Abstract
Diabetic nephropathy is a growing health concern with characteristic sterile inflammation. As the underlying mechanisms of this inflammation remain poorly defined, specific therapies targeting sterile inflammation in diabetic nephropathy are lacking. Intriguingly, an association of diabetic nephropathy with inflammasome activation has recently been shown, but the pathophysiological relevance of this finding remains unknown. Within glomeruli, inflammasome activation was detected in endothelial cells and podocytes in diabetic humans and mice and in glucose-stressed glomerular endothelial cells and podocytes in vitro. Abolishing Nlrp3 or caspase-1 expression in bone marrow–derived cells fails to protect mice against diabetic nephropathy. Conversely, Nlrp3-deficient mice are protected against diabetic nephropathy despite transplantation of wild-type bone marrow. Pharmacological IL-1R antagonism prevented or even reversed diabetic nephropathy in mice. Mitochondrial reactive oxygen species (ROS) activate the Nlrp3 inflammasome in glucose or advanced glycation end product stressed podocytes. Inhibition of mitochondrial ROS prevents glomerular inflammasome activation and nephropathy in diabetic mice. Thus, mitochondrial ROS and Nlrp3-inflammasome activation in non-myeloid-derived cells aggravate diabetic nephropathy. Targeting the inflammasome may be a potential therapeutic approach to diabetic nephropathy.
Keywords: diabetic nephropathy, endothelial cell, inflammasome, mitochondrial ROS, Nlrp3, podocyte
Diabetic nephropathy is the leading cause of end-stage renal disease in adults, putting an enormous burden on affected individuals and health-care systems. Current therapies are insufficient, necessitating the search for new therapeutic strategies in diabetic nephropathy. Inflammatory processes have been identified as potential modulators of diabetic nephropathy. Accumulation of macrophages or T cells has been demonstrated in humans with and in rodent models of diabetic nephropathy.1, 2 The extent of inflammatory cell accumulation in the kidney is associated with the decline of renal function, suggesting a causative link.1, 2, 3, 4 Indeed, inhibition of inflammatory cell recruitment into the kidney has been shown to be protective in experimental diabetic nephropathy.5, 6 Local generation of pro-inflammatory cytokines, such as interleukin 1β (IL-1β), contributes to inflammatory cell recruitment.3, 7 Cytokines are thought to be released from activated immune cells.3, 7 However, renal cells, such as podocytes, endothelial cells, or mesangial cells, can likewise secrete pro-inflammatory cytokines and may thus aggravate diabetic nephropathy.8, 9, 10, 11 However, whether non-myeloid-derived glomerular resident cells promote diabetic nephropathy by secreting cytokines and how cytokine secretion from these cells may be controlled remain unknown.
The release of the pro-inflammatory cytokines IL-1β and IL-18 is controlled by the inflammasome. Various danger signals, including mitochondrial reactive oxygen species (ROS), activate the Nlrp3 (nucleotide-binding domain and leucine-rich repeat pyrin 3 domain) inflammasome.12, 13 Upon detecting cellular stress, Nlrp3 recruits and assembles with ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and caspase-1, resulting in caspase-1 activation, maturation of IL-1β and IL-18, and promotion of inflammatory cell recruitment.14 Although initially thought to be restricted to immune cells it has been recently shown that the inflammasome can be functional in nonimmune cells, including podocytes.11, 13, 15 In addition, inflammasome activation has lately been demonstrated in diabetic nephropathy,16 but the pathophysiological relevance and the mechanism of inflammasome activation in diabetic nephropathy remain elusive. Likewise, the potential involvement of glomerular resident cells, such as podocytes or endothelial cells, for inflammasome activation in diabetic nephropathy remains unknown. To address these questions we evaluated the role of inflammasome activation in glomerular cells in the current study. Answering these questions may be of therapeutic relevance, as the inflammasome is amendable to therapeutic interventions.17
RESULTS
The onset of diabetic nephropathy is associated with renal inflammasome activation
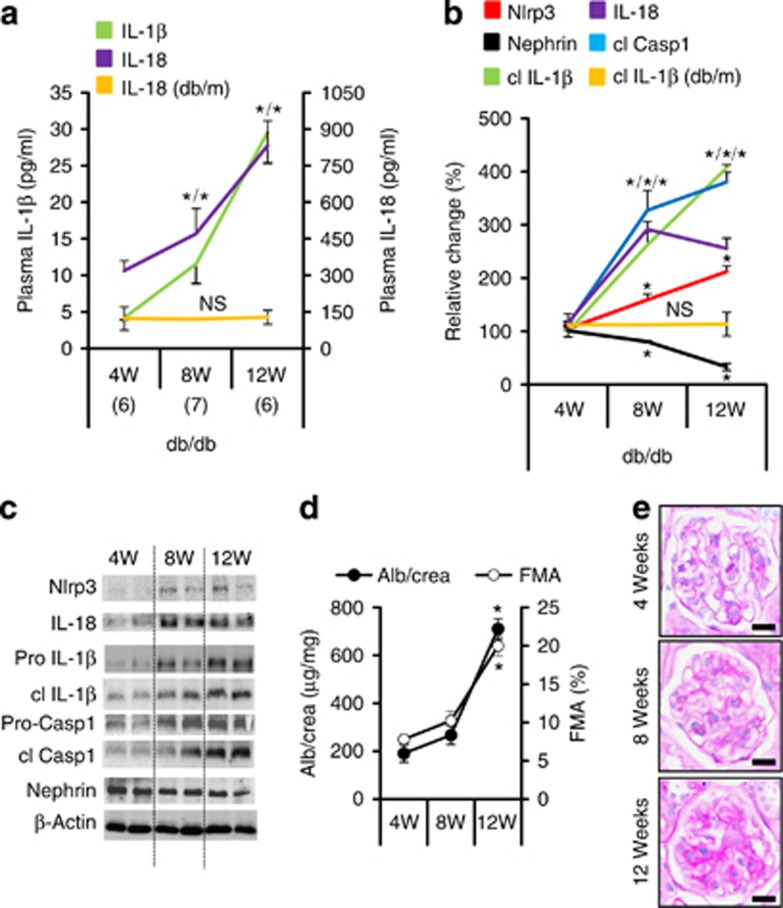
To evaluate the relevance of the inflammasome in diabetic nephropathy we analyzed diabetic mice of various ages. Levels of (cleaved) IL-1β and IL-18 were low in plasma (Figure 1a) and hardly detectable in renal cortex extracts (Figure 1b and c) of 4-week-old db/db mice, but significantly increased in 8- and 12-week-old db/db mice. In parallel, Nlrp3 and cleaved caspase-1 levels increased, while nephrin expression declined in renal cortex extracts of db/db mice with age (Figure 1b and c). Thus, inflammasome activation occurs at an early stage of nephropathy in db/db mice.18 Albuminuria and glomerular extracellular matrix accumulation (FMA, fractional mesangial area) were significantly increased in 12, but not in 8-week-old db/db mice (Figure 1d and e and Supplementary Figure S1a online), suggesting that inflammasome activation precedes and may hence be mechanistically linked with diabetic nephropathy. Changes suggestive of nephropathy or inflammasome activation were not apparent in nondiabetic db/m control mice (Figure 1a and b and Supplementary Figure S1d–f online).
Figure 1.
