat ovarian follicles. Reproduction. 2002;123:891–898. [PubMed] [Google Scholar]
- 61.Olatinwo MO, Bhat GK, Stah CD, Mann DR. Impact of gonadotropin administration on folliculogenesis in prepubertal ob/ob mice. Mol Cell Endocrinol. 2005;245:121–127. doi: 10.1016/j.mce.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Wise PM. Neuroendocrine modulation of the “menopause”: insights into the aging brain. Am J Physiol. 1999;277:E965–E970. doi: 10.1152/ajpendo.1999.277.6.E965. [DOI] [PubMed] [Google Scholar]
- 63.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 64.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 65.Yeh J, Kim B, Peresie J, Liang YJ, Arroyo A. Serum and ovarian Mullerian inhibiting substance, and their decline in reproductive aging. Fertil Steril. 2007;87:1227–1230. doi: 10.1016/j.fertnstert.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Matt DW, Dahl KD, Sarkissian A, Sayles TE. Apparent absence of negative feedback in middle-aged persistent-estrous rats following luteinizing hormone-releasing hormone agonist treatment: relation to plasma inhibin and 17 beta-estradiol. Biol Reprod. 1993;48:333–339. doi: 10.1095/biolreprod48.2.333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.