Abstract
Background and Aims In flowering plants, fertilization relies on the delivery of the sperm cells carried by the pollen tube to the ovule. During the tip growth of the pollen tube, proper assembly of the cell wall polymers is required to maintain the mechanical properties of the cell wall. Xyloglucan (XyG) is a cell wall polymer known for maintaining the wall integrity and thus allowing cell expansion. In most angiosperms, the XyG of somatic cells is fucosylated, except in the Asterid clade (including the Solanaceae), where the fucosyl residues are replaced by arabinose, presumably due to an adaptive and/or selective diversification. However, it has been shown recently that XyG of Nicotiana alata pollen tubes is mostly fucosylated. The objective of the present work was to determine whether such structural differences between somatic and gametophytic cells are a common feature of Nicotiana and Solanum (more precisely tomato) genera.
Methods XyGs of pollen tubes of domesticated (Solanum lycopersicum var. cerasiforme and var. Saint-Pierre) and wild (S. pimpinellifolium and S. peruvianum) tomatoes and tobacco (Nicotiana tabacum) were analysed by immunolabelling, oligosaccharide mass profiling and GC-MS analyses.
Key Results Pollen tubes from all the species were labelled with the mAb CCRC-M1, a monoclonal antibody that recognizes epitopes associated with fucosylated XyG motifs. Analyses of the cell wall did not highlight major structural differences between previously studied N. alata and N. tabacum XyG. In contrast, XyG of tomato pollen tubes contained fucosylated and arabinosylated motifs. The highest levels of fucosylated XyG were found in pollen tubes from the wild species.
Conclusions The results clearly indicate that the male gametophyte (pollen tube) and the sporophyte have structurally different XyG. This suggests that fucosylated XyG may have an important role in the tip growth of pollen tubes, and that they must have a specific set of functional XyG fucosyltransferases, which are yet to be characterized.
Keywords: plant cell wall, GC-MS, gametophyte, immunolabelling, oligosaccharide mass profiling, OLIMP, pollen tube, tomato, Solanum lycopersicum, S. peruvianum; S. pimpinellifolium, xyloglucan, XyG, Nicotiana tabacum.
INTRODUCTION
In flowering plants, sexual reproduction requires the mating of the sperm cells, carried by the pollen tube, with the ovule. After adhesion and rehydration on the stigma, the pollen grain germinates and produces a tube that grows within the stigma and style through the transmitting tract tissue. During the journey, interactions with the female tissues occur promoting the fast tip-polarized pollen tube growth and guidance (Johnson and Lord, 2006; Mollet et al., 2007; Cheung and Wu, 2008). Finally, attracted by the synergid cells, the pollen tube bursts and releases the two sperm cells, allowing the double fertilization and the formation of seeds (Franklin-Tong, 2010; Palanivelu and Tsukamoto, 2012).
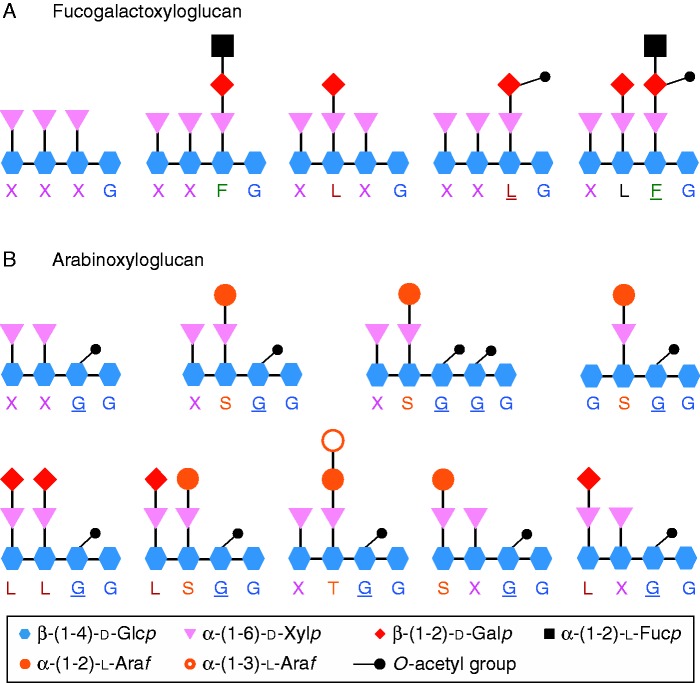
The primary cell wall confers many functions such as cell adhesion, signal transduction as well as physical active barriers against micro-organisms (Durand et al., 2009; Fry, 2011). The cell wall of pollen tubes in most flowering species is mainly composed of three types of polysaccharides: β-glucans, hemicellulose and different pectin motifs including homogalacturonan, xylogalacturonan, rhamnogalacturonan-I (RG-I) and RG-II (Dardelle et al., 2010; Chebli et al., 2012; Mollet et al., 2013; Dumont et al., 2014). Cellulose, a β-(1→4)-linked glucan, is generally present in low amounts. In contrast, the main glucan found in the pollen tube cell wall is callose, a β-(1→3)-linked glucan (Qin et al., 2012; Mollet et al., 2013). In vegetative organs of eudicots, the main hemicellulose is xyloglucan (XyG) representing 20–25 % (dry weight) of the primary cell wall, whereas it accounts for only 2 % in certain monocots (Hsieh and Harris, 2009, Fry, 2011). XyG is linked to cellulose microfibrils by hydrogen bonds that regulate cell expansion, avoid aggregation of cellulose microfibrils and increase the strength of the primary cell wall (Hayashi and Kaida, 2010; Scheller and Ulvskov, 2010; Popper et al., 2011). XyG is made of a β-(1→4)-glucan backbone that can be substituted by different side chains (Scheller and Ulvskov, 2010) named by one letter according to the nomenclature proposed by Fry et al. (1993) (Fig. 1). G is the non-substituted glucosyl (Glc) residue, X is the α-d-xylose (Xyl) linked to the C-6 of the β-d-Glc. Then, in most eudicots, the Xyl residue can be branched on the C-2 residue by a galactose (Gal) (L), itself substituted by a fucosyl (Fuc) residue (F) forming the fucogalactoXyG (Fig. 1A). Surprisingly, very little is known concerning the XyG composition of pollen tubes. Using the OLIMP (OLIgosaccharide Mass Profiling) method (Lerouxel et al., 2002), Dardelle et al. (2010) have shown that the XyG of Arabidopsis thaliana (Malvid, Brassicaceae) pollen tubes was highly fucosylated and O-acetylated as compared with the XyG found in vegetative organs. The main fragments detected were XXFG and XLFG, both being O-acetylated as indicated by the underlined letter (Dardelle et al., 2010) (Fig. 1A). These data suggested an important role for these two structural features during pollen tube growth and possibly in the cell wall interaction with the female tissues. Interestingly, in the lamiid clade (euasterids I) (including the Solanaceae with Nicotiana sp. and Solanum sp.) (APG III, 2009), XyG found in vegetative organs is not decorated with Fuc residues, and Gal was only detected in tomato (Sims et al., 1996; York et al., 1996; Jia et al., 2003, 2005, Hoffman et al., 2005). Instead, XyG displays abundant Araf-(1→2)-Xylp (S) and Araf-(1→3)-Araf-(1→2)-Xylp (T) oligosaccharides linked to the glucan backbone and was named arabinoXyG (Fig. 1B) (York et al., 1996; Jia et al., 2003, 2005; Hoffman et al., 2005). In tomato and tobacco, the most abundant fragments were XXGG and XSGG (Fig. 1B) (York et al., 1996; Sims et al., 1996; Vincken et al., 1997; Jia et al., 2003, 2005; Hsieh and Harris, 2009). Recently, Lampugnani et al. (2013) showed clear structural differences in the composition of XyG in Nicotiana alata pollen tubes. They revealed that, in contrast to the vegetative organs, the cell wall was most exclusively a fucogalactoXyG type.
FIG. 1.
