Abstract
Objective
To evaluate safety, tolerability, seizure frequency, and regional variations in treatment responses with the AMPA antagonist, perampanel, in a large extension study during up to 3 years of treatment.
Methods
Patients ≥12 years old with partial-onset seizures despite treatment with 1–3 antiepileptic drugs at baseline completed a perampanel phase III trial and entered extension study 307 (NCT00735397). Patients were titrated to 12 mg/day (or their individual maximum tolerated dose) during the blinded conversion period, followed by open-label maintenance. Exposure, safety (adverse events [AEs], vital signs, weight, electrocardiography [ECG], laboratory values) and seizure outcomes were analyzed; key measures were assessed by geographic regions.

Results
Among 1,216 patients, median exposure was 1.5 years (range 1 week to 3.3 years), with >300 patients treated for >2 years. Treatment retention was 58.5% at cutoff. AEs reported in ≥10% of patients were dizziness, somnolence, headache, fatigue, irritability, and weight increase. Only dizziness and irritability caused discontinuation in >1% of patients (3.9% and 1.3%, respectively). The only serious AEs reported in >1% of patients were epilepsy-related (convulsion, 3.0%; status epilepticus, 1.1%). No clinically relevant changes in vital signs, ECG or laboratory parameters were seen. After titration/conversion, responder rate and median percentage change from baseline in seizure frequency were stable: 46% for both measures at 9 months (in 980 patients with ≥9 months' exposure) and 58% and 60%, respectively, at 2 years (in the 337 patients with 2 years' exposure). Median percentage reduction in frequency of secondarily generalized (SG) seizures ranged from 77% at 9 months (N = 422) to 90% at 2 years (N = 141). Among the 694 patients with maintenance data ≥1 year, 5.3% were seizure-free for the entire year.
Significance
No new safety signals emerged during up to 3 years of perampanel exposure in 39 countries. Seizure responses remained stable, with marked reductions, particularly in SG seizures.
Keywords: Epilepsy, Antiepilepsy drugs, AMPA receptor, Antagonist, Seizure freedom
Perampanel is a noncompetitive AMPA receptor antagonist recently approved in the United States and Europe for the adjunctive treatment of partial-onset seizures. More than 1,200 patients with pharmacoresistant partial-onset seizures completed three phase III trials of perampanel1–3 and enrolled into an extension treatment study (307, NCT00735397). An interim report described tolerability during initial study treatment4; however, less than one half of patients had >1 year of perampanel exposure, and it is important to evaluate long-term safety, tolerability, and retention during large exposures over several years for new therapies. We examined 1-year data for >800 patients and 2-year data for >300 patients, with an extensive review of safety, tolerability, and retention during up to 3 years of exposure, as well as additional seizure outcomes including seizure freedom and frequency of secondarily generalized (SG) seizures. This global extension study included patients from 39 countries, permitting country groupings and providing preliminary insight into safety and seizure outcomes in regions with different ethnicities, practice styles, and antiepileptic drug (AED) use.
Methods
Participants
Patients who had completed one of the three phase III perampanel trials (studies 304, 305, and 306) were eligible to participate (N = 1,264). Core study inclusion criteria have been reported previously.1–3 Patients were ≥12 years old with partial-onset seizures (simple or complex, with or without secondary generalization), inadequately controlled despite treatment with 1–3 approved AEDs. The first patients entered the extension in October 2008; the cutoff date for this analysis was October 2011.
Patients from 249 centers in 39 countries4 were grouped into five geographical regions: (1) North America; (2) Latin America; (3) Europe, Middle East (Israel), and South Africa (collectively named the EMEA region); (4) Indo–Pacific; and (5) China–Pacific (see Table S1). Groupings were determined primarily by geography, although the countries in the groups had similar socioeconomic and ethnicity composition. Three small-enrolling countries were roughly grouped geographically to avoid creating a small “other” group: South Africa (N = 8) and Israel (N = 32) had predominantly Caucasian and mixed-race subjects and are grouped into the EMEA region; Australia (N = 46) is grouped into the Indo–Pacific region. Indo–Pacific and China–Pacific countries were grouped based on geographic proximity. Outcome measures are reported for the whole group and by regional country groupings.
Study design and interventions
The design of extension study 307 (NCT00735397)4 consists of a 16-week blinded conversion period and a subsequent long-term open-label treatment period. Patients entering the extension were up-titrated 2 mg every 2 weeks from the dose on which they finished the core study (or from 2 mg for patients previously on placebo) to 12 mg/day or their individual maximum tolerated doses of perampanel. Dose adjustments of perampanel, and concomitant AEDs, were allowed at the investigator's discretion after the end of the conversion period. Patients who could not tolerate their dose could be down-titrated at the investigator's discretion; those who could not tolerate at least 2 mg/day were discontinued. Perampanel was given once daily, at bedtime.
Concomitant AEDs classified a priori as enzyme-inducing were carbamazepine, phenobarbital, phenytoin, and primidone. However, an analysis of the phase III pharmacokinetics/pharmacodynamics (PK/PD) a posteriori showed that only carbamazepine, oxcarbazepine, and phenytoin actually caused a metabolic induction, thereby increasing clearance.5
The trial protocol was reviewed by national regulatory authorities and independent ethics committees/institutional review boards. Patients provided informed consent prior to participating in the study.
Outcomes
Visits were every 4 weeks during the blinded conversion period, and every 3 months thereafter.
Data analysis
Data were summarized with descriptive statistics. The safety and the intent-to-treat (ITT) analysis sets were determined from times of initial exposure in core and extension studies with specific outcome definitions, including adverse events (AEs).
