Abstract
Immunization of neonates is problematic because of the immaturity of their immune system and the presence of maternal antibodies, both of which affect B cell responses. We tested the effects of co-administration of measles vaccine with a combination of TLR-3 (pI:C) and TLR-9 (ODN2216, optimized for human TLR-9) agonists on the ability to induce an effective immune response in neonatal cotton rats. TLR-9 expression in cotton rat lymphocytes was at the same low level as in human lymphocytes, which is in contrast to mice that express higher levels. TLR-3 expression levels were comparable between cotton rats, mice and humans. A combination of TLR-3 and TLR-9 agonists synergistically induced high levels of type I interferon in neonatal spleen cells and higher levels of IL-10 as compared to adult spleen cells. Previously, it was shown that type I interferon stimulates B cell generation and antibody secretion in vitro and in vivo, and that IL-10 has immunomodulatory effects. The simultaneous induction of both type I interferon and IL-10 indicated that this immunization regimen could be both effective and safe. Neonatal cotton rats did not generate neutralizing antibodies after measles vaccination in the first week of life (although a T cell response was detectable). However, co-administration of the TLR-3 and TLR-9 agonist combination with measles vaccine in neonatal cotton rats induced neutralizing antibody responses comparable to those after adult immunization. This immunization regimen was also effective in neonatal cotton rats in the presence of natural maternal antibodies, although antibody titers were lower than after immunization in the absence of maternal antibodies.
Keywords: maternal antibodies, neonatal immunization, measles vaccine, neutralizing antibodies, TLR agonists, cotton rat
Introduction
Maternal antibodies present in the circulation of neonates and infants inhibit the generation of antibody responses after immunization. This phenomenon has been documented after vaccination of both animals and humans with subunit as well as live-attenuated vaccines. One of the best documented examples of inhibition of vaccination by maternal antibodies is measles immunization. In both patients and cotton rats, which are a relevant model of measles virus pathogenesis, it was demonstrated that B cell proliferation and antibody secretion are suppressed by maternal antibodies ([1], for review [2]) although T cell responses are often detectable [3], [4], [5], [6], [7], [8]. Mechanisms of the suppression of B cell responses by maternal antibodies include neutralization of live-attenuated vaccines (and thereby reduction of antigen production) and epitope-masking (thereby interfering with recognition of antigen by B cells). Neutralization of live-attenuated vaccines is not likely because the immune response against protein vaccines is also inhibited by maternal antibodies, and B cell responses can be inhibited by non-neutralizing antibodies as well [9]. Neutralizing antibodies can only inhibit B cell responses if the constant region of the antibody is able to bind to the FcγIIB receptor (CD32) on the B cell surface [9]. Epitope masking predicts that the B cell epitopes are blocked by antibodies and inhibition of B cell responses increases in an antibody concentration-dependent manner. In contrast to predictions from this model, we demonstrated that a mix of antibodies with the same final concentration as a single antibody was more suppressive compared to single antibody treatment. This could be due to negative signaling after cross-linking of CD32 (FcγRIIB) with the B cell receptor (BCR) by a measles vaccine/maternal IgG complex [9]. Inhibition via CD32 is thought to require oligomerization of CD32, which is accomplished by cross-linking of CD32 with the BCR through complex formation of the vaccine with antibodies recognizing different epitopes on the measles vaccine. The inhibitory effect of CD32 oligomerization can be overcome by B cell stimulation, for example, via the induction of interferon α (IFNα) through a combination of TLR-3 and TLR-9 agonists [1]. IFNα binds to two receptors on the B cell surface, CD21 and IFRA [1] leading to amplification of the stimulatory signal, which overcomes the inhibitory effect of maternal antibodies in vivo.
In neonates, immunization is hampered not only by maternal antibodies but also by the immaturity of the immune system, which does not allow vaccination to the same degree as in adult animals or humans (for review [10] and [11]). However, in contrast to the inhibition by maternal antibodies that specifically targets the B cell response; the immaturity of the neonatal immune system affects cell types of both the innate and adaptive immune systems. Therefore, immunization of neonates often results in a detectable T cell response but not in an antibody response.
In this study, we asked whether a combination of TLR-3 and TLR-9 agonists would synergistically induce IFNα in neonatal cells, stimulate the neonatal B cell response, and whether stimulation would be strong enough to overcome B cell inhibition by maternal antibodies.
Materials and Methods
Cotton rats
Inbred cotton rats (Sigmodon hispidus) were purchased from Harlan Laboratories, Inc., Indianapolis). Breeding pairs were kept according to established procedures [13]. For the induction of natural maternal antibodies, 4 to 6 week-old female cotton rats were immunized subcutaneously with a 100μl volume of 105 pfu of MeV (Schwarz strain) before pairing. The titer of maternal antibodies in pups born from immunized dams was a Neutralizing Titer (NT) of 48±12. Neonates were immunized subcutaneously with 100μl of PBS containing TLR agonists at various concentrations and 105 pfu of MeV (Schwarz strain). Unless otherwise noted, four animals per group were used.