Graphic representation of the main endo-glucanase-generated fragments released from (A) fucogalactoXyG and (B) arabinoXyG. The structures of XyG fragments are shown according to the one-letter code nomenclature proposed by Fry et al. (1993). Underlined and bold letters represent O-acetylated side chains. The main arabinoXyG fragments found in tobacco and tomato cultured cells and/or leaves are from Jia et al. (2003) and Hoffmann et al. (2005).
The present work attempted to determine by immunolabelling, OLIMP and gas chromatography–mass spectrometry (GC-MS) if XyG found in pollen tubes of other Solanaceae species shared a similar structural feature. Most of the investigated species were from the Solanum genus. They included domesticated tomatoes such as S. lycopersicum var. Saint-Pierre and S. lycopersicum var. cerasiforme ‘wva106’ (cherry tomato), and wild species S. pimpinellifolium and S. peruvianum that retraced the diversity, evolution and selection. In addition, another species from the Nicotiana genus (N. tabacum) was also investigated.
Here, we show that XyG in the pollen tube cell wall of N. tabacum is structurally similar to that found in N. alata (Lampugnani et al., 2013) and the relative level of fucosylated fragments was about 62 %. In contrast, XyG found in pollen tubes of tomato species is composed of arabinosylated and fucogalactosylated XyG. Finally, the relative abundance of fucosylated XyG was significantly lower in the domesticated than in the wild species, but, in all the cases, it did not exceed 20 %. Our data suggest that tomato pollen tubes have specific and functional XyG fucosyltransferases and that fucosylated XyG might be important for the tip growth of the male gametophyte within the female tissues.
MATERIALS AND METHODS
Plant growth
Wild tomatoes Solanum pimpinellifolium LA1589 and S. peruvianum CMV-INRA, and domesticated tomatoes S. lycopersicum var. cerasiforme ‘West Virginia 106’ (wva106) and S. lycopersicum var. Saint-Pierre (Truffaut, France) seeds were germinated in the dark for 5 d at 20 °C on wet cotton, transferred to sterilized soil and cultured in a growth chamber. All species were grown with a photoperiod of a 16 h light/8 h dark cycle at 25 and 22 °C during the light and dark period, respectively. Relative humidity was maintained at 60 %, and plants were watered every 2 d.
Cell suspension culture
The tomato (S. lycopersicum ‘dombito’) cell suspension was initiated from hypocotyl sections of seedlings (Muschitz et al., 2009). Cells were harvested by filtration after 5 d of growth and chemically fixed with 4 % formaldehyde in PBS (phosphate-buffered saline: 8 g L−1 NaCl, 0·29 g L−1 KCl, 1·45 g L−1 Na2HPO4, 0·24 g L−1 KH2PO4) for immunolabelling or washed with and stored in 70 % ethanol (EtOH) at 4 °C for biochemical analyses.
Pollen tube culture
Pollen grains were collected from freshly dehisced anthers, and the stamens of three flowers (tobacco) or five flowers (tomato) were submerged in 5 mL of BK medium [1·62 mm H3BO3, 1·25 mm Ca(NO3)2·4H2O, 2·97 mm KNO3 and 1·65 mm MgSO4·7H2O] (Brewbaker and Kwack, 1963) containing 15 % sucrose. Pollen grains were suspended in the liquid medium by vortex, and the stamens were removed with tweezers. Pollen tubes were grown at 22 °C in the dark for 6 h under agitation. During this period, pollen tubes are actively growing. For immunolocalization experiments, pollen tubes were chemically fixed according to Dardelle et al. (2010). For XyG analysis, 3 vols of EtOH were added to the suspension and pollen tubes were separated from the medium and ungerminated pollen grains by filtration using a 50 µm nylon mesh filter. After several washes with 70 % EtOH, pollen tubes were stored at 4 °C in 70 % EtOH.
Immunolocalization of XyG epitopes
LM15 (Plant Probes, UK) and CCRC-M1 (CCRC, University of Georgia, USA) monoclonal antibodies (mAbs) were used to detect XXXG motifs (Marcus et al., 2008), and α-Fuc-(1→2)-β-Gal (Puhlmann et al., 1994) epitopes, respectively. Pollen tubes and the cell suspension were immunolabelled as described by Dardelle et al. (2010) and Follet-Gueye et al. (2012). Controls were performed by omitting the primary antibody. Samples were observed with a Leica DMLB microscope under bright field and fluorescence illumination with fluorescein isothiocyanate (FITC) filter sets (absorption, 485–520 nm; emission, 520–560 nm). Images were digitally acquired with a Leica DFC300FX camera at the same exposure time.
Cell wall extraction
Pollen tubes, cells or leaves were homogenized with a glass potter and treated three times with 70 % EtOH at 70 °C. The insoluble residues were then incubated with a chloroform/methanol mixture (1:1, v/v) and acetone to yield the cell wall extracts.
Xyloglucan analysis
Preparation of xyloglucan fragments.
A 1–5 mg aliquot of the cell wall extract was incubated overnight at 37 °C under agitation at 180 rpm in 500 µL of endo-(1–4)-β-d-glucanase (5 U, EC 3.2.1.4; Megazyme) prepared in 10 mm ammonium acetate buffer, pH 5. After the addition of 1·5 mL of 96 % EtOH and centrifugation (10 min at 8000 g), the supernatant was evaporated to concentrate the XyG oligomers. XyG from tamarind seeds (Megazyme) was treated similarly in parallel and used as a control for the digestion step.
Extraction of the hemicellulose-enriched fraction.
The enzyme-resistant pellets or the crude cell wall extracts were treated with 0·5 % ammonium oxalate at 100 °C for 1 h to remove the pectins. After centrifugation, the pellet was treated overnight with 4 m KOH supplemented with 20 mm NaBH4 to extract the hemicellulose-enriched fraction. The solution was neutralized with acetic acid to pH 5·5, centrifuged and the supernatant was dialysed against ddH2O. The hemicellulose-enriched fraction was freeze-dried and submitted to a new endo-(1–4)-β-d-glucanase digestion or treated for monosaccharide composition and glycosyl-linkage analysis.
MALDI-TOF MS of oligosaccharides.
A voyager DE-Pro matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer (Applied Biosystems) equipped with a 337 nm nitrogen laser was used to analyse XyG fragments. Mass spectra were collected in the reflector delayed extraction mode using 2,5-dihydroxybenzoic acid (Sigma-Aldrich) as matrix. The matrix, freshly dissolved at 5 mg mL−1 in a solution composed of 70:30 acetonitrile/0·1 % trifluoroacetic acid (TFA), was mixed with the water-solubilized oligosaccharides in a ratio 1:1 (v/v). The spectra were recorded in a positive mode, using an acceleration voltage of 20 000 V with a delay time of 100 ns and >50 % of the laser energy. They were externally calibrated using commercially available mixtures of peptides and proteins (ProteoMass™ MALDI Calibration Kit, Sigma-Aldrich). Laser shots were accumulated for each spectrum in order to obtain an acceptable signal to noise ratio (sum of two spectra of 1000 shots per spectrum). Values are expressed as relative percentage and are means ± s.d. from MALDI-TOF spectra obtained after endo-glucanase digestion of the cell wall from three independent biological samples.
Structural characterization of oligosaccharides by MALDI-TOF/TOF MS.
In order to confirm the structure and the presence of fucosyl residues, oligosaccharides were permethylated as described by Mathieu-Rivet et al. (2013). The eluates were spotted in DHB matrix 5 mg mL−1 onto a MALDI plate and analysed by the Autoflex III time-of-flight (TOF/TOF) mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with a frequency-tripled Nd:YAG laser emitting at 355 nm, a LIFT cell, a FlexControl (3.3) and FlexAnalysis (3.3) software package (Bruker Daltonics) in positive reflector mode.
Monosaccharide composition by gas chromatography–flame ionization detection (GC-FID).
Hemicellulose-enriched fractions were hydrolysed by 2 m TFA for 2 h at 110 °C. Monosaccharides were then derivatized with 1 m methanol-HCl (Supelco) at 80 °C overnight followed by a mixture of hexamethyldisiloxan:trimethyldisiloxan:pyridine (3:1:9, Supelco) at 110 °C for 20 min. After drying, derivatives were dissolved in 1 mL of cyclohexane and injected in the 3800 GC system equipped with a CP-Sil5-CB column (Agilent Technologies). The gradient of temperature was from 120 to 160 °C at 10 °C min−1, 160 to 220 °C at 1·5 °C min−1 and 220 to 280 °C at 20 °C min−1. Quantification was based on the internal standard and response factors previously determined for each monosaccharide.