The efficacy analysis group comprises all patients who received at least one dose of perampanel in the extension and had valid seizure data during perampanel treatment (either core study or extension); the safety analysis group comprises all patients who received at least one dose of perampanel in the extension and had at least one post-dose safety assessment in the extension. Pre-perampanel baseline was calculated over the baseline period of the core study for patients who were randomized to perampanel in the core studies, and over the entire double-blind study duration for patients randomized to placebo in the core studies.
Safety outcomes
AEs were reported if they began on or after the day of the first dose of perampanel (in the core study or extension), occurred up to 30 days after last dose, or began before perampanel exposure but increased in severity during treatment. Vital signs, clinical laboratory parameters, and electrocardiography (ECG) recordings were assessed, including body mass index (BMI) changes (definitions of abnormal values in Table S2).
Seizure outcomes
Seizure outcomes were based on patient- or carer-recorded seizure diary data, which recorded all simple partial (with or without motor signs), complex partial, or SG seizures; all patients were educated in the same standardized fashion at each visit in the core study and extension. Because the trials began before the 2010 International League Against Epilepsy (ILAE) terminology was issued, the 1989 ILAE classifications were used. Primary outcomes in the core studies were median percentage change in seizure frequency (all seizure types)/28 days, relative to pre-perampanel baseline, and responder rate (percentage of patients with ≥50% reduction in seizure frequency/28 days relative to pre-perampanel baseline). Other prespecified endpoints reported here are median percentage change in frequency of SG seizures/28 days (relative to pre-perampanel baseline) and seizure freedom. Seizure-free rates were calculated as a percentage of the number of patients with data available during the extension maintenance period only (i.e., from week 17 onward), and were based on the number of patients experiencing no partial-onset seizures during the defined period.
Results
Disposition
Of the 1,218 patients who entered the extension study (96.4% of the 1,264 who completed the three core studies),4 1,216 were included in the safety analysis group and 1,217 in the efficacy analysis group. The safety analysis group for each region was: North America, N = 259; Latin America, N = 132; EMEA, N = 540; Indo–Pacific, N = 149; and China–Pacific, N = 136. Overall, 41.5% of the safety analysis group had discontinued treatment at cutoff for this analysis.
General and regional demographics
Baseline demographics for the 1,216 patients in the current safety analysis group were similar to the pivotal and interim reports.1–4 Overall, 610 male and 606 female patients were included with a mean age of 34.3 years (range 12–76). Mean age was lowest in Indo–Pacific (29.8 years; range 12–61) and highest in North America (36.1 years; range 12–73). Mean BMI at baseline was high (25.0 kg/m2), ranging from 23.0 kg/m2 in China–Pacific to 26.9 kg/m2 in North America. Racial variation was representative of the geographical regions (Table S3). There were 124 adolescents (aged ≥12 to ≤17 years during the baseline period of the core studies) who continued into extension treatment. The four most common concomitant AEDs in all regions were carbamazepine (used by 34.0% of all patients), valproic acid (33.3%), lamotrigine (31.3%), and levetiracetam (29.2%). Carbamazepine and valproic acid use was lowest in North America (used by 21.2% and 14.3% of patients, respectively) and highest in Latin America (48.5%, carbamazepine) and China–Pacific (51.5%, valproic acid). Lamotrigine and levetiracetam were more commonly used in North America and EMEA than in other regions, but the variation was moderate: lamotrigine was used by 19.7–34.3% of patients across regions; levetiracetam was used by 16.7–35.1% (Table S3).
The majority of patients (87%) were taking two or three AEDs at baseline (ranging from 81.1% to 89.9%); 48.4% of patients were taking AEDs that were classified a priori as inducing (ranging from 40.9% in North America to 69.1% in Indo–Pacific). A history of SG seizures was reported in 71% (ranging from 65% to 75%) of patients, and the median frequency of partial-onset seizures was 11.2/28 days at baseline (range 9.0–14.0, Table S3).
Exposure
A maximum perampanel daily dose of 10 or 12 mg/day was reached by 1,122 patients (92.3%), and the mean (± standard deviation [SD]) daily dose across the open-label maintenance period was 10.6 mg (±2.25). The mean (±SD) daily doses across the maintenance period by region were the following: North America, 10.6 (±2.20); Latin America, 10.8 (±2.04); EMEA, 10.7 (±2.23); Indo–Pacific, 11.0 (±2.03); and China–Pacific, 9.8 (±2.65) mg/day.
Median duration of exposure to perampanel was 78.4 weeks, or approximately 1.5 years (range 1 week to 3.3 years), totaling 1,803 patient-years. Exposure was similar across regions, with median duration ranging from 1.3 years in North America to 1.7 years in Indo–Pacific. Overall, a large majority of patients (71.3%, N = 867) received >52 weeks of treatment with perampanel; 30.0% (N = 365) received >100 weeks; and 10.7% (N = 130) received >124 weeks of treatment. Varying enrollment dates account for minor variation in exposure across the regions.
