Summary
Background
Factor VIII (FVIII), a procoagulant cofactor, plays a crucial role in the intrinsic coagulation cascade. A causal association between elevated FVIII levels and venous thrombosis incidence has been established; no such association has been confirmed with arterial thrombosis.
Objective
The independent role of elevated FVIII levels in arteriolar thrombosis was evaluated in a mouse model to determine the thrombogenic potential of elevated levels of FVIII.
Methods
The in vitro thrombogenic effect of elevated FVIII levels was examined using thrombin-antithrombin (TAT) complex generation and thromboelastography (TEG) assays. The thrombogenic potential of acute and extended elevation of circulating FVIII levels was assessed using ferric chloride induced injury of the cremaster arterioles.
Results
The rate of TAT complex formation, and the final concentration of TAT complexes, significantly increased as FVIII levels were elevated from 100% to 400% FVIII activity. TEG analysis of fibrin and clot formation showed that as FVIII levels were elevated, the time to initial fibrin formation decreased and the rate of fibrin formation increased. The acute elevation of circulating FVIII to 400% FVIII activity resulted in significantly decreased times to vessel occlusion. Prolonged elevation of FVIII activity did not significantly affect time to vessel occlusion.
Conclusion
Acute elevations in FVIII levels result in a non-linear thrombogenic effect, with non-significant increases in thrombogenic risk within the physiological range (FVIII levels up to 200%). Prolonged elevation of plasma FVIII did not further increase the thrombogenic potential of elevated FVIII levels.
Keywords: Animal Models, Arterial Thrombosis, Factor VIII, Intravital Microscopy
Introduction
Factor VIII (FVIII) is an essential cofactor for blood coagulation. It circulates in the plasma complexed with von Willebrand factor (VWF), which prevents the premature proteolysis of FVIII and the binding of FVIII to phospholipid membrane surfaces (1,2). Following endothelial damage, coagulation is initiated through the exposure of tissue factor to activated factor VII (3). The extrinsic pathway of haemostasis subsequently generates trace amounts of thrombin that are sufficient for the propagation and amplification of coagulation through the intrinsic and common pathways (4). Thrombin proteolytically cleaves three sites within FVIII, activating FVIII and dissociating it from VWF (5). Activated FVIII (FVIIIa) interacts with activated factor IX to form the intrinsic tenase complex, resulting in a subsequent secondary and amplified burst of thrombin formation (6,7).
Thrombosis can occur within both arteries and veins. Arterial thrombosis most often arises as a result of atherosclerotic plaque rupture or inappropriate platelet activation, resulting in the formation of platelet-rich clots (8,9). In contrast, venous thrombosis arises as a result of alterations to the vessel wall, alterations in blood flow, or alterations in blood composition and results in the formation of fibrin-rich clots (9).
The role of elevated FVIII levels in the development of venous thrombosis has been thoroughly examined epidemiologically (10–15). Through these epidemiological studies, it has been well established that elevated FVIII levels are a strong independent risk factor for the development of venous thrombosis. Elevated FVIII levels are an independent risk factor for both the risk of a single venous thrombotic incident and the risk of recurring venous thrombotic events (11,15). In addition, FVIII has been shown to play a significant role in the development of venous thrombosis in a mouse model (16). In contrast, although epidemiological associations between elevated FVIII levels and arterial thrombosis have been demonstrated, a causative pathogenic role has not been established (17–19). In addition, there have been limited evaluations of the effects of FVIII elevation and arterial thrombosis in biological whole animal models (20,21).
The objective of this study was to examine the thrombogenic potential of FVIII in vitro and in a standardized animal model, investigating the independent effect of elevated FVIII levels on arterial thrombosis. We hypothesized that quantitative variability in FVIII levels would contribute to the development of arterial thrombosis in a concentration dependent manner. We also wished to evaluate whether there were differences in the thrombogenic potential of acute versus extended elevations of FVIII. The thrombogenic effect of variously elevated FVIII levels was examined both in vitro and in vivo, through the measurement of thrombin generation by quantification of thrombin-antithrombin complex formation, thromboelastography, and ferric chloride induced cremaster arteriolar injury.
Methods
Animals
Eight to ten week old C57Bl/6 normal and C57Bl/6 FVIII exon 16-disrupted (FVIII−/−) mice (22,23) were used for all experiments. All mouse protocols were reviewed and approved by the Queen’s University Animal Care Committee.
Recombinant Human FVIII
Recombinant human FVIII (rhFVIII; Kogenate FS, Bayer) was used in the elevation of FVIII levels in all in vitro and in vivo studies. The lyophilized FVIII was dissolved in distilled water, according to the manufacturer’s instructions, to a concentration of 200 IU/ml and stored at −80°C until use.
FVIII Activity Measurement
Plasma FVIII activity was assessed using a one-stage FVIII clotting assay on an automated coagulometer (General Diagnostics Coag-A-Mate), using human FVIII deficient plasma (Precision Biologics) and TriniCLOT PT HTF reagent (Kordia). The FVIII levels were measured against a human plasma standard.
Thrombin-Antithrombin Complex Generation and Measurement
Immediately prior to thrombin-antithrombin (TAT) complex generation, recombinant human FVIII was added directly to C57Bl/6 FVIII−/− mouse platelet poor plasma. Plasma samples were incubated at 37°C for two minutes. Diluted tissue factor (1:30000 final dilution, ~3 pM; Innovin) was added to the plasma and the samples incubated for 2 minutes. Calcium chloride (0.20 M) was added to the plasma. At specific time points, quenching solution (50 mM EDTA, 10 mM L-benzamidine in 2 mM HEPES, 150 mM NaCl, pH 7.4, 10 mM D-Phe-Pro-Arg chloromethylketone in 0.01 N HCl) was added to inhibit the formation of thrombin. The samples were vortexed for 10 seconds and centrifuged for 3 minutes at 15000g; the supernatants were frozen at −80°C until tested (24). The concentration of TAT complexes was quantified via a TAT enzyme-linked immunosorbent assay (ELISA) kit (Affinity Biologics). FVIII levels were measured by one-stage FVIII clotting assay to confirm FVIII activity in the samples.