Glycosyl-linkage analysis by gas chromatography–electron ionization (GC-EI) mass spectrometry.
Hemicellulose-enriched extracts were modified to their partially methylated alditol acetate monosaccharides as described by the glycotechnology core resource of San Diego (http://glycotech.ucsd.edu/protocols/07_Comp_Analysis_by_Alditol_Rev2.pdf) and Plancot et al. (2014). Derivatives were then injected in a GC-EI mass spectrometer composed of a Hewlett-Packard 6890 series gas chromatograph coupled with an Autospec mass spectrometer of EBE geometry (Micromass, Manchester, UK) equipped with an Opus 3.1 data system. Chromatographic separations were obtained using a Zebron Z5-MSi (30 m, 0·25 mm id, 0·25 µm film thickness, Phenomenex) capillary column. Helium was the carrier gas and the flow-rate was 0·8 mL min−1. The temperature programming started at 100 °C for 1 min, ramped to 160 °C at 10 °C min−1, then to 220 °C at 2 °C min−1 and finally to 270 °C at 15 °C min−1 (maintained at 270 °C for 1 min). The temperature of the injector, the interface and the lines was 250 °C. Injections of 0·5 µL of samples were performed in splitless mode. The EI mass spectra were recorded using an electron energy of 70 eV, an acceleration voltage of 8 kV and a resolving power of 1000. The trap current was 200 µA and the magnet scan rate was 1 s per decade over a m/z range 600–38. The temperature of the ion source was 250 °C.
Statistical analysis
Comparison of the relative abundance of fucosylated XyG fragments between the different species was determined by one-way analysis of variance (ANOVA; P < 0·05) with GraphPad InStat version 3.1 (GraphPad Software, San Diego, CA, USA, www.graphpad.com).
RESULTS
Immunolocalization of XyG in tomato pollen tubes and cell suspension
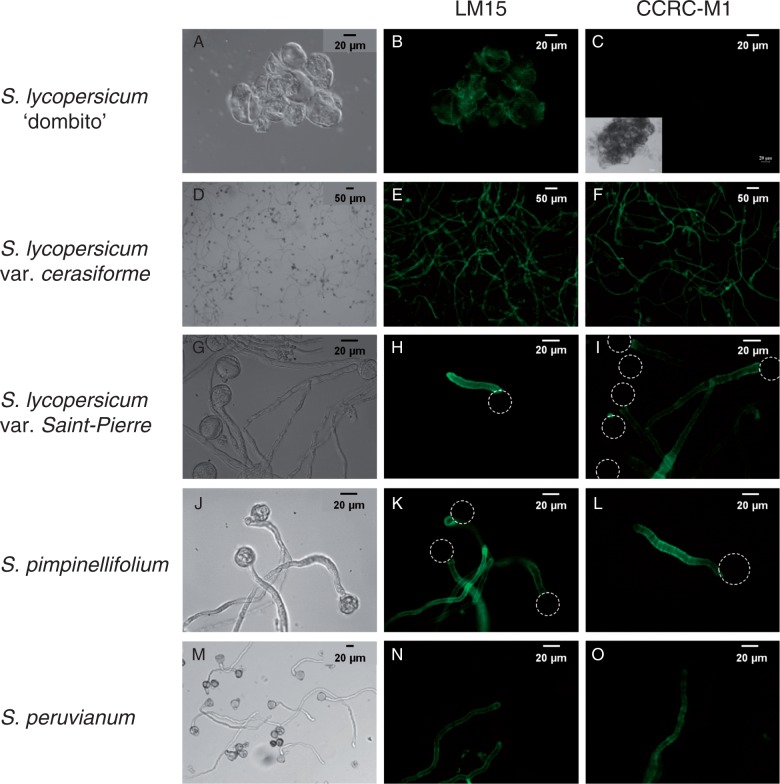
Using a well-described germination medium (Brewbaker and Kwack, 1963), the pollen germination rate of S. lycopersicum var. cerasiforme ‘wva106’ and var. Saint-Pierre was consistently >80 % and pollen tubes were actively growing, reaching over 700 µm in length after 6 h of growth (Fig. 2D, G). Germination rates of S. pimpinellifolium and S. peruvianum reached 71 and 32 %, respectively. The means of the pollen tube length were 630 ± 310 and 260 ± 180 µm, with some pollen tubes reaching over 1 mm (Fig. 2J, M). In the cell walls of the tomato cell suspension (Fig. 2B) and pollen tubes (Fig. 2E, H, K, N), a signal was detected by probing with the LM15 mAb. On the other hand, the CCRC-M1 mAb did not label the cell wall of the tomato cell suspension (Fig. 2C). In contrast, pollen tube cell walls of all the tomato species were labelled in the tip region and in the shank of the tubes (Fig. 2F, I, L, O), suggesting the presence of fucosylated XyG.
Fig. 2.
Immunolocalization of xylosylated XyG with LM15 and fucosylated XyG with CCRC-M1 in tomato suspension-cultured cells and pollen tubes. Brightfield and fluorescent images of (A–C) S. lycopersicum ‘dombito’ cell suspension, (D–F) S. lycopersicum var. cerasiforme ‘wva106’, (G–I) S. lycopersicum var. Saint-Pierre, (J–L) S. pimpinellifolium and (M–O) S. peruvianum pollen tubes. Dotted circles indicate the location of the pollen grain.
MALDI-TOF MS analysis of XyG from tomato leaves and cell suspension
XyG from S. lycopersicum var. cerasiforme leaves contained predominantly the arabinosylated motifs with the main fragment at m/z 1127 (XSGG/SXGG) (31 ± 1·5 %) (Fig. 3; Supplementary Data Fig, S1B, Table S1). The four main fragments (m/z 995, 1127, 1157 and 1289) accounted for > 80 % of the total ion count (Fig. 3; Fig. S1B, Table S1). No fucosylated but several galactosylated motifs such as LLGG (m/z 1319) were detected (Fig. 3; Fig. S1B, Table S1). No significant differences were found between the domesticated species [i.e. S. lycopersicum var. Saint-Pierre (Fig. S1A) and S. lycopersicum var. cerasiforme (Fig. S1B)] and the wild species [i.e. S. pimpinellifolium (Fig. S1C) and S. peruvianum (Fig. 3; Fig. S1D)]. In S. lycopersicum ‘dombito’ suspension-cultured cells, the same motifs were detected with an additional ion at m/z 1169 in relatively high proportion (21·2 ± 0·4 %) that may correspond to TGGG (Fig. S2, Table S1).
Fig. 3.
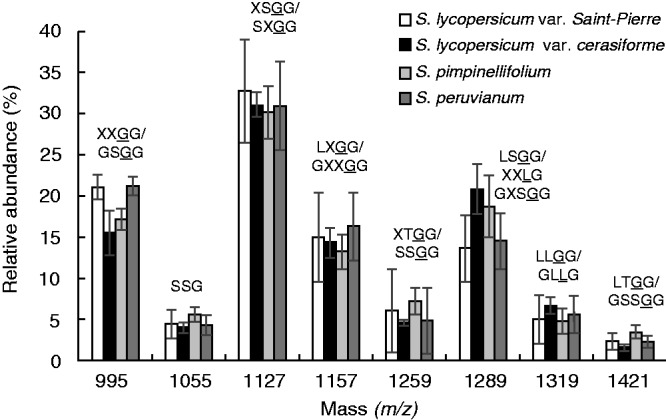
Comparison of the oligosaccharide profile of the main endo-glucanase-sensitive XyG fragments between S. lycopersicum var. Saint-Pierre, S. lycopersicum var. cerasiforme ‘wva106’, S. pimpinellifolium and S. peruvianum leaves. The possible structures of XyG fragments are shown according to the nomenclature proposed by Fry et al. (1993) and shown in Fig. 1. The structures in bold were characterized in S. lycopersicum leaves and cell suspension by York et al. (1996), Jia et al. (2003) and Hoffman et al. (2005). Underlined structures represent O-acetylated side chains. The mean relative abundance is the average of three independent biological samples (n = 3 ± s.d.).