Inflammasome activation is associated with the onset of diabetic nephropathy in db/db mice. IL-1β and IL-18 plasma levels (a) and tissue levels of Nlrp3 (nucleotide-binding domain and leucine-rich repeat pyrin 3 domain) and cleaved IL-1β (cl IL-1β) in renal cortex extracts (b, c) are increased in db/db mice at 8 weeks of age (compared with 4 weeks of age) and are further increased at age 12 weeks. Tissue levels of cleaved caspase-1 (cl Casp1) and IL-18 increase between age 4 and 8 weeks and then remain elevated (b, c). Nephrin expression is reduced at 8 and decreases further at 12 weeks of age (b, c). These changes are associated with a significant increase in albuminuria (Alb) and the fractional mesangial area (FMA) in 12- but not in 8-week-old db/db mice (d, e). Markers of inflammasome activation (plasma IL-18, a; cleaved IL-1β, b) remain normal in nondiabetic db/m control mice. Mean value±s.e.m. Number of mice (a–e) in each group is shown in parentheses in a; representative immunoblots in (c) and representative periodic acid–Schiff–stained glomeruli in e, scale bar=20 μm; *P<0.05. FMA, fractional mesangial area; IL, interleukin; NS, not significant.
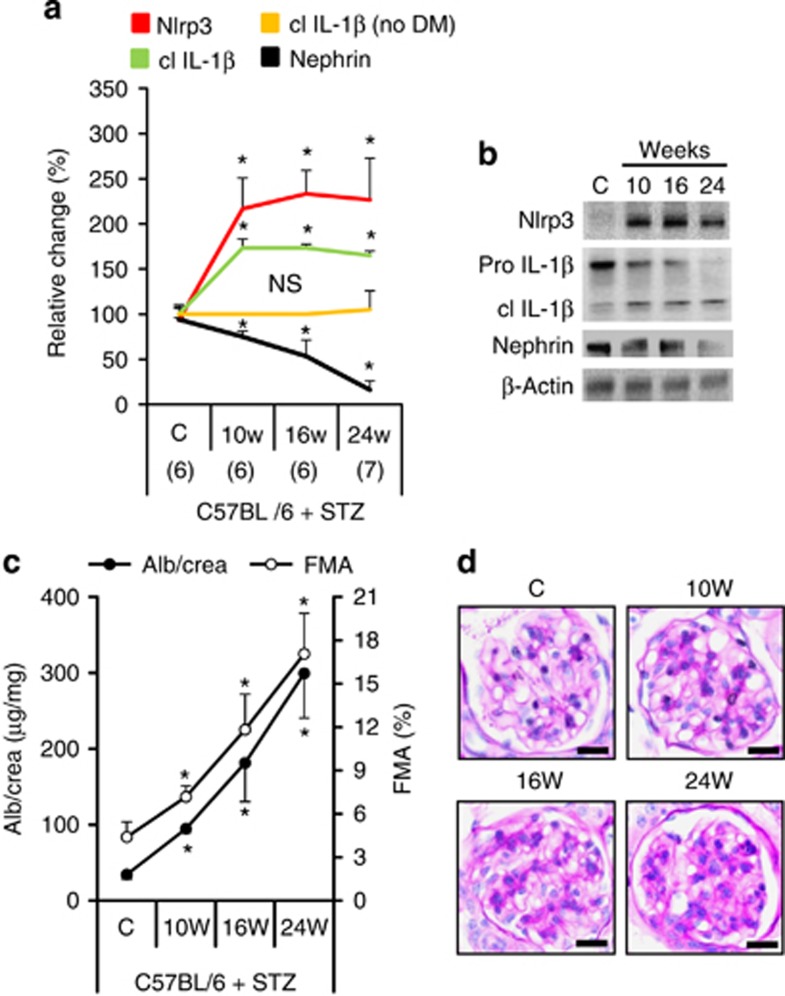
The renal inflammasome activation observed in db/db mice could reflect insulin resistance and obesity19, 20 and may thus be independent of renal pathology. To address this possibility we analyzed mice with persistent hyperglycemia in the absence of obesity or insulin resistance (streptozotocin (STZ) model). In these mice albuminuria and loss of nephrin expression were likewise associated with an early increase of Nlrp3 and cleaved IL-1β levels in the renal cortex (Figure 2 and Supplementary Figure S2 online), whereas inflammasome activation was not observed in nondiabetic control mice (Figure 2a and Supplementary Figure S2d online). Hence, renal inflammasome activation is independent of obesity and insulin resistance.
Figure 2.
Inflammasome activation is associated with the onset of diabetic nephropathy in streptozotocin (STZ)-treated C57BL/6 mice. Tissue levels of Nlrp3 (nucleotide-binding domain and leucine-rich repeat pyrin 3 domain) and cleaved interleukin-1β (cl IL-1β) increase, whereas expression of nephrin declines in renal cortex extracts of diabetic C57BL/6 mice (STZ model; a, b). Inflammasome activation is paralleled by an increase in albuminuria and fractional mesangial area (FMA, c, d; overviews for d in Supplementary Figure S2a online). Cleaved IL-1β remains normal in nondiabetic C57BL/6 control mice (no DM, a). Mean value±s.e.m. Number of mice (a–d) in each group is shown in parentheses in a; representative immunoblots in b and periodic acid Schiff-stained glomeruli in d, scale bar=20 μm; *P<0.05. DM, diabetic mice; NS, not significant.
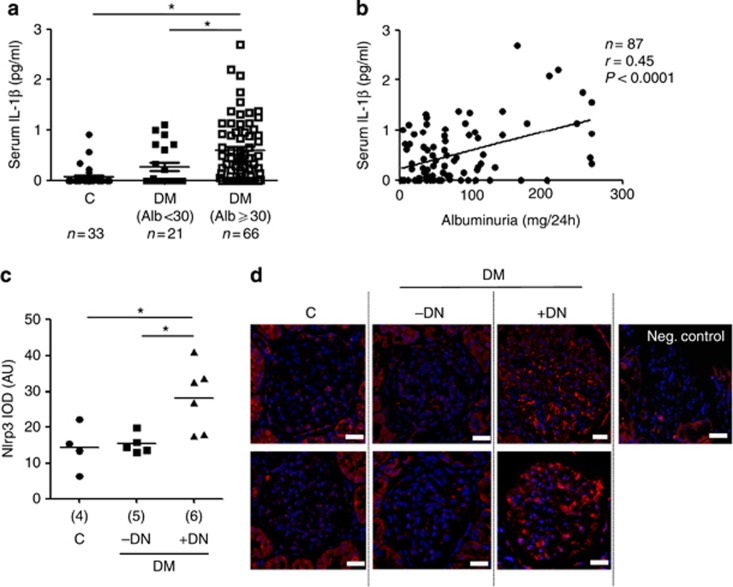
Next, we analyzed markers of inflammasome activation in type 2 diabetic patients in a pilot study. In albuminuric diabetic patients, serum IL-1β levels were significantly increased compared with diabetic patients without albuminuria or nondiabetic controls (Figure 3a) and the degree of albuminuria correlated with serum IL-1β (Figure 3b). This correlation remained stable after correcting for age, sex, and disease duration. Furthermore, Nlrp3 expression was on average increased in glomeruli of albuminuric diabetic patients compared to diabetic patients without albuminuria or nondiabetic controls (Figure 3c and d).
Figure 3.
Inflammasome activation in human diabetic patients. Serum interleukin (IL)-1β levels are increased in albuminuric diabetic patients when compared to nondiabetic controls and non-albuminuric diabetic patients (a) and serum IL-1β levels in diabetic patients correlate with albuminuria (b). Nlrp3 (nucleotide-binding domain and leucine-rich repeat pyrin 3 domain) expression is increased in glomeruli of diabetic patients with diabetic nephropathy (DM+DN) as compared to nondiabetic controls (C) or diabetic patients without diabetic nephropathy (DM−DN) (c, d). Scatter diagram (c) and two representative images each (d) of glomeruli from indicated groups; immunofluorescence labeling for Nlrp3 (red), 4,6-diamidino-2-phenylindole counterstain (blue), and a negative control using a nonspecific primary antibody (Neg. control); scale bar=20 μm. Scatter diagrams representing individual data points and mean (horizontal line (a, c); *P<0.05. AU, arbitrary units; DM, diabetic mice; IOD, integrated optical density.
