Abstract
Most studies on the nuclear retinoid-X receptor (RXR) have focused on its role as a heterodimeric partner but less about its own activation pattern during development and the distribution of potential endogenous ligands. The aim of this study is to visualize the distribution of activated RXRα in live transgenic Xenopus laevis embryos across a wide range of developmental stages. We adopted a nuclear receptor–Gal4 fusion/upstream activation sequence-based reporter system for our assay. Strong activation of the RXRα ligand-binding domain was observed in a segment of the spinal cord just posterior to the hindbrain. This activation is first detected in neurula stage embryos and persists up to swimming tadpole stages, after which activation strongly declines. Addition of exogenous ligands, such as 9-cis retinoic acid or all-trans retinoic acid, expands the activation of RXR throughout the spinal cord but not in the brain, whereas the RXR-specific ligand LG268 expanded the Gal4–RXR activation into the brain and olfactory epithelia. Treatment with the RAR-specific ligand 4-(E-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl)benzoic acid or thyroid hormone had no effect on Gal4–RXR activation, whereas these compounds significantly increased their corresponding Gal4/receptor fusion proteins under similar conditions. Embryos expressing a Gal4–RXR fusion protein with a deletion in the ligand-dependent activation domain (AF2) show no reporter gene activation. The results shown in this paper reveal a specific activation pattern for Gal4–RXRα specifically in the developing spinal cord and suggest the existence of RXR ligand “hot-spots” in this region.
Keywords: Gal4, transgenesis, Xenopus laevis, nuclear receptor
Retinoic acid (RA), a metabolite of vitamin A, is a potent signaling molecule important for proper vertebrate development (1). RA deficiency or excess causes severe defects in the developing central nervous system (CNS), heart, and limbs. Thus, a tight balance of RA synthesis and degradation must be maintained. Two isomers of RA are high-affinity ligands for two members of the nuclear receptor family, retinoic acid receptors (RARs) and retinoid-X receptors (RXRs) (2–4). RXRs bind exclusively to the 9-cis-isomer of RA, (5–9), whereas RARs interact with both 9-cis and all-trans isomers of RA (10, 11). RXR occupies a central position as a heterodimerization partner with RAR as well as with other members of the nuclear receptor superfamily. In some cases, RXR appears to be a completely silent partner (such as in RXR/thyroid hormone receptor complexes), a conditionally silent partner with certain other nuclear receptors (e.g., RXR/RAR), and with still others, RXR appears to be fully able to be activated by its cognate ligand irrespective of the ligand occupancy of its partner (12).
Vitamin A (retinol) is acquired via the diet and can be converted into retinol in cells. Retinol is a key metabolite required for the visual process but also serves as the precursor for all-trans RA (atRA) biosynthesis. atRA is synthesized by a two-step process involving the conversion of vitamin A (retinol) to retinaldehyde by the activity of alcohol or retinol dehydrogenase, followed by the conversion of retinaldehyde to atRA by retinaldehyde dehydrogenase (13, 14). A proposed enzymatic isomerization of the all-trans isomer yields its 9-cis isomer. The regulation of this key enzymatic step controls the relative 9-cis/all-trans ratio within the cell and therefore may regulate the RXR and RAR pathways. Despite a wealth of knowledge regarding atRA synthesis, far less is known about endogenous 9-cis RA distribution and its role as a physiological activator of RXR. 9-cis RA has been very difficult to detect in vivo, and the enzyme responsible for the atRA to 9-cis RA conversion step remains elusive. More recently, a lipid abundant in the adult mammalian brain, docosahexaenoic acid, has been identified as a potential ligand for RXR, and still other potential ligands been proposed based on x-ray crystallographic results (15, 16).
To determine the activation pattern of RXR in the nervous system of the developing embryo in both time and space, we applied the binary Gal4/upstream activation sequence (UAS) system (17) in transgenic Xenopus laevis embryos. We cointegrated an expression vector for the yeast Gal4 DNA-binding domain fused to the ligand-binding domain of RXRα with a UAS-based GFP reporter gene. Expression of the reporter gene indicates the activation of the RXR ligand-binding domain. In live transgenic embryos, we show that RXR is transiently activated in the same region and time when primary motor neurons develop in the X. laevis spinal cord, just posterior to the hindbrain. This activation was completely reliant on the ligand-dependent activation domain AF-2. Our results provide “real-time” information about the regional distribution of the activated RXRα in the CNS and thus the location of a potentially important endogenous ligand. Furthermore, this technique can be applied to localizing the active form of orphan nuclear receptors and their putative ligands, which may be synthesized transiently and in highly localized areas of developing embryos.
Materials and Methods
Chemicals. Liebovitz L-15, OptiMEM, and Lipofectamine 2000 were purchased from Invitrogen Life Technologies. Charcoal-stripped FBS was obtained from HyClone (Logan, UT). atRA, 9-cis RA, and 4-(E-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl)benzoic acid (TTNPB) were obtained from Sigma. The RXR selective agonist LG100268 and antagonist LG100208 were kindly provided by M. Leibowitz (Ligand Pharmaceuticals, San Diego). Pfu DNA polymerase was obtained from Stratagene.