Thromboelastography
A thromboelastograph coagulation analyzer 5000 (Haemoscope Corp.) was used for all thromboelastography (TEG) experiments. In light of plasma volume constraints, these studies were performed on human, and not mouse, plasma samples. Immediately before samples were assayed by TEG, recombinant human FVIII (rhFVIII) was added directly to human FVIII deficient platelet-poor plasma (Precision Biologics). Plasma was added to the TEG cup and incubated at 37°C for two minutes. Diluted tissue factor (1:30000 final dilution, ~3 pM; Innovin) was added to the plasma and incubated for 2 minutes at 37°C. Finally, calcium chloride (0.20 M) was added to the plasma and the TEG immediately started. Samples were run for 90 minutes. Factor VIII activity in the spiked samples was confirmed by one-stage FVIII clotting assay.
Blood Collection from Mice
Mice were anaesthetized with isofluorane/oxygen. Blood samples were obtained, using uncoated microhematocrit capillary tubes (Fisher Scientific), via the retro-orbital plexus and mixed with one-tenth volume 3.2% sodium citrate. Samples were centrifuged, for five minutes, at 11000g at room temperature; the resulting platelet-poor plasma was stored at −80°C until tested.
Ferric Chloride-Induced Thrombosis
Intravital microscopy was performed with a trinocular Wild-Leitz ELR-intravital microscope (Leica Microsystems Canada) that was fitted with both transmitted (50 W halogen) and fluorescence (50 W mercury incidence) light accessories, as well as an epifluorescent filter cube 540/25 (528–553 nm). A Hamamatsu ORCA ER video camera was used to capture images of thrombosis formation. Analysis of the formed thrombi and fluorescence intensity accumulation was performed using Image ProPlus, Version 6.0 (Media Cybernetics). To facilitate visualization of the formation of the in situ thrombus, platelets were fluorescently labeled in vivo through the injection of rhodamine 6G (25 mg/kg; Sigma-Aldrich).
Ferric chloride injury was induced as described previously (25). Briefly, male mice were anaesthetized with an intraperitoneal injection of ketamine/xylazine/atropine. The right external jugular vein was cannulated and the cremaster muscle exteriorized. The muscle was superfused with 37°C saline solution. Arterioles ranging in size from 50 to 75 µm were selected for observation. The application of 10% ferric chloride soaked filter paper for three minutes was used to induce vessel injury. Following vessel injury, the muscle was flushed with 37°C saline, and the injured area in a single arteriole observed for 40 minutes. The time to vessel occlusion with thrombus and accumulated fluorescence intensity at 5 minute intervals were documented.
Elevation of Circulating FVIII Levels
ACUTE ELEVATION OF FVIII LEVELS
Circulating FVIII levels were acutely and transiently elevated in FVIII−/− mice with the infusion of rhFVIII, following the surgery preparation for intravital microscopy and ten minutes prior to ferric chloride-induced injury to the cremaster arterioles. The amount of FVIII required to elevate circulating FVIII levels to approximately 100% FVIII activity was calculated according to the manufacturer’s dosage recommendations, and plasma FVIII levels were confirmed by measurement of FVIII activity in a subset of mice. Factor VIII levels of approximately 200% and 400% were achieved with the infusion of correspondingly increased amounts of rhFVIII, and again plasma FVIII levels were checked in a subset of mice.
EXTENDED ELEVATION OF FVIII LEVELS
Longer term elevations of FVIII levels (3 weeks) were achieved in male C57Bl/6 normal mice though hydrodynamic tail vein injections (25) of the pSC11 plasmid (26) containing B-domain deleted murine Fviii cDNA (27) and the strong synthetic, liver specific ET promoter (28).
Statistical Analysis
Data are presented as mean values plus or minus SEM. The student t test, one-way analysis of variance (ANOVA) with Tukey’s post-test, and two-way ANOVA were performed for statistical analysis. Vessel occlusion times exceeding 40 minutes were recorded as 40 minutes.
Results
Thrombin-Antithrombin Complex Formation
Thrombin generation, and thus potential thrombogenicity, can be evaluated indirectly through the measurement of thrombin-antithrombin (TAT) complexes (29). FVIII levels were elevated in mouse platelet poor C57Bl/6 FVIII−/− plasma, and coagulation stimulated through the addition of tissue factor and calcium chloride. The time course of TAT complex formation over 25 minutes was assessed, and the final concentration of formed TAT complexes determined. In addition, the propagation rate of TAT complex formation was determined from the slope of the TAT complex formation curve from 5 minutes to 15 minutes.
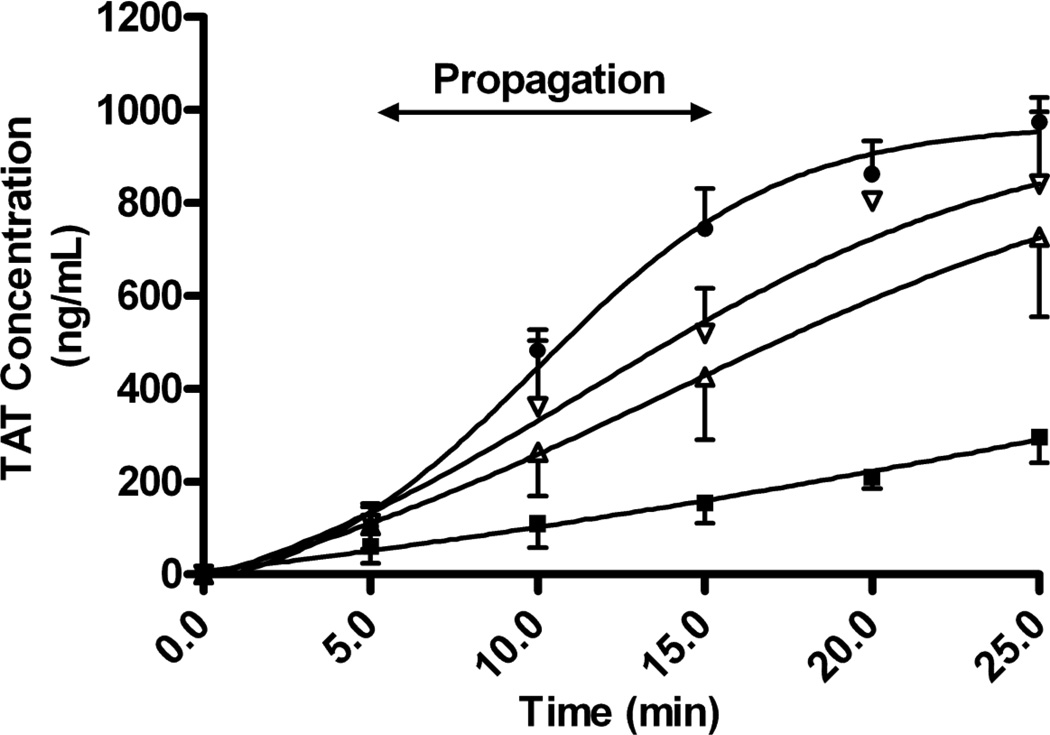
The rate with which TAT complexes formed and the concentration of TAT complexes at the final time point were increased in samples as FVIII levels were elevated from 0% FVIII activity to 400% FVIII activity (Figure 1). Elevation of FVIII levels above 0% FVIII activity resulted in significantly increased TAT complex propagation rates (P<.01, one-way ANOVA). Variability of the TAT assay results in the six replicate experiments was small, and while there was a trend for increased TAT complex generation with increasing FVIII concentrations, the propagation rate of TAT complex generation was not statistically significant with the elevation of FVIII levels from 100% to 200% (32.0 ng/ml/min and 40.9 ng/ml/min, respectively; P>.05, Tukey’s post test) or with the elevation of FVIII levels from 200% to 400% (40.9 ng/ml/min and 64.1 ng/ml/min, respectively; P>.05, Tukey’s post test). The propagation rate of TAT complex generation was only statistically increased between the 100% and 400% FVIII activity samples (P<.05, Tukey’s post test).