MALDI-TOF MS analysis of XyG from tomato pollen tubes
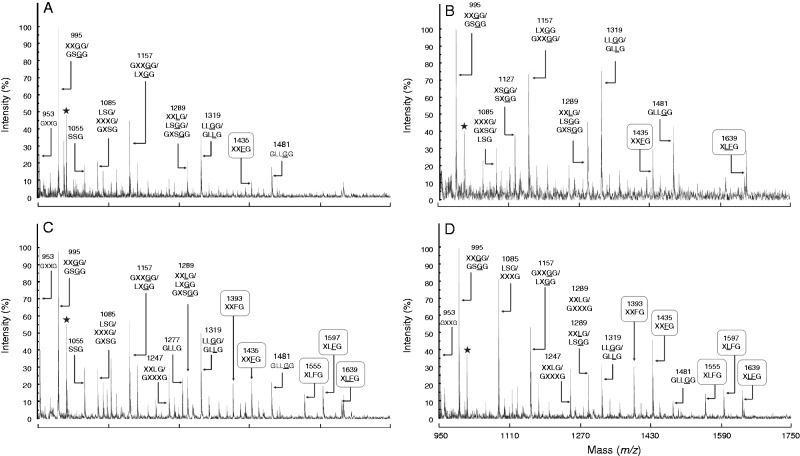
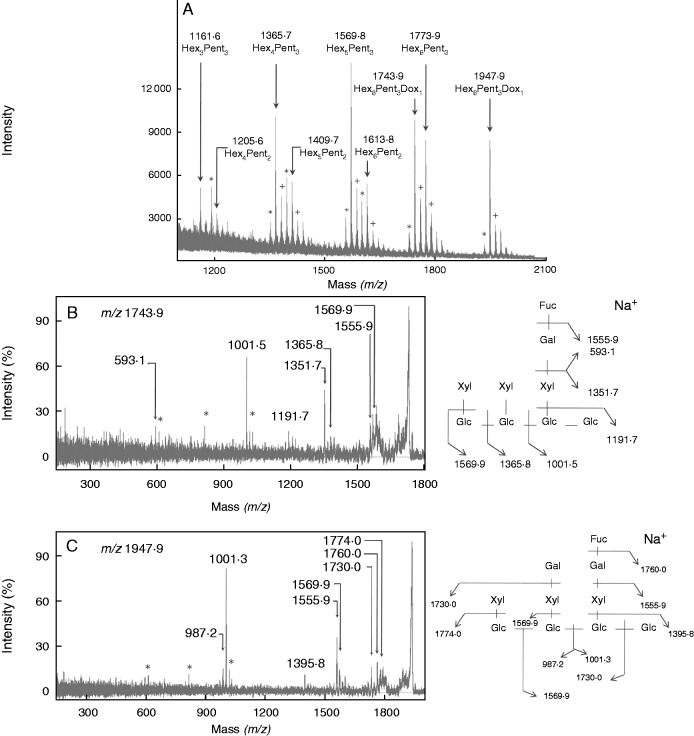
Four categories of XyG oligosaccharides (arabinosylated, xylosylated, galactosylated and fucosylated motifs) were found in tomato pollen tubes (Figs 4 and 5). The main motifs were XXGG/GSGG (m/z 995) (Figs 4 and 5; Supplementary Data Table S2). MALDI-TOF/TOF MS of the ion at m/z 1085 (corresponding to m/z 1365 after permethylation) confirmed the mixture of LSG and XXXG (Fig. S3A). Similarly, the ion at m/z 1289 (corresponding to m/z 1569 after permethylation) was composed of LSGG and XXLG structures in S. peruvianum pollen tubes (Fig. S3B). A new ion at m/z 1409 was observed in the hemicellulose-enriched fraction that may correspond to the bi-galactosylated motif (XLLG) (Fig. S4). Galactose residues were shown to be important for the interaction of XyG with cellulose microfibrils (Whitney et al., 2006), thus it is possible that galactosylated XyG was less accessible to the enzyme degradation in the cell wall residue. The main fucosylated fragments were XXFG (m/z 1435) and XLFG (m/z 1597) in all the investigated species (Figs 4 and 5; Table S2). An additional fucosylated motif XXFG (m/z 1393) was found in the XyG from wild species (Fig. 4C, D). The structure of the two oligosaccharides was confirmed by MALDI-TOF/TOF MS of the permethylated fragments (m/z 1743·9 and 1947·9) in S. peruvianum pollen tubes (Fig. 6). The sum of the relative abundance of fucosylated fragments ranged from 5·3 ± 1 % in S. lycopersicum var. Saint-Pierre to 19·4 ± 2·6 % in S. peruvianum (Fig. 5; Table S2). Solanum lycopersicum var. cerasiforme and S. pimpinellifolium displayed intermediate abundance of 6·5 ± 1·4 and 12·9 ± 1·7 %, respectively (Fig. 5; Table S2). Statistical analyses indicated that the relative abundance of fucosylated XyG motifs was significantly higher in S. peruvianum and S. pimpinellifolium compared with S. lycopersicum sp. (Fig. 5; Table S2).
Fig. 4.
MALDI-TOF MS of endo-glucanase-generated XyG fragments from the cell wall residues of (A) S. lycopersicum var. Saint-Pierre, (B) S. lycopersicum var. cerasiforme ‘wva106’, (C) S. pimpinellifolium and (D) S. peruvianum pollen tubes. The possible structures of XyG fragments are shown according to the nomenclature proposed by Fry et al. (1993) and shown in Fig. 1. In (D), the structures in bold were characterized in this study. Underlined structures represent O-acetylated side chains. The observed ions are mainly [M + Na]+ adducts. Black stars represent unassigned fragments.
Fig. 5.
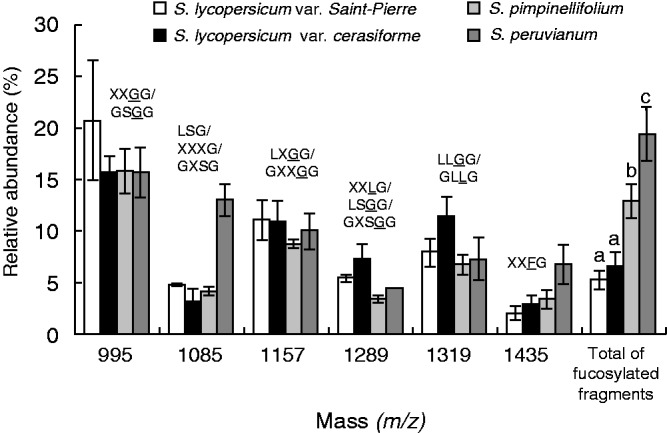
Comparison of the oligosaccharide profile of the main endo-glucanase-sensitive XyG fragments between S. lycopersicum var. Saint-Pierre, S. lycopersicum var. cerasiforme ‘wva106’, S. pimpinellifolium and S. peruvianum pollen tubes. The possible structures of XyG fragments are shown according to the nomenclature proposed by Fry et al. (1993) and shown in Fig. 1. Underlined structures represent O-acetylated side chains. The mean relative abundance is the average of three independent biological samples (n = 3 ± s.d.). Different letters indicate significant differences (P < 0·05).
Fig. 6.
Structural characterization of the fucosylated XyG oligosaccharides (XXFG and XLFG) from S. peruvianum pollen tubes. (A) MALDI-TOF MS spectrum of permethylated oligosaccharides. Ions are in the [M+Na]+ adduct except for those annotated with + ([M + K]+ adduct). Ions labelled by * correspond to underpermethylated ions. (B) MALDI-TOF/TOF MS of the precursor ion at m/z = 1743·9 ([M + Na]+ adduct) of the permethylated Hex5Pent3Dox1 structure (XXFG) and the corresponding fragmentation pattern. (C) MALDI-TOF/TOF MS of the precursor ion at m/z = 1947·9 ([M + Na]+ adduct) of the permethylated Hex6Pent3Dox1 structure (XLFG) and the corresponding fragmentation pattern. Hex, hexose; Pent, pentose; Dox, deoxyhexose; Glc, glucose; Gal, galactose; Xyl, xylose; Fuc, fucose; *, double fragmentation ion (in B and C).