Retention
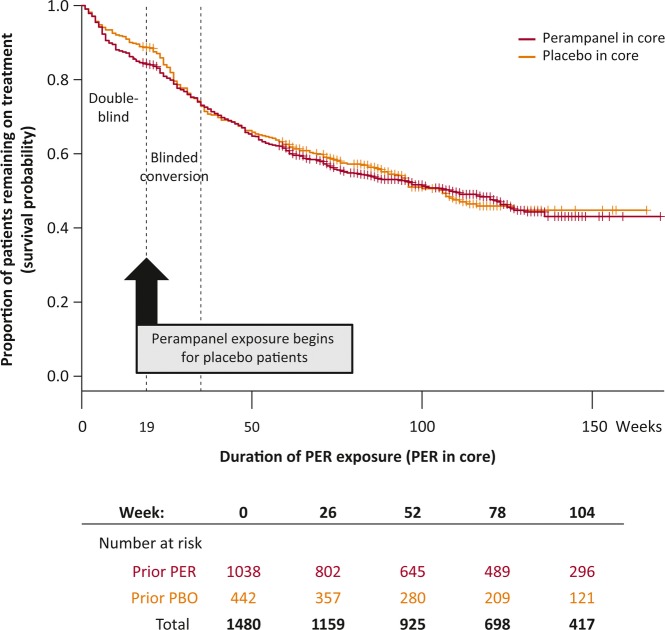
The proportion remaining on treatment over time (survival probability) from the start of the core studies is shown in Figure 1. Retention is shown for patients receiving perampanel during the core and conversion periods. Retention proportions were similar after blinded conversion periods for patients initially or later treated with perampanel. Discontinuation percentages by region were the following: 28.9% in Indo–Pacific, 32.6% in Latin America, 43.4% in China–Pacific, 43.5% in EMEA, and 48.3% in North America. The most common primary reasons for discontinuation were subject choice (13.8%), AEs (12.9%), and inadequate therapeutic effect (11.6%); inadequate therapeutic effect as a primary reason for discontinuation was highest in North America (21.6%) and lowest in Indo–Pacific (3.4%).
Figure 1.
Kaplan–Meier curve of retention, by previous treatment group in the core studies. Survival probability (patients ongoing as a proportion of the patients at risk [patients at risk determined by study entry and data cutoff]) assessed by previous treatment group. Study time is shown in weeks on the x-axis; exposure to perampanel began at week 0 for patients randomized to perampanel treatment in the core studies, and from week 19 for patients randomized to placebo in the core studies. Patients who did not enter the extension are censored at the end of the 19-week core study (vertical lines), and patients who were ongoing at time of cutoff are also censored (vertical lines, at the corresponding exposure duration they had reached). PER, perampanel; PBO, placebo.
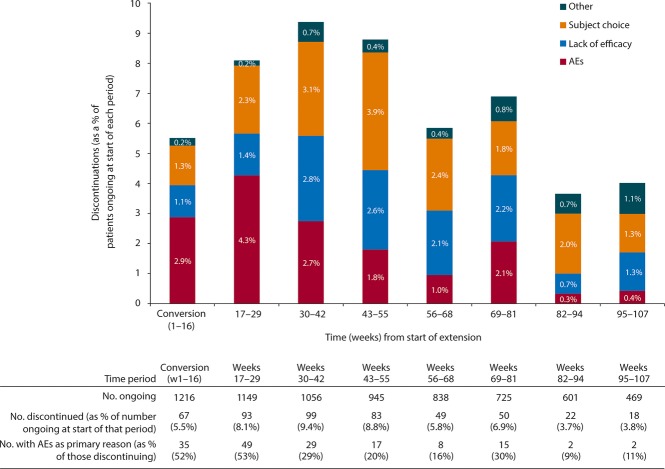
Figure 2 shows the primary reasons for discontinuation (of study or of study drug) over time. The proportion of patients discontinuing increased from 5.5% during the 16-week conversion period to 9.4% in weeks 30–42, and decreased to <4% by week 82.
Figure 2.
Reasons for discontinuations during perampanel treatment duration. Discontinuations during extension study conversion period (weeks 1–16) and in 13-week periods during maintenance are shown for the safety group (N = 1,216; all patients who received at least one dose of perampanel in the extension and had at least one post-dose safety assessment in the extension). Other includes “lost to follow-up,” “missing,” and “admin/other.” AEs, adverse events.
The proportion discontinuing due to AEs peaked in weeks 17–29 (49 patients discontinuing), and few patients discontinued due to AEs after week 55. Across the entire period of perampanel exposure, only two AEs led to discontinuation in >1% of the overall patient population: dizziness (48/1,216, 3.9%) and irritability (16/1,216, 1.3%). Other AEs that occurred in more than three discontinuing patients in any time interval were ataxia (n = 4) and headache (n = 4) during the conversion period (0.3% of patients each), and abnormal behavior (n = 5, 0.4%) and aggression (n = 5, 0.4%) during weeks 17–29. The main AEs leading to discontinuations in each region were the following: North America, dizziness (n = 11 [4.2%]) and ataxia (n = 8 [3.1%]); Latin America, dizziness (n = 2 [1.5%]) with all other AEs listed <1%; EMEA, dizziness (n = 16 [3.0%]) and vertigo (n = 6 [1.1%]); Indo–Pacific: dizziness (n = 4 [2.7%]) and somnolence (n = 3 [2.0%]); and China–Pacific, dizziness (n = 15 [11.0%]) and irritability (n = 6 [4.4%]). A total of 67 patients (5.5%) discontinued treatment due to psychiatric symptoms: 26 patients (10.0%) from North America, 27 patients (5.0%) from EMEA, 6 patients (4.0%) from Indo–Pacific, 5 patients (3.7%) from China–Pacific, and 3 patients (2.3%) from Latin America.
Tolerability and safety
At least one AE was reported by 1,110 patients (91.3%) during the entire duration of adjuvant perampanel treatment, and in 80.2% of these patients (890/1,110) AEs were mild or moderate (Table 1). There was no clear pattern in the regional incidence of total AEs: reported incidence ranged from 86.5% in EMEA to 98.8% in North America. Overall, AEs led to withdrawal in 195 patients (16%), dose reduction in 483 (39.7%), and dose interruption in 47 (3.9%).
