Abstract

Charged intermediates and reagents are ubiquitous in organic transformations. The interaction of these ionic species with chiral neutral, anionic, or cationic small molecules has emerged as a powerful strategy for catalytic, enantioselective synthesis. This review describes developments in the burgeoning field of asymmetric ion-pairing catalysis with an emphasis on the insights that have been gleaned into the structural and mechanistic features that contribute to high asymmetric induction.
Keywords: anion-binding catalysis, asymmetric catalysis, chiral anions, ion pairs, phase-transfer catalysis
1. Introduction
All chemical reactions involve some degree of charge polarization, and many archetypal organic transformations proceed via the intermediacy of at least one discrete, charged species. Controlling the stereochemical outcome of reactions of charged reagents and intermediates is a long-standing goal in organic synthesis, and the identification of efficient, catalytic methods to do so represents a current frontier of the field. This review focuses on two general approaches that have emerged for asymmetric catalysis of reactions proceeding via charged intermediates or reagents: (i) ion-pairing with a charged, chiral catalyst and (ii) noncovalent binding to the intermediate ion pair by a chiral, neutral catalyst (Figure 1).
Figure 1.

Types of asymmetric ion-pairing catalysis.
In order to frame the challenges that exist in designing enantioselective catalysts that operate by ion-pairing mechanisms, a brief description of the physical principles underlying ion pairs is appropriate. The concept of ion pairs as distinct chemical entities was introduced in 1926 by Bjerrum.[1] Anslyn and Dougherty provide a modern physical-organic textbook definition:
“An ion pair is defined to exist when a cation and anion are close enough in space that the energy associated with their electrostatic attraction is larger than the thermal energy (RT) available to separate them. This means that the ions stay associated longer than the time required for Brownian motion to separate noninteracting species.”[2]
Coulomb’s law [Eq. (1)] describes the attractive potential energy (E) between two ions of opposite charge (q1 and q2). The magnitude of the electrostatic interaction is inversely related to the distance between the ions (r) and the dielectric constant of the medium (ε). Strong ion-pairing interactions are thus favored at short distances and in nonpolar solvents.
| (1) |
Solvation effects are crucially important in understanding the behavior of ion pairs, which can be classified into three different types based on the extent to which the ions are solvated (Figure 2). An ion pair with a common solvation shell and no solvent molecules between the two ions is termed a contact ion pair. When the ions are separated by a shared solvation shell, or when they each have their own solvation shell, they are termed solvent-shared and solvent-separated ion pairs, respectively. Contact ion pairs are energetically favorable in nonpolar solvents of low dielectric constant, whereas the solvent-shared and solvent-separated ion pair types are more prevalent in solvents of higher dielectric constant.[3] The ions of contact pairs have a stronger influence on the environment around each other; therefore, for reactions in which selectivity is controlled largely by an ion-pairing interaction, higher selectivity is generally observed in nonpolar solvents.
Figure 2.

Types of ion pairs.
The goal in asymmetric ion-pairing catalysis is to identify synthetic, chiral molecules that can induce high enantioselectivity in reactions of discrete ionic species. Small-molecule catalysts, by their very nature, rely on a limited number of interactions to control stereoselectivity. In such systems, the level of transition structure organization that is necessary for high stereoinduction is often achieved through highly directional catalyst-substrate interactions. For example, covalent iminium catalysis and Lewis acid catalysis – two approaches that rely chiefly on strong, directional interactions – are broadly successful approaches to enantioselective activation of carbonyl derivatives (Figure 3). Hydrogen-bond donor catalysts rely on interactions that are generally weaker and less directional in nature, yet a growing number of highly effective chiral catalysts of this type have also been reported.[4]
Figure 3.

Comparative directionality of catalyst-substrate interactions of common asymmetric catalysis strategies (Ln* = chiral ligand; B*H = chiral Brønsted acid; X* = chiral counterion)
While covalent, Lewis acid, and H-bond donor catalysts have clear utility in enantioselective synthesis, their application to reactions of charged intermediates is often not straightforward, and it is in this context that ion-pairing catalysis emerges as a most attractive strategy. The fact that ion-pairing interactions are inherently less directional than covalent or hydrogen-bonding interactions underlies the challenge in designing stereoselective catalysts that operate by this principle. Yet, as outlined in this review, a variety of highly enantioselective small-molecule catalysts have been identified that proceed through the basic mechanisms outlined in Figure 1. The success of these systems can be ascribed to the fact that the catalysts incorporate secondary structural elements capable of inducing specific attractive or repulsive interactions in the stereoselectivity-determining transition structures, in a manner reminiscent of their far more complex, enzymatic counterparts.
Here we provide an analysis of the four classes of asymmetric ion-pairing catalysis defined in Figure 1, with emphasis placed on the conceptual and mechanistic underpinnings of key illustrative reactions. This field has a relatively long history in phase-transfer catalysis with chiral cationic or cation-binding catalysts.[5] In contrast, ion-pairing catalysis with chiral anionic[6] and anion-binding[7] catalysts is a much newer field of endeavor, but research in this area has progressed at a particularly rapid pace in the last five years.[8] The aim of this review is to tie together these different activation modes to help shed light on the common features that enable highly enantioselective transformations of reactive, charged species with small-molecule chiral catalysts, with the ultimate goal of providing design principles for future discovery efforts.
2. Chiral Cation-Directed Catalysis
The history of ion-pairing catalysis as an important approach in asymmetric synthesis can be traced to the 1984 report by Merck scientists on the use of a chiral quaternary ammonium salt as a highly efficient and enantioselective phase-transfer catalyst for the C-methylation of indanones (Scheme 1).[5i] This result revealed for the first time that high enantioselectivity can be achieved solely through electrostatic and other noncovalent interactions of a charged intermediate with a chiral, ionic catalyst. Since that seminal discovery, a remarkable number of methods that utilize chiral quaternary ammonium and phosphonium ion catalysts have been identified. These catalysts have been shown to induce high levels of enantioselectivity in a wide range of reactions, including enolate alkylation, Michael, Aldol, Mannich, and Darzens reactions, as well as epoxidations and aziridinations. The large number of excellent reviews on chiral cation-directed catalysis is a testament to this field’s success.[5a–h]
Scheme 1.

First reported enantioselective phase-transfer-catalyzed alkylation of indanone derivatives.
Because extensive secondary literature exists on asymmetric catalysis with onium ions, this review will focus only on selected systems that have yielded mechanistic and conceptual insight into the basis for enantioselectivity.
2.1. Quaternary Ammonium Cations
Quaternary ammonium salts are the largest and most well-studied class of chiral phase-transfer catalysts (Figure 4). Building on the pioneering work by the Merck research group,[5i] O’Donnell and co-workers reported a similar cinchona alkaloid-derived ammonium salt for the enantioselective synthesis of α-amino acids under phase-transfer conditions.[9] N-benzyl cinchonidinium chloride (1b) was found to effect the alkylation of the benzophenone imine of glycine tert-butyl ester (4) with moderate levels of enantioselectivity (Scheme 2a). This synthetically useful alkylation of 4 has served as a benchmark reaction for the development of new enantioselective phase-transfer catalysts.
Figure 4.
Structures of chiral ammonium phase-transfer catalysts.
Scheme 2.
(a) Enantioselective alkylation of glycine ester 4 catalyzed by various quaternary ammonium ions and (b) the interfacial mechanism for phase-transfer catalysis.
Two general mechanistic descriptions have been proposed for phase-transfer-catalyzed reactions using aqueous bases, differing in the location of the substrate in the deprotonation event – either at the interface of the aqueous and organic phases (interfacial mechanism), or in the organic phase (extraction mechanism).[10] For enolate alkylation reactions, experimental support for the interfacial mechanism was provided by Merck scientists (Scheme 2b).[11] Specifically, the alkylation of glycine ester 4 was shown to proceed via α-deprotonation of the ester at the aqueous/organic interface by metal hydroxide. The resulting metal enolate remains at the interface until ion exchange with the chiral catalyst cation (Q*+) generates key lipophilic ion pair intermediate 5. After diffusion into the organic phase, this chiral ion pair reacts with an alkyl halide, affording the optically active monoalkylated product and regenerating the catalyst.
Through the study of catalyst structure-activity/selectivity relationships, several highly selective N-alkyl cinchona alkaloid-derived phase-transfer catalysts (1) have been identified for the glycinate Schiff base alkylation methodology. A particularly strong dependence of catalyst performance on the identity of the N(1)-arylmethyl substituent has been noted.[12] In 1997, Corey[13] and Lygo[14] independently reported that replacement of the N-benzyl group of 1b with a bulky N-9-anthracenylmethyl group resulted in the highly efficient and enantioselective catalysts 1c–d (Scheme 2a). Subsequently, Park and Jew took advantage of the positive influence of sterically bulky N(1)-substituents to develop dimeric and trimeric cinchona alkaloid-derived catalysts linked via the quinuclidinium nitrogen. These multimeric catalysts provide dramatically improved levels of asymmetric induction in alkylations of 4 compared to less sterically congested, monomeric catalysts such as 1b.[5e]
The basis for enantioselectivity in the alkylation of glycine derivative 4 catalyzed by N-anthracenylmethyl ammonium salts 1c and 1d has been probed by several research groups. The quaternary ammonium ion of these catalysts can be envisioned at the center of a tetrahedron composed of the four carbon atoms adjacent to the bridgehead nitrogen (Figure 5a).[13] Based on an X-ray crystal structure of the p-nitrophenoxide salt of 1d, Corey and co-workers propose that three of the faces of this tetrahedron are blocked by the quinuclidine, quinoline, and anthracenyl groups. The remaining face, however, is sufficiently open to allow for close contact between the enolate anion and ammonium cation.26 Pochapsky and co-workers provided experimental support for preferred anion occupancy at this face of the ammonium ion with the observation of specific interionic NOE correlations of a cinchona alkaloid-derived ammonium• borohydride ion pair in solution.[15] The higher selectivity observed with the N-anthracenylmethyl catalysts 1c–d versus N-benzyl catalyst 1b is attributed to more effective differentiation of the tetrahedron faces.
Figure 5.

(a) Tetrahedron stereoselectivity model for cinchona alkaloid-derived catalysts and the proposed enolate approach based on (b) the optimal geometry for attractive R3N+–C–H•••X bonds in the [Me3NH•CH2COOMe] complex at the MP2/6-311++G** level of theory.
Studies by the Reetz and Houk research groups have provided insight into the noncovalent interactions that result in highly structured enolate•ammonium catalyst contact ion pairs. Reetz and co-workers used molecular orbital calculations to determine that the positive charge of tetraalkylammonium cations is delocalized to a significant extent onto the α-carbon atoms.[16] This charge distribution is consistent with the stabilizing R3N+–C–H•••−O–C=C hydrogen bonds identified in several X-ray structures of tetrabutylammonium•enolate ion pairs.[17] Using MP2 calculations with a large basis set, Houk and Cannizzaro evaluated the geometries and interaction energies of such hydrogen bonds using [Me3NH•CH2COOMe] as a model ion pair (Figure 5b).[18] The most stable complex identified has the plane containing the enolate parallel to the tetrahedral face of the trimethylammonium cation defined by the hydrogens of three parallel C-H bonds α to the quaternary nitrogen. Based on this computational model, Houk proposes a mechanism for the alkylation reaction in which the Z-enolate of 4[19] binds in a parallel fashion to the open face of the cinchonidinium, leaving the si face exposed for electrophilic attack (Figure 5a).[20] This arrangement also allows for π-stacking between the electron-rich phenyl substituents of the enolate substrate and the electron-deficient anthracenyl unit of the catalyst. The high enantioselectivity observed in the alkylation of glycine derivative 4, catalyzed by cinchonidinium salts 1c–d, is thus ascribed to a highly ordered catalyst-enolate complex stabilized primarily by N+–C–H•••X hydrogen-bonding and π-stacking interactions. This complex leaves only one face of the nucleophile exposed to alkylation.
The effect on enantioselectivity of varying the electronic properties of the quinuclidinium N(1)-arylmethyl substituents has been investigated. Park and co-workers prepared several fluorinated N-benzylcinchonidinium salts and identified 2′,3′,4′-trifluorophenyl analog 1e as a highly enantioselective catalyst for the alkylation of glycine ester 4 (Figure 6).[21] The fluorine substituent in the 2′-position of the benzyl group was found to be of particular importance for achieving high enantioselectivity. The authors propose that the 2′-F substituent is involved in internal hydrogen bonding via a water molecule, which results in a more rigid catalyst conformation (Figure 6). This model was corroborated by the success of catalysts containing 2′-N-oxopyridine (1g) or 2′-cyanophenyl (1h) moieties;[22] these groups have previously been established, through a series of X-ray crystal structures, to form internal hydrogen-bonding networks similar to those of fluorophenyl substituents.[23]
Figure 6.

Proposed internal hydrogen-bonding via a water molecule for catalysts 1e–h and enantioselectivity in the benzylation of 4.
Even though cinchona alkaloid derivatives have been applied with great success as asymmetric phase-transfer catalysts, these natural product-derived molecular frameworks are amenable to only limited structural modification. This restriction has provided an impetus for the development of fully synthetic catalyst structures that are easier to modify and diversify. Towards this goal, the research groups of Maruoka[24] and Shibasaki[25] have developed N-spiro C2-symmetric quaternary ammonium catalysts (2) and two-centered tartrate-derived diammonium salts (3), respectively (Figure 4).[26] Carefully optimized derivatives of these catalysts promote the alkylation of glycine ester 4, as well as other transformations, in a highly enantioselective fashion (Scheme 2a).[27] Maruoka and coworkers found that the steric and electronic nature of the 3,3′-binaphthyl substituents (Ar) of 2 had a dramatic effect on reactivity and enantioselectivity in the alkylation of 4.[27b, 28] In particular, the catalyst bearing 3,4,5-trifluorophenyl groups determined to be optimal; the beneficial effect of electron-deficient aromatic substituents could be a consequence of an attractive π-π interaction between the catalyst and phenyl groups of substrate 4. X-ray crystallographic analysis of the PF6− salt of 2 revealed that the two 3,4,5-trifluorophenyl groups create a well-defined pocket by blocking two sides of the central ammonium cation (Figure 7). While the usual caution must be exercised in deriving mechanistic interpretation of selectivity effects based solely on solid state catalyst structures,[29] Maruoka and co-workers advance the intriguing proposal that it is this pocket that restricts binding of the E-enolate of 4 to conformations where the si-face is shielded by the binaphthyl and trifluorophenyl moieties. This binding orientation leaves the re-face accessible to alkyl halides, in agreement with the observed sense of enantioinduction in alkylations of 4.
Figure 7.

X-ray crystal structure of N-spiro binaphthyl catalyst (2)•PF6− (adapted from reference 28).
To gain insight into the interactions that produce organized binding of tartrate-derived diammonium catalyst 3 to the enolate of 4, Shibasaki and co-workers carried out a computational analysis of the complex.[25a] The optimized structure suggests that the Z-enolate is associated to the catalyst through a network of hydrogen bonds between the methylene groups adjacent to the ammonium nitrogens and the enolate (Figure 8). Thus, N+–C–H•••X hydrogen bonds are proposed to serve as the primary organizational interactions of enolate ion-pair complexes formed with both cinchona alkaloid