Monosaccharide composition and glycosyl-linkage analysis of the hemicellulose-enriched fractions from tomato pollen tubes
The GC-FID and GC-EI MS analyses of the hemicellulose-enriched fractions from S. peruvianum pollen tubes showed the typical glycosyl-linkages found in fucogalactoXyG (Table 1). These included the 4-Glcp, 4,6-Glcp, t-Xylp, 2-Xylp, t-Galp, 2-Galp and t-Fucp residues. In S. pimpinellifolium, we could detect 4-Glcp, 4,6-Glcp, t-Xylp, 2-Xylp and t-Araf, but not t-Fucp, 2-Galp and t-Galp (Table 1), probably due to the lower level of XyG fucosylation. Fucosyl residues accounted for 0·9 mol % in S. peruvianum and 0·6 mol % in S. pimpinellifolium. In addition, other polymers were also detected, such as the prominent β-glucan found in pollen tube cell walls (i.e. callose) composed of 3-Glcp and the pectic RG-I polymer made of Rha, GalA, Gal (t-Galp, 4- and 6-linked) and Ara (t-Araf and 5-Araf) residues (Table 1).
Table 1.
Monosaccharide composition and glycosyl-linkage analysis of S. peruvianum and S. pimpinellifolium hemicellulose-enriched pollen tube fractions
| S. peruvianum | S. pimpinellifolium | |
|---|---|---|
| Monosaccharide composition* (mol%) | ||
| Glc | 47·2 | 52·1 |
| Xyl | 15·8 | 13·2 |
| Gal | 14·1 | 13·1 |
| Ara | 13·9 | 13·8 |
| Fuc | 0·9 | 0·6 |
| GalA | 1·6 | 1·5 |
| Rha | 2·1 | 2 |
| Man | 4·4 | 3·8 |
| Detected glycosyl-linkage† | ||
| 3-Glcp (callose) | 3-Glcp (callose) | |
| 4-Glcp (XyG) | 4-Glcp (XyG) | |
| 4,6-Glcp (XyG) | 4,6-Glcp (XyG) | |
| t-Xylp (XyG) | t-Xylp (XyG) | |
| 2-Xylp (XyG) | 2-Xylp (XyG) | |
| t-Galp (XyG, RG-I, AGP) | ND | |
| 2-Galp (XyG) | ND | |
| 4-Galp (RG-I) | 4-Galp (RG-I) | |
| 6-Galp (RG-I, AGP) | 6-Galp (RG-I, AGP) | |
| t-Araf (XyG, RG-I, AGP) | t-Araf (XyG, RG-I, AGP) | |
| 5-Araf (RG-I) | 5-Araf (RG-I) | |
| t-Fucp (XyG) | ND | |
*Determined by GC and expressed as mol%. Ara, arabinose; Fuc, fucose; Gal, galactose; Glc, glucose; GalA, galacturonic acid; Rha, rhamnose; Man, mannose; Xyl, xylose.
†Determined by GC-MS of partially methylated alditol acetates. t-Araf denotes 1,4-di-O-acetyl-1-deuterio-2,3,5-tri-O-methyl-D-arabinitol, etc. Polymers that contain these glycosyl-linkages are indicated. XyG, xyloglucan; RG-I, rhamnogalacturonan-I; AGP, arabinogalactan proteins. In bold are the glycosyl-linkages found in XyG. ND, not detected.
MALDI-TOF/MS, monosaccharide composition and glycosyl-linkage analyses of the XyG from N. tabacum pollen tubes
Spectra obtained from the cell wall residues of N. tabacum pollen tubes showed the dominance of fucosylated motifs (Supplementary Data Fig. S5A, Table S3) as reported in N. alata (Lampugnani et al., 2013). The main fragments were the O-acetylated XXFG (m/z 1435) and XLFG (m/z 1639) (29·7 ± 1·9 and 23 ± 3 %, respectively) (Table S3). In the hemicellulose-enriched fraction, the same motifs were found, i.e. XXFG (m/z 1393) and XLFG (m/z 1555), but in their de-acetylated forms as expected with the KOH treatment (Fig. S5B, Table S3). Finally, all the glycosyl-linkages found in fucogalactoXyG were also detected, and the fucosyl residue accounted for 1·4 mol % (Table S4).
DISCUSSION
In our previous work, we showed that the XyG in A. thaliana pollen tubes was highly fucosylated (Dardelle et al., 2010) and hypothesized that this structural feature may have an important role in sexual plant reproduction. In the present study, using immunolocalization, OLIMP (Lerouxel et al., 2002; Obel et al., 2009; Günl et al., 2011), GC and GC-MS analyses, we have investigated the structure of XyG of pollen tube cell walls of several Solanaceae species taking advantage of the fact that XyG of somatic cells/organs in this family was shown to lack fucosyl residues (Sims et al., 1996; York et al., 1996; Jia et al., 2003, 2005; Hoffman et al., 2005). The reason for and significance of this difference in the XyG structure between the lamiid clade and the other angiosperms are not known, but it has been suggested that it may be related to an adaptive and/or selective diversification (Del Bem and Vincentz, 2010).
XyG from the leaves of wild and domesticated tomato species is structurally similar
Analyses of XyG from the leaf and cell suspension of the domesticated species S. lycopersicum are consistent with previous works described in the literature. First, the cell wall of tomato cell suspension was labelled with the mAb LM15 that recognizes epitopes present in fucogalactoXyG and arabinoXyG. In contrast, no signal was observed with CCRC-M1, a mAb that binds to α-Fuc-(1→2)-β-Gal epitopes found in fucosylated XyG (Puhlmann et al., 1994). Similar results were shown previously in tobacco suspension-cultured cells (Chevalier et al., 2010) and organs including roots and leaves (Balestrini et al., 1996; Brennan and Harris, 2011). Secondly, the relative abundance of the oligosaccharides analysed by MALDI-TOF MS from S. lycopersicum is in agreement with previous data obtained by chromatography and spectroscopy (York et al., 1996; Jia et al., 2003, 2005; Hoffman et al., 2005). Altogether, the data indicate that XyG is not fucosylated, but it is mostly arabinosylated and can be galactosylated in the tomato species.
The data also indicate that the structures of XyG from the leaf of wild and domesticated tomato species are comparable. They suggest that the successive selections, primarily performed for improving the fruit traits, have not affected the XyG structure of the vegetative organs. In contrast, Zhang et al. (2012) reported that the culm cell walls of wild and domesticated rice species contain structural variations in the arabinoxylan structure with an increased level of xylan backbone in the domesticated species. The authors suggested that these structural changes of cell wall xylans may have helped the cultivated plants to increase their erect growth habit compared with the less upright growth habit of the wild species.