Inflammasome inhibition ameliorates diabetic nephropathy in mice
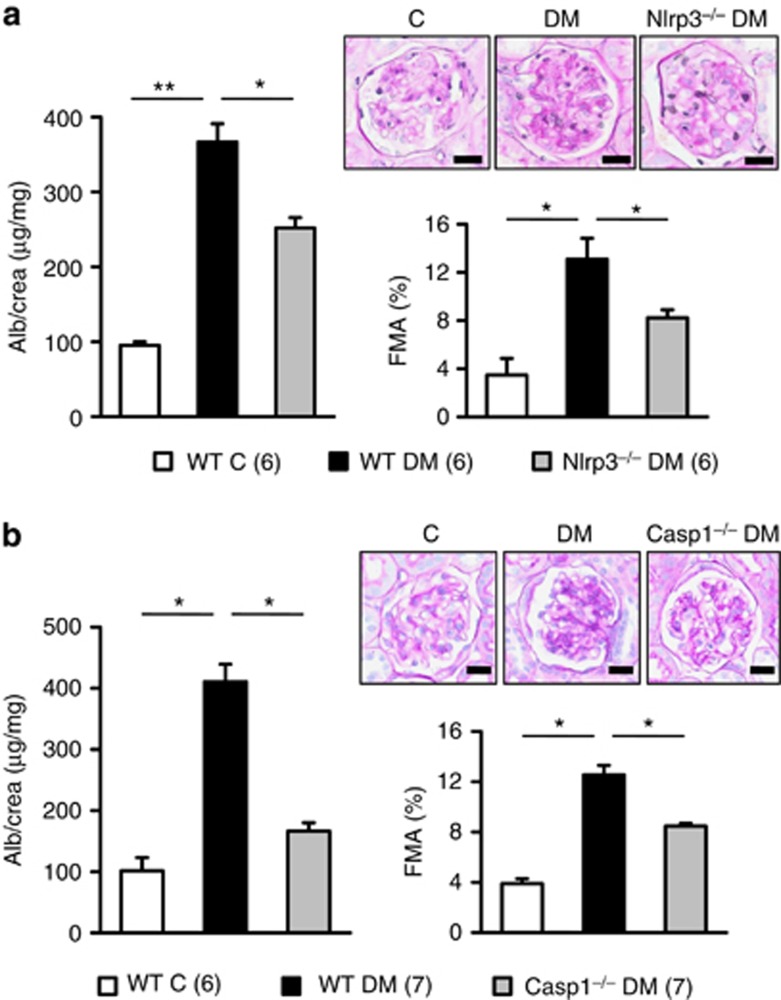
To explore the functional relevance of inflammasome activation in diabetic nephropathy we induced persistent hyperglycemia in uninephrectomized Nlrp3−/− or caspase-1−/− mice using STZ. Nlrp3 or caspase-1 deficiency ameliorated albuminuria and the FMA without affecting the body weight or blood glucose levels (Figure 4 and Supplementary Figure S3 online), establishing a functional role of inflammasome activation in diabetic nephropathy.
Figure 4.
Nlrp3 (nucleotide-binding domain and leucine-rich repeat pyrin 3 domain) or caspase-1-deficiency ameliorates diabetic nephropathy (DN). Albuminuria and fractional mesangial area (FMA) are significantly reduced in Nlrp3−/− (a) or caspase-1−/− (b) diabetic mice in comparison with wild-type diabetic mice (WT DM). Mean value±s.e.m. Number of mice (a, b) in each group is shown in parentheses in a; representative periodic acid Schiff-stained glomeruli; scale bar (a, b)=20 μm; *P<0.05, **P<0.01.
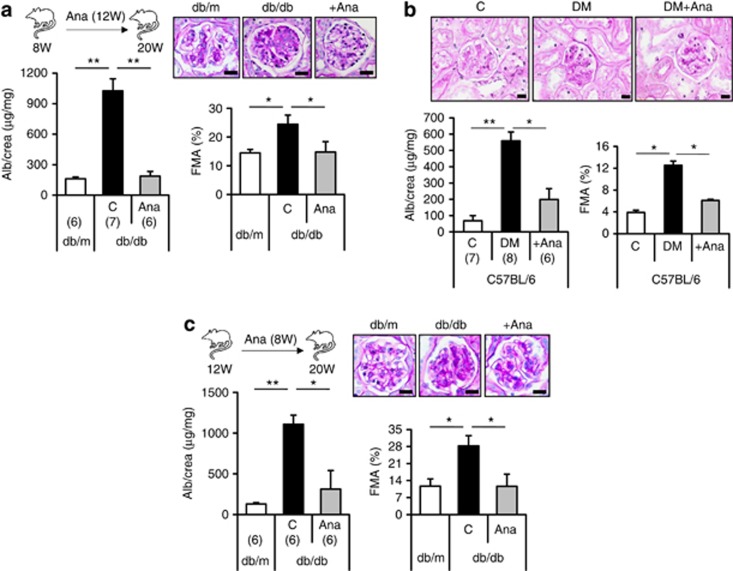
Pathological sequelae of inflammasome activation are frequently amendable to therapeutic approaches.17 Application of the IL-1 receptor antagonist anakinra in hyperglycemic mice with insulin resistance (db/db mice) or insulinopenia (STZ model) reduced albuminuria and FMA (Figure 5a and b). Treatment with anakinra (for 12 weeks) reduced the body weight and blood glucose levels in db/db mice, but not in insulinopenic mice (STZ model, Supplementary Figure S4a–c online), illustrating that anakinra can ameliorate diabetic nephropathy independent of its metabolic effects. Of note, in 12-week-old db/db mice with established nephropathy18 (and current data) the intervention with anakinra for 8 weeks normalized albuminuria and FMA (Figure 5c). Thus, inhibition of IL-1 receptor signaling can not only prevent, but also reverse, diabetic nephropathy in mice.
Figure 5.
Inhibition of the inflammasome with anakinra (Ana) ameliorates or reverses diabetic nephropathy. Initiating anakinra (interleukin (IL)-1 receptor antagonist) treatment in 8-week-old db/db mice (a) or diabetic (streptozotocin (STZ) model) uninephrectomized C57BL/6 mice (b) prevents albuminuria (Alb) and extracellular matrix accumulation (fractional mesangial area, FMA) over a period of 12 weeks (db/db) or 8 weeks (C57BL/6 + STZ), respectively. Initiating anakinra treatment in 12–week-old db/db mice and hence after establishment of experimental diabetic nephropathy anakinra normalizes albuminuria and FMA (c). Mean value±s.e.m. Number of mice in each group is shown in parentheses in a–c; representative periodic acid–Schiff–stained glomeruli; scale bar (a–c)=20 μm. *P<0.05, **P<0.01. DM, diabetic mice.
Hyperglycemia triggers inflammasome activation in glomerular cells
Whether inflammasome activation occurs in glomerular cells in diabetic nephropathy and the potential implications of this remained unknown hitherto. Using confocal microscopy we observed partial colocalization of Nlrp3 or cleaved caspase-1 with podocytes and glomerular endothelial cells in histological sections of diabetic humans or mice, respectively (Figure 6a and b, Supplementary Figure S5a online). In 12-week-old db/db mice cleaved caspase-1 localized predominately to glomeruli (Supplementary Figure S5b online).
Figure 6.