Measles virus
The Schwarz vaccine strain of measles virus was grown and titrated on Vero cells as described [14] with MEM/10% fetal calf serum. Infected cells were pelleted, resuspended in PBS and frozen. After thawing, cell debris was pelleted by centrifugation at 4°C at 15,000 ×g for 15 minutes. Supernatant was aliquoted, stored in liquid nitrogen and one aliquot was tested by plaque assay.
Immunohistochemical determination of TLR-3 and TLR-9 expression in spleen sections
Rabbit anti-human CD283 (TLR-3) and CD289 (TLR-9) polyclonal antibodies (Serotec) were confirmed to cross-react with cotton rat TLR-3 and TLR-9 on paraffin embedded cotton rat spleen sections. Subsequently, immunohistochemistry on spleens (three per group) from neonatal (3-day old) and adult cotton rats (7-week old), C57BL/6 and BALB/C mice (6-week old), and humans (20- to 40-year old accident victims; obtained from National Disease Research Interchange, Philadelphia) was performed as described [15]. The positive percentage of cells expressing TLR3 and TLR 9 from stained tissue section areas was scored by Aperio image scope positive pixel counter algorism (Aperio) and expressed as the total number of positive pixels divided by the total number of pixels.
TLR agonists
Polyinosinic-polycytidylic acid (poly(I:C) was purchased from Sigma (St. Louis, MO) and ODN 2216 from Invivogen (San Diego, CA).
Type I interferon induction and measurement by bioassay
Spleen cells were plated in triplicate in 96-well plates at 2×105 cells/well with different concentrations of TLR agonists, and the type interferon was measured as described [16]. IFN-α/β concentrations in samples were expressed as units with 1 unit being the equivalent of 1pg of IFN-α. The threshold of detection was 16 units.
Quantitative polymerase chain reaction to measure interleukin 6 and interleukin 10
Quantitative polymerase chain reaction was performed as described [16]. The primer sequences for IL-6 were 5′-TTGGCACACTTAGGCACAGC-3′ (forward) and 5′-CAAAAGGACTGGCCGAGGAC-3′ (reverse), and the plasmid pCR-script Amp SK(+)-Cotton rat IL-6 [9] was used as standard. The primer sequences for IL-10 were 5′- CATGCTTCGAGAGCTTCGGG-3′ (forward) and 5′- AGCGCTGCAATTGTCTCCTC-3′ (reverse), and the plasmid pCR-script Amp SK(+)-Cotton rat IL-10 was used as standard.
Antibody neutralization assay
Neutralizing antibody titers were determined as described [17]. Cotton rat serum samples were two-fold serially diluted and incubated with 50 pfu MeV strain NSE for one hour at 37°C in a 96-well. One hour post-incubation 104 Vero cells were added per well. Five days post-infection cytopathic effect (CPE) was determined microscopically. The titer was defined as the reciprocal of the last protective serum dilution, as calculated from duplicate measurements.
B cell ELISPOT assay
For the B cell ELISPOT assay [18], mediastinal lymph node cells, bone marrow cells or spleens from measles virus immune cotton rats four to eight weeks after s.c. immunization with 105 pfu of MeV (Schwarz strain) were used. 96 well plates (Millipore) were coated with gradient-purified, UV-inactivated MeV antigen in sodium carbonate buffer (pH 9.6) overnight at 4ºC. Serially diluted lymphoid cells were plated and cultured at 37°C in an incubator. After overnight incubation, plates were washed with PBS/0.05% Tween 20. Plates were incubated with rabbit anti-cotton rat IgG (Virion Systems) and subsequently with goat anti-rabbit alkaline-phosphatase conjugated IgG (Zymed) in PBS/10% cotton rat serum. For development of spots, plates were washed three times with PBS and TMB-HK (high kinetic 3,3′,5,5′-tetramethylbenzidine, Moss, Inc.) substrate was added. Plates were incubated at room temperature for 5 minutes and spots were counted using an ELISPOT plate reader (CTL).
T cell proliferation assay
To test for MeV-specific T cell proliferation, 100μl of 10μg/ml of gradient purified, UV-inactivated MeV (Edmonston B strain) in 200mM NaCO3 buffer (pH 9.6) was coated onto 96-well plates at 4°C overnight. Plates were washed twice with PBS and spleen cells from immunized animals were plated onto the MeV-coated and untreated wells. Two days later, spleen cells were labeled with 3H-thymidine (0.5 μCi/well) for 20 hours, then harvested and measured as described [19]. The stimulation index was calculated as counts per minute (cpm) of MeV-stimulated wells divided by the cpm of control (unstimulated) wells. The stimulation index of naïve animals is typically not higher than 2.