XyG of tomato pollen tubes is arabinosylated and fucosylated
Pollen tubes from all investigated species were significantly labelled with CCRC-M1, indicating the presence of epitopes associated with fucosylated XyG as shown in A. thaliana (Freshour et al., 2003; Dardelle et al., 2010; Chebli et al., 2012) and N. alata (Lampugnani et al., 2013) pollen tubes. The presence of fucosylated XyG in tomato pollen tubes was confirmed by MALDI-TOF MS, MALDI-TOF/TOF MS and GC-MS. The main XyG oligosaccharide (XXFG) found in tomato pollen tubes is also the principal motif detected in A. thaliana (Dardelle et al., 2010), N. tabacum and N. alata (Lampugnani et al., 2013) pollen tubes. The main difference compared with A. thaliana is the presence of the double O-acetylated XLFG fragment (m/z 1639), previously described in bilberry and peach fruits (Hilz et al., 2007; Lahaye et al., 2012). The relative abundance of fucosylated fragments was 52 % in A. thaliana (Dardelle et al., 2010), 62 % in N. tabacum (Supplementary Data Table S3) and <20 % in tomato pollen tubes (Fig. 7). In addition, the typical glycosyl-linkages were characteristic of fucogalactoXyG. Despite slight differences in the relative abundance of the fucosylated motifs between N. alata (Lampugnani et al., 2013) and N. tabacum pollen tubes, XyG is exclusively of the fucogalactoXyG type. In contrast, tomato pollen tube cell walls are composed of arabino- and fucogalactoXyGs. This uncommon mixture of arabinosylated and fucosylated XyG was reported once before in the leaf cell wall of Nerium oleander (lamiids, Gentianales), suggesting that this early divergent order may bridge an evolutionary gap between plants with Fuc–Gal and Ara substitution of the XyG in this clade (Hoffman et al., 2005). Whether these two populations of XyG coexist in the cell wall or whether there is only one type of polymer that is branched with both Ara and Fuc residues is not known.
Fig. 7.
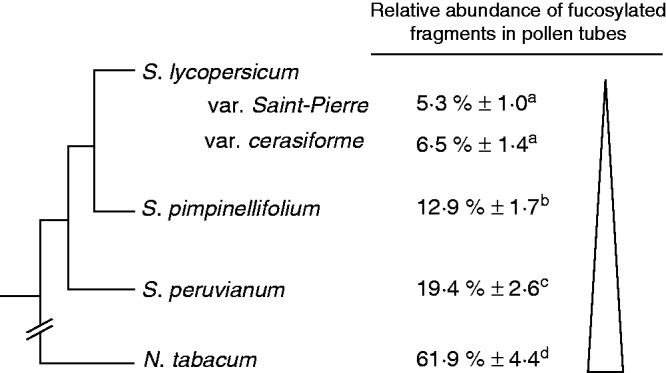
Comparison of the relative abundance of fucosylated XyG oligosaccharides found in pollen tubes of N. tabacum and the tomato species. The schematic tree was based on the work of Rodriguez et al. (2009), Nakazato et al. (2010) and Bedinger et al. (2011). Different letters indicate significant differences (P < 0·05).
XyG of pollen tubes from wild tomatoes is more fucosylated than that from the domesticated species
The evolution of tomato species has been highly explored due to their agronomic and economic importance (Baudry et al., 2001; Tanksley, 2004; Rodriguez et al., 2009; Nakazato et al., 2010; Bedinger et al., 2011). To date, it has been assumed that S. lycopersicum, the domesticated species, shares a common ancestor with S. pimpinellifolium and S. peruvianum, both described as wild species. However, S. pimpinellifolium was proposed to be the closest wild ancestor of the domesticated S. lycopersicum var. cerasiforme, the cherry tomato (Fig. 7) (Nesbitt and Tanksley, 2002; Bai and Lindhout, 2007; Ranc et al., 2008). It appears that there is a gradient in the abundance of fucosylated XyG motifs between the wild tomatoes (S. peruvianum and S. pimpinellifolium) and the domesticated S. lycopersicum (Fig. 7). The biological significance of the reduction in the abundance of fucosylated XyG in the cell wall of pollen tubes from the domesticated tomatoes is not known, but it may reflect an evolutionary trait and/or a selective pressure. Interestingly, differences in the structure and size of the stigma, style and transmitting tissue and in the composition of the exudates exist between the different species (Bedinger et al., 2011). The different levels of XyG fucosylation may possibly contribute to adapt the pollen tube interaction and growth in the different pistil structures.
Tomato pollen tubes must have specific and functional XyG fucosyltransferases
The presence of fucosylated XyG in pollen tubes indicates that pollen must have specific and functional XyG fucosyltransferases. The transcriptome of N. alata pollen grains revealed the presence of most related genes involved in XyG biosynthesis such as the β-(1→4)-d-glucansynthase, α-(1→6)-d-xylosyltransferase and β-(1→2)-d-galactosyltransferase (Lampugnani et al., 2013). However, the RNA-Seq analysis of Lampugnani et al. (2013) failed to detect any RNA contigs that were predicted to encode α-(1→2)-l-fucosyltransferases, while they showed that the cell wall of N. alata pollen tubes contains most exclusively fucosylated XyG. In the S. lycopersicum ‘Heinz’ genome (Tomato Genome Consortium, 2012), three genes (Solyc07g047920.1, Solyc06g061210.2 and Solyc03g115830.1) are predicted to encode XyG galactoside α-2-fucosyltransferases, and Solyc07g047920.1 is expressed in flowers (SOL genomics network, Mueller et al., 2005; http://ted.bti.cornell.edu/cgi-bin/TFGD/digital/search.cgi?ID=D004). This suggests that this gene may be a good candidate for the transfer of GDP-fucose onto the galactosyl residues in tomato pollen tube cell walls, but functional studies are required to confirm this hypothesis.
XyG is important in tip-polarized cells and can have unusual composition
Studies of mutants impaired in XyG xylosyltransferases (xxt1/xxt2), in A. thaliana that lacks detectable XyG (Cavalier et al., 2008) and in Oryza sativa, a grass species with low XyG content (Wang et al., 2014), showed no obvious growth defect of the mutant plants except abnormal growth of root hairs, which are also tip-polarized cells. Interestingly, the pollen tube of the xxt1/xxt2 double mutant was not labelled with the anti-XyG mAb, CCRC-M1 and LM15, and displayed severe phenotype and growth defects along with a slight decrease of the stiffness of the pollen tube (Draeger, 2012). These studies and others (Zabotina et al., 2008) clearly indicate that XyG, even in a low amount, is of prime importance for tip-growing cells and particularly pollen tubes.
The unexpected XyG structure in pollen tube cell walls found in N. alata (Lampugnani et al., 2013), N. tabacum and Solanaceae species indicates that tip-polarized cells have specific structural cell wall features. Peña et al. (2012) have also found an unusual type of XyG containing galacturonic acid residues in A. thaliana root hairs. Similarly, in the tip-growing moss Physcomitrella patens, XyG also contains galacturonic acid (Peña et al., 2008). This sugar residue has not been detected in A. thaliana (Dardelle et al., 2010) and N. alata (Lampugnani et al., 2013) pollen tubes nor in the Solanaceae species investigated in the present study but it reinforces the idea that tip-polarized cells have specific cell wall structural properties adapted to their fast intrusive growth, i.e. between the soil particles for the root hair or between the transmitting tract cells for the pollen tube.
Role of XyG in the mechanical properties of the cell wall
The impact of the structural differences (Ara-Xyl vs. Fuc-Gal-Xyl side chains) on the architecture of the cell wall, on the interaction between the polymers in the cell wall network and on the mechanical properties of the cell wall is not clear. However, by expressing tomato XyG arabinosyltransferases in the A. thaliana double mutant mur3.1/xlt2 that lacks galactosylated XyG, Schultink et al. (2013) showed that the transgenic plants were able to produce the arabinosylated form of XyG, a structure not found in wild-type plants. This resulted in the partial restoration of the growth phenotype and mechanical properties of the cell wall in the double mutant, suggesting that XyG galactosylation and arabinosylation may fulfil similar functions (Schultink et al., 2013).
The role of fucosyl residues in XyG is still not clear. The fact that most eudicots have fucosylated XyG suggested that this structural feature may give a possible selective advantage for the plants (Hoffman et al., 2005). However, mutation in AtFUT1 encoding a XyG fucosyltransferase or in MUR3 encoding a XyG galactosyltransferase induced an absence of a fucosyl residue or a Fuc–Gal motif on the XyG, but the mutant plants showed normal growth habit and wall strength (Vanzin et al., 2002; Madson et al., 2003; Perrin et al., 2003), suggesting that the plants may compensate by modifying other cell wall structural features. In addition, it has been reported that fucosyl residues were not of prime importance for the interaction of XyG with cellulose microfibrils, in contrast to the galactosyl residues (Peña et al., 2004; Whitney et al., 2006). Park and Cosgrove (2012a, b) have shown by analysing the cell wall biochemical properties in the xxt1/xxt2 mutant and cucumber hypocotyl treated with specific hydrolases that only a minor XyG component was important for the interaction with cellulose microfibrils and the cell wall mechanics. Thus, the presence of fucosyl residues and O-acetyl groups on the XyG may then modulate its interaction with cellulose microfibrils in the pollen tube cell wall, allowing the tube growth and elongation.