Figure 1. Effect of elevated FVIII levels on the formation of TAT complexes.
FVIII levels were elevated in C57Bl/6 FVIII−/− plasma through the addition of rhFVIII: 0% FVIII activity (■), 100% FVIII activity (▲), 200% FVIII activity (▼), and 400% FVIII activity (♦). Coagulation was stimulated through the addition of tissue factor and calcium chloride. The concentration of formed TAT complexes was quantified using TAT complex ELISA. The propagation rate, calculated from the slope between 5 minutes and 15 minutes of the TAT complex generation curve, was significantly increased in samples with 400% FVIII activity, when compared to samples with 100% FVIII activity (P<.05, Tukey’s post test). The error bars are representative of the standard error of the mean of 6 experiments.
The TAT complex concentration determined at the final time point was significantly increased in the samples with 400% FVIII activity when compared to the TAT complex concentration in the samples with 100% FVIII activity (974.7±52.8 ng/ml and 726.2±170.4 ng/ml, respectively; P<.05, Tukey’s post test). Nevertheless, in samples with a 200% FVIII level, the TAT complex concentration at the final time point was not significantly increased when compared to samples with a FVIII level of 100% (840.9±156.5 ng/ml and 726.2±170.4 ng/ml, respectively; P>.05, Tukey’s post test) and was similarly not significantly decreased when compared to samples with a FVIII level of 400% (P>.05, Tukey’s post test).
Thromboelastography Evaluation of the Thrombogenic Effect of Elevated FVIII
Thromboelastography (TEG), a global coagulation assay (30), was performed to examine the thrombogenicity of FVIII elevations in FVIII deficient plasma. Due to sample volume limitations, the thrombogenic effect of FVIII elevations was assessed in commercially available human FVIII deficient plasma (Precision Biologics) rather than in plasma from mice. Of note, assays of TAT complex formation in human plasma gave similar results to those obtained in mouse plasma (data not shown).
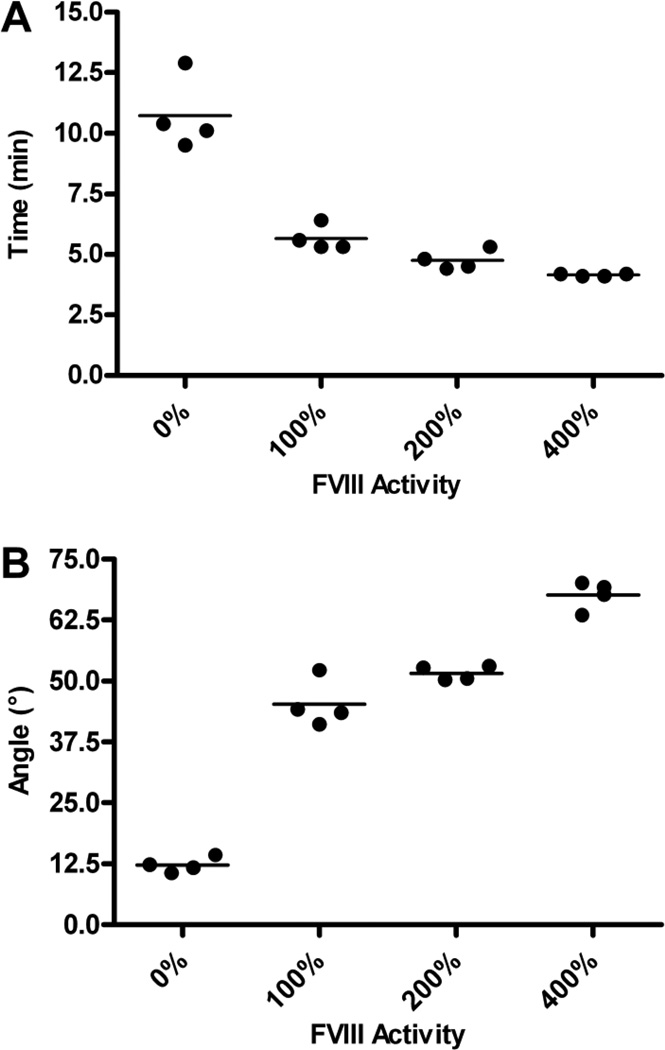
Two TEG parameters were assessed: R-value and α-angle. The R-value, which is indicative of the time to initial fibrin formation (31), was significantly decreased in the presence of FVIII when compared to samples in which FVIII was absent (Figure 2A; P<.001, one way ANOVA). The elevation of FVIII to 200% resulted in a significant decrease in the R-value when compared to samples with a FVIII level of 100% (4.75±0.20 min and 5.65±0.26 min, respectively; P<.05, Tukey’s post test). Furthermore, samples with 400% FVIII activity had significantly decreased R-values compared to samples with 200% FVIII (4.15±0.03 min and 4.75±0.20 min, respectively; P<.001).