Statistical Analysis
Multiple comparison analysis was performed by one-way analysis of variance (ANOVA) using Graph Pad InStat 3 for Windows (GraphPad Software). The number of asterisks denotes the level of statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
The level of TLR-3 and TLR-9 expression in neonatal and adult cotton rat spleens is comparable to levels detected in human spleens
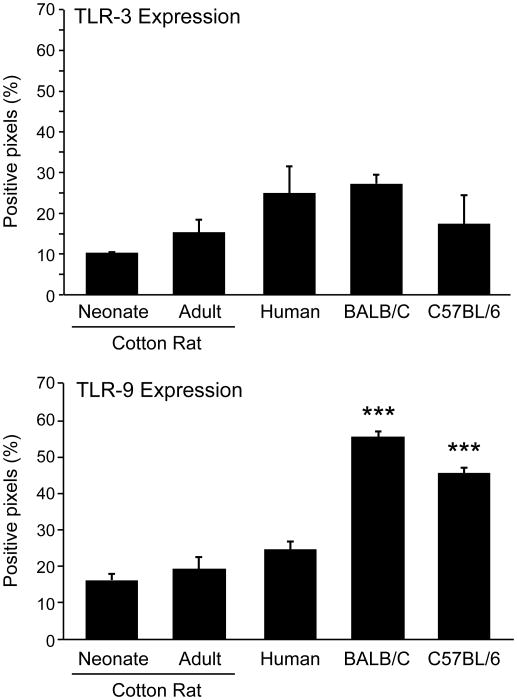
Previously, we showed that the synergistic action of TLR-3 and TLR-9 agonists results in the secretion of interferon α, resulting in enhanced B cell and antibody responses after vaccination with measles virus in adult cotton rats [1]. Now, we wanted to test whether this mode of immunization would also result in secretion of IFN α, and subsequently in an increased antibody response in neonatal cotton rats. In order to verify that neonatal cotton rats express TLR-3 and TLR-9 at levels comparable to adult animals, immunohistochemistry for these receptors was performed on sections of spleens from neonates (3 days old) and adult (7-week old) cotton rats. For comparison, sections from adult human spleens and adult mouse spleens (BALB/C and C57BL/6 strains) were used. After staining with sera specific for TLR-3 and TLR-9, slides were analyzed with the Aperio image scope positive pixel counter algorithm. Spleens from both neonatal and adult cotton rats expressed levels of TLR-3 comparable to each other and C57BL/6 mice (Figure 1). Differences in expression levels in human and BALB/C mouse spleens failed to reach statistical significance. In agreement with previous reports, TLR-9 expression levels were high in mouse spleens, and low in spleens from humans (Figure 1; p< 0.001). Similar to spleens from humans, neonatal as well as adult cotton rat spleens expressed low levels of TLR-9, which differed significantly from expression levels in mouse spleens (p<0.001).
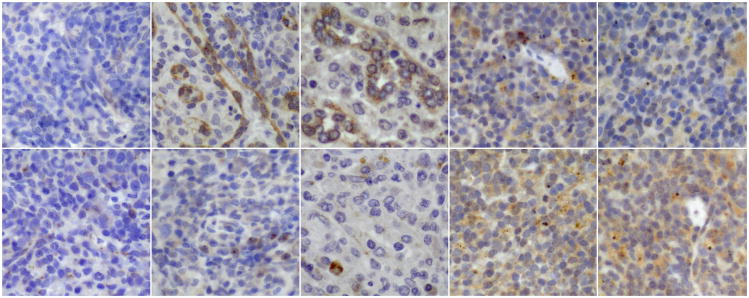
Figure 1. TLR-3 and TLR-9 expression in cotton rat, human and mouse spleen cells.
Paraffin-embedded sections of spleens (three per group) from neonatal cotton rats (3 days old), adult cotton rats (7 weeks), adult humans (20 to 40 years old), BALB/C and C57BL/6 mice (6 weeks old) were stained with cross-reactive polyclonal sera specific for human TLR-3 and TLR-9.
A. TLR-3 specific stain (brown) is shown in the top panel, and TLR-9 specfic stain (brown) in the bottom panel (400×). From left to right neonatal and adult cotton rat spleen, human spleen, BALB/C and C57BL/6 mouse spleen.
B. Positive pixels were quantified with the Aperio image scope positive pixel counter algorism. The number of asterisks denotes the level of statistical significance (***p < 0.001).
Combination of TLR-3 and TLR-9 agonists stimulates IFNα and IL-10 expression in neonatal spleen cells
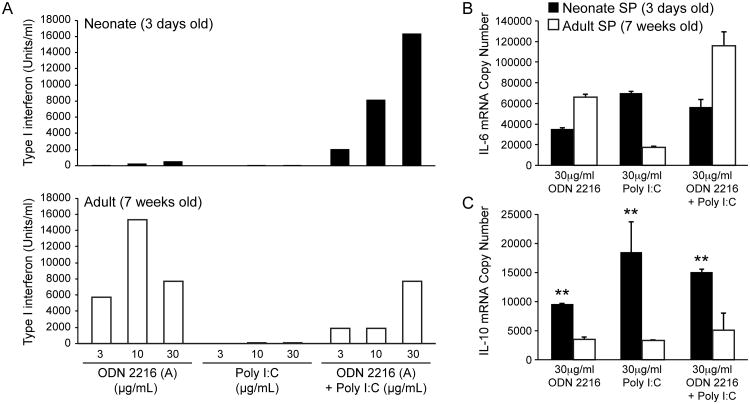
After determining expression levels of TLR-3 and TLR-9 on neonatal cotton rat spleens, we tested the response to stimulation with TLR-3 and TLR-9 agonists. Previously, the induction of IFNα by these treatments was tested on plasmacytoid dendritic cells derived from bone marrow of adult animals. However, the generation of dendritic cells involves a culture period of one week during which dendritic cells mature. To avoid possible experimental bias due to in vitro maturation, the stimulatory capacity of ODN 2216, pI:C or ODN 2216 and pI:C was tested on spleen cells from adult (7-week old) and neonatal (3-day old) cotton rats. For adult spleen cells, ODN 2216 and the combination ODN 2216/pI:C induced IFNα although no additive effect was seen for the combination (Figure 2A). In contrast, neonatal spleen cells were stimulated to a very small degree by ODN 2216 or pI:C alone. The combination of ODN 2216/pI:C, however, stimulated high levels of IFNα secretion. In contrast, all treatments induced the expression of IL-6 (Figure 2B) and IL-10 mRNA (Figure 2C) in adult and neonatal spleen cells. Furthermore, IL-10 induction was significantly increased in neonatal spleen cells versus adult spleen cells (p<0.01).