To conclude, the biological significance of the differences observed in the XyG structure between the male gametophyte and the sporophyte is unclear, but the finding suggests that fucosylation of XyG, even in low abundance, is an important feature allowing mechanical stability of the cell wall during the tip-polarized growth of the pollen tube and/or its interaction with the pistil. Finally, the completion of the detailed annotation of S. pimpinellifolium and other genomes and transcriptomic data should provide more insight into the evolution of the Solanaceae and in the discovery of new genes (Ranjan et al., 2012) that may allow the functional characterization of XyG fucosyltransferases specific to tomato pollen.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: MALDI-TOF MS of endo-glucanase-generated XyG fragments from S. lycopersicum var. Saint-Pierre, S. lycopersicum var. cerasiforme, S. pimpinellifolium and S. peruvianum leaves. Figure S2: MALDI-TOF MS of endo-glucanase-generated XyG fragments from S. lycopersicum ‘dombito’ cell suspension. Figure S3: structural characterization of LSG and LSGG/XXLG oligosaccharides from S. peruvianum pollen tubes by MALDI-TOF/TOF MS. Figure S4: MALDI-TOF MS of endo-glucanase-generated XyG fragments from the hemicellulose-enriched extract of S. lycopersicum var. cerasiforme pollen tubes. Figure S5: MALDI-TOF MS of endo-glucanase-generated XyG fragments from the cell wall extract and the hemicellulose-enriched extract of N. tabacum pollen tubes. Table S1: relative abundance of XyG oligosaccharides released after endo-glucanase digestion of the cell wall of S. lycopersicum ‘dombito’ cell suspension and S. lycopersicum var. cerasiforme leaves. Table S2: relative abundance of XyG oligosaccharides released after endo-glucanase digestion of the cell wall residue and the hemicellulose-enriched extracts from tomato pollen tubes. Table S3: relative abundance of XyG oligosaccharides released after endo-glucanase digestion of the cell wall residue and the hemicellulose-enriched extract from N. tabacum pollen tubes. Table S4: monosaccharide composition and glycosyl-linkage analysis of N. tabacum hemicellulose-enriched pollen tube extract.
ACKNOWLEDGEMENTS
The authors are grateul to Hélène Burck (Génétique et amélioration des fruits et légumes, INRA Avignon UR1052, France) for the gift of wild tomato seeds, to Dr Pierre Baldet (Physiologie et Biotechnologie Végétales, INRA Bordeaux UMR 619, France) for providing the cherry tomato ‘wva106’ seeds, to Dr Aurélie Muschitz (University of Limoges, France) for the tomato cell suspension, to Maxime Grare for the culture of tomato pollen tubes and several of the MALDI-TOF MS analyses, to Christelle Leroux, Marie Dumont and Dr Abderrakib Zahid for technical support, and to PRIMACEN (Regional Platform for Cell Imaging of the Upper-Normandy region), part of the Institute for Research and Innovation in Biomedicine (IRIB), for the use of equipment. We are grateful to the Upper Normandy region, the Research Network ‘Végétal, Agronomie, Sol, Innovation’ (VASI) Haute Normandie, the University of Rouen, the Labex SynOrg (ANR-11-LABX-0029) and the European Regional Development Fund (ERDF 31708) for financial support. VASI is also greatly acknowledged for the doctoral fellowship (to F.D.).
LITERATURE CITED
- APG III. 2009. An update of the Angiosperm Phylogeny Group for the orders and families of flowering plants: APGIII. Botanical Journal of the Linnean Society 161: 105–121. [Google Scholar]
- Bai YL, Lindhout P. 2007. Domestication and breeding of tomatoes: what have we gained and what can we gain in the future? Annals of Botany 100: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini R, Hahn MG, Faccio A, et al. 1996. Differential localization of carbohydrate epitopes in plant cell walls in the presence and absence of arbuscular mycorrhizal fungi. Plant Physiology 111: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry E, Kerdelhue C, Innan H, Stephan W. 2001. Species and recombination effects on DNA variability in the tomato genus. Genetics 158: 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedinger PA, Chetelat RT, McClure B, et al. 2011. Interspecific reproductive barriers in the tomato clade: opportunities to decipher mechanisms of reproductive isolation. Sexual Plant Reproduction 24: 171–187. [DOI] [PubMed] [Google Scholar]
- Brennan M, Harris PJ. 2011. Distribution of fucosylated xyloglucans among the walls of different cell types in monocotyledons determined by immunofluorescence microscopy. Molecular Plant 4: 144–156. [DOI] [PubMed] [Google Scholar]
- Brewbaker JL, Kwack BH. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany 50: 859–865. [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, et al. 2008. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. The Plant Cell 20: 1519–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y, Kaneda M, Zerzour R, Geitmann A. 2012. The cell wall of the Arabidopsis thaliana pollen tube – spatial distribution, recycling and network formation of polysaccharides. Plant Physiology 160: 1940–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM. 2008. Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annual Review of Plant Biology 59: 547–572. [DOI] [PubMed] [Google Scholar]
- Chevalier L, Bernard S, Ramdani Y, et al. 2010. Subcompartment localization of the side chain xyloglucan-synthesizing enzymes within Golgi stacks of tobacco suspension-cultured cells. The Plant Journal 64: 977–989. [DOI] [PubMed] [Google Scholar]
- Dardelle F, Lehner A, Ramdani Y, et al. 2010. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiology 153: 1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bem LE, Vincentz MGA. 2010. Evolution of xyloglucan-related genes in green plants. BMC Evolutionary Biology 10: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger C. 2012. Genetic, biochemical, and biomechanical study of plant cell wall dynamics in pollen tubes. PhD thesis, University of Zurich. [Google Scholar]
- Dumont M, Lehner A, Bouton S, et al. 2014. The cell wall pectic polymer rhamnogalacturonan-II is required for proper pollen tube elongation: implication of a putative sialyltransferase-like protein. Annals of Botany (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand C, Vicre-Gibouin M, Follet-Gueye ML, et al. 2009. The organization pattern of root border-likes cells of Arabidopsis thaliana is dependent on cell wall homogalacturonan. Plant Physiology 150: 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follet-Gueye ML, Mollet JC, Vicré-Gibouin M, et al. 2012. Immuno-glyco-imaging in plant cells: localization of cell wall carbohydrate epitopes and their biosynthesizing enzymes. In: Delghani H, ed. Applications of immunocytochemistry. Rijeka: InTech, 297–320. [Google Scholar]
- Franklin-Tong N. 2010. Plant fertilization: bursting pollen tubes! Current Biology 20: R681–R683. [DOI] [PubMed] [Google Scholar]
- Freshour G, Bonin CP, Reiter WD, et al. 2003. Distribution of fucose-containing xyloglucans in cell walls of mur1 mutant of Arabidopsis thaliana. Plant Physiology 131: 1602–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC. 2011. Cell wall polysaccharide composition and covalent crosslinking. Annual Plant Review 41: 1–42. [Google Scholar]
- Fry SC, York WS, Albersheim P, et al. 1993. An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiologia Plantarum 89: 1–3. [Google Scholar]
- Günl M, Kraemer F, Pauly M. 2011. Oligosaccharide Mass Profiling (OLIMP) of cell wall polysaccharides by MALDI-TOF/MS. Methods in Molecular Biology 15: 43–54. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kaida R. 2010. Functions of xyloglucan in plant cells. Molecular Plant 4: 17–24. [DOI] [PubMed] [Google Scholar]
- Hilz H, de Jong LE, Kabel MA, et al. 2007. Bilberry xyloglucan – novel building blocks containing beta-xylose within a complex structure. Carbohydrate Research 342: 170–181. [DOI] [PubMed] [Google Scholar]
- Hoffman M, Jia Z, Pena MJ, et al. 2005. Structural analysis of xyloglucans in the primary cell walls of plants in the subclass Asteridae. Carbohydrate Research 340: 1826–1840. [DOI] [PubMed] [Google Scholar]
- Hsieh YSY, Harris PJ. 2009. Xylogucans of monocotyledons have diverse structures. Molecular Plant 2: 943–965. [DOI] [PubMed] [Google Scholar]
- Jia Z, Qin Q, Darvill AG, York WS. 2003. Structure of the xyloglucan produced by suspension-cultured tomato cells. Carbohydrate Research 338: 1197–1208. [DOI] [PubMed] [Google Scholar]