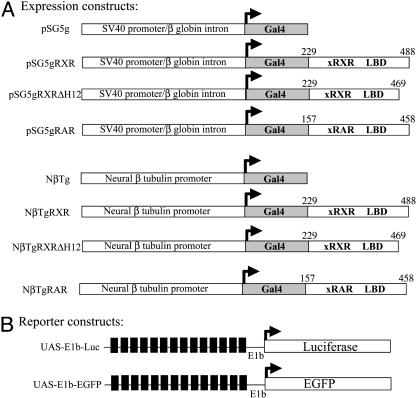
DNA Constructs. pSG5gRXR was cloned as follows: DNA encoding the RXRα ligand-binding domain (LBD) (amino acids 229–488) was amplified by PCR using Pfu DNA polymerase from X. laevis RXRα cDNA (gift of B. Blumberg, University of California, Irvine) by using primers flanked by EcoRI and BglII sites. This fragment was subcloned in-frame into pSG5-Gal4 vector (pSG5g) (gift of M. Privalsky, University of California, Davis). pSG5gRXRΔH12 was created by inverse PCR using primers flanking amino acid codons corresponding to helix 12 (amino acids 469–488) and containing BglII sites. The PCR product was digested with BglII and religated. pSG5 gRAR was cloned as above, by amplifying the xRARα cDNA corresponding to amino acids 157–458. Neural-β tubulin (NβTg), NβTgRXR, NβTgRAR, and NβTgRXRΔH12 constructs were created as follows: DNA sequences containing the gal4 or gal4 fusions were amplified by PCR of the corresponding pSG5-based constructs. The PCR primers flanked the coding sequences to be amplified and contained ClaI and NotI sites at their 5′ ends. PCR products were digested and ligated downstream of the Xenopus NβT vector (gift of N. Marsh-Armstrong, Johns Hopkins Medical Institute, Baltimore). The reporter construct 14XUAS-E1b-EGFP was provided by S. Fraser (California Institute of Technology, Pasadena) (18). The reporter construct 14XUAS-E1b-Luc was created by subcloning the UAS and E1b minimal promoter sequences in front of the luciferase cDNA in the pGL2 Basic vector (Promega). All clones were verified by DNA sequencing (University of California, Davis, sequencing facility).
Transient Transfection Assays. X. laevis XLA cells were maintained and transfected as described (19). A total of 0.1 μg of pSG5g, pSG5gRXR, pSG5gRXRΔH12, or pSG5gRAR was cotransfected with: 0.1 μg pCS2+ β-galactosidase (a gift of D. Turner, University of Michigan, Ann Arbor), 0.1 μg of 14XUAS-E1b-luciferase, and miwCAT to yield a final amount of 1 μg of transfected DNA per well. Cells were harvested after 48 h of ligand or vehicle treatment, and cell extracts were assayed for luciferase and β-galactosidase activity (19). Results are shown in triplicate from a representative experiment, and each experiment was repeated at least two additional times.
Transgenic X. laevis. Transgenic X. laevis embryos were produced essentially as described (20, 21). In each reaction, 100 ng of each linearized DNA construct was used. Embryos were allowed to develop in 0.1× MMR (1× MMR/0.1 M NaCl/2mMKCl/1mM MgSO4/2 mM CaCl2/5 mM Hepes/0.1 mM EDTA, pH 7.8) for 2 days at 18°C and then transferred to room temperature in 0.1× MMR. Enhanced GFP (EGFP) expression was examined throughout development by using a fluorescence dissecting stereomicroscope (Leica MZFLIII). Images were captured by using an Optronics LE-750 digital camera. Large images were assembled by using photoshop (Adobe Systems, San Jose, CA). For ligand treatments, transgenic tadpoles at the indicated stages were treated with 100 nM 3,5,5′-triiodothyronine (T3), 1 μM each of 9-cis RA, atRA, LG100268, or TTNPB, or 1 or 10 μM LG100208 as indicated for 3 days. Tadpoles were allowed to develop at room temperature, and EGFP expression was assessed daily. To genotype transgenic animals, genomic DNA was isolated from tadpole tails as described (22) and amplified with EGFP or Gal4 expression-vector-specific primers.
Immunohistochemistry. Embryos and tadpoles were fixed and processed for histology as described (23). Ten-micrometer sections were incubated with 1:100 diluted monoclonal antibody against EGFP (Torrey Pines Biolabs, Houston) and 1:250 diluted alkaline phosphatase secondary antibody (Sigma).
Results
In Vitro Analysis of Gal4/UAS System in Transfected X. laevis Cells. To monitor the activation pattern of RXRα in live embryos, a binary Gal4 fusion protein/UAS reporter system was adopted (17, 24, 25). The expression vectors carry the coding region of yeast Gal4 DNA-binding domain (DBD) alone or fused in-frame to the coding region of RXR or RAR ligand binding domains. The simian virus 40 (SV40) promoter in pSG5-based plasmids was used in transfection experiments, and NβT-based plasmids were used in transgenic animals (Fig. 1A). The reporter vectors contain UAS, a minimal promoter (E1b) (18), and either luciferase or EGFP reporter genes (Fig. 1B).
Fig. 1.
Schematic diagram of expression and reporter constructs. (A) Gal4 fusion protein expression constructs. The Gal4 DNA-binding domain (amino acids 1–147) was fused in-frame with the ligand-binding domain of X. laevis RXRα (gRXR, amino acids 229–488), RXRα ΔH12 (gRXRΔH12, amino acids 229–469), or RARα (gRAR, amino acids 157–458) under the control of a ubiquitously expressed SV40 early promoter (pSG5) for transfection experiments or the X. laevis neural-β tubulin (NβT) promoter for transgenic experiments. (B) UAS reporter constructs. The EGFP reporter construct UAS-E1b-EGFP contains 14 Gal4 upstream activation sequences (UAS, black boxes) and a minimal promoter (E1b) driving the expression of EGFP. UAS-E1b-Luc is identical to UAS-E1b-EGFP except luciferase is the reporter gene.