Figure 2. Cytokine induction by TLR-3 (pI:C) and TLR-9 (ODN2216) agonists in neonatal and adult cotton rat spleen cells.
Neonatal (day 3 old) and adult (week 7 old) spleen cells were stimulated with different concentrations of pI:C, ODN 2216 or a combination of both (1:1 ratio). A. Supernatants were tested for the presence of type I interferon by bioassay. (A representative experiment of three is shown). B. IL-6 mRNA was quantified by PCR from cell pellets. C. IL-10 mRNA was quantified by PCR from cell pellets. The number of asterisks denotes the level of statistical significance (**p < 0.01).
Combination of TLR-3 and TLR-9 agonists leads to increased antibody and B cell responses in neonatal cotton rats
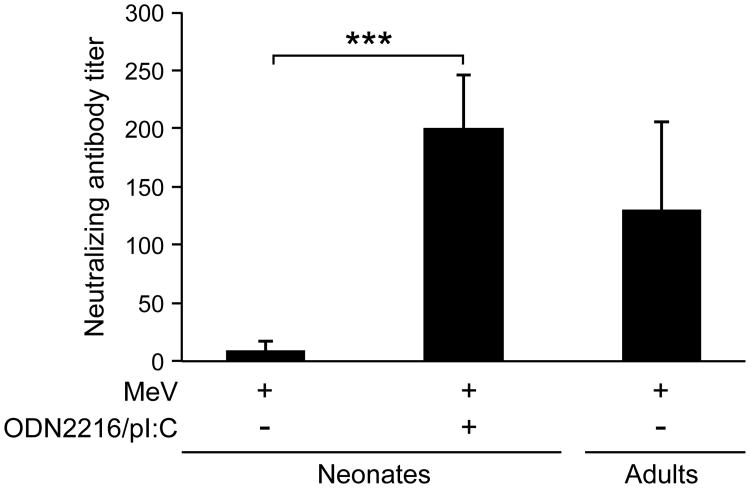
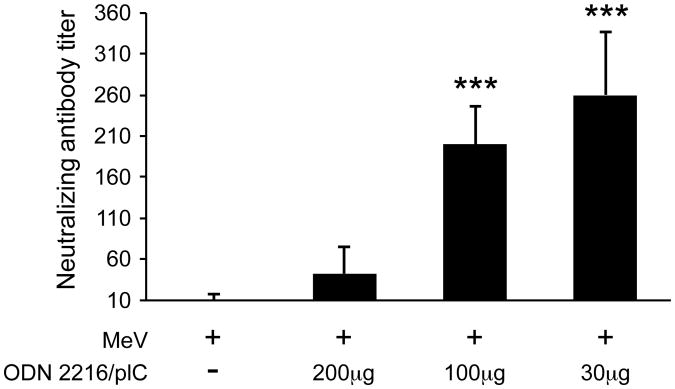
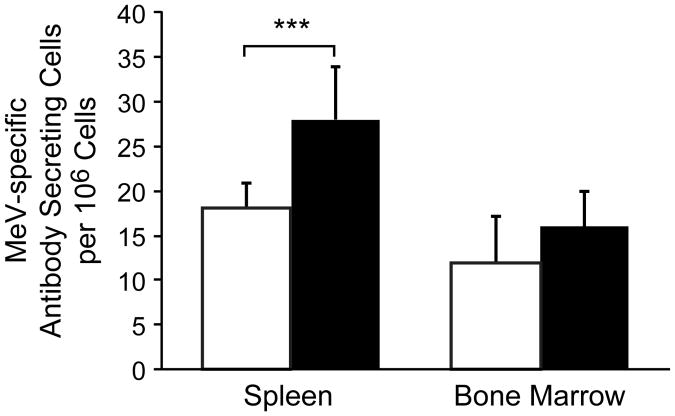
In order to test whether MeV (Schwarz strain) vaccination is able to induce an immune response in neonates, 3-day old cotton rats were immunized s.c. with MeV. After seven weeks, a T cell response (stimulation index of 15±4) had developed but only barely detectable levels of neutralizing antibodies were observed (NT of 10±7) (Figure 3). In contrast, the same immunization regimen led to high levels of neutralizing antibodies in adult cotton rats (NT of 130±75). These data are in agreement with previous reports that immunization of neonates induces a measurable T cell response but only a marginal antibody response [11]. Immunization of neonatal cotton rats with MeV (Schwarz strain) and 100μg of a combination of ODN 2216/pI:C induced a T cell response (SI of 15±2) and neutralizing antibody levels (NT of 200±46) comparable to those seen in adult animals with MeV alone. The dose of 100μg of the combination of ODN 2216/pI:C was derived from prior immunization of adult cotton rats. In order to define optimal amounts, neonatal cotton rats were immunized with MeV (Schwarz strain) and 200μg, 100μg or 30μg of the combination of ODN 2216/pI:C. After seven weeks, neutralizing antibodies were detectable in all animals immunized with the combination plus MeV, but doses of 100μg and 30μg stimulated antibody responses significantly better than 200μg (Figure 4). A dose of 30μg was slightly better than a dose of 100μg probably corresponding with the lower body weight of neonates. In adult animals, immunization with the combination of ODN 2216/pI:C and MeV led to an increased B cell response compared to immunization with MeV alone, which could be detected in spleen cells by day 12 post immunization. After immunization of neonatal cotton rats, the number of MeV-specific B cells was higher in the spleens of animals immunized with 30μg of ODN 2216/pI:C plus MeV versus animals immunized with MeV alone (Figure 5). In bone marrow, the difference failed to reach statistical significance.