Inflammasome activation in resident glomerular cells in diabetic nephropathy. Nlrp3 (nucleotide-binding domain and leucine-rich repeat pyrin 3 domain; red) colocalizes with markers for podocytes (synaptopodin, green, left) and endothelial cells (PECAM, green, right) in glomeruli of microalbuminuric diabetic patients (a), scale bar=20 μm. In glomeruli of 20-week-old db/db mice, cleaved caspase-1 (red) colocalizes with markers for podocyte (synaptopodin, green, left) and endothelial cell (CD34, green, right; b, scale bar=20 μm). Representative images of glomeruli obtained by confocal microscopy (Zeiss LSM 4 Pascal microscope, Carl Zeiss, Jena, Germany) and higher magnification of areas indicated by white dotted lines; yellow reflects colocalization (a, b). In mouse glomerular endothelial cells (GENC) and human podocytes (hPodo) glucose (Gluc, 25 mmol/l, 24 h) induces Nlrp3 and cleaved interleukin-1β (cl IL-1β) in comparison to control (C, phosphate-buffered saline) or mannitol (M, 25 mmol/l mannitol)-treated cells (c); representative immunoblots, β-actin as loading control. In primary podocytes isolated from C57BL/6 wild-type (WT), but not from caspase-1–deficient (casp1−/−) mice, cl IL-1β can be readily detected (d). Glucose (G, 25 mmol/l), but not mannitol (M, 25 mmol/l), induces cl IL-1β in WT, but not in caspapse-1–deficient podocytes (d); representative immunoblots, β-actin as loading control.
In vitro glucose, but not mannitol, induced Nlrp3 expression cell-autonomously and cleaved IL-1β in glomerular endothelial cells and podocytes (Figure 6c). Furthermore, cleaved IL-1β was readily observed in primary podocytes isolated from C57BL/6 wild-type mice and was induced following glucose, but not mannitol, treatment (Figure 6d). Of note, only pro-IL-1β, but not cleaved IL-1β, could be detected in primary podocytes isolated from caspase-1−/− mice (Figure 6d), consistent with cell-autonomous maturation of IL-1β in podocytes.
Inflammasome deficiency in bone marrow–derived cells fails to ameliorate early stages of diabetic nephropathy
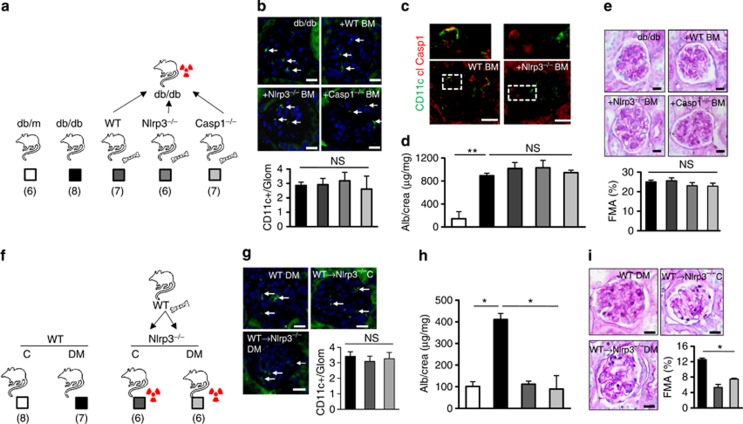
To address the pathogenic relevance of inflammasome activation in myeloid vs. non-myeloid-derived cells, we next conducted bone marrow transplantation experiments. First, Nlrp3−/−, caspase-1−/−, or wild-type (control) bone marrow was transplanted into lethally irradiated db/db mice (age 8 weeks, Figure 7a). This had no impact on body weight, blood glucose levels (Supplementary Figure S6 online), or the glomerular frequency of CD11c+ cells (Figure 7b). Of note, in db/db mice transplanted with Nlrp3−/− bone marrow no cleaved caspase-1 could be detected in glomerular CD11c+ cells (Figure 7c). In comparison to control db/db mice albuminuria and FMA increased to the same extend in db/db mice transplanted with Nlrp3−/− or caspase-1−/− bone marrow after 12 weeks (Figure 7d and e). Thus, Nlrp3- or caspase1-deficiency in bone marrow–derived cells does not ameliorate diabetic nephropathy in mice.
Figure 7.
Non-myeloid-derived cells are sufficient to promote diabetic nephropathy. Following transplantation of Nlrp3−/− or Casp1−/− bone marrow into db/db mice (a: experimental approach) the frequency of CD11c+ cells in glomeruli is similar to that in control db/db mice (b), immunofluorescence, CD11c: green; 4,6-diamidino-2-phenylindole (DAPI) counterstain, but no cleaved caspase-1 (cl Casp1) can be detected in CD11c+ cells (c), representative immunofluorescence following transplantation of Nlrp3−/− bone marrow; cleaved caspase-1: red; CD11c: green; colocalization: yellow; white dotted lines indicate areas shown at higher magnification above. Albuminuria and FMA remain high in db/db mice despite transplantation with Nlrp3−/− or caspase-1−/− bone marrow (d, e). Conversely, diabetic and uninephrectomized Nlrp3−/− mice are protected from nephropathy despite transplantation of Nlrp3+/+ bone marrow (f: experimental approach). The frequency of CD11c+ cells in glomeruli is similar in experimental groups (g); CD11c: green; DAPI counterstain, but albuminuria and FMA remain normal in diabetic Nlrp3−/− mice despite reconstitution with Nlrp3+/+ bone marrow (h, i). Mean value±s.e.m. Number of mice (a–e and f–i) in each group is shown in parentheses in a and f; representative periodic acid–Schiff–stained glomeruli in e and i; scale bar=20 μm (b, c, e, g, and i); white arrows in b and g indicate CD11c+ cells; *P<0.05; **P<0.01. BM, bone marrow; DM, diabetic mice; FMA, fractional mesangial area; IL, interleukin; Nlrp3, nucleotide-binding domain and leucine-rich repeat pyrin 3 domain; NS, not significant; WT, wild type.
Next, we conducted the reverse experiment transplanting wild-type bone marrow into Nlrp3−/− mice to evaluate the role of the Nlrp3 inflammasome in glomerular resident cells (Figure 7f). As Nlrp3−/− mice are not readily available on a db/db background we used the STZ model. Following transplantation of wild-type bone marrow into Nlrp3−/− mice, body weight, blood glucose levels, and the presence of CD11c+ cells in glomeruli (Figure 7g, Supplementary Figure S6 online) were not altered. Of note, albuminuria and FMA remained normal in diabetic and nondiabetic Nlrp3−/− mice despite reconstitution with wild-type bone marrow (Figure 7h and i). These data suggest that inflammasome activation primarily in renal resident cells contributes to diabetic nephropathy.
Inflammasome activation by mitochondrial ROS in diabetic mice
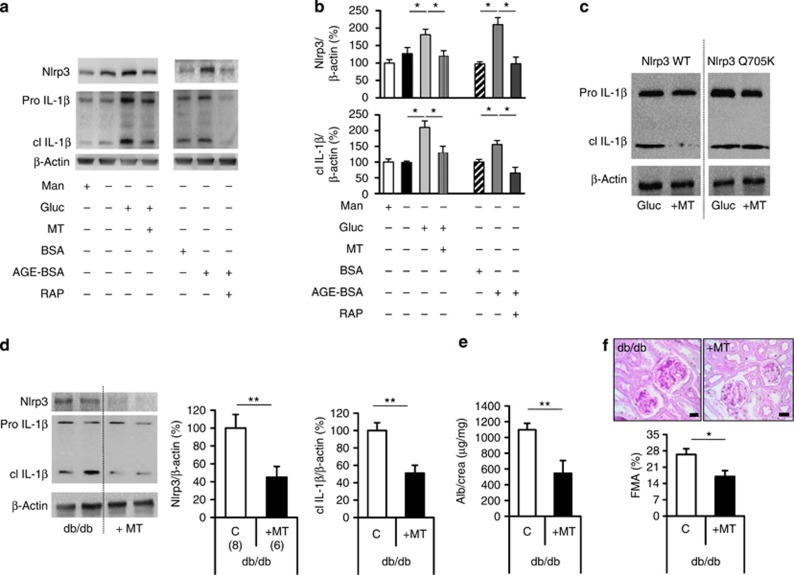
Mitochondrial ROS promote diabetic nephropathy21, 22 and can trigger Nlrp3-inflammasome activation.13, 23 To evaluate whether mitochondrial ROS are mechanistically linked with glomerular Nlrp3-dependent inflammasome activation we used the mitochondria-targeted antioxidant MitoTempo, a superoxide dismutase mimetic that accumulates in mitochondria.24 MitoTempo reduced mitochondrial ROS (Supplementary Figure S7a online) and in parallel Nlrp3 levels and IL-1β activation in glucose-stressed podocytes (Figure 8a and b).