Figure 2. Effect of elevated FVIII levels on time to clot initiation and rate of clot formation.
FVIII levels were elevated in human FVIII deficient plasma through the addition of rhFVIII. Coagulation was stimulated through the addition of tissue factor and calcium chloride and two TEG parameters examined: R-value and α angle. (A) Time to clot initiation. Elevated FVIII levels significantly decreased the R-value, which is indicative of time to clot initiation (P<.001, one way ANOVA). Each symbol represents one sample. (B) Rate of clot formation. Elevated FVIII levels significantly increased the α angle, which is indicative of the rate of fibrin formation and an indirect measure of the rate of clot formation (P<.001, one way ANOVA). Each symbol represents one sample.
The α angle reflects the rate of fibrin formation (31). Elevation of FVIII levels resulted in significantly increased α angles and, thus, significantly increased clot formation rates, when compared to samples with 0% FVIII activity (Figure 2B; P<.001, one way ANOVA). The elevation of FVIII levels from 100% to 200% did not significantly increase the α angle (45.3±2.4° and 49.7±1.7°, respectively; P>.05, Tukey’s post test). In contrast, there was a significant increase in α angle when FVIII levels were increased from 100% to 400% (45.3±2.4° and 67.6±1.5°, respectively; P<.001, Tukey’s post test). Additionally, the elevation of FVIII levels from 200% to 400% also resulted in a significant increase in the rate of fibrin formation (51.7±0.7° and 67.6±1.5°, respectively; P<.001, Tukey’s post test).
Thrombogenic Effect of Acute FVIII Elevations in vivo
The in vivo thrombogenic effect of FVIII elevation was examined using a cremaster arteriolar injury model, in which real-time thrombus growth can be quantified. Circulating FVIII levels were acutely and transiently elevated in C57Bl/6 FVIII−/− male mice through FVIII protein (rhFVIII) infusion. Elevation of FVIII levels to approximately 100% FVIII activity was confirmed in a separate set of mice that did not undergo surgery or ferric chloride induced injury.
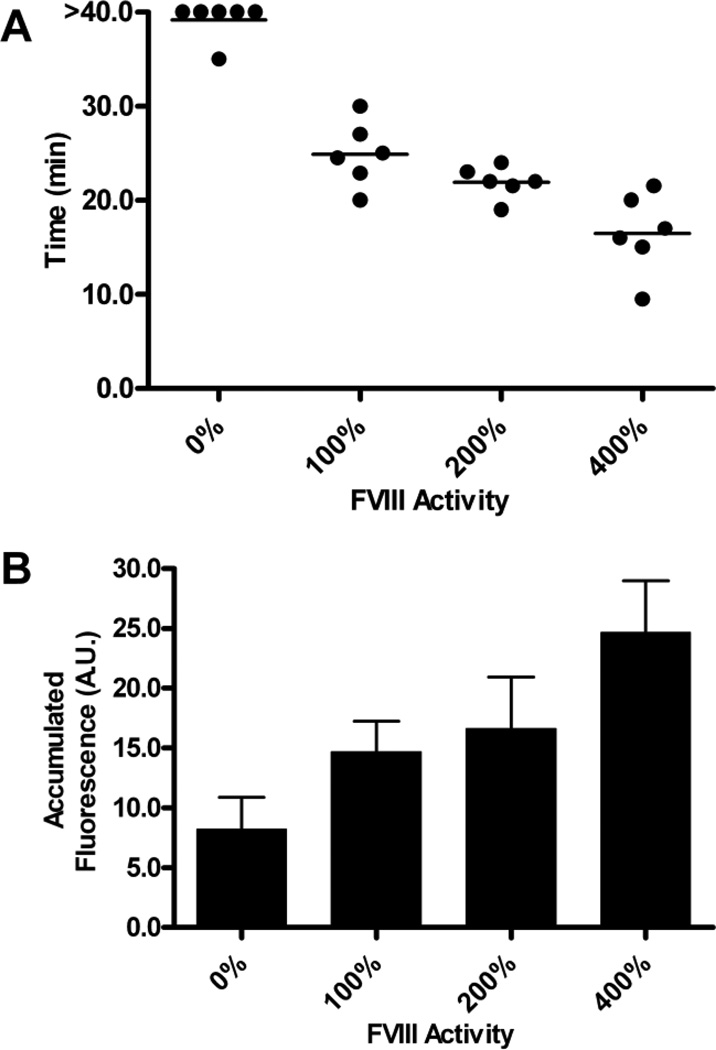
Following ferric chloride induced injury of arterioles within the cremaster, the time to vessel occlusion was assessed (Figure 3A). Elevation of circulating FVIII levels resulted in significantly decreased times to occlusion (P<.01, one way ANOVA). C57Bl/6 FVIII−/− mice with FVIII levels raised to 100% (102.0±4.63%; N=5) had a mean time to occlusion that was similar to normal C57Bl/6 mice (24.9±1.4 min and 23.9±1.2 min, respectively; P>.05, t test). Elevation of circulating FVIII levels to 200% (193.4±3.6%; N=5) resulted in times to vessel occlusion (21.9±0.7 min) that were not significantly decreased when compared to those mice with FVIII levels of 100% (P>.05; Tukey’s post test). In contrast, when FVIII levels were elevated to 400% (397.9±2.4%; N=5), the mean time to occlusion (16.5±1.7 min) was significantly decreased when compared to mice with FVIII levels of 100% and 200% (P<.01 and P<.05, respectively, Tukey’s post test).
Figure 3. Time to vessel occlusion and platelet accumulation at sites of endothelial damage in mice with acutely elevated circulating FVIII levels.
Cremaster muscle arterioles (50–75 µm in diameter) were injured through the application of 10% ferric chloride for 3 minutes. Circulating FVIII levels were elevated in FVIII−/− mice through the infusion of full-length rFVIII, prior to the application of ferric chloride. Vessels were observed for 40 minutes. (A) Time to vessel occlusion. Mice with 400% FVIII activity had vessels that occluded significantly more quickly than mice with 100% FVIII activity (P<.05, Tukey’s post test). Each symbol represents one mouse. (B) Total fluorescence accumulation. The area under the curve of fluorescence intensity was determined as a surrogate measure of platelet accumulation. Total fluorescent accumulation was not significantly increased in mice with 400% FVIII activity, when compared to mice with 100% FVIII activity. The error bars are representative of the standard error of the mean of 6 experiments.