Figure 3. Combination of TLR-3 and TLR-9 agonists induces neutralizing antibodies in neonatal cotton rats.
Neonatal cotton rats (3 days old) were immunized s.c. with 105 pfu measles vaccine virus (Schwarz strain) alone or in combination with 100μg of pI:C/ODN 2216 (1:1 ratio). Adult cotton rats (7 week old) were immunized with measles virus, only. After 7 weeks, neutralizing antibodies were determined from serum. The number of asterisks denotes the level of statistical significance (***p < 0.001).
Figure 4. Induction of neutralizing antibodies in neonatal cotton rats is dose dependent.
Neonatal cotton rats (3 days old) were immunized s.c. with 105 pfu measles vaccine (Schwarz strain) alone, or in combination with 200, 100 or 30μg of pI:C/ODN2216 (1:1 ratio). After 7 weeks, neutralizing antibody titers were determined from serum. The number of asterisks denotes the level of statistical significance (***p < 0.001).
Figure 5. Increase in B cell response in neonatal cotton rats with TLR-3 and TLR-9 agonists.
Neonatal cotton rats (3 days old) were immunized s.c. with 105 pfu measles vaccine (Schwarz strain) alone, or in combination with 30μg of pI:C/ODN2216 (1:1 ratio). 12 days later, B cell numbers in spleen and bone marrow were measured by ELISPOT. The number of asterisks denotes the level of statistical significance (***p < 0.001).
Measles virus vaccine combined with ODN 2216/pI:C leads to the induction of neutralizing antibodies in the presence of maternal antibodies
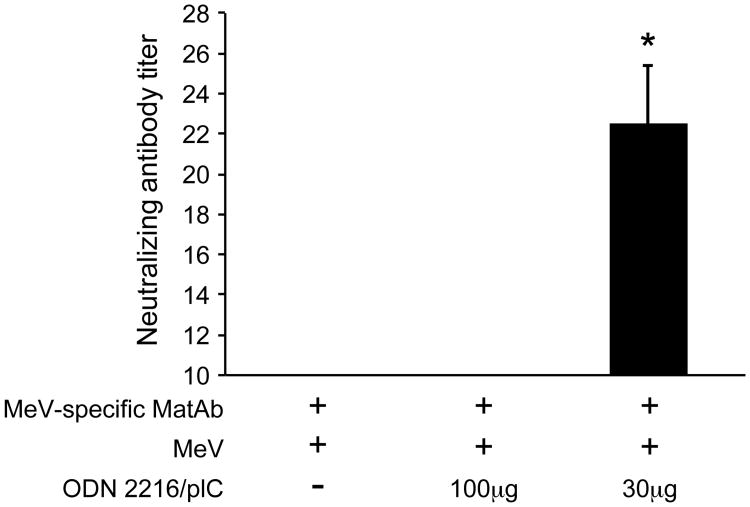
The induction of neutralizing antibodies in neonatal mice indicates that the combination of ODN 2216/pI:C induces a more mature B cell response than MeV vaccination alone. In order to test whether this immunization regimen would increase the stimulation of the neonatal B cell response in the presence of maternal antibodies, dams were immunized with MeV prior to mating. Three-day old pups with an average maternal antibody NT of 48±12 were immunized with MeV alone, MeV plus 100μg of ODN 2216/pI:C or MeV plus 30μg of ODN 2216/pI:C. After 7 weeks, neutralizing antibody titers were tested in the immunized pups. No neutralizing antibodies were detectable in animals immunized with MeV or MeV plus 100μg of ODN 2216/pI:C. However, in animals immunized with MeV plus 30μg of ODN 2216/pI:C, protective levels of neutralizing antibodies were detected.
