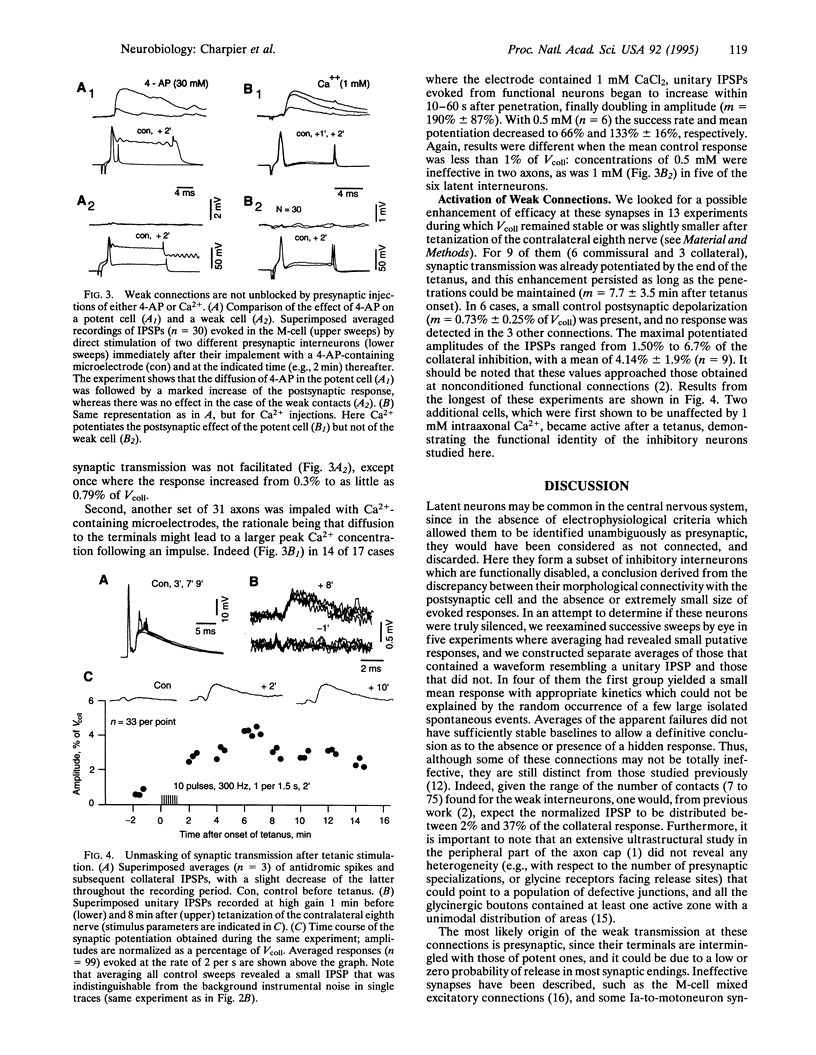
Abstract
Simultaneous pre- and postsynaptic recordings from identified glycinergic inhibitory interneurons and the Mauthner cell showed that 25% of the afferents produced no or extremely small postsynaptic responses. Morphological determination of the number of contacts made by these cells on the Mauthner cell revealed a connectivity similar to that of functional neurons which always produce clear inhibitory postsynaptic potentials, suggesting that most of the endings, made by weak interneurons are silent. Intraaxonal injection of 4-aminopyridine or Ca2+ greatly enhanced transmission at functional connections but did not modify those which were ineffective. However, after eighth nerve tetanic stimuli, transmission at the weak connections was unmasked or enhanced for prolonged periods and was twice as likely to be potentiated, with a 6-fold greater mean enhancement than the potent ones. This result provides additional support for long-term potentiation of inhibitory synapses. Furthermore, weakly functional junctions represent a "reserve" pool which can be critical for the expression of plasticity within a network, and, consequently, for setting the threshold of reflex activities such as the escape reaction mediated by the Mauthner cell.