Table 1.
AE overview, by region, during total exposure to perampanel (core studies and extension)
| Region | ||||||
|---|---|---|---|---|---|---|
| Overall | North America | Latin America | EMEA | Indo–Pacific | China–Pacific | |
| N | 1,216 | 259 | 132 | 540 | 149 | 136 |
| Incidence of AEs, n (%) | ||||||
| Any AE | 1,110 (91.3) | 256 (98.8) | 124 (93.9) | 467 (86.5) | 132 (88.6) | 131 (96.3) |
| Severe AEs | 220 (18.1) | 70 (27.0) | 27 (20.5) | 81 (15.0) | 22 (14.8) | 20 (14.7) |
| Treatment-related AEs | 993 (81.7) | 229 (88.4) | 109 (82.6) | 414 (76.7) | 115 (77.2) | 126 (92.6) |
| SAEs | 227 (18.7) | 66 (25.5) | 13 (9.8) | 94 (17.4) | 30 (20.1) | 24 (17.6) |
| Deaths | 5 (<1.0) | 0 | 1 (<1.0) | 3 (<1.0) | 1 (<1.0) | 0 |
| AEs leading to study drug adjustment | 606 (49.8) | 130 (50.2) | 74 (56.1) | 250 (46.3) | 58 (38.9) | 94 (69.1) |
| Dose reduction | 483 (39.7) | 108 (41.7) | 61 (46.2) | 195 (36.1) | 43 (28.9) | 76 (55.9) |
| Dose interruption | 47 (3.9) | 14 (5.4) | 7 (5.3) | 11 (2.0) | 5 (3.4) | 10 (7.4) |
| Discontinuation | 195 (16.0) | 50 (19.3) | 17 (12.9) | 79 (14.6) | 19 (12.8) | 30 (22.1) |
| Incidence of individual AEs occurring in ≥10% of overall population, n (%) | ||||||
| Dizziness | 569 (46.8) | 131 (50.6) | 68 (51.5) | 225 (41.7) | 43 (28.9) | 102 (75.0) |
| Somnolence | 258 (21.2) | 50 (19.3) | 41 (31.1) | 99 (18.3) | 32 (21.5) | 36 (26.5) |
| Headache | 222 (18.3) | 66 (25.5) | 25 (18.9) | 70 (13.0) | 26 (17.4) | 35 (25.7) |
| Fatigue | 159 (13.1) | 61 (23.6) | 4 (3.0) | 63 (11.7) | 14 (9.4) | 17 (12.5) |
| Irritability | 140 (11.5) | 44 (17.0) | 22 (16.7) | 38 (7.0) | 11 (7.4) | 25 (18.4) |
| Weight increased | 132 (10.9) | 52 (20.1) | 20 (15.2) | 35 (6.5) | 11 (7.4) | 14 (10.3) |
AE, adverse event; EMEA, Europe, Middle East and Africa; SAEs, serious adverse events.
Analysis carried out in all patients who received at least one dose of perampanel in the extension and had at least one post-dose safety assessment in the extension.
Individual AEs
Individual AEs that occurred in ≥10% of patients during perampanel exposure were dizziness, somnolence, headache, fatigue, irritability, and weight increase (Table 1). The two most common AEs across all of the regional groupings were dizziness and somnolence, with the exception of North America, where dizziness (50.6%) and headache (25.5%) were the most common (Table 1).
Individual AEs in the “nervous system” system organ class (SOC) that occurred in <10% and >2% of patients were convulsion (7.9%), ataxia (6.6%), balance disorder (5.7%), dysarthria (4.6%), tremor (2.8%), and memory impairment (2.6%). AEs in the “psychiatric” SOC (<10% and >2%) were depression (5.4%), insomnia (5.3%), aggression (5.1%), anxiety (5.0%), and mood swings (2.1%) (see Tables S4 and S5 for detailed AE listings).
Serious AEs and deaths
Overall, serious AEs (SAEs) occurred in 227 patients (18.7%) over this long exposure period. The number (%) of patients with SAEs by region were North America, 66 (25.5%); Indo–Pacific, 30 (20.1%); China–Pacific, 24 (17.6%); EMEA, 94 (17.4%); and Latin America, 13 (9.8%). Similar to data across the three core trials, only two individual SAEs were seen in >1% of patients overall, and these were epilepsy-related: convulsion (n = 36, 3.0%) and status epilepticus (n = 13, 1.1%).
Of the 1,216 patients in the safety analysis, 47 (3.9%) had at least one psychiatric SAE (10 of these patients had more than one different psychiatric SAE). Twenty of these patients (42.6%) had histories of previous psychiatric disorders. An SAE of aggression (including aggressive behaviors and outbursts or increases in aggressivity) occurred in 12 patients overall (1.0%); psychotic disorder and suicidal ideation each occurred in six patients (0.5%); affective disorder, depression, and suicide attempt each occurred in four patients (0.3%); paranoia in three patients (0.2%); and abnormal behavior, acute psychosis, agitation, and disorientation each occurred in two patients (0.2%). For 4 of the 12 patients with an SAE of aggression, the day of onset of aggression symptoms occurred later than the end of the conversion period. None of the 12 patients reporting an SAE of aggression had aggression reported in their medical history. Four of the 12 had either anxiety, depression, cognitive delay, or intellectual disability reported in their previous medical history; one patient had anger management problems, anxiety disorder, mood swings, depression, and memory problems reported, and one patient had oppositional defiant disorder, learning disorder, depression, and behavior disorder reported. Aggression resolved in 11 of the 12 patients (status was not recorded in one patient), with 7 withdrawing from perampanel treatment, 4 continuing on stable doses, and one with reduced perampanel dose. Seven of the 12 with an SAE of aggression reported no other psychiatric SAE during perampanel exposure.