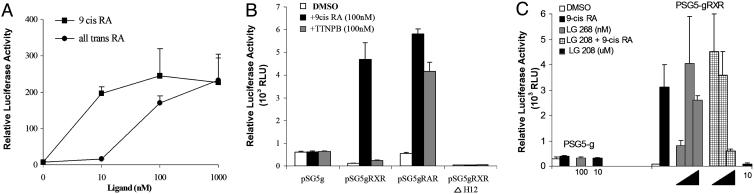
Transcriptional activity of the Gal4RXRα fusion protein (gRXR) was tested by transient transfection assays in X. laevis kidney epithelial XLA cells (Fig. 2), which express endogenous RXRα, RARα, and RARγ (A. Kanamori, personal communication). Basal activity was observed in cells expressing Gal4 alone (gDBD) in the presence or absence of ligand treatment (Fig. 2B). Cells that expressed the gRXR fusion protein strongly repressed basal expression in the absence of added ligand (Fig. 2B). The RXR ligand, 9-cis RA, and its isomer, atRA, significantly increased activity of gRXR as expected (Fig. 2A). Activation of gRXR by atRA may be the result of partial isomerization of atRA into 9-cis RA (11), because significantly higher concentrations of atRA than 9-cis RA are required to fully activate gRXR (Fig. 2A). Given that both atRA and 9-cis RA can activate RAR, the activated gRXR might be modulated through activation of its partner, RAR. Several observations argue against this hypothesis. First, an RAR-specific agonist TTNPB dramatically increased activation of the gRAR fusion protein, but not the gRXR fusion protein (Fig. 2B). Addition of T3 also does not activate gRXR in these cells (data not shown) despite the fact that these cells also express endogenous functional thyroid hormone receptors (19, 26). Second, deletion of helix 12 from RXRα LBD sequence (gRXRΔH12) abolished activation of gRXR by 9-cis RA, although repression was not affected (Fig. 2B). Helix 12 is necessary for coactivator recruitment in a ligand dependent fashion, even though 9-cis RA can still bind to this mutated receptor (27), further suggesting that activation of gRXR is by binding to a specific ligand. Next, the synthetic RXR agonist LG100268 and antagonist LG100208 were examined to further investigate gRXR activation properties (28–31). As shown in Fig. 2C, LG100268 (LG268) is a potent RXR agonist, and induced luciferase expression in a dose-dependent manner. LG100208 (LG208) inhibits 100 nM 9-cis RA induction of gRXR, but only at relatively high ligand concentrations (10 μM, Fig. 2C).
Fig. 2.
gRXR fusion proteins regulate UAS reporter genes in transfected XLA cells. Relative luciferase activity was used to quantitate the transcriptional responsiveness of the gRXR or gRAR fusion proteins in response to added ligands. (A) Cells transfected with pSG5gRXR and treated with increasing concentrations of 9-cis RA (squares) or atRA (circles). (B) Cells transfected with pSG5g, pSG5gRXR, pSG5gRAR, or pSG5gRXRΔH12 with UAS-E1b-Luc and treated with vehicle alone (DMSO, white bars), 100 nM 9-cis RA (black bars), or RAR selective agonist TTNPB (striped bars) as indicated. (C) Cells transfected with PSG5g or PSG5gRXR and UAS-E1b-Luc and treated with vehicle alone (DMSO), 9-cis RA (100 nM), LG100268 (1, 10, and 100 nM LG268, as indicated), or 9-cis RA with increasing concentrations of LG100208 (0.1, 1, and 10 μM LG208, as indicated). LG268 increased luciferase activity, whereas LG 208 inhibits 9-cis RA effect only at 10 μM. Error bars represent mean ± SEM of triplicates from a representative experiment.
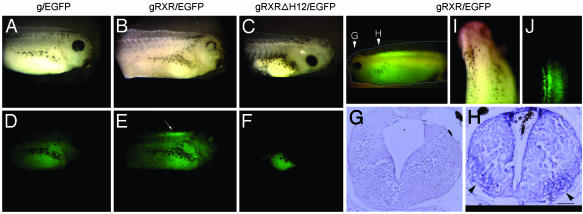
In Vivo Activation of RXRα in the Developing X. laevis CNS. To localize a potential activating ligand for RXRα in the CNS, we created transgenic X. laevis expressing the gRXR fusion protein by using the NβT promoter. The NβT gene is expressed exclusively in CNS of X. laevis, mainly in postmitotic cells (21, 32, 33). Using these constructs together with the reporter gene 14XUAS-E1b-EGFP (Fig. 1) allows us to visualize the reporter gene activity in live embryos. If an endogenous RXRα ligand were present, then we expected to see reporter gene activation without exogenous ligand addition. Indeed, EGFP expression was detected only in transgenic embryos carrying the NβTgRXR construct (Fig. 3 E, H, and J), but not the NβTg construct (Fig. 3D) or the NbTgRXRΔH12 construct expressing the helix 12 deleted RXR LBD (Fig. 3F). The latter result strongly suggests that activation of RXRα in the spinal cord depends on direct ligand-dependent activation of the LBD and not via heterodimerization with an endogenous nuclear receptor such as RAR. Integration of both expression and reporter transgenes were verified by PCR genotyping (data not shown). gRXR-dependent EGFP expression was localized to the most anterior region of the spinal cord parallel to somites 1–6 (Fig. 3 E and J). Our findings are highly consistent with earlier studies of embryonic RXR signaling in mouse, showing activated RXRα in the developing spinal cord (17). This pattern was further confirmed by immunohistochemical analysis, showing that the highest level of activated RXRα is observed in the ventral horn of the spinal cord (Fig. 3H). EGFP expression was not observed in the brain (Fig. 3G). Interestingly, in cross sections of gRXR tadpole spinal cord (Fig. 3H), EGFP staining was localized at the same region where retinaldehydrogenase 1 expression was reportedly found, which may suggest a correlation between RXR ligand “hot spots” and retinaldehydrogenase 1 activity (34).