Full text
PDF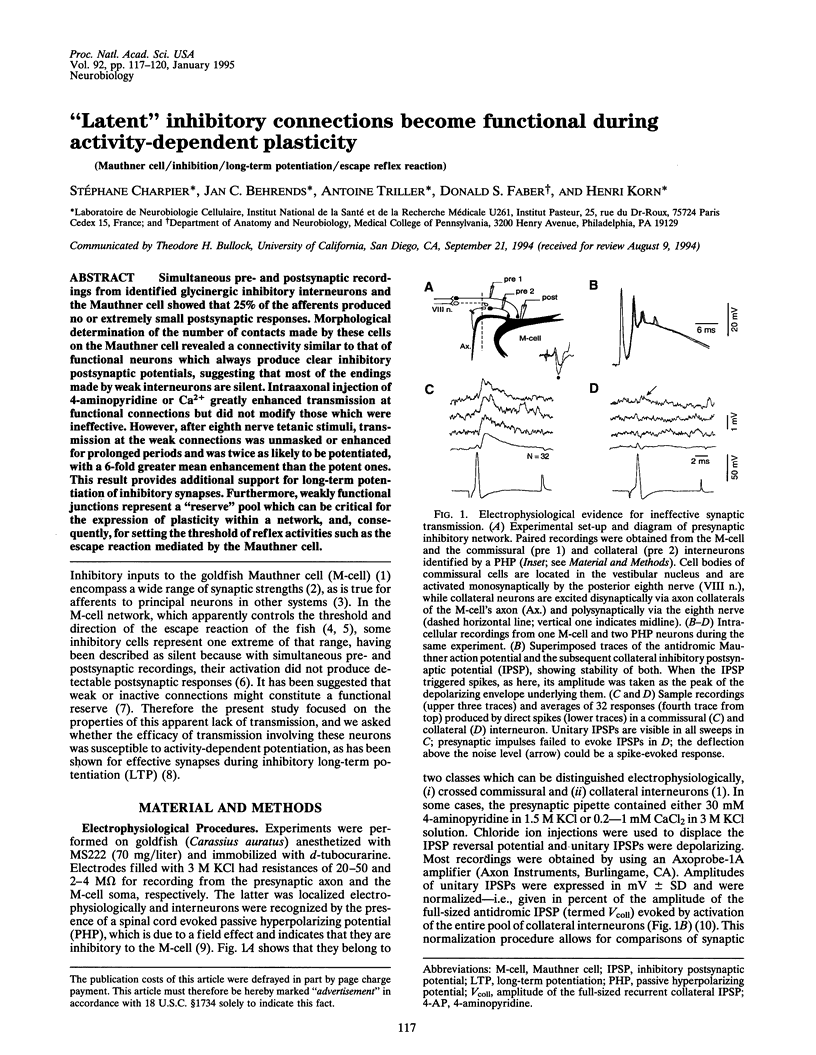


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S., Behrends J. C., Chang Y. T., Sur C., Korn H. Synchronous bursting in a subset of interneurons inhibitory to the goldfish Mauthner cell: synaptic mediation and plasticity. J Neurophysiol. 1994 Aug;72(2):531–541. doi: 10.1152/jn.1994.72.2.531. [DOI] [PubMed] [Google Scholar]
- Henneman E., Lüscher H. R., Mathis J. Simultaneously active and inactive synapses of single Ia fibres on cat spinal motoneurones. J Physiol. 1984 Jul;352:147–161. doi: 10.1113/jphysiol.1984.sp015283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler N. A., Shirke A. M., Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993 Dec 9;366(6455):569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. Modifications to synaptic transmission at group Ia synapses on cat spinal motoneurones by 4-aminopyridine. J Physiol. 1981 Dec;321:111–126. doi: 10.1113/jphysiol.1981.sp013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Kandel E. R. Synaptic transmission: a bidirectional and self-modifiable form of cell-cell communication. Cell. 1993 Jan;72 (Suppl):1–30. doi: 10.1016/s0092-8674(05)80025-x. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Iwakiri M. Long-term modification of inhibitory synaptic transmission in developing visual cortex. Neuroreport. 1993 Jul;4(7):907–910. doi: 10.1097/00001756-199307000-00017. [DOI] [PubMed] [Google Scholar]
- Korn H., Faber D. S. An electrically mediated inhibition in goldfish medulla. J Neurophysiol. 1975 Mar;38(2):452–471. doi: 10.1152/jn.1975.38.2.452. [DOI] [PubMed] [Google Scholar]
- Korn H., Faber D. S., Triller A. Probabilistic determination of synaptic strength. J Neurophysiol. 1986 Feb;55(2):402–421. doi: 10.1152/jn.1986.55.2.402. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Korn H., Oda Y., Faber D. S. Long-term potentiation of inhibitory circuits and synapses in the central nervous system. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):440–443. doi: 10.1073/pnas.89.1.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A., Stratford K., Jack J. Quantal analysis of excitatory synaptic action and depression in hippocampal slices. Nature. 1991 Mar 28;350(6316):344–347. doi: 10.1038/350344a0. [DOI] [PubMed] [Google Scholar]
- Lin J. W., Faber D. S. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. II. Plasticity of excitatory postsynaptic potentials. J Neurosci. 1988 Apr;8(4):1313–1325. doi: 10.1523/JNEUROSCI.08-04-01313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Walton K., Bohr V. Synaptic transmission in squid giant synapse after potassium conductance blockage with external 3- and 4-aminopyridine. Biophys J. 1976 Jan;16(1):83–86. doi: 10.1016/S0006-3495(76)85664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Malenka R. C., Nicoll R. A. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Malinow R. Transmission between pairs of hippocampal slice neurons: quantal levels, oscillations, and LTP. Science. 1991 May 3;252(5006):722–724. doi: 10.1126/science.1850871. [DOI] [PubMed] [Google Scholar]
- Miles R. Variation in strength of inhibitory synapses in the CA3 region of guinea-pig hippocampus in vitro. J Physiol. 1990 Dec;431:659–676. doi: 10.1113/jphysiol.1990.sp018353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I., Korn H. Serotonergic facilitation of quantal release at central inhibitory synapses. J Neurosci. 1991 Nov;11(11):3359–3370. doi: 10.1523/JNEUROSCI.11-11-03359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y. Fine structure of the synaptic endings on the Mauthner cell of the goldfish. J Comp Neurol. 1974 Aug 15;156(4):379–402. [PubMed] [Google Scholar]
- Redman S., Walmsley B. Amplitude fluctuations in synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:135–145. doi: 10.1113/jphysiol.1983.sp014885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Clements J. D., Westbrook G. L. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993 Oct 29;262(5134):754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The presence of ineffective synapses and the circumstances which unmask them. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):361–372. doi: 10.1098/rstb.1977.0048. [DOI] [PubMed] [Google Scholar]




