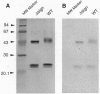
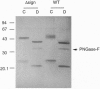
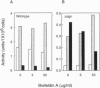
Abstract
A mutant of human gamma-glutamyl transpeptidase (EC 2.3.2.2, a membrane-bound enzyme of importance in glutathione metabolism) that differs from the wild type by deletion of the putative signal peptide/anchor domain (amino acid residues 1-27) was expressed in insect cells using a baculovirus system. In contrast to the wild-type enzyme--which, as expected, was mainly cell-associated--the mutant enzyme was secreted into the medium. The mutant and wild-type enzymes were purified and found to exhibit virtually identical catalytic properties. The mutant enzyme was glycosylated and processed into two subunits, as found for the wild-type enzyme. Brefeldin A inhibited secretion of the mutant enzyme and led to its accumulation in cells. The findings indicate that gamma-glutamyl transpeptidase can be targeted to the endoplasmic reticulum in a manner that does not involve function of an amino-terminal "signal/anchor" domain and that this domain is involved primarily in a membrane anchoring function. Another region of the enzyme may function as a signal domain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barouki R., Finidori J., Chobert M. N., Aggerbeck M., Laperche Y., Hanoune J. Biosynthesis and processing of gamma-glutamyl transpeptidase in hepatoma tissue culture cells. J Biol Chem. 1984 Jun 25;259(12):7970–7974. [PubMed] [Google Scholar]
- Bird P., Gething M. J., Sambrook J. The functional efficiency of a mammalian signal peptide is directly related to its hydrophobicity. J Biol Chem. 1990 May 25;265(15):8420–8425. [PubMed] [Google Scholar]
- Blochberger T. C., Sabatine J. M., Lee Y. C., Hughey R. P. O-linked glycosylation of rat renal gamma-glutamyltranspeptidase adjacent to its membrane anchor domain. J Biol Chem. 1989 Dec 5;264(34):20718–20722. [PubMed] [Google Scholar]
- Capraro M. A., Hughey R. P. Processing of the propeptide form of rat renal gamma-glutamyltranspeptidase. FEBS Lett. 1983 Jun 27;157(1):139–143. doi: 10.1016/0014-5793(83)81132-6. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Kaufman R. J. Analysis of synthesis, processing, and secretion of proteins expressed in mammalian cells. Methods Enzymol. 1990;185:577–596. doi: 10.1016/0076-6879(90)85046-q. [DOI] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Fujii J., Taniguchi N. Significance of Arg-107 and Glu-108 in the catalytic mechanism of human gamma-glutamyl transpeptidase. Identification by site-directed mutagenesis. J Biol Chem. 1993 Feb 25;268(6):3980–3985. [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T., Matsuda Y., Katunuma N. The conversion of the precursor form of gamma-glutamyltranspeptidase to its subunit form takes place in brush border membranes. Biochem Biophys Res Commun. 1983 Jul 29;114(2):889–895. doi: 10.1016/0006-291x(83)90864-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laperche Y., Bulle F., Aissani T., Chobert M. N., Aggerbeck M., Hanoune J., Guellaën G. Molecular cloning and nucleotide sequence of rat kidney gamma-glutamyl transpeptidase cDNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):937–941. doi: 10.1073/pnas.83.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991 Nov 1;67(3):601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Meek R. L., Walsh K. A., Palmiter R. D. The signal sequence of ovalbumin is located near the NH2 terminus. J Biol Chem. 1982 Oct 25;257(20):12245–12251. [PubMed] [Google Scholar]
- Muesch A., Hartmann E., Rohde K., Rubartelli A., Sitia R., Rapoport T. A. A novel pathway for secretory proteins? Trends Biochem Sci. 1990 Mar;15(3):86–88. doi: 10.1016/0968-0004(90)90186-f. [DOI] [PubMed] [Google Scholar]
- Nash B., Tate S. S. In vitro translation and processing of rat kidney gamma-glutamyl transpeptidase. J Biol Chem. 1984 Jan 10;259(1):678–685. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Papandrikopoulou A., Frey A., Gassen H. G. Cloning and expression of gamma-glutamyl transpeptidase from isolated porcine brain capillaries. Eur J Biochem. 1989 Aug 15;183(3):693–698. doi: 10.1111/j.1432-1033.1989.tb21100.x. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E., Heisterkamp N., Groffen J. Cloning and nucleotide sequence of human gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8840–8844. doi: 10.1073/pnas.85.23.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M., Tomiyoshi R., Kuroiwa T., Mihara K., Omura T. Functions of signal and signal-anchor sequences are determined by the balance between the hydrophobic segment and the N-terminal charge. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):16–19. doi: 10.1073/pnas.89.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamuro D., Yamazoe M., Matsuda Y., Kangawa K., Taniguchi N., Matsuo H., Yoshikawa H., Ogasawara N. The primary structure of human gamma-glutamyl transpeptidase. Gene. 1988 Dec 15;73(1):1–9. doi: 10.1016/0378-1119(88)90307-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400–419. doi: 10.1016/s0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981 Sep 25;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Hitoi A., Matsuda Y., Tsuji A., Katunuma N., Kobata A. Structural studies of the carbohydrate moieties of rat kidney gamma-glutamyltranspeptidase. An extremely heterogeneous pattern enriched with nonreducing terminal N-acetylglucosamine residues. J Biol Chem. 1983 Jan 25;258(2):1098–1107. [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]