- Jia Z, Cash M, Darvill AG, York WS. 2005. NMR characterization of endogenously O-acetylated oligosaccharides isolated from tomato (Lycopersicon esculentum) xyloglucan. Carbohydrate Research 340: 1818–1825. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Lord EM. 2006. Extracellular guidance cues and intracellular signaling pathways that direct pollen tube growth. In: Malho R, ed. The pollen tube: a cellular and molecular perspective, Vol. 3 Heidelberg: Springer, 223–242. [Google Scholar]
- Lahaye M, Falourd X, Quemener B, et al. 2012. Cell wall polysaccharide chemistry of peach genotypes with contrasted textures and other fruit traits. Journal of Agricultural and Food Chemistry 60: 6594–6605. [DOI] [PubMed] [Google Scholar]
- Lampugnani ER, Moller IE, Cassin A, et al. 2013. In vitro grown pollen tubes of Nicotiana alata actively synthesise a fucosylated xyloglucan. PLoS One 8: e77140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouxel O, Choo TS, Séveno M, et al. 2002. Rapid structural phenotyping of plant cell wall mutants by enzymatic oligosaccharide fingerprinting. Plant Physiology 130: 1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson M, Dunand C, Li X, et al. 2003. The MUR3 gene of Arabidopsis encodes a xyloglucan galactosyltransferase that is evolutionarily related to animal exostosins. The Plant Cell 15: 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SE, Verhertbruggen Y, Herve C, et al. 2008. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biology 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Rivet E, Scholz M, Arias C, et al. 2013. Exploring the N-glycosylation pathway in Chlamydomonas reinhardtii unravels novel complex structures. Molecular and Cellular Proteomics 12: 3160–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet JC, Faugeron C, Morvan H. 2007. Cell adhesion, separation and guidance in compatible plant reproduction. Annual Plant Review 25: 69–90. [Google Scholar]
- Mollet JC, Leroux C, Dardelle F, Lehner A. 2013. Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants 2: 107–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller LA, Solow TH, Taylor N, et al. 2005. The SOL Genomics Network: a comparative resource for Solanaceae biology and beyond. Plant Physiology 138: 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschitz A, Faugeron C, Morvan H. 2009. Response of cultured tomato cells subjected to excess zinc: role of cell wall in zinc compartmentation. Acta Physiologiae Plantarum 31: 1197–1204. [Google Scholar]
- Nakazato T, Warren DL, Moyle LC. 2010. Ecological and geographic modes of species divergence in wild tomatoes. American Journal of Botany 97: 680–693. [DOI] [PubMed] [Google Scholar]
- Nesbitt TC, Tanksley SD. 2002. Comparative sequencing in the genus lycopersicon: implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics 162: 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel N, Erben V, Schwarz T, et al. 2009. Microanalysis of plant cell wall polysaccharides. Molecular Plant 2: 922–932. [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Tsukamoto T. 2012. Pathfinding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization. WIREs Developmental Biology 1: 96–113. [DOI] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ. 2012a. Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiology 158: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ. 2012b. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiology 158: 1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña MJ, Ryden P, Madson M, Smith AC, Carpita NC. 2004. The galactose residues of xyloglucan are essential to maintain mechanical strength of the primary cell walls in Arabidopsis during growth. Plant Physiology 134: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña MJ, Darvill AG, Eberhard S, York WS, O’Neill MA. 2008. Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from xyloglucans synthesised by hornworts and vascular plants. Glycobiology 18: 891–904. [DOI] [PubMed] [Google Scholar]
- Peña MJ, Kong Y, York WS, O’Neill MA. 2012. A galacturonic acid-containing xyloglucan is involved in Arabidopsis root hair tip growth. The Plant Cell 24: 4511–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RM, Jia Z, Wagner TA, et al. 2003. Analysis of xyloglucan fucosylation in Arabidopsis. Plant Physiology 132: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plancot B, Vanier G, Maire F, et al. 2014. Structural characterization of arabinoxylans from two African plant species Eragrostis nindensis and Eragrostis tef using various mass spectrometric methods. Rapid Communications in Mass Spectrometry 28: 908–916. [DOI] [PubMed] [Google Scholar]
- Popper ZA, Michel G, Hervé C, et al. 2011. Evolution and diversity of plant cell walls: from algae to flowering plants. Annual Review of Plant Biology 62: 567–590. [DOI] [PubMed] [Google Scholar]
- Puhlmann J, Bucheli E, Swain MJ, et al. 1994. Generation of monoclonal antibodies against plant cell wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1→2)-linked fucosyl-containingepitope. Plant Physiology 104: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Ting D, Shieh A, McCormick S. 2012. Callose plug deposition patterns vary in pollen tubes of Arabidopsis thaliana ecotypes and tomato species. BMC Plant Biology 12: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranc N, Muños S, Santoni S, Causse M. 2008. A clarified position for Solanum lycopersicum var. cerasiforme in the evolutionary history of tomatoes (Solanaceae). BMC Plant Biology 8: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A, Ichihashi Y, Sinha NR. 2012. The tomato genome: implications for plant breeding, genomics and evolution. Genome Biology 13: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez F, Wu F, Ané C, Tanksley S, Spooner DM. 2009. Do potatoes and tomatoes have a single evolutionary history, and what proportion of the genome supports this history? BMC Evolutionary Biology 9: 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P. 2010. Hemicelluloses. Annual Review of Plant Biology 61: 263–289. [DOI] [PubMed] [Google Scholar]
- Schultink A, Cheng K, Park YB, Cosgrove DJ, Pauly M. 2013. The identification of two arabinosyltransferases from tomato reveals functional equivalency of xyloglucan side chain substituents. Plant Physiology 163: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims IM, Munro SL, Currie G, Craik D, Bacic A. 1996. Structural characterisation of xyloglucan secreted by suspension-cultured cells of Nicotiana plumbaginifolia. Carbohydrate Research 293: 147–172. [DOI] [PubMed] [Google Scholar]
- Tanksley SD. 2004. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. The Plant Cell 16: S181–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium. 2012. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzin GF, Madson M, Carpita NC, et al. 2002. The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proceedings of the National Academy of Sciences, USA 99: 3340–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincken JP, York WS, Beldman G, Voragen AGJ. 1997. Two general branching patterns of xyloglucan, XXXYG and XXYGG. Plant Physiology 114: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li S, Ng S, et al. 2014. Mutation in xyloglucan 6-xylosytransferase results in abnormal root hair development in Oryza sativa. Journal of Experimental Botany 65: 4149–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SEC, Wilson E, Webster J, et al. 2006. Effects of structural variation in xyloglucan polymers on interactions with bacterial cellulose . American Journal of Botany 93: 1402–1414. [DOI] [PubMed] [Google Scholar]
- York WS, Kumar Kolli VS, Orlando R, Albersheim P, Darvill AG. 1996. The structures of arabinoxyloglucans produced by solanaceous plants. Carbohydrate Research 285: 99–128. [DOI] [PubMed] [Google Scholar]
- Zabotina OA, van de Ven WT, Freshour G, et al. 2008. Arabidopsis XXT5 gene encodes a putative alpha-1,6-xylosyltransferase that is involved in xyloglucan biosynthesis. The Plant Journal 56: 101–115. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Song XQ, Yu BS, et al. 2012. Identification of quantitative trait loci affecting hemicellulose characteristics based on cell wall composition in a wild and cultivated rice species. Molecular Plant 5: 162–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.