Five patients died during the 1,803 patient-years of exposure; no deaths were considered related to study medication by investigators. Two deaths were due to intracerebral hemorrhage and a passenger dying in a motor vehicle crash. Three deaths appeared linked to seizures occurring during treatment: head injury during a seizure, sudden unexplained death in epilepsy (SUDEP), and sudden death of cause unknown (possibly SUDEP; the patient had been seizure-free for 10 months) in a patient with cardiovascular disease.
Laboratory values, vital signs, and ECG
There was a low incidence of markedly abnormal laboratory test values (0–5.7%). As in the previous report,4 occurrence of aspartate aminotransferase or alanine aminotransferase >3 times the upper limit of normal (ULN) was <1%, and creatine phosphokinase >5 times the ULN was 2.1%. Few patients had abnormal lipid profiles: high cholesterol was seen in 25 (2.1%) of 1,202 patients and high triglycerides in 9 (<1%) of 1,202 patients during the entire period of perampanel exposure. The occurrence of markedly low or high systolic or diastolic blood pressure was <2%. There were no clinically important changes in ECG parameters: no patients had a corrected QT duration of >500 msec during perampanel treatment (whether corrected using Fridericia's formula, QTcF, or Bazett's formula, QTcB), and an increase in the maximum QTc duration of >60 msec from baseline was seen in <1% of patients.
The incidence of markedly low serum sodium levels and neutrophil and white blood cell counts at any time during perampanel exposure was 3.7% (n = 45), 5.7% (n = 69), and 3.0% (n = 36), respectively. Of the 45 patients with low sodium, 44 were taking concomitant carbamazepine or oxcarbazepine.
Body weight
An increase in weight of >7% from baseline occurred in 460 (37.8%) of 1,216 patients across the perampanel treatment duration, and loss of >7% occurred in 134 (11.0%) of 1,216 patients. Weight data were calculated separately for adults and adolescents (as adolescents would be expected to gain weight). There were 1,090 adults with weight data at any time during perampanel exposure (820 with 1-year data and 271 with 2-year data); and 124 adolescents (94 with 1-year data and 43 with 2-year data). In adults, mean change from baseline weight (71.6 kg) was +1.90 kg at 1 year and +2.19 kg at 2 years. In adolescents, mean change from baseline weight (57.2 kg) was +4.35 kg at 1 year and +6.10 kg at 2 years. Of the adults, 17.3% (189/1,090) were obese (BMI > 30 mg/m2) at baseline, 20.5% at 1 year and 17.7% at 2 years.
Seizure outcomes
Responder rate and percentage change by longevity cohorts
Seizure outcomes were analyzed in cohorts of patients with the same minimum duration of exposure: patients with ≥6 months of exposure (N = 1,090, 89.6% of the 1,217 ITT patients); patients with ≥9 months of exposure (N = 980); patients with ≥12 months of exposure (N = 874); and patients with ≥24 months of exposure (N = 337). This analysis permitted change in seizure outcomes over time to be explored without being confounded by changes in number of patients evaluated at each time point.
In the four subsets of patients with different treatment durations, the patterns of change in seizure frequency (Fig. 3A) and responder rate (Fig. 3B) are comparable at similar time points. Most responses occurred in the 1–13-week and 14–26-week time periods, as perampanel was uptitrated. For example, patient responder rates were similar across the four exposure groups—32–35% during weeks 1–13 of treatment and 42–48% during 14–26 weeks of treatment. Subsequent seizure responses were stable for the smaller groups of patients with longer exposures, with responder rates ranging from 52% (weeks 27–39) to 58% (weeks 92–104), although these proportions are more influenced by study dropouts. A similar pattern for responder rates was observed across the geographic regions, with a trend for responder rates to be highest in the Indo–Pacific region and lowest in Latin America (Fig. 4).
Figure 3.
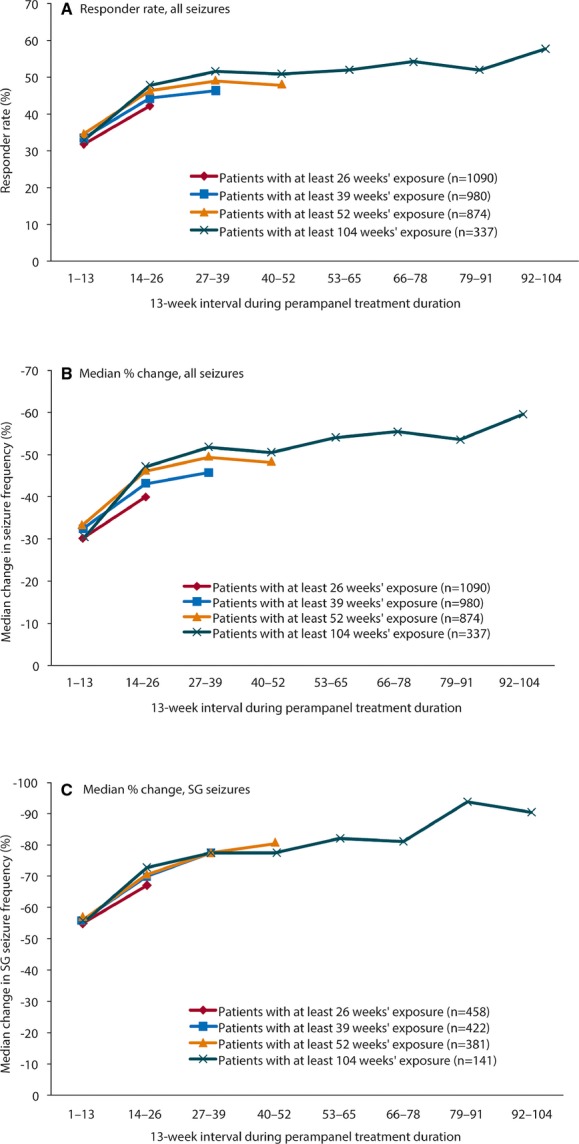
Seizure outcomes for each 13-week period from first exposure to perampanel, by treatment duration cohort. Perampanel treatment duration starts from first perampanel exposure, either in the core study or extension, except for patients with a gap in perampanel exposure of >14 days between the core study and the extension, for whom duration is based on the extension study. (A) Responder rate (percentage of patients with ≥50% reduction in seizure frequency/28 days from pre-perampanel baseline); (B) median percentage change in seizure frequency from pre-perampanel baseline; and (C) median percentage change in SG seizure frequency from pre-perampanel baseline in patients with SG seizures during baseline. Analysis carried out in all patients who received at least one dose of perampanel in the extension and had valid seizure data during perampanel treatment (either core study or extension). SG, secondarily generalized.