Fluorescence intensity analysis was performed at 5-minute intervals as a surrogate measure of platelet accumulation within the injured vessel. Total fluorescence accumulation was determined, through the examination of the area under the fluorescence intensity curve. Elevation of circulating FVIII levels above 0% FVIII activity resulted in significantly increased platelet accumulation following vessel damage (Figure 3B; P<.05, one-way ANOVA). Interestingly, elevation of FVIII levels to 200% and 400% resulted in an insignificant increase in the total platelet accumulation, when compared to the total platelet accumulation seen in mice with a FVIII level of 100% (P>.05, Tukey’s post test).
Thrombogenic Effect of Extended Elevation of FVIII Levels
Finally, to evaluate the thrombogenic potential of a longer term elevation of FVIII, FVIII levels in C57Bl/6 mice were elevated through the hydrodynamic delivery of the pSC11-ET-mFviii plasmid, containing the murine B-domain deleted FVIII cDNA (Table 1). Peak FVIII levels were observed at day 7. Mean FVIII levels on day 21, the time point at which the thrombogenic effect of the elevated FVIII levels was evaluated using intravital microscopy, were 315±30% FVIII activity. VWF antigen (VWF:Ag) was also examined and, while it was decreased compared to unmanipulated C57Bl/6 mice, was within normal range. Sequential TAT assays were performed alongside the FVIII activity and VWF antigen assays and showed a small decrease between the day 7 (105.4 ng/mL) and day 21 (91.9 ng/ml) time points following the FVIII transgene injection.
Table 1. Plasma FVIII Activity, VWF Antigen, and Circulating TAT concentration following hydrodynamic delivery of the pSC11-ET-mFViii plasmid to C57Bl/6 normal mice.
Results represent mean values +/− SEM (N=4 mice).
| Day 7 | Day 14 | Day 21 | |
|---|---|---|---|
| FVIII (% Activity) | 535.3±76.9 | 353.7±69.0 | 314.2±29.5 |
| VWF:Ag (U/ml) | 0.71±0.24 | 0.61±0.15 | 0.56±0.17 |
| TAT (ng/ml) | 105.4±58.1 | N/A | 91.9±41.2 |
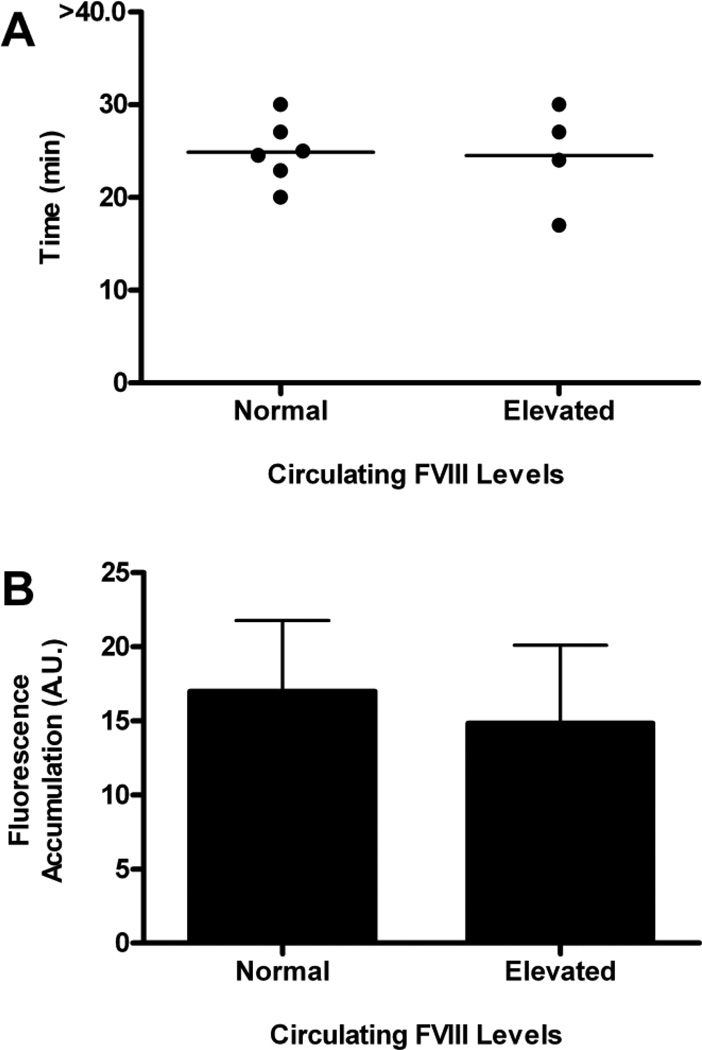
The thrombogenic potential of the extended elevation of FVIII levels was assessed using the ferric chloride-induced arteriolar injury model. C57Bl/6 mice that had FVIII levels elevated to greater than 300% FVIII activity had a mean time to vessel occlusion that was similar to that seen in unmanipulated C57Bl/6 mice (Figure 5A; 24.5±2.8 min versus 23.9±1.2 min; P>.05, t test). Total fluorescence accumulation, determined from the area under the fluorescence intensity curve, revealed that platelet accumulation at the site of arteriolar damage in C57Bl/6 normal mice with elevated FVIII levels was similar to the amount of platelet accumulation in normal C57Bl/6 mice (Figure 5B), as well as the mice that had FVIII levels acutely elevated to 200% activity (P>.05, one-way ANOVA).
Discussion
Epidemiological studies have established that elevated FVIII levels are a strong independent risk factor for the development of venous thrombosis (11,15). Associations between FVIII elevation and arterial thrombosis have also been established in epidemiological studies, but a direct pathogenic role for elevated FVIII levels in arterial thrombosis has not been documented (17–19). In addition, limited biological animal model examinations of the thrombogenic potential of elevated FVIII levels have been evaluated (20,21). In this study, we have demonstrated that while elevations of FVIII do indeed increase thrombin generation in vitro, as would be expected, this is not a linear association. Furthermore, in a ferric chloride induced arteriolar injury model, in which FVIII is transiently elevated in FVIII−/− mice through the infusion of recombinant human FVIII, there is no significant increase in thrombogenicity when FVIII levels are transiently elevated from 100 to 200% and no evidence of enhanced platelet accumulation between 100 and 400% FVIII levels. Finally, extended elevations of FVIII activity did not increase in vivo arteriolar thrombogenicity in this study.