Fig. 3.
A gRXR fusion protein expressed in the nervous system activates GFP expression specifically in the rostral spinal cord. Transgenic X. laevis tadpoles were created with UAS-E1b-EGFP along with one of the following expression vectors: NβTg (A and D;g/EGFP), NβTgRXR (B and E; gRXR/EGFP), or NβT gRXRΔH12 (C and F; gRXRΔH12). Bright field images are shown in A–C; fluorescent images are shown in D–F. Endogenous activation of gRXR was observed only in embryos carrying gRXR/EGFP transgene, and not in g/EGFP alone or mutated gRXRΔH12/EGFP. (G and H) Overlaid picture of light and fluorescent images. Cross sections of midbrain (G) and rostral spinal cord (H) of gRXR/EGFP tadpole stained with an anti-EGFP antibody. (I and J) Dorsal view of light (I) and fluorescence (J) images. (Bar, 100 μm.) This pattern was observed in all positive embryos, with few intensity changes along the spinal cord.
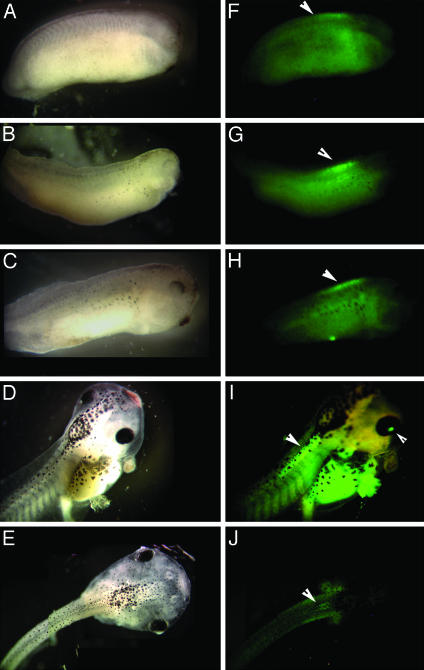
An important advantage of studying RXR signaling in transgenic X. laevis is that external development allows a precise monitoring of the timing and location of activated RXRα. Using EGFP as the reporter, we were able to follow the activation of gRXR in a spatial and temporal pattern throughout Xenopus nervous system development in live embryos. As shown in Fig. 4, the earliest EGFP expression was localized to the developing spinal cord during late neurula stages (Fig. 4F). As the embryo develops, EGFP expression was exclusively limited to the most anterior part of spinal cord (Fig. 4 G–I) except in stage 42 where EGFP expression is also detected in the eye (Fig. 4I). Expression of EGFP fell to undetectable levels in the spinal cord and eye by the time the embryos reached swimming and feeding tadpole stages (Fig. 4J). In addition, screening mature tadpoles (stage 46 and through metamorphosis) showed no further expression of EGFP in the CNS (data not shown).
Fig. 4.
Developmental activation of the gRXR fusion protein in the X. laevis spinal cord. EGFP expression is specifically induced in the rostral spinal cord of NβTgRXR/UAS-E1b-EGFP transgenic embryos beginning at stage 24 (A and F), and continuing through stages 26 (B and G), 35 (C and H), and 42 (D and I, overlaid image), but disappears by stage 46 (E and J). Bright field images are shown in A–E; fluorescent images are shown in F–J. Arrows indicate location of fluorescence induced above background autofluorescence, including both spinal cord and eye (in I).
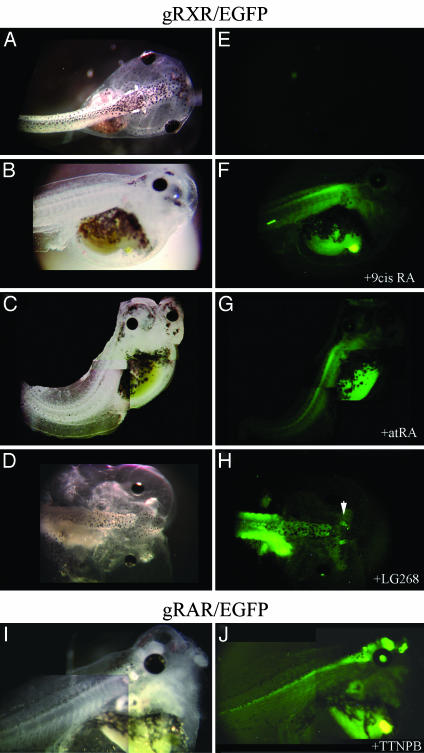
Activation of RXRα in the Embryonic Nervous System Induced by Exogenous Ligands. To verify that the reporter gene observed in the spinal cord is caused by specific activation of RXR, we further examined the activation pattern of gRXR by using exogenous ligands. First, EGFP-positive transgenic tadpoles were treated with high doses of exogenous 9-cis RA or atRA. Strong EGFP expression was evenly distributed throughout the spinal cord of gRXR tadpoles treated with either ligand (1 μM, Fig. 5 F and G). High doses of 9-cis RA and atRA caused major deformations in tadpole development (Fig. 5 B and C), along with the observed posteriorizing effect on EGFP expression. No EGFP expression was detected in the brain (Fig. 5 F and G), even though the NβT promoter is highly active there (Fig. 5J and data not shown). However, activation of gRXR may also be modulated through one of the nonpermissive or permissive nuclear receptor RXR partners. To address this issue, tadpoles carrying the gRXR and UAS-EGFP transgenes (scored as positive by fluorescence and/or PCR genotyping) were treated with various nuclear receptor agonists and antagonists. First, we examined RAR-specific ligand, TTNPB, the thyroid hormone receptor-specific ligand, T3, and RXR-specific synthetic agonist LG268 and antagonist LG208. Although TTNPB induced strong activation of gRAR in the retina, olfactory epithelia, and brain and along the spinal cord (Fig. 5J), no expression of EGFP was observed with TTNPB or T3 treatment in embryos carrying gRXR/EGFP transgene (data not shown). Treatment of gRXR/EGFP embryos before stage 36 with 10 μM RXR antagonist, LG208, had a toxic effect, and all of the embryos died within a day (data not shown). With lower concentrations of LG208 (0.1 or 1 μM), activation of RXR was abolished when tadpoles reached swimming stage, but a firm conclusion is difficult because the signal eventually declines normally. Finally, positive gRXR/EGFP tadpoles were treated with 1 μM of the RXR-specific ligand LG268, and showed modestly induced EGFP expression in the olfactory epithelia and brain, and maintains EGFP expression in the rostral spinal cord (Fig. 5H). Morphological changes were also observed in these tadpoles, including head truncations roughly similar to the effects of 9-cis RA and atRA (Fig. 5 B–D).