Figure 8.
Mitochondrial reactive oxygen species cause inflammasome activation and promote diabetic nephropathy in db/db mice. Glucose (Gluc, 25 mmol/l for 24 h) and AGE-BSA (200 μg/ml) induce Nlrp3 expression and IL-1β cleavage in mouse podocytes (a, b). Inhibition of the receptor RAGE using RAP (RAGE antagonist peptide, 10 μmol/l for 24 h) was sufficient to prevent the AGE-BSA-dependent induction of Nlrp3 and IL-1β cleavage (a, b). The mitochondrial-targeted antioxidant MitoTempo (MT) reduces glucose-induced Nlrp3 expression and IL-1β cleavage in mouse (a, b) and human (c) podocytes, respectively (a–c). Following transfection of human podocytes with a constitutive active human Nlrp3 mutant (Q705K), MitoTempo (10 μm) fails to suppress IL-1β cleavage (b); representative immunoblots of at least three independent repeat experiments (a, c) and bar graph (b) summarizing densitometric results. Treatment of 8-week-old db/db mice with MitoTempo (MT for 8 weeks) reduces Nlrp3 expression and IL-1β cleavage in renal cortex tissue extracts (d); representative immunoblots of Nlrp3, IL-1β, and β-actin and bar graph summarizing results, as well as albuminuria (e) and FMA (f) in comparison with phosphate-buffered saline-treated db/db controls (C: controls); mean value±s.e.m. Number of mice (d–f) in each group is shown in parentheses in d; representative periodic acid–Schiff–stained glomeruli in f; scale bar (e)=20 μm; *P<0.05; **P<0.01. FMA, fractional mesangial area; IL, interleukin; Nlrp3, nucleotide-binding domain and leucine-rich repeat pyrin 3 domain.
Enhanced glycolytic flux is sufficient to increase mitochondrial ROS, but glucose-modified proteins such as AGEs (advanced glycation endproducts) may likewise induce mitochondrial ROS generation through a RAGE (receptor for AGEs)-dependent mechanism.25 Indeed, AGE-BSA induced Nlrp3 expression and IL-1β cleavage in podocytes, an effect that was prevented by RAGE inhibition (Figure 8a and b). Thus, glucose and glucose-induced metabolites may synergistically contribute to ROS-dependent inflammasome activation, in particular in the in vivo situation. Of note, MitoTempo failed to reduce levels of cleaved IL-1β in glucose-stressed human podocytes transfected with the constitutive active human Nlrp3 mutant Q705K,26 indicating ROS triggered Nlrp3-dependent IL-1β maturation in podocytes (Figure 8c).
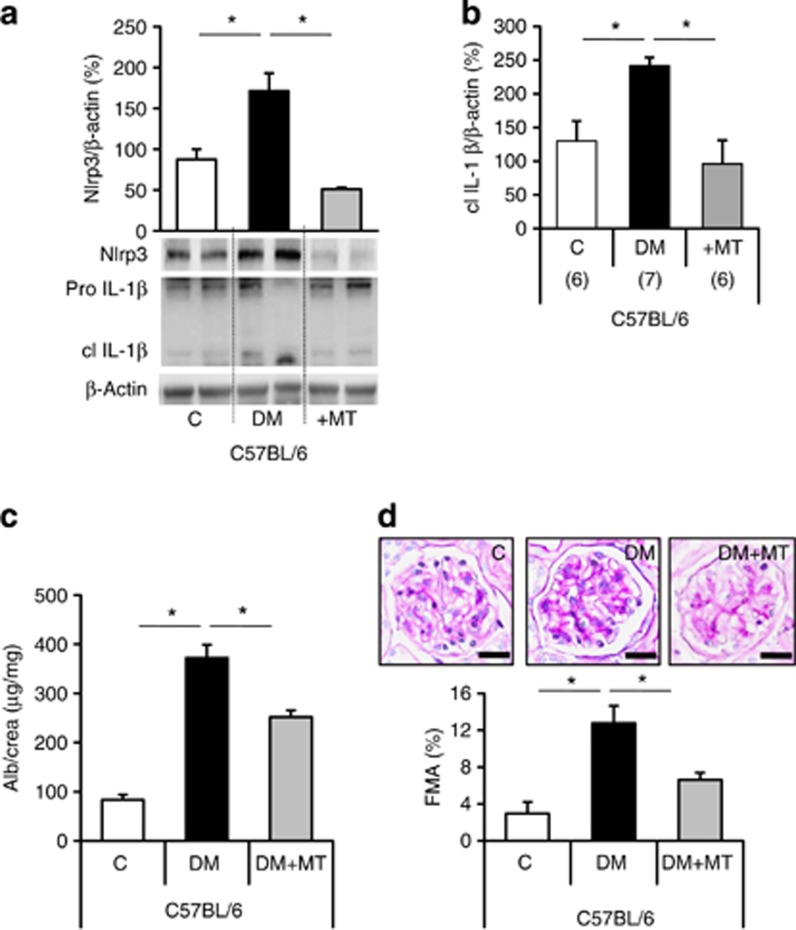
To evaluate the role of mitochondrial ROS for inflammasome activation in vivo we next treated db/db and uninephrectomized diabetic C57BL/6 mice (STZ model, STZ mice) with MitoTempo. MitoTempo treatment had no effect on body weight or blood glucose levels (Supplementary Figure S7b online). In db/db mice, MitoTempo treatment markedly reduced levels of Nlrp3, cleaved IL-1β, albuminuria, and FMA in comparison with control db/db mice (Figure 8d–f). Likewise, indices of diabetic nephropathy and inflammasome activation were reduced in MitoTempo-treated STZ mice when compared with phosphate-buffered saline-treated diabetic controls (Figure 9a–d).
Figure 9.
MitoTempo protects against diabetic nephropathy in diabetic C57BL/6 mice. Treatment of diabetic uninephrectomized (DM, STZ model) C57BL/6 mice (DM) with MitoTempo (+MT, for 8 weeks) reduces levels of Nlrp3 and cleaved IL-1β in renal cortex tissue extracts (a): bottom: representative immunoblots of Nlrp3, IL-1β, and β-actin; a and b: bar graphs summarizing results, albuminuria (Alb) (c), and FMA (d) in comparison with phosphate-buffered saline-treated diabetic controls (DM). Nondiabetic C57BL/6 mice served as controls (C); mean value±s.e.m. Number of mice (a–d) in each group is shown in parentheses in b; representative periodic acid–Schiff–stained glomeruli in d; scale bar=20 μm; *P<0.05. DM, diabetic mice; FMA, fractional mesangial area; IL, interleukin; Nlrp3, nucleotide-binding domain and leucine-rich repeat pyrin 3 domain.