Discussion
Immunization of newborns and infants is unsuccessful for two reasons: the immature immune system is not sufficiently stimulated by vaccines, and maternal antibodies inhibit the generation of immune (mainly B cell) responses. Both phenomena have been studied in children immunized with the measles virus vaccine. These studies have clearly demonstrated the inhibitory effect of maternal antibodies (for review [20]). With respect to the relative immaturity of the immune system in early in life, T cell responses develope after immunization [21], [5], whereas the ability to generate antibody responses develops later and improves over the first 12 months of life [22], [23], [5], [3]. These results are similar to data from other vaccination studies [11] and significant for all infectious diseases where neutralizing antibodies are crucial for protection, such as MeV infection. Since the B cell and antibody response in neonates is markedly more inhibited than the T cell response, the focus of our approach was on stimulating the neonatal B cell response. In animals with a mature immune system in which MeV-specific antibodies inhibit the B cell response, the B cell response is restored by the induction of high levels of type I interferon through a combination of TLR-3 (pI:C) and TLR-9 (ODN2216) agonists. The strong stimulatory action of IFN α is due to the fact that it utilizes both CD21 and IFNRA as functional receptors for B cell stimulation [1].
Both agonists (pI:C and ODN 2216) have been tested on human neonatal cells alone but not in combination. In comparison to adult cells, neonatal cord blood cells stimulated with pI:C [24] as well as neonatal plasmacytoid dendritic cells (pDC) stimulated with ODN 2216 [25] responded with reduced secretion of IFNα [26], but maturation could be improved with ODN 2216 based on expression of cell surface markers [27]. In a longitudinal study it was shown that TLR-9 agonist responses of ex vivo blood samples improved within the first 12 months of life [28]. A study with neonatal dendritic cells [29] confirmed the concept that a combination of TLR agonists can stimulate neonatal cells (in this case TLR-3/TLR-8 and TLR-4/TLR8). In mice, type B CpG oligodeoxynucleotides (ODN) have been tested for vaccination of neonates. Whereas type A ODN (to which ODN 2216 belongs) mainly induce secretion of IFNα by pDC, type B ODN bind directly to B cells and stimulate B cell proliferation. In neonatal mice with and without maternal antibodies, immunization with type B ODN either had no effect on antibody production [30] or induced only a small increase [31], [32], [33]. These data are in agreement with our findings in cotton rats. In an ELISPOT assay, type B ODN stimulated B cells but was without effect in stimulating antibody responses after MeV vaccination in the presence of maternal antibodies (data not shown). The combination of pI:C and ODN 2216, however, induced synergistically IFNα, and stimulated the generation of neutralizing antibodies in 3-day old cotton rats to levels comparable to immunization of adult animals.
Experimentally, the first week of life of rodents is considered to be comparable to the first four weeks of life of a newborn baby with an extremely immature immune system and no antibody response [10]. Weeks two to four in rodents are thought to reflect months 2 to 12 of the developing human immune system. This general scheme seems to be applicable to cotton rats, in that a T cell response can be generated during the first week of life in the absence of an antibody response [34], [35], and that antibody responses are still impaired in comparison to adult animals at day 21 [36]. Another interesting parallel between cotton rats and humans is the low expression level of TLR-9. In humans, pDC, neutrophils and some B cells express TLR-9 [37]. In mice, all myeloid cells (including dendritic cells) and B cells express TLR-9 [37]. The functional significance of these differences is not understood, and analysis is made difficult by the fact that mRNA/protein expression does not equal function. For example, human neutrophils express TLR-9 but do not respond to TLR-9 agonists [38]. For this reason it is difficult to assess whether the lower level of TLR-9 expression in cotton rats is meaningful in functional terms.
The immaturity of the immune system is only part of the challenges for immunization of neonates, the other challenge being the presence of maternal antibodies. The combination of pI:C/ODN 2216 was able to induce neutralizing antibodies after immunization of 3-day old cotton rats in the presence of natural maternal antibodies. Based on former studies, the level of protection in cotton rats is comparable to humans [39], [19], and a neutralization titer of 10 to 20 protects against infection [40]. However, in comparison with neonates who were immunized in the absence of maternal antibodies, immunization success was markedly reduced. If this immunization scheme would be applied to humans it might require a second immunization. In the specific case of MeV vaccination, this would not pose a problem as a second immunization at the age of 5 years is already recommended.
In summary, the combination of TLR-3 (pI:C) and TLR-9 (ODN2216) agonists induces a B cell response comparable to adults in the absence of maternal antibodies and still induces protective levels of neutralizing antibodies in the presence of maternal antibodies. The combination of these two agonists induces type I interferon as a stimulatory cytokine for B cell activation and IL-10 with potentially immunoregulatory function controlling unwanted inflammation.
Figure 6. Co-immunization with TLR-3 and TLR-9 agonists induces neutralizing antibodies after immunization in the presence of maternal antibodies.