Fig. 5.
Exogenous ligand treatment expands GFP expression in gRXR fusion protein expressing embryos. Bright field (A–D and I) and fluorescent images (E–H and J) of transgenic embryos created with NβTgRXR (A–H) or NβTgRAR (I and J) expression vectors and the UAS-E1b-EGFP reporter gene. No expression of reporter gene was observed in untreated swimming tadpoles (A and E). Reporter gene expression was seen in gRXR tadpoles that were treated with exogenous 1 μM 9-cis RA (B and F), atRA (C and G), or LG100268 (D and H). TTNPB induced activation only in gRAR transgene embryos (I and J). Embryos were monitored daily, and activation was observed after 3 days of treatment.
Discussion
In this paper, we describe the spatial and temporal activation of RXRα in live transgenic embryos by using a binary Gal4 fusion protein/UAS reporter gene system. We found that the RXRα LBD is transiently active in a specific region of the X. laevis spinal cord, parallel to somites 1–6. Activation begins during neurulation and terminates as the tadpole reaches feeding and swimming stages. Our results therefore indicate the presence of a highly localized RXR ligand “hot-spot” in or near the rostral spinal cord of the developing embryo, an observation that we now show to be evolutionarily conserved between frog and mouse (17). Although the identity of physiologically relevant endogenous RXR ligand or ligands remains to be established, we can conclude from these and previous studies that a source of ligand exists in the anterior region of the spinal cord in vertebrates. This ligand is transiently synthesized in this region and, hence, activates RXR in a spatial and temporal manner. In this study, we used the NβT promoter that drives expression of gRXR in postmitotic cells of the central nervous system (21, 32, 33), whereas in a previous study, expression of gRXR was mostly located in proliferating cells by using the nestin promoter (17). In both cases, activation of gRXR was found in the ventral region of the spinal cord, indicating either a ligand source in both regions or diffusion of the ligand from one region to the other. Interestingly, we did not observe endogenous activation of a Gal4/RAR fusion protein in our system by using the NβT promoter unless the RAR-specific ligand TTNPB was added to the rearing media (Fig. 5J). This finding suggests a divergence between RAR and RXR signaling in immature versus postmitotic neurons.
The following observations support our conclusion that gRXR is selectively activated by a transiently produced ligand. First, activated gRXR lasts only for few days (Fig. 4). Second, deletion of helix 12, which is responsible for ligand dependent coactivator recruitment to the LBD, completely abolished activation of gRXR (Fig. 3). The mutated gRXR shows no activation in either transfected cells and in transgenic embryos (Figs. 2 and 3). This finding also suggests direct ligand activation dependence in the LBD of RXR because deletion of AF-2 domain does not interfere with heterodimerization with other nuclear receptors like RAR (35, 36). Consistent with previous results, mutations introduced in the ligand-dependent activation function 2 of mice RXRα also suggested an active ligand-binding role in RXRα signaling (37).
It is unlikely that activation of gRXR is a result of heterodimerization with another permissive or nonpermissive nuclear receptor partner. Because RAR ligand TTNPB and thyroid hormone receptor ligand T3 failed to activate gRXR in embryos carrying gRXR/EGFP transgene, whereas they were able to potently induce activation of their own cognate receptor Gal4 fusion proteins (Fig. 5J and data not shown). We also show that both 9-cis RA and atRA induced activation of gRXR, when the fusion protein was activated at lower concentrations of 9-cis RA than atRA (Fig. 2). This effect has been observed with mammalian RXRs and has been previously explained by partial spontaneous or enzyme-dependent isomerization of atRA into 9-cis RA (5). In addition, the RXR-specific synthetic agonist LG268 induced activation of gRXR in transfected cells (Fig. 2C), and expands the activation of gRXR in transgenic embryos (Fig. 5H). Although the RXR antagonist LG208 dramatically inhibited gRXR activation in transfection experiments (Fig. 2C), no conclusion using this compound can be made from transgenic embryo experiments. First, at the most effective inhibitory concentration (10 μM), all of the embryos died. At LG208 concentrations <10 μM, gRXR activation was indeed abolished over time; however, untreated animals also show decreased activation as the animal matures. Although we cannot exclude a putative heterodimerization of RXR with Nurr1, a permissive nuclear receptor that was found abundant in the nervous system (38), overall our results show selective activation of gRXR by a transiently produced endogenous ligand.