Two in vitro assays were performed to examine the thrombogenic effect of FVIII elevations: TAT complex formation and thromboelastography. The evaluation of TAT complex formation allows for the indirect assessment of coagulation activation and thrombin generation and has been validated for use in mouse plasma (32). In contrast, thromboelastography quantifies the global hemostatic effect of elevated FVIII levels in the presence of normal levels of all other blood proteins, through the evaluation of viscoelastic changes that occur during coagulation (30). Although these two assays measure thrombogenicity under different conditions, the two assays have been shown to be complementary when used to assess whole blood (24).
Following the stimulation of coagulation through the addition of tissue factor and calcium chloride, the rate with which TAT complexes formed and the final TAT complex concentration were increased as FVIII levels were elevated. However, of note, both the propagation rate and the final TAT complex concentrations were only significantly increased in samples with FVIII levels of 400% as opposed to 100%. Elevation of FVIII levels from 100% to 200% and from 200% to 400% resulted in insignificant increases in TAT complex formation. A similar non-linear thrombogenic response to FVIII elevation was observed in samples evaluated by TEG, where the rate with which fibrin formation occurred (α angle) was significantly increased when FVIII levels were elevated from 200% to 400%, but not when levels were elevated from 100% to 200%. In summary, the in vitro results from the TAT and TEG assays both indicate that the thrombogenic potential of FVIII elevation likely does not increase in a linear manner. Rather, it appears that while FVIII elevation does increase the thrombogenic potential in these in vitro systems, large increments in FVIII, beyond physiological concentrations, are required to produce this effect.
In order to examine the thrombogenic potential of elevated FVIII levels in the presence of platelets and flowing blood, intravital microscopy was performed and ferric chloride-induced cremaster arteriolar damage assessed. As FVIII levels were elevated through the infusion of FVIII, the time to arteriolar occlusion significantly decreased. However, elevations of FVIII from 100 to 200% did not result in a significant decrease in time to vessel occlusion. Furthermore, we observed no increase in platelet accumulation in this thrombosis model at FVIII levels between 100 and 400%. Thus, as circulating FVIII levels were elevated, thrombosis occurred more quickly at levels greater than 200%, but the size of the thrombotic occlusions remained similar.
This latter finding is consistent with the recent observations of Machlus et al, who examined the role of elevated FVIII activity in an animal protocol involving vascular injury of varying intensity. In this carotid artery injury model, the thrombi in both control mice and mice with elevated FVIII activity did not differ significantly in size (21). In contrast, Kawasaki et al found that photochemical injury to the carotid artery resulted in significantly enhanced thrombus size in the presence of elevated FVIII levels {{214}}. The reason for these conflicting results may arise from the different injury methods used and/or the different mouse genotype. Kawasaki et al examined the effect of FVIII elevation in FVB mice, whereas this study, and that of Machlus et al, used mice on a C57Bl/6 background. In addition, the mechanism through which thrombosis is initiated may differ between photochemically induced injury and ferric chloride induced injury to the vessels of choice {{487}}.
While both this study and that of Machlus et al used ferric chloride to injure the vasculature, the severity of ferric chloride induced injury differed. In their study, Machlus et al found that elevated FVIII decreases the time to vessel occlusion when the degree of injury to the carotid artery was moderate (2 minutes injury time) but not when the degree of injury was severe (3 minute injury time). In contrast, we found that elevated FVIII levels enhance arterial thrombosis following severe injury (3 minutes injury time) of the cremaster muscle arterioles {{987}}. Importantly, injury of the cremaster arterioles is initiated through the application of ferric chloride soaked filer paper to the cremaster tissue, whereas injury of the carotid artery is initiated through the application of ferric chloride soaked filter paper directly on the artery. The results obtained using these two different injury models in two different vascular beds need to be interpreted with caution, and it is possible that the extent of subendothelial exposure could be similar in this study and that of Machlus et al.
Although ferric chloride vessel injury provides a reproducible and quantifiable model for the examination of arteriolar thrombosis formation, it, like all other established small animal in vivo thrombosis models, has limitations. This mode of injury results in severe endothelial damage, exposing collagen within the subendothelial matrix and provoking platelet accumulation through a VWF-mediated mechanism (33,34). Importantly though, ferric chloride induced injury of arterioles models acute thrombosis observed following vascular injury. The denudation of the endothelium exposes smooth muscle cell tissue factor and results in the formation of platelet-rich thrombi similar to those seen in patients with arterial clots (35,36).
In order to assess the thrombogenic potential of extended elevations of FVIII levels, hydrodynamic injections of a mouse FVIII expression plasmid were performed. These injections resulted in significant increases in circulating FVIII activity in the mice for the three weeks prior to the intravital microscopy analysis. In contrast to the experiments in which FVIII was elevated transiently, the extended elevation of FVIII to levels of approximately 300% did not significantly affect either determinant of thrombogenicity (time to occlusion and platelet accumulation). The extended elevation of FVIII activity resulted in times to occlusion that were the same as C57Bl/6 mice with 100% FVIII activity.
It is unclear as to why extended elevation of FVIII did not significantly affect the time to vessel occlusion. Indeed, it was thought that the extended elevation of FVIII might be more reflective of the relationship between FVIII and thrombosis documented in vivo. The transient elevation of FVIII through the infusion of recombinant FVIII, in contrast, would be more indicative of the situation that would arise in hemophiliac patients receiving supratherapeutic levels of FVIII. It is important to recognize that a number of potential ‘confounders’ of these studies should be kept in mind. Most importantly, the FVIII being expressed in the hydrodynamic studies is different from that used in the FVIII infusion studies. The infused FVIII is a full-length human recombinant FVIII while the hydrodynamically-delivered transgene encodes a B-domain deleted murine FVIII. Furthermore, the fact that the murine BDD FVII is being expressed predominantly from hepatocytes may also influence the procoagulant activity of the protein. Finally, the profile of FVIII levels will be very different between the two systems: relatively stable in the transgenic studies and transiently elevated in the infusion study. Thus, for several reasons, caution should be used in directly comparing the in vivo hemostatic effects of these two proteins under these different conditions.