Neonatal cotton rats from measles virus-immune dams were immunized s.c. on day 3 after birth with 105 pfu measles vaccine (Schwarz strain) alone, or in combination with 100 or 30μg of pI:C/ODN2216 (1:1 ratio). After 7 weeks, neutralizing antibodies were determined from serum. The number of asterisks denotes the level of statistical significance (*p < 0.05).
Acknowledgments
We thank Dr. Kathleen Hayes-Ozello for editorial review of the manuscript and Drs. Mi Young Kim and Michael Oglesbee for providing mouse spleens.
References
- 1.Kim D, Niewiesk S. Synergistic Induction of Interferon alpha through TLR-3 and TLR-9 Agonists Identifies CD21 as Interferon alpha Receptor for the B Cell Response. PLoS Pathog. 2013;9:e1003233. doi: 10.1371/journal.ppat.1003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naniche D. Human immunology of measles virus infection. In: Griffin DE, Oldstone MBA, editors. Measles - Pathogenesis and Control. Heidelberg: Springer Verlag; 2009. pp. 151–171. [DOI] [PubMed] [Google Scholar]
- 3.Gans HA, Arvin AM, Galinus J, Logan L, DeHovitz R, et al. Deficiency of the humoral immune response to measles vaccine in infants immunized at age 6 months. JAMA. 1998;280:527–532. doi: 10.1001/jama.280.6.527. [DOI] [PubMed] [Google Scholar]
- 4.Gans HA, Maldonado Y, Yasukawa LL, Beeler J, Audet S, et al. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J Immunol. 1999;162:5569–5575. [PubMed] [Google Scholar]
- 5.Gans H, Yasukawa L, Rinki M, DeHovitz R, Forghani B, et al. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. JInfect Dis. 2001;184:817–826. doi: 10.1086/323346. [DOI] [PubMed] [Google Scholar]
- 6.Gans H, DeHovitz R, Forghani B, Beeler J, Maldonado Y, et al. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21:3398–3405. doi: 10.1016/s0264-410x(03)00341-4. [DOI] [PubMed] [Google Scholar]
- 7.Siegrist CA, Barrios C, Martinez X, Brandt C, Berney M, et al. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur J Immunol. 1998;28:4138–4148. doi: 10.1002/(SICI)1521-4141(199812)28:12<4138::AID-IMMU4138>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Pueschel K, Tietz A, Carsillo M, Steward M, Niewiesk S. Measles virus-specific CD4 T-cell activity does not correlate with protection against lung infection or viral clearance. Journal of Virology. 2007;81:8571–8578. doi: 10.1128/JVI.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D, Huey D, Oglesbee M, Niewiesk S. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood. 2011;117:6143–6151. doi: 10.1182/blood-2010-11-320317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 11.Randolph D. The Neonatal Adaptive Immune System. NeoReviews. 2005;6:e454–e462. [Google Scholar]
- 12.Pueschel K, Tietz A, Carsillo M, Steward M, Niewiesk S. Measles virus-specific CD4 T-cell activity does not correlate with protection against lung infection or viral clearance. J Virol. 2007;81:8571–8578. doi: 10.1128/JVI.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green MG, Huey D, Niewiesk S. The cotton rat (Sigmodon hispidus) as an animal model for respiratory tract infections with human pathogens. Lab Anim (NY) 2013;42:170–176. doi: 10.1038/laban.188. [DOI] [PubMed] [Google Scholar]
- 14.Pfeuffer J, Püschel K, ter Meulen V, Schneider-Schaulies J, Niewiesk S. Extent of measles virus spread and immune suppression differentiates between wildtype and vaccine strains in the cotton rat model (Sigmodon hispidus) J Virol. 2003;77:150–158. doi: 10.1128/JVI.77.1.150-158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrula C, Zimmerman B, Nadella P, Shu S, Rosol T, et al. Expression of tumor invasion factors determines systemic engraftment and induction of humoral hypercalcemia in a mouse model of adult T-cell leukemia. Veterinary Pathology. 2009;46:1003–1014. doi: 10.1354/vp.08-VP-0254-N-FL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Martinez-Sobrido L, Choi C, Petroff N, Garcia-Sastre A, et al. Induction of type I interferon secretion through recombinant Newcastle disease virus expressing measles virus hemagglutinin stimulates antibody secretion in the presence of maternal antibodies. J Virol. 2011;85:200–207. doi: 10.1128/JVI.01624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weidinger G, Ohlmann M, Schlereth B, Sutter G, Niewiesk S. Vaccination with recombinant modified vaccinia virus Ankara protects against measles virus infection in the mouse and cotton rat model. Vaccine. 2001;19:2764–2768. doi: 10.1016/s0264-410x(00)00531-4. [DOI] [PubMed] [Google Scholar]
- 18.Niewiesk S, Götzelmann M, Ter Meulen V. Selective in vivo suppression of T lymphocyte responses in experimental measles virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4251–4255. doi: 10.1073/pnas.060012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlereth B, Germann PG, ter Meulen V, Niewiesk S. DNA vaccination with the hemagglutinin and the fusion proteins, but not the nucleocapsid protein protects against experimental measles virus infection. J Gen Virol. 2000;81:1321–1325. doi: 10.1099/0022-1317-81-5-1321. [DOI] [PubMed] [Google Scholar]