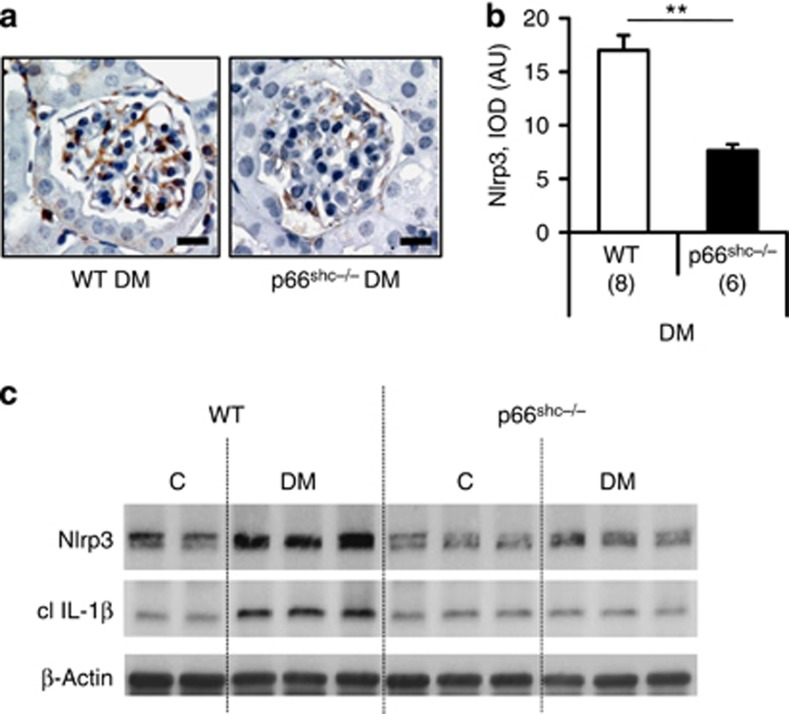
Genetic ablation of p66Shc reduces mitochondrial ROS and protects mice from diabetic nephropathy.21 Again, persistent hyperglycemia failed to induce Nlrp3 expression and IL-1β cleavage in p66Shc deficient diabetic mice (Figure 10a–c), supporting a critical role of mitochondrial ROS for inflammasome activation in diabetic nephropathy.
Figure 10.
Deficiency of the redox-protein p66Shc reduces inflammasome activation in diabetic nephropathy. In diabetic mice lacking the redox-protein p66Shc levels of Nlrp3 (a–c) and cleaved IL-1β (c) are reduced in comparison with diabetic wild-type (WT) mice (streptozotocin model, 24 weeks of hyperglycemia). Immunohistochemical detection of Nlrp3 in glomeruli of diabetic WT and p66Shc−/− mice (a), Nlrp3-positive cells detected by HRP-DAB reaction, brown; hematoxylin counterstain, blue; scale bar=20 μm; bar graph summarizing results of integrated optical density (IOD) of positive DAB stain (b); mean value±s.e.m. Number of mice (a–c) in each group is shown in parentheses in b, and representative immunoblots in c; **P<0.01. AU, arbitrary units; DM, diabetic mice; IL, interleukin; Nlrp3, nucleotide-binding domain and leucine-rich repeat pyrin 3 domain.
DISCUSSION
Diabetic nephropathy is considered an inflammatory disease, and several reports recently demonstrated inflammasome activation in association with diabetic nephropathy. In the current study we establish that inflammasome activation is causatively linked with diabetic nephropathy. IL-1 receptor antagonism or inhibition of mitochondrial ROS, an established activator of the Nlrp3 inflammasome, ameliorates diabetic nephropathy in mice, suggesting that interventions targeting this mechanism of sterile inflammation may be a feasible therapeutic approach in diabetic nephropathy.
Although originally thought to be specific for the innate immune system, inflammasome activation and signaling has recently been described in cells not belonging to the immune system.13, 15, 27 Previous to the currently observed hyperglycemia-associated inflammasome activation in podocytes, similar observations have been made in mice with hyperhomocysteinanemia or diet-induced obesity.8, 11, 28 In addition to these reports we observed inflammasome activation in glomerular endothelial cells, suggesting that inflammasome activation in various kidney cells may contribute to diabetic nephropathy. Indeed, kidney-restricted silencing of ASC, an adaptor protein required for the inflammasome complex, attenuated proteinuria, albuminuria, and glomerular sclerosis.8, 11, 28 This approach, however, targets all cells within the kidney, including inflammatory cells. Thus, although demonstrating a role of inflammasome activation in glomerular disease, the question as to whether inflammasome activation in resident glomerular cells, such as podocytes or endothelial cells, contributes to glomerular sclerosis remained unresolved in these studies.
To address this question we conducted bone marrow–transplantation experiments, which demonstrated that inflammasome activation in renal resident cells, but not in bone marrow–derived cells, is sufficient to trigger diabetic nephropathy in mice. We acknowledge that the chosen method, bone marrow transplantation, has limitations and that cell-specific inactivation of inflammasome regulators such as Nlrp3 or caspase-1 would be preferred. Cell-specific deletion would likewise enable a researcher to specifically address the role of inflammasome activation in glomerular and tubular cells. However, mice with conditional inactivation of Nlrp3 or caspase-1 are currently not readily available. In addition, inflammasome activation occurred at least in podocytes and endothelial cells. Hence, genetic inflammasome inactivation in multiple renal cell types will be required to address the functional role of inflammasome activation in resident glomerular cells vs. bone marrow–derived cells. Considering these obstacles, we believe that the chosen method constitutes a reasonable approach. In view of the current data, more sophisticated experimental approaches to clarify the role of inflammasome activation in renal resident cells are now justified and required.
Following transplantation of Nlrp3-deficient bone marrow we failed to detect CD11c+ cells with signs of inflammasome activation in renal glomeruli, indicating efficient replacement of renal dendritic cells. This is consistent with the replacement of innate immune cells in tissues such as skin, lung, or liver following bone marrow transplantation.29, 30, 31, 32 While persistence of some Nlrp3 or caspase-1-expressing innate immune cells in the kidney despite bone marrow transplantation cannot be excluded, the current data argue against a role of inflammasome activation in cells actively recruited from the bone marrow into the kidney during the disease course. This, however, does not exclude a pathogenetic function of bone marrow–derived cells in the course of diabetic nephropathy. Bone marrow–derived cells may aggravate the disease progression independent of the inflammasome, as previously proposed.6, 7, 33, 34 In addition, we cannot exclude that inflammasome activation in bone marrow–derived cells contributes to the later disease stages or interstitial tissue damage. On the basis of available data it appears possible that inflammasome activation in renal resident cells including glomerular cells triggers recruitment of bone marrow–derived cells, which then aggravate the disease progression. This possibility has to be experimentally evaluated.
Inflammasome inhibition may be superior to blocking inflammatory cell recruitment1, 5 as IL-1β and IL-18 impair podocyte and endothelial function independent of inflammatory cell recruitment.35 Whether modulation of noncanonical functions of Nlrp3, such as the Nlrp3-dependent modulation of Smad2/3 phosphorylation by transforming growth factor-β in tubular cells,36 will constitute an additional benefit of inflammasome inhibition remains to be evaluated.
The identification of mitochondrial ROS as the Nlrp3 activator in diabetic glomeruli provides a mechanistic link between ROS and sterile inflammation in diabetic nephropathy.12, 21, 22, 37 ROS-dependent activation of the Nlrp3 inflammasome has been recently described in monocytes and macrophages from diabetic patients,38 indicating that this pathway is also functional in non-renal cells. Mitochondrial ROS have been proposed as a common pathway mediating Nlrp3 activation.12, 37 Mitochondrial ROS dissociate TxNIP from thioredoxin, and free TxNIP can bind to and activate Nlrp3.12, 13, 39 In addition, accumulation of dysfunctional, ROS-generating mitochondria is sufficient to activate the Nlrp3 inflammasome.12, 13 Although we used two independent approaches to evaluate the relevance of mitochondrial ROS (MitoTempo- and p66Shc-deficient mice), tools to specifically inhibit ROS in glomerular cells were not available to us. Hence, stringent evidence pinpointing a causative role of glomerular-derived mitochondrial ROS is still pending.