A logical candidate for an RXR ligand is the often-cited 9-cis RA. The CNS, including the spinal cord, is a major site of retinoid action (39–42). It was found that the rostral spinal cord and hindbrain highly express retinaldehydrogenase 2, the enzyme involved in the conversion of retinol into atRA, as well as the cellular retinoic acid-binding protein (CRABP) (34, 43, 44), two key players in retinoid signaling. However, subsequent isomerization to 9-cis RA is poorly understood (45). In addition, CYP26, which is responsible for the breakdown of RA and, hence, deactivation of RAR or RXR, was found to be expressed in the brain of early embryos (46–48). CYP26 expression may explain the lack of gRXR activation in the brain and the inability of exogenous retinoic acid isomers to induce reporter gene expression anterior to the spinal cord, whereas the synthetic ligand LG268 is able to do so. Although endogenous concentrations of retinoids have been measured in early X. laevis embryos (49), other studies in frogs and mice have failed to show significant levels of 9-cis RA in the developing embryo (50, 51). These findings raise questions about a physiological role for endogenous 9-cis RA as a physiological RXR ligand. It is still formally possible that, in places where atRA is very highly concentrated, such as in the developing embryonic spinal cord, 9-cis RA can be derived from nonenzymatic atRA isomerization, and thus serve an important role in development. Besides 9-cis RA, recent studies screening for biologically active RXR ligands revealed additional candidates such as docosahexaenoic acid (DHA) (15). DHA, a long-chain polyunsaturated fatty acid, was isolated as a potential RXR ligand from adult mouse brain, and found to influence neural function through activation of an RXR signaling pathway (15).
The spatiotemporal activation of gRXR correlates with a functional role for liganded RXR in the development of motor neurons in the CNS. In X. laevis, primary motor neurons first form within the rostral spinal cord at stage 24/25, coinciding closely with the onset of gRXR activation (52). Peak motor neuron density in newly hatched tadpoles is observed in the same region as our observed gRXR activation (53). In addition, Hb9, a homeobox gene involved in motor neuron specification, is expressed in the X. laevis nervous system in a highly similar spatiotemporal fashion (54). Like our reporter gene activity regulated by gRXR, Hb9 expression strongly declines at swimming tadpole stages. A potential role for activated RXR in motor neuron development is further supported by other studies in mice showing a strong connection between retinoid signaling and the specification and proliferation of motorneurons (17, 41, 55–58). Synthetic RXR-specific agonists increase motor neuron number in mouse spinal cord explants, whereas RXR-specific antagonists have the opposite effect. In contrast, RAR-specific activation appears to be primarily involved in controlling motor neuron subtype identity (58, 59).
In summary, transgenesis in X. laevis is a powerful technique where reporter gene activation by Gal4 fusion proteins can be monitored in each step of development in the F0 generation. We propose that the activation of gRXR is induced by a specific ligand that is being synthesized early in neurula stages and ceases to be made when tadpoles acquire swimming ability, suggesting a phylogenetically conserved role for activated RXRα in the enhancement of motor neuron number during spinal cord development. The ability to visualize RXR activation in a specific time and place in a readily accessible embryo available in large quantities should greatly facilitate attempts to identify a physiologically relevant ligand for RXR in the developing anterior spinal cord.
Acknowledgments
We thank Hayung Yang, Yvette Chu, and Eric Charles Gonzales Peterson for technical assistance, and especially Eric Neff for help with transgenic X. laevis production. We also gratefully acknowledge Dr. Phyllis Wise for her generous support during the final stages of this work, and Dr. Mark Leibowitz (Ligand Pharmaceuticals) for his kind gifts of the LG100268 and LG100208 compounds. We also thank members of the Center for Comparative Medicine pathology laboratory at the University of California, Davis, for their help with sectioning. This work was supported by National Institutes of Health Grant DK55511 and a University of California, Davis, Center for Environmental Health Sciences pilot project grant.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RA, retinoic acid; RAR, RA receptor; RXR, retinoid-X receptor; atRA, all-trans RA; TTNPB, 4-(E-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl)-benzoic acid; NβT, neural β tubulin; EGFP, enhanced GFP; T3, 3,5,5′-triiodothyronine; LBD, ligand-binding domain.