Figure 4.
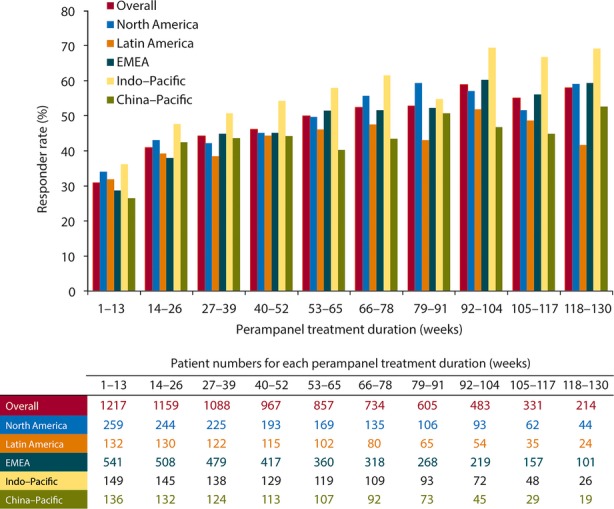
Responder rate during perampanel treatment duration, overall and by region. Responder rate (percentage of patients with ≥50% reduction in seizure frequency/28 days from pre-perampanel baseline), by 13-week exposure periods. Perampanel treatment duration starts from first perampanel exposure, either in the core study or extension, except for patients with a gap in perampanel exposure of >14 days between the core study and the extension, for whom duration is based on the extension study. Analysis carried out in all patients who received at least one dose of perampanel in the extension and had valid seizure data during perampanel treatment (either core study or extension). EMEA, Europe, Middle East, and Africa.
The pattern of improvement was similar for SG seizures in the subset of patients in whom this seizure type was present at baseline (n = 458 with ≥26 weeks of exposure; Fig. 3C). As for all seizures, the greatest reductions in SG seizures were seen over the first 26 weeks, as doses were up-titrated. Thereafter, reduction in frequency of SG seizures reached 90% in patients continuing treatment to weeks 92–104 (2 years; Fig. 3C). Of patients in the ITT analysis set, 9% (113/1,217) did not have SG seizures during the trial baselines, but had these during the extension treatment period. Overall, approximately 20% of patients who had a history of secondary generalization had no SG seizures during the 6-week baseline.
Seizure freedom
The seizure-free rate (all partial-onset seizure types) over the first 6 months of the extension maintenance period was 4.9% (45/918 patients), and over the last 6 months for patients who had at least 2 years of data the proportion was 10.6% (15/141) (Table 2). The number (and percentage) of patients who had a minimum of 1 year of extension maintenance data and were seizure-free for the entire year were the following: North America, n = 6 (4.9%); Latin America, n = 1 (1.3%); EMEA, n = 19 (6.4%); Indo–Pacific, n = 5 (4.7%); and China–Pacific, n = 6 (6.5%).
Table 2.
Seizure freedom in the extension study maintenance period
| Patient population (N) | Seizure-free for entire duration, n (%) | Seizure-free for last 6 months of that period, n (%) |
|---|---|---|
| ≥6 months of extension maintenance data (N = 918) | 45 (4.9) | – |
| ≥1 year of extension maintenance data (N = 694) | 37 (5.3) | 53 (7.6) |
| ≥2 years of extension maintenance data (N = 141) | 4 (2.8) | 15 (10.6) |
Analysis carried out in all patients who received at least one dose of perampanel in the extension and had valid seizure data during perampanel treatment (either core study or extension).