- 20.Griffin DE. Immune responses during measles virus infection. In: Billeter M, ter Meulen V, editors. Measles virus. Berlin: Springer; 1995. pp. 117–134. [Google Scholar]
- 21.Gans HA, Yasukawa LL, Alderson A, Rinki M, Dehovitz R, et al. T cell immunity to measles viral proteins in infants and adults after measles immunization. Viral Immunol. 2004;17:298–307. doi: 10.1089/0882824041310522. [DOI] [PubMed] [Google Scholar]
- 22.Kumar ML, Johnson CE, Chui LW, Whitwell JK, Staehle B, et al. Immune response to measles vaccine in 6-month-old infants of measles seronegative mothers. Vaccine. 1998;16:2047–2051. doi: 10.1016/s0264-410x(98)00083-8. [DOI] [PubMed] [Google Scholar]
- 23.Nair N, Gans H, Lew-Yasukawa L, Long-Wagar AC, Arvin A, et al. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J Infect Dis. 2007;196:1339–1345. doi: 10.1086/522519. [DOI] [PubMed] [Google Scholar]
- 24.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 25.De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103:1030–1032. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 26.Vosters O, Lombard C, Andre F, Sana G, Sokal EM, et al. The interferon-alpha and interleukin-10 responses in neonates differ from adults, and their production remains partial throughout the first 18 months of life. Clin Exp Immunol. 2010;162:494–499. doi: 10.1111/j.1365-2249.2010.04267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold MC, Donnelly E, Cook MS, Leclair CM, Lewinsohn DA. Purified neonatal plasmacytoid dendritic cells overcome intrinsic maturation defect with TLR agonist stimulation. Pediatr Res. 2006;60:34–37. doi: 10.1203/01.pdr.0000220352.13547.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, et al. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLos One. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68:813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Bjarnarson SP, Adarna BC, Benonisson H, Del Giudice G, Jonsdottir I. The adjuvant LT-K63 can restore delayed maturation of follicular dendritic cells and poor persistence of both protein- and polysaccharide-specific antibody-secreting cells in neonatal mice. J Immunol. 2012;189:1265–1273. doi: 10.4049/jimmunol.1200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pihlgren M, Tougne C, Schallert N, Bozzotti P, Lambert PH, et al. CpG-motifs enhance initial and sustained primary tetanus-specific antibody secreting cell responses in spleen and bone marrow, but are more effective in adult than in neonatal mice. Vaccine. 2003;21:2492–2499. doi: 10.1016/s0264-410x(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 32.Weeratna RD, Brazolot Millan CL, McCluskie MJ, Siegrist CA, Davis HL. Priming of immune responses to hepatitis B surface antigen in young mice immunized in the presence of maternally derived antibodies. FEMS Immunol Med Microbiol. 2001;30:241–247. doi: 10.1111/j.1574-695X.2001.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 33.Chelvarajan RL, Raithatha R, Venkataraman C, Kaul R, Han SS, et al. CpG oligodeoxynucleotides overcome the unresponsiveness of neonatal B cells to stimulation with the thymus-independent stimuli anti-IgM and TNP-Ficoll. Eur J Immunol. 1999;29:2808–2818. doi: 10.1002/(SICI)1521-4141(199909)29:09<2808::AID-IMMU2808>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Wong DT, Ogra PL. Neonatal respiratory syncytial virus infection: role of transplacentally and breast milk-acquired antibodies. J Virol. 1986;57:1203–1206. doi: 10.1128/jvi.57.3.1203-1206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svet-Moldavsky GJ, Svet-Moldasky IA. Sarcomas in cotton rats inoculated with Rous virus. Science. 1964;143:54–55. doi: 10.1126/science.143.3601.54. [DOI] [PubMed] [Google Scholar]
- 36.Prince GA, Jenson AR, Horswood RL, Camargo E. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978;93:771–792. [PMC free article] [PubMed] [Google Scholar]
- 37.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 38.Trevani AS, Chorny A, Salamone G, Vermeulen M, Gamberale R, et al. Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol. 2003;33:3164–3174. doi: 10.1002/eji.200324334. [DOI] [PubMed] [Google Scholar]
- 39.Schlereth B, Rose KJ, Buonocore L, ter Meulen V, Niewiesk S. Successful vaccine-induced seroconversion by single dose immunization in the presence of measles virus specific maternal antibodies. J Virol. 2000;74:4652–4657. doi: 10.1128/jvi.74.10.4652-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlereth B, Buonocore L, Tietz A, ter Meulen V, Rose JK, et al. Successful mucosal immunization of cotton rats in the presence of measles virus-specific antibodies depends on degree of attenuation of vaccine vector and virus dose. J Gen Virol. 2003;84:2145–2151. doi: 10.1099/vir.0.19050-0. [DOI] [PubMed] [Google Scholar]