Mitochondrial ROS do not only mediate harmful effects but are also required for maintaining cellular homeostasis.40 This dual function may limit the therapeutic efficacy of mitochondrial-targeted ROS inhibition. Rather, cell-damaging pathways initiated by ROS, but amendable to therapeutic interventions, ought to be identified. Mitochondrial ROS activate the inflammasome, and inhibition of inflammasome signaling using anakinra provided nephroprotection and even reversed diabetic nephropathy in the current study, whereas inhibition of mitochondrial ROS using MitoTempo was only partially protective. Thus, inflammasome inhibition, which is already clinically established,17 constitutes a feasible therapeutic target.
Inflammasome inhibition in diabetic patients may provide additional benefits due to its metabolic effects.19, 20, 41 Indeed, we observed a reduction in body weight and blood glucose levels in db/db mice. Yet, anakinra conveyed nephropection not only in db/db mice, but also in mice with STZ-induced insulinopenia and hyperglycemia (reflecting type 1 diabetes). Hence, nephroprotection by inflammasome inhibition is at least partially independent of these metabolic effects. The efficacy, feasibility, and safety of inflammasome inhibition in patients with diabetic nephropathy, potentially using small molecule inhibitors,42 remains to be evaluated.
The current findings extend recent studies imputing an immune modulatory function to podocytes.43, 44, 45, 46 Thus, besides inflammasome activation8, 11 (and current study) podocytes are competent to signal via TLR,44, 45 to modulate complement,43, 46, 47 and to activate naive T cells following antigen presentation.45 The ability of podocytes to sense danger signals and to initiate an immune response may render the glomerular filtration barrier not only sensitive to inflammatory disease, but in addition endow the kidney, which continuously controls a large portion of the body's blood, with an immune-surveillance function, reminiscent of the dual function of teleost's kidneys.48 These immunological functions may be pathologically relevant not only in infectious or autoimmune diseases.49 Rather, the current and previous studies50 suggest that sterile inflammation in the kidney may be of pathogenic relevance in the context of metabolic diseases. This may lay ground for novel therapeutic approaches to nephropathies associated with metabolic diseases.
MATERIALS AND METHODS
See online Supplementary materials for additional information.
Mice
Caspase1−/−, db/db (Lepr db/db), and nondiabetic db/m control mice (C57BL/6J background) were obtained from Jackson Laboratories, Bar Harbor, ME. p66shc−/− and Nlrp3−/− mice have been previously described.21, 51, 52 In the current study we used littermates that have been backcrossed for at least 10 generations on a C57BL/6 or C57BL/6J background. All animal experiments were conducted following standards and procedures approved by the local Animal Care and Use Committee (Landesverwaltungsamt Halle, Germany).
Diabetic nephropathy models
Two different STZ-dependent models of diabetic nephropathy were used in the current study. A long-term model (24 weeks) following a previously published protocol21, 46, 53, 54 was used in genetically modified p66Shc−/− mice and their controls. In these mice, persistent hyperglycemia was induced by intraperitoneal administration of 60 mg/kg STZ freshly dissolved in 0.05 mol/l sterile sodium citrate at pH 4.5 for 5 consecutive days in 8-week-old mice, as described.21, 46, 53, 54
In addition we used the unilateral nephrectomy model of diabetic nephropathy.21, 55 This short-term model is particularly useful to test therapeutic interventions. Briefly, 8-week-old mice were anesthetized with pentobarbital (1 mg/kg body weight, intraperitoneally). A dorso-lumbar incision (approximately 1 cm) was made and the ureter, the renal artery, and renal vein were ligated and subsequently severed. The kidney was removed and the incision was sutured. Two weeks after surgery, diabetes was induced by injections of STZ (intraperitoneally, 40 mg/kg body weight, freshly dissolved in 0.05 mol/l sterile sodium citrate, pH 4.5) for 5 consecutive days. Age-matched control mice received 100 μl phosphate-buffered saline intraperitoneally for 5 consecutive days.
In both models mice were considered diabetic if blood glucose levels were above 300 mg/dl 16 d after the last STZ injection.21, 53, 54 Blood glucose levels were determined from blood samples taken from the tail vein by using ACCU-CHEK glucose strips. In the first 3 weeks after the onset of diabetes, blood glucose values were measured three times per week, and thereafter once per week.21, 53, 54 Mice with blood glucose levels above 500 mg/dl received 1–2 U of insulin (Lantus) to avoid excessive and potentially lethal hyperglycemia.21, 53, 54 Blood and tissue samples were obtained after 24 weeks (long-term model) or 8 weeks (short-term model) of persistent hyperglycemia in diabetic mice. Age-matched littermates served as controls.
In vivo intervention studies
A subset of mice was treated with the IL-1R antagonist (Anakinra, 10 mg/kg, intraperitoneally)56 or the mitochondria-targeted antioxidant MitoTempo (700 μg/kg body weight per day; subcutaneous).57
Interventions with Anakinra or MitoTempo were initiated in 8-week-old db/db mice to determine its preventive effect. In uninephrectomized, STZ-treated mice, treatment with Anakinra or MitoTempo was initiated 2 weeks after establishment of stable hyperglycemia and continued for 8 weeks. Treatment with anakinra was initiated in 12-week-old db/db mice to determine its effect after onset of diabetic nephropathy. Control mice were injected with phosphate-buffered saline.
Analyses of human samples
Patients (N=87) with type 2 diabetes, according to the American Diabetes Association criteria,58 were recruited from the diabetes outpatient clinic at the University Hospital Heidelberg. Microalbuminuria was diagnosed if at least two separate urine samples out of three consecutive urine samples (albumin excretion rate AER⩾30 mg/24 h) were positive. Nondiabetic control subjects (N=33) were recruited at the outpatient clinic of the University Hospital Heidelberg and Magdeburg. Nondiabetic controls had either stable and minor osteoporosis or stable and well-controlled thyroid function (e.g., after thyreoidectomy). Nondiabetic controls were on vitamin D and calcium substitution only (osteoporosis patients) or thyroid hormones, but no other medication. All patients and controls were Caucasian. The study complied with the Declaration of Helsinki; all patients entered the study according to the guidelines of the local ethics committees after giving informed consent (Ethic-Committee-No: 204/2004).
Statistical analysis
The data are summarized as the mean±s.e.m. (standard error of the mean). Statistical analyses were performed with Student's t-test and analysis of variance. Post-hoc comparisons of analysis of variance were corrected with the method of Tukey. For the patient data, Pearson's correlation coefficient (r) was determined and the two-tailed P-value is given. The correlation was corrected for age, sex, disease duration, and estimated glomerular filtration rate by multiple regression analysis. StatistiXL (www.statistixl.com) and Prism 5 (www.graphpad.com) software were used for statistical analyses. Statistical significance was accepted at values of P<0.05.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (IS 67/2–4 to BI; TH 1789/1-1 to MT, BI 1281/3-1 to PPN), the EFSD (European Foundation for the Study of Diabetes, BI), the DDS (Deutsche Diabetes Stiftung; BI), Hopp Stiftung (PPN) and the Stiftung für Pathobiochemie und Molekulare Diagnostik (BI and FB), and an ERC StG and a BioSysNet Research group (OG). We thank Kathrin Deneser, Julia Judin, Juliane Friedrich, Rene Rudat, and Rumiya Makarova for excellent technical support.
Author contributions
KS, FB, and WD designed, performed, and interpreted experiments; HW, ShK, RS, JW, CW, and MMA conducted cloning work and mouse experiments; St K collected and analyzed patient data; S St assisted in ex vivo analyses; KR, OG, and PS provided reagents and assisted with data analyses; HJG, VS, and SP provided histological sections of human kidney biopsies and assisted with data analyses; PPN assisted with the collection and analyses of patient data and in preparing the manuscript; TM interpreted data, and BI designed and interpreted the experimental work and prepared the manuscript.