Discussion
This long-term safety study showed that perampanel was well tolerated as adjunctive treatment for pharmacoresistant partial-onset seizures during up to 3 years of exposure with a median daily dose of 10.6 mg/day. Across the disparate regions that contributed to the phase III studies, the severity and refractoriness of the patients' epilepsy were similar: most patients had two to three partial-onset seizures per week and were receiving treatment with two or three other AEDs. AEs were most frequent during dose titration, most commonly dizziness, somnolence, and headache. Individual SAEs were uncommon during long exposure, and included seizure events and psychiatric symptoms. Patients with initial placebo treatment had seizure responses similar to initially treated patients, and seizure responses for both groups were stable over intermediate to long exposures (6 months to 3 years). These results confirm the safety and seizure response data seen in the 19-week placebo-controlled trials,6 and stability of treatment over the long term. The mortality rate observed in the trial is within the range expected for pharmacoresistant epilepsy patients over this time period.7,8
The analysis by regional groupings showed sustained seizure outcomes over time, with little regional variation. The total proportions of patients with treatment-emergent AEs were similar across the five regional groups. Dizziness—the most commonly reported individual AE across all regions—was reported by 75% of the 136 patients in China–Pacific and by only 28.9% of the 149 patients in the Indo–Pacific group. Discontinuation rates were highest in North America (48.3%), where the rates of AEs, severe AEs, and SAEs were slightly higher than in other regions. These regional differences may be influenced by variations in the use of enzyme-inducing AEDs across the regions. Enzyme-inducing AEDs (carbamazepine, oxcarbazepine, and phenytoin) reduce perampanel plasma concentrations two to threefold.5,9 Discontinuation rates were lowest in the Indo–Pacific region (28.9%), where overall use of enzyme-inducing AEDs was the highest (Table S1) and AE rates were among the lowest (Table 1). The proportion of significant AEs associated with discontinuations was similar across the Indo–Pacific, Latin America, and EMEA regions (12.8%, 12.9%, and 14.6%, respectively). Discontinuations due to AEs were slightly higher in North America (19.3%) and also in China–Pacific (22.1%), where discontinuations because of dizziness were particularly high in the China–Pacific and North America regions (11.0% and 4.2%, respectively) and irritability (4.4%) was higher in China–Pacific than in other regions. Such variations in AE reporting and in retention are also seen in other studies10 and may reflect differences in culture, attitudes, investigator relations, and variability with small group sizes. Other regional differences in enrolled patients (in addition to differences in exposures to enzyme-inducing AEDs) are likely, but were not clear in the study.
The number of patients with SAEs over the treatment periods was low, and only dizziness and irritability caused discontinuation in >1% of patients (3.9% and 1.3%, respectively). The proportion of patients discontinuing treatment was greatest approximately 6–12 months after starting extension treatment (weeks 30–55), with subject choice the most common reason. Perampanel was titrated up by 2 mg every 2 weeks from placebo or blinded-treatment doses to the maximum tolerated dose; this is a more gradual schedule than the 1-week titration increments in the core studies.1–3 This titration schedule was well tolerated, with most patients reaching 10 mg/day doses and only 4.3% discontinuing treatment due to AEs in the 12 weeks following titration (weeks 17–29).
Psychiatric SAEs were observed in 47 patients overall (3.9%) during up to 170 weeks of perampanel exposure. This compares with 0.9% of patients who reported psychiatric SAEs in the placebo arm during the much shorter 19-week controlled study periods.6 Patients with active psychotic illness (i.e., taking antipsychotic drugs) and those with a suicide attempt within the past 2 years were excluded from the study; however, patients with stable depression were included and 43% (20 of 47) of participants with psychiatric SAEs had prior histories of psychiatric illness. Overall, the proportions with psychiatric symptoms were similar to population studies of patients with epilepsy.11
The most common events in a detailed analysis of psychiatric AEs in the core phase III trials based on MedDRA Standardized Medical Query were irritability (7% 8 mg; 12% 12 mg perampanel vs. placebo 3%), aggression (2% 8 mg; 3% 12 mg perampanel vs. 1% placebo), and anger (1% 8 mg; 3% 12 mg perampanel vs. 0.2% placebo).6 The highest rates of onset were during the first 6 weeks of treatment. Five (0.5%), 5 (0.5%), and 4 (0.4%) patients discontinued during the core phase III trials due to irritability, aggression, and anger, respectively.
Aggression was reported as an AE in 62 patients (5.1%) during the much longer extension study. The proportion of patients discontinuing treatment due to psychiatric AEs, however, was low in the extension study: In weeks 17–29, psychiatric events leading to discontinuation in >3 patients were aggression (five patients [0.4%]), abnormal behavior (five patients [0.4%]), and irritability (six patients [0.5%]).
By region, psychiatric disorders as a reason for discontinuation was highest in North America (10.0%) compared with other geographic regions (range Latin America 2.3% to EMEA 5.0%). This may be related to differences in enrolled patients or cultural differences in reporting such events, which would be of interest to explore in future analyses.
Aggression was reported as an SAE in 1.0% of patients during extension treatment. This incidence of aggression (as an AE and as an SAE) over the long term, once the longer observation period is considered, is consistent with, or lower than, that in the core studies.
Changes in body weight in the long-term extension study were difficult to interpret because of the absence of a control group and the high proportion of patients who were overweight or obese at baseline (17.3% of adults were obese [BMI >30 kg/m2] at baseline), particularly in the adolescent patients. During the 19 weeks of the core studies, the average weight gain was 1.2 kg with perampanel, versus 0.4 kg with placebo.6 Patients with 2 years of extension treatment had a mean increase of 2.2 kg. After adjusting for exposure periods, this change is similar to other AEDs not typically associated with weight gain.12 Patients were requested to take perampanel at bedtime with snacks, which may have also contributed to weight changes.
The extension study has limitations inherent to open treatment in which there are no placebo data available to compare risks for specific symptoms and outcomes. It is difficult, for example, to determine specific risks for psychiatric symptoms such as aggression in patients with medically resistant epilepsy who are at relatively high risk for these complications. Another limitation is the difficulty in interpreting AEs and seizure responses over long treatment periods (up to 3.3 years) when patients have different exposures due to entering the study at different times (mean exposure 1.5 years); some patients discontinue treatment and enrich results at such long time points. Nonetheless, the majority continued treatment >1 year and the AE and safety results may characterize open treatment with perampanel over several years in patients with treatment-resistant partial-onset seizures. Moreover, the efficacy results were stable at similar time points when patients were grouped by exposure periods, suggesting stable responses over 6-month to >2-year treatment periods. In addition, a large proportion of patients (up to 90%) had marked reduction in occurrence of SG seizures, a particularly disabling seizure type. Another limitation was that because most patients were taking 10 or 12 mg perampanel, we were not able to explore dose–response relationships of side effects, and blood levels of perampanel were not assessed in the adult population. Several country geographic groupings were performed that represented a compromise between geographic location, ethnicities, and practice patterns.