The etiology of arterial thrombosis is complex and multifactorial, with arterial thrombosis arising as a result of multiple genetic and environmental factors. Thus, the lack of thrombotic effect associated with the extended elevation of FVIII may be due, in part, to the absence of additional risk factors. Had the effect of longer term elevation of FVIII been examined in the context of atherosclerotic vasculature, a significant decrease in time to vessel occlusion may have been observed. Thus, it is possible that extended elevation of FVIII does act as a significant prothrombotic stimulus but that it does not act as one independently.
It is possible that FVIII plays a role in the maintenance of VWF homeostasis and the presence of extended elevation of FVIII results in the adjustment of the hemostatic system to prevent the occurrence of inappropriate thrombosis. It has been shown in vitro, under shear stress conditions, that FVIII accelerates the cleavage of VWF by ADAMTS13. Specifically, FVIII preferentially accelerates the cleavage of high molecular weight multimers of VWF, which are those that are more hemostatically active (37). In addition, it has been shown that the presence of platelets synergistically accelerates the proteolysis of VWF multimers, predicting that the presence of elevated FVIII levels in vivo would act to decrease the presence of circulating high molecular weight multimers of VWF, decreasing the risk of inappropriate thrombosis (38). Recently, Cao et al showed that the hydrodynamic delivery of FVIII elevated circulating FVIII to supraphysiological levels, with no significant effect on VWF antigen levels, which resulted in the decreased ratio of high molecular weight multimers to low molecular weight multimers (39). Thus, it is possible that the extended elevation of circulating FVIII may have resulted in accelerated proteolysis of VWF, minimizing the thrombotic potential of the FVIII.
In this series of complementary in vitro and in vivo studies, we have demonstrated that while elevations of FVIII do result in increased thrombogenicity, the details of this effect are complex. First, within the physiological range of FVIII levels between 100 and 200%, there is no significant increase in thrombogenicity in any of the in vitro or in vivo assay systems. Second, the in vivo tests of thrombogenicity showed no evidence of enhanced platelet accumulation with increasing FVIII and modest evidence of accelerated thrombus development with FVIII levels of 400%. Finally, we also found no evidence for a significant prothrombotic influence after 3 weeks with FVIII levels greater than 300%. These results are also supported in part by recent observations relating to a lack of effect of increased FVIII on thrombus size (21). Thus, overall, there is very little evidence in these studies to incriminate a linear increase in thrombogenic risk with increasing levels of FVIII, and it would appear that FVIII levels have to reach substantially greater than 200% before there is any evidence of an increased risk. These results suggest that the contribution of transient FVIII increases to the likelihood of arterial thrombogenesis is small, and that while clinical replacement therapies need to aim for normal or modestly elevated FVIII levels, large transient FVIII increments are likely not a major thrombotic risk factor. In addition, it appears that extended elevation of circulating FVIII levels does not independently increase arterial thrombotic risk, although it may act as a significant prothrombotic stimulus in the presence of additional genetic or environmental risk factors for arterial thrombosis.
Figure 4. Time to vessel occlusion and platelet accumulation following endothelial damage in mice with extended elevation of circulating FVIII levels.
Injury of cremaster muscle arterioles (50–75 µm in diameter) was induced through the application of 10% ferric chloride soaked filter paper and injured arterioles observed for 40 minutes. FVIII levels were elevated in C57Bl/6 normal mice through hydrodynamic injection of the pSC11-ET-mFviii-plasmid, containing the murine B-domain deleted FVIII cDNA. (A) Time to vessel occlusion. Extended elevation of FVIII levels did not significantly decrease time to vessel occlusion. Each symbol represents one mouse. (B) Total fluorescence accumulation. Platelet accumulation was determined from the area under the fluorescence intensity curve. Total platelet accumulation was not significantly increased in mice with elevated FVIII levels. The error bars are representative of the standard error of the mean (N=4–6 mice).
Extra Table.
What is known on this topic?
|
What this paper adds?
|
Acknowledgements
This study was supported by funds from the Heart and Stroke Foundation of Ontario (NA6386). MG is the recipient of a Queen’s University Graduate Scholarship and the Dr. Robert J. Wilson Graduate Fellowship. DL holds a Canada Research Chair in Molecular Hemostasis. We thank Luigi Naldini for the synthetic ET promoter. We thank Kate Sponagle for her help with the mouse breeding and maintenance.
Footnotes
Contribution: MG designed, performed, analyzed, and interpreted research, performed statistical analysis, and wrote the manuscript; JM performed research; and DL designed research, interpreted data, and wrote the manuscript.
Conflicts of Interest: the authors have no conflicts of interest.