References
- 1.Means, A. L. & Gudas, L. J. (1995) Annu. Rev. Biochem. 64, 201–233. [DOI] [PubMed] [Google Scholar]
- 2.Giguere, V., Ong, E. S., Segui, P. & Evans, R. M. (1987) Nature 330, 624–629. [DOI] [PubMed] [Google Scholar]
- 3.Petkovich, M., Brand, N. J., Krust, A. & Chambon, P. (1987) Nature 330, 444–450. [DOI] [PubMed] [Google Scholar]
- 4.Mangelsdorf, D. J., Ong, E. S., Dyck, J. A. & Evans, R. M. (1990) Nature 345, 224–229. [DOI] [PubMed] [Google Scholar]
- 5.Heyman, R. A., Mangelsdorf, D. J., Dyck, J. A., Stein, R. B., Eichele, G., Evans, R. M. & Thaller, C. (1992) Cell 68, 397–406. [DOI] [PubMed] [Google Scholar]
- 6.Levin, A. A., Sturzenbecker, L. J., Kazmer, S., Bosakowski, T., Huselton, C., Allenby, G., Speck, J., Kratzeisen, C., Rosenberger, M., Lovey, A., et al. (1992) Nature 355, 359–361. [DOI] [PubMed] [Google Scholar]
- 7.Kastner, P., Grondona, J. M., Mark, M., Gansmuller, A., LeMeur, M., Decimo, D., Vonesch, J. L., Dolle, P. & Chambon, P. (1994) Cell 78, 987–1003. [DOI] [PubMed] [Google Scholar]
- 8.Zhang, X. K., Lehmann, J., Hoffmann, B., Dawson, M. I., Cameron, J., Graupner, G., Hermann, T., Tran, P. & Pfahl, M. (1992) Nature 358, 587–591. [DOI] [PubMed] [Google Scholar]
- 9.Mangelsdorf, D. J., Borgmeyer, U., Heyman, R. A., Zhou, J. Y., Ong, E. S., Oro, A. E., Kakizuka, A. & Evans, R. M. (1992) Genes Dev. 6, 329–344. [DOI] [PubMed] [Google Scholar]
- 10.Allegretto, E. A., McClurg, M. R., Lazarchik, S. B., Clemm, D. L., Kerner, S. A., Elgort, M. G., Boehm, M. F., White, S. K., Pike, J. W. & Heyman, R. A. (1993) J. Biol. Chem. 268, 26625–26633. [PubMed] [Google Scholar]
- 11.Allenby, G., Bocquel, M. T., Saunders, M., Kazmer, S., Speck, J., Rosenberger, M., Lovey, A., Kastner, P., Grippo, J. F., Chambon, P., et al. (1993) Proc. Natl. Acad. Sci. USA 90, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willy, P. J. (1997) in Hormones and Signaling, ed. O'Malley, B. (Academic, New York), pp. 307–358.
- 13.Duester, G. (1996) Biochemistry 35, 12221–12227. [DOI] [PubMed] [Google Scholar]
- 14.Duester, G. (2000) Eur. J. Biochem. 267, 4315–4324. [DOI] [PubMed] [Google Scholar]
- 15.de Urquiza, A. M., Liu, S., Sjoberg, M., Zetterstrom, R. H., Griffiths, W., Sjovall, J. & Perlmann, T. (2000) Science 290, 2140–2144. [DOI] [PubMed] [Google Scholar]
- 16.Egea, P. F., Mitschler, A. & Moras, D. (2002) Mol. Endocrinol. 16, 987–997. [DOI] [PubMed] [Google Scholar]
- 17.Solomin, L., Johansson, C. B., Zetterstrom, R. H., Bissonnette, R. P., Heyman, R. A., Olson, L., Lendahl, U., Frisen, J. & Perlmann, T. (1998) Nature 395, 398–402. [DOI] [PubMed] [Google Scholar]
- 18.Koster, R. W. & Fraser, S. E. (2001) Dev. Biol. 233, 329–346. [DOI] [PubMed] [Google Scholar]
- 19.Furlow, J. D. & Brown, D. D. (1999) Mol. Endocrinol. 13, 2076–2089. [DOI] [PubMed] [Google Scholar]
- 20.Amaya, E. & Kroll, K. L. (1999) Methods Mol. Biol. 97, 393–414. [DOI] [PubMed] [Google Scholar]
- 21.Marsh-Armstrong, N., Huang, H., Berry, D. L. & Brown, D. D. (1999) Proc. Natl. Acad. Sci. USA 96, 14389–14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, H., Marsh-Armstrong, N. & Brown, D. D. (1999) Proc. Natl. Acad. Sci. USA 96, 962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furlow, J. D., Berry, D. L., Wang, Z. & Brown, D. D. (1997) Dev. Biol. 182, 284–298. [DOI] [PubMed] [Google Scholar]
- 24.Kozlova, T. & Thummel, C. S. (2002) Development (Cambridge, U.K.) 129, 1739–1750. [DOI] [PubMed] [Google Scholar]
- 25.Hartley, K. O., Nutt, S. L. & Amaya, E. (2002) Proc. Natl. Acad. Sci. USA 99, 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furlow, J. D. & Kanamori, A. (2002) Endocrinology 143, 3295–3305. [DOI] [PubMed] [Google Scholar]
- 27.Budhu, A. S. & Noy, N. (2000) Biochemistry 39, 4090–4095. [DOI] [PubMed] [Google Scholar]
- 28.Boehm, M. F., Zhang, L., Zhi, L., McClurg, M. R., Berger, E., Wagoner, M., Mais, D. E., Suto, C. M., Davies, J. A., Heyman, R. A., et al. (1995) J. Med. Chem. 38, 3146–3155. [DOI] [PubMed] [Google Scholar]
- 29.Repa, J. J., Turley, S. D., Lobaccaro, J. A., Medina, J., Li, L., Lustig, K., Shan, B., Heyman, R. A., Dietschy, J. M. & Mangelsdorf, D. J. (2000) Science 289, 1524–1529. [DOI] [PubMed] [Google Scholar]
- 30.Canan Koch, S. S., Dardashti, L. J., Hebert, J. J., White, S. K., Croston, G. E., Flatten, K. S., Heyman, R. A. & Nadzan, A. M. (1996) J. Med. Chem. 39, 3229–3234. [DOI] [PubMed] [Google Scholar]
- 31.Canan Koch, S. S., Dardashti, L. J., Cesario, R. M., Croston, G. E., Boehm, M. F., Heyman, R. A. & Nadzan, A. M. (1999) J. Med. Chem. 42, 742–750. [DOI] [PubMed] [Google Scholar]
- 32.Marsh-Armstrong, N., Huang, H., Remo, B. F., Liu, T. T. & Brown, D. D. (1999) Neuron 24, 871–878. [DOI] [PubMed] [Google Scholar]
- 33.Schlosser, G., Koyano-Nakagawa, N. & Kintner, C. (2002) Dev. Dyn. 225, 485–498. [DOI] [PubMed] [Google Scholar]
- 34.Zhao, D., McCaffery, P., Ivins, K. J., Neve, R. L., Hogan, P., Chin, W. W. & Drager, U. C. (1996) Eur. J. Biochem. 240, 15–22. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, X. K., Salbert, G., Lee, M. O. & Pfahl, M. (1994) Mol. Cell. Biol. 14, 4311–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, S. K., Na, S. Y., Kim, H. J., Soh, J., Choi, H. S. & Lee, J. W. (1998) Mol. Endocrinol. 12, 325–332. [DOI] [PubMed] [Google Scholar]
- 37.Mascrez, B., Mark, M., Dierich, A., Ghyselinck, N. B., Kastner, P. & Chambon, P. (1998) Development (Cambridge, U.K.) 125, 4691–4707. [DOI] [PubMed] [Google Scholar]
- 38.Wallen-Mackenzie, A., Mata de Urquiza, A., Petersson, S., Rodriguez, F. J., Friling, S., Wagner, J., Ordentlich, P., Lengqvist, J., Heyman, R. A., Arenas, E. & Perlmann, T. (2003) Genes Dev. 17, 3036–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durston, A. J., Timmermans, J. P., Hage, W. J., Hendriks, H. F., de Vries, N. J., Heideveld, M. & Nieuwkoop, P. D. (1989) Nature 340, 140–144. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz i Altaba, A. & Jessell, T. (1991) Genes Dev. 5, 175–187. [DOI] [PubMed] [Google Scholar]
- 41.Maden, M. (2002) Nat. Rev. Neurosci. 3, 843–853. [DOI] [PubMed] [Google Scholar]
- 42.Niederreither, K., Vermot, J., Schuhbaur, B., Chambon, P. & Dolle, P. (2000) Development (Cambridge, U.K.) 127, 75–85. [DOI] [PubMed] [Google Scholar]
- 43.Ruberte, E., Friederich, V., Morriss-Kay, G. & Chambon, P. (1992) Development (Cambridge, U.K.) 115, 973–987. [DOI] [PubMed] [Google Scholar]
- 44.Dekker, E. J., Vaessen, M. J., van den Berg, C., Timmermans, A., Godsave, S., Holling, T., Nieuwkoop, P., Geurts van Kessel, A. & Durston, A. (1994) Development (Cambridge, U.K.) 120, 973–985. [DOI] [PubMed] [Google Scholar]
- 45.Romert, A., Tuvendal, P., Simon, A., Dencker, L. & Eriksson, U. (1998) Proc. Natl. Acad. Sci. USA 95, 4404–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollemann, T., Chen, Y., Grunz, H. & Pieler, T. (1998) EMBO J. 17, 7361–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii, H., Sato, T., Kaneko, S., Gotoh, O., Fujii-Kuriyama, Y., Osawa, K., Kato, S. & Hamada, H. (1997) EMBO J. 16, 4163–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Roos, K., Sonneveld, E., Compaan, B., ten Berge, D., Durston, A. J. & van der Saag, P. T. (1999) Mech. Dev. 82, 205–211. [DOI] [PubMed] [Google Scholar]
- 49.Kraft, J. C., Schuh, T., Juchau, M. & Kimelman, D. (1994) Proc. Natl. Acad. Sci. USA 91, 3067–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ulven, S. M., Gundersen, T. E., Sakhi, A. K., Glover, J. C. & Blomhoff, R. (2001) Dev. Dyn. 222, 341–353. [DOI] [PubMed] [Google Scholar]
- 51.Blumberg, B., Bolado, J., Jr., Derguini, F., Craig, A. G., Moreno, T. A., Chakravarti, D., Heyman, R. A., Buck, J. & Evans, R. M. (1996) Proc. Natl. Acad. Sci. USA 93, 4873–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Mier, P., van Rheden, R. & ten Donkelaar, H. J. (1985) Anat. Embryol. 172, 311–324. [DOI] [PubMed] [Google Scholar]
- 53.Roberts, A., Walford, A., Soffe, S. R. & Yoshida, M. (1999) J. Comp. Neurol. 411, 472–486. [DOI] [PubMed] [Google Scholar]
- 54.Saha, M. S., Miles, R. R. & Grainger, R. M. (1997) Dev. Biol. 187, 209–223. [DOI] [PubMed] [Google Scholar]
- 55.Rossant, J., Zirngibl, R., Cado, D., Shago, M. & Giguere, V. (1991) Genes Dev. 5, 1333–1344. [DOI] [PubMed] [Google Scholar]
- 56.Colbert, M. C., Linney, E. & LaMantia, A. S. (1993) Proc. Natl. Acad. Sci. USA 90, 6572–6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCaffery, P. & Drager, U. C. (1994) Proc. Natl. Acad. Sci. USA 91, 7194–7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sockanathan, S. & Jessell, T. M. (1998) Cell 94, 503–514. [DOI] [PubMed] [Google Scholar]
- 59.Sockanathan, S., Perlmann, T. & Jessell, T. M. (2003) Neuron 40, 97–111. [DOI] [PubMed] [Google Scholar]