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S1. Age dependent changes in db/db and db/dm mice.
Figure S2. Age dependent changes in streptozotocin treated and control C57BL/6 mice.
Figure S3. Body weight and blood glucose in Nlrp3 or caspase-1 deficient C57BL/6 mice.
Figure S4. Body weight and blood glucose in anakinra treated mice.
Figure S5. Inflammasome activation in resident glomerular cells.
Figure S6. Effect of bone-marrow transplantation experiments on body weight and blood glucose.
Figure S7. Mitochondrial ROS cause inflammasome activation and promote diabetic nephropathy.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
Absent files uploaded 08th August 2014.
References
- Chow F, Ozols E, Nikolic-Paterson DJ, et al. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Ping F, Mu W, et al. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 2006;11:226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- Lim AK, Ma FY, Nikolic-Paterson DJ, et al. Lymphocytes promote albuminuria, but not renal dysfunction or histological damage in a mouse model of diabetic renal injury. Diabetologia. 2010;53:1772–1782. doi: 10.1007/s00125-010-1757-1. [DOI] [PubMed] [Google Scholar]
- Moriya R, Manivel JC, Mauer M. Juxtaglomerular apparatus T-cell infiltration affects glomerular structure in Type 1 diabetic patients. Diabetologia. 2004;47:82–88. doi: 10.1007/s00125-003-1253-y. [DOI] [PubMed] [Google Scholar]
- Awad AS, Kinsey GR, Khutsishvili K, et al. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am J Physiol Renal Physiol. 2011;301:F1358–F1366. doi: 10.1152/ajprenal.00332.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ozols E, et al. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005;16:1711–1722. doi: 10.1681/ASN.2004070612. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ma FY, et al. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- Abais JM, Zhang C, Xia M, et al. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal. 2013;18:1537–1548. doi: 10.1089/ars.2012.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemir ZI, Stein H, Dworacki G, et al. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritides. Kidney Int. 1997;52:393–403. doi: 10.1038/ki.1997.346. [DOI] [PubMed] [Google Scholar]
- Tesch GH, Yang N, Yu H, et al. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrol Dial Transplant. 1997;12:1109–1115. doi: 10.1093/ndt/12.6.1109. [DOI] [PubMed] [Google Scholar]
- Zhang C, Boini KM, Xia M, et al. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 2012;60:154–162. doi: 10.1161/HYPERTENSIONAHA.111.189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production. Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, et al. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- Lorenz G, Darisipudi MN, Anders HJ. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant. 2014;29:41–48. doi: 10.1093/ndt/gft332. [DOI] [PubMed] [Google Scholar]
- Feldmeyer L, Keller M, Niklaus G, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Yang SM, Ka SM, Wu HL, et al. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-kappaB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia. 2014;57:424–434. doi: 10.1007/s00125-013-3115-6. [DOI] [PubMed] [Google Scholar]
- Mitroulis I, Skendros P, Ritis K. Targeting IL-1beta in disease; the expanding role of NLRP3 inflammasome. Eur J Intern Med. 2010;21:157–163. doi: 10.1016/j.ejim.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- Stienstra R, van Diepen JA, Tack CJ, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock F, Shahzad K, Wang H, et al. Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci USA. 2012;110:648–653. doi: 10.1073/pnas.1218667110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- Heid ME, Keyel PA, Kamga C, et al. Mitochondrial Reactive oxygen species induces NLRP3-Dependent lysosomal damage and inflammasome activation. J Immunol. 2013;191:5230–5238. doi: 10.4049/jimmunol.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova AE, Bikineyeva AT, Budzyn K, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan MT, Thorburn DR, Penfold SA, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;4:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Sarndahl E, Andersson H, et al. The Q705K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1beta and IL-18 production. PLoS One. 2012;7:e34977. doi: 10.1371/journal.pone.0034977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, et al. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boini KM, Xia M, Abais JM, et al. Activation of inflammasomes in podocyte injury of mice on the high fat diet: Effects of ASC gene deletion and silencing. Biochim Biophys Acta. 2014;1843:836–845. doi: 10.1016/j.bbamcr.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale RP, Sparkes RS, Golde DW. Bone marrow origin of hepatic macrophages (Kupffer cells) in humans. Science. 1978;201:937–938. doi: 10.1126/science.356266. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa M, Ginhoux F, Wang XN, et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med. 2009;206:371–385. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ED, Ramberg RE, Sale GE, et al. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976;192:1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- Vielhauer V, Anders HJ, Mack M, et al. Obstructive nephropathy in the mouse: progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol. 2001;12:1173–1187. doi: 10.1681/ASN.V1261173. [DOI] [PubMed] [Google Scholar]
- Wada T, Furuichi K, Sakai N, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- Kusuhara M, Isoda K, Ohsuzu F. Interleukin-1 and occlusive arterial diseases. Cardiovasc Hematol Agents Med Chem. 2006;4:229–235. doi: 10.2174/187152506777698335. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang X, Chun J, et al. Inflammasome-independent NLRP3 augments TGF-beta signaling in kidney epithelium. J Immunol. 2013;190:1239–1249. doi: 10.4049/jimmunol.1201959. [DOI] [PubMed] [Google Scholar]
- Misawa T, Takahama M, Kozaki T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- Mirza RE, Fang MM, Weinheimer-Haus EM, et al. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63:1103–1114. doi: 10.2337/db13-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Xia L, Goldberg H, et al. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem. 2013;288:6835–6848. doi: 10.1074/jbc.M112.419101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes RP, Weissmann N, Schroder K. Redox-mediated signal transduction by cardiovascular Nox NADPH oxidases. J Mol Cell Cardiol. 2014;73C:70–79. doi: 10.1016/j.yjmcc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Juliana C, Fernandes-Alnemri T, Wu J, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Wang Y, Chang A, et al. Mouse podocyte complement factor H: the functional analog to human complement receptor 1. J Am Soc Nephrol. 2007;18:1157–1166. doi: 10.1681/ASN.2006101125. [DOI] [PubMed] [Google Scholar]
- Banas MC, Banas B, Hudkins KL, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–713. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwich A, Burkard M, Olke M, et al. Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol. 2013;24:906–916. doi: 10.1681/ASN.2012020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Vinnikov I, Shahzad K, et al. The lectin-like domain of thrombomodulin ameliorates diabetic glomerulopathy via complement inhibition. Thromb Haemost. 2012;108:1141–1153. doi: 10.1160/TH12-07-0460. [DOI] [PubMed] [Google Scholar]
- Puri TS, Quigg RJ. The many effects of complement C3- and C5-binding proteins in renal injury. Semin Nephrol. 2007;27:321–337. doi: 10.1016/j.semnephrol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Iliev DB, Thim H, Lagos L, et al. Homing of Antigen-Presenting Cells in Head Kidney and Spleen—Salmon Head Kidney Hosts Diverse APC Types. Front Immunol. 2013;4:137. doi: 10.3389/fimmu.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- Abais JM, Xia M, Li G, et al. Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia. Free Radic Biol Med. 2014;67:211–220. doi: 10.1016/j.freeradbiomed.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Poeck H, Bscheider M, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Isermann B, Vinnikov IA, Madhusudhan T, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- Madhusudhan T, Wang H, Straub BK, et al. Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood. 2011;119:874–883. doi: 10.1182/blood-2011-07-365973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Bernabe P, D'Alessandro-Gabazza CN, Toda M, et al. Exogenous activated protein C inhibits the progression of diabetic nephropathy. J Thromb Haemost. 2012;10:337–346. doi: 10.1111/j.1538-7836.2012.04621.x. [DOI] [PubMed] [Google Scholar]
- Abbate A, Salloum FN, Vecile E, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- Luo M, Guan X, Luczak ED, et al. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Absent files uploaded 08th August 2014.