References
- 1.Vlot AJ, Koppelman SJ, van den Berg MH, et al. The affinity and stoichiometry of binding of human factor VIII to von Willebrand factor. Blood. 1995;85:3150–3157. [PubMed] [Google Scholar]
- 2.Lollar P, Hill-Eubanks DC, Parker CG. Association of the factor VIII light chain with von Willebrand factor. J Biol Chem. 1988;263:10451–10455. [PubMed] [Google Scholar]
- 3.Monroe DM, Hoffman M, Roberts HR. Transmission of a procoagulant signal from tissue factor-bearing cell to platelets. Blood Coagul Fibrinolysis. 1996;7:459–464. doi: 10.1097/00001721-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Butenas S, van 't Veer C, Mann KG. Evaluation of the initiation phase of blood coagulation using ultrasensitive assays for serine proteases. J Biol Chem. 1997;272:21527–21533. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 5.Eaton D, Rodriguez H, Vehar GA. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986;25:505–512. doi: 10.1021/bi00350a035. [DOI] [PubMed] [Google Scholar]
- 6.van Dieijen G, Tans G, Rosing J, et al. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981;256:3433–3442. [PubMed] [Google Scholar]
- 7.Orfeo T, Brummel-Ziedins KE, Gissel M, et al. The nature of the stable blood clot procoagulant activities. J Biol Chem. 2008;283:9776–9786. doi: 10.1074/jbc.M707435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voetsch B, Loscalzo J. Genetic determinants of arterial thrombosis. Arterioscler Thromb Vasc Biol. 2004;24:216–229. doi: 10.1161/01.ATV.0000107402.79771.fc. [DOI] [PubMed] [Google Scholar]
- 9.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bank I, Libourel EJ, Middeldorp S, et al. Elevated levels of FVIII:C within families are associated with an increased risk for venous and arterial thrombosis. J Thromb Haemost. 2005;3:79–84. doi: 10.1111/j.1538-7836.2004.01033.x. [DOI] [PubMed] [Google Scholar]
- 11.Kraaijenhagen RA, in't Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost. 2000;83:5–9. [PubMed] [Google Scholar]
- 12.Schambeck CM, Grossmann R, Zonnur S, et al. High factor VIII (FVIII) levels in venous thromboembolism: role of unbound FVIII. Thromb Haemost. 2004;92:42–46. doi: 10.1160/TH04-02-0063. [DOI] [PubMed] [Google Scholar]
- 13.Koster T, Blann AD, Briet E, et al. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–155. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 14.Tsai AW, Cushman M, Rosamond WD, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) Am J Med. 2002;113:636–642. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 15.Kyrle PA, Minar E, Hirschl M, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. 2000;343:457–462. doi: 10.1056/NEJM200008173430702. [DOI] [PubMed] [Google Scholar]
- 16.Chauhan AK, Kisucka J, Lamb CB, et al. von Willebrand factor and factor VIII are independently required to form stable occlusive thrombi in injured veins. Blood. 2007;109:2424–2429. doi: 10.1182/blood-2006-06-028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortellaro M, Boschetti C, Cofrancesco E, et al. The PLAT Study: hemostatic function in relation to atherothrombotic ischemic events in vascular disease patients. Principal results. PLAT Study Group. Progetto Lombardo Atero-Trombosi (PLAT) Study Group. Arterioscler Thromb. 1992;12:1063–1070. doi: 10.1161/01.atv.12.9.1063. [DOI] [PubMed] [Google Scholar]
- 18.Tracy RP, Arnold AM, Ettinger W, et al. The relationship of fibrinogen and factors VII and VIII to incident cardiovascular disease and death in the elderly: results from the cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:1776–1783. doi: 10.1161/01.atv.19.7.1776. [DOI] [PubMed] [Google Scholar]
- 19.Meade TW, Cooper JA, Stirling Y, et al. Factor VIII, ABO blood group and the incidence of ischaemic heart disease. Br J Haematol. 1994;88:601–607. doi: 10.1111/j.1365-2141.1994.tb05079.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki T, Kaida T, Arnout J, et al. A new animal model of thrombophilia confirms that high plasma factor VIII levels are thrombogenic. Thromb Haemost. 1999;81:306–311. [PubMed] [Google Scholar]
- 21.Machlus KR, Lin FC, Wolberg AS. Procoagulant activity induced by vascular injury determines contribution of elevated factor VIII to thrombosis and thrombus stability in mice. Blood. 2011;118:3960–3968. doi: 10.1182/blood-2011-06-362814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi L, Lawler AM, Antonarakis SE, et al. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 23.Bi L, Sarkar R, Naas T, et al. Further characterization of factor VIII-deficient mice created by gene targeting: RNA and protein studies. Blood. 1996;88:3446–3450. [PubMed] [Google Scholar]
- 24.Rivard GE, Brummel-Ziedins KE, Mann KG, et al. Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. J Thromb Haemost. 2005;3:2039–2043. doi: 10.1111/j.1538-7836.2005.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golder M, Pruss CM, Hegadorn C, et al. Mutation-specific hemostatic variability in mice expressing common type 2B von Willebrand disease substitutions. Blood. 2010;115:4862–4869. doi: 10.1182/blood-2009-11-253120. [DOI] [PubMed] [Google Scholar]
- 26.Shi CX, Graham FL, Hitt MM. A convenient plasmid system for construction of helper-dependent adenoviral vectors and its application for analysis of the breast-cancer-specific mammaglobin promoter. J Gene Med. 2006;8:442–451. doi: 10.1002/jgm.867. [DOI] [PubMed] [Google Scholar]
- 27.Ye P, Thompson AR, Sarkar R, et al. Naked DNA transfer of Factor VIII induced transgene-specific, species-independent immune response in hemophilia A mice. Mol Ther. 2004;10:117–126. doi: 10.1016/j.ymthe.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Amendola M, Venneri MA, Biffi A, et al. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol. 2005;23:108–116. doi: 10.1038/nbt1049. [DOI] [PubMed] [Google Scholar]
- 29.Boisclair MD, Lane DA, Wilde JT, et al. A comparative evaluation of assays for markers of activated coagulation and/or fibrinolysis: thrombin-antithrombin complex, D-dimer and fibrinogen/fibrin fragment E antigen. Br J Haematol. 1990;74:471–479. doi: 10.1111/j.1365-2141.1990.tb06337.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Donnell J, Riddell A, Owens D, et al. Role of the Thrombelastograph as an adjunctive test in thrombophilia screening. Blood Coagul Fibrinolysis. 2004;15:207–211. doi: 10.1097/00001721-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Reikvam H, Steien E, Hauge B, et al. Thrombelastography. Transfus Apher Sci. 2009;40:119–123. doi: 10.1016/j.transci.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Frank RD, Weber J, Dresbach H, et al. Role of contact system activation in hemodialyzer-induced thrombogenicity. Kidney Int. 2001;60:1972–1981. doi: 10.1046/j.1523-1755.2001.00009.x. [DOI] [PubMed] [Google Scholar]
- 33.Ni H, Denis CV, Subbarao S, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denis C, Methia N, Frenette PS, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brill A. A ride with ferric chloride. J Thromb Haemost. 2011;9:776–778. doi: 10.1111/j.1538-7836.2011.04238.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Miller C, Swarthout RF, et al. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–713. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao W, Krishnaswamy S, Camire RM, et al. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proc Natl Acad Sci U S A. 2008;105:7416–7421. doi: 10.1073/pnas.0801735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skipwith CG, Cao W, Zheng XL. Factor VIII and platelets synergistically accelerate cleavage of von Willebrand factor by ADAMTS13 under fluid shear stress. J Biol Chem. 2010;285:28596–25603. doi: 10.1074/jbc.M110.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao W, Sabatino DE, Altynova E, et al. Light Chain of Factor VIII Is Sufficient for Accelerating Cleavage of von Willebrand Factor by ADAMTS13 Metalloprotease. J Biol Chem. 2012 doi: 10.1074/jbc.M112.390690. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]