In conclusion, no new major safety problems were detected during relatively long exposures to perampanel (over 1,803 patient-years). Reductions in seizure frequency were stable over treatment periods of up to 3 years. In patients with SG seizures, up to 90% reduction in frequency of SG seizures was observed. Seizure freedom lasting at least 1 year was achieved by 5% of patients. Safety and seizure responses were similar across a large number of geographic regions and ethnicities, and retention was high, averaging 58.5% over the different regions.
Acknowledgments
Medical writing support in the development of this manuscript was provided by Kate Carpenter of Choice Healthcare Solutions and was funded by Eisai Europe Ltd.
Disclosure or Conflict of Interest
MB, MG, and JZ are employees of Eisai. DS was an employee of Eisai during the data collection, analysis, and writing of this manuscript. GK has been an investigator for Eisai, UCB Pharma, Sunovion, Upsher Smith, Vertex, and the National Institutes of Health (NIH), and has acted as a consultant for Eisai (fees paid to Johns Hopkins University). EBM has received speaker or consultancy fees from Eisai, Janssen-Cilag, Lundbeck, and UCB. In addition, she has also received research grants from Bial, Eisai, and UCB. JFC has been an investigator for UCB Pharma, Sunovion, Upsher Smith, and Eisai. PK has received speaker's honoraria from GlaxoSmithKline, Sanofi-aventis, and UCB Pharma, and has served on scientific advisory boards for GlaxoSmithKline and Eisai. His institution has received research funding from Eisai, GlaxoSmithKline, Johnson & Johnson, Pfizer, UCB Pharma, Hong Kong Research Grants Council, Health and Health Services Research Fund, Innovation and Technology Fund, RMH Neuroscience Foundation, University of Melbourne and the NIH. EP has received research grants from the European Union, the Italian Medicines Agency, the Italian Ministry of Health, and the Italian Ministry for Education, University and Research. In addition, he has received speaker's or consultancy fees and/or research grants from Bial, Eisai, GlaxoSmithKline, Lundbeck, Medichem, Pfizer, Sun Pharma, Supernus, UCB Pharma, Upsher-Smith, and Vertex. JS has received research funding from NIH and Mayo Clinic Foundation and also receives research grant support from Eisai Inc. and Visualase Inc. XW has no fees, grants, or honoraria to declare. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Countries included in the five geographic regions.
Table S2. Definitions.
Table S3. Baseline patient demographics and clinical characteristics.
Table S4. AEs occurring in <10% and ≥5% of the overall population, by region.
Table S5. AEs occurring in ≥1% of patients in specific SOCs, over entire duration of perampanel exposure (core studies and extension).
References
- 1.French JA, Krauss GL, Biton V, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79:589–596. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54:117–125. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 3.Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78:1408–1415. doi: 10.1212/WNL.0b013e318254473a. [DOI] [PubMed] [Google Scholar]
- 4.Krauss GL, Perucca E, Ben-Menachem E, et al. Perampanel, a selective, noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, as adjunctive therapy for refractory partial-onset seizures: interim results from phase III, extension study 307. Epilepsia. 2013;54:126–134. doi: 10.1111/j.1528-1167.2012.03648.x. [DOI] [PubMed] [Google Scholar]
- 5.Laurenza A, Ferry J, Hussein Z. Population pharmacokinetics and pharmacodynamics of perampanel: a pooled analysis from three phase III trials. Epilepsy Curr. 2012;12(Suppl. 1):2.231. Abstract. [Google Scholar]
- 6.Steinhoff BJ, Ben-Menachem E, Ryvlin P, et al. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia. 2013;54:1481–1489. doi: 10.1111/epi.12212. [DOI] [PubMed] [Google Scholar]
- 7.Mohanraj R, Norrie J, Stephen LJ, et al. Mortality in adults with newly diagnosed and chronic epilepsy: a retrospective comparative study. Lancet Neurol. 2006;5:481–487. doi: 10.1016/S1474-4422(06)70448-3. [DOI] [PubMed] [Google Scholar]
- 8.Silanpää M, Jalava M, Kaleva O, et al. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 9.Gidal B, Ferry J, Majid O, et al. Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia. 2013;54:1490–1497. doi: 10.1111/epi.12240. [DOI] [PubMed] [Google Scholar]
- 10.Perucca P, Gilliam FG. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11:792–802. doi: 10.1016/S1474-4422(12)70153-9. [DOI] [PubMed] [Google Scholar]
- 11.Tellez-Zenteno J, Patten S, Jette N, et al. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–2344. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 12.Baulac M, Leon T, O'Brien T, et al. A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partial-onset seizures. Epilepsy Res. 2010;91:10–19. doi: 10.1016/j.eplepsyres.2010.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article:
Countries included in the five geographic regions.
Table S2. Definitions.
Table S3. Baseline patient demographics and clinical characteristics.
Table S4. AEs occurring in <10% and ≥5% of the overall population, by region.
Table S5. AEs occurring in ≥1% of patients in specific SOCs, over entire duration of perampanel exposure (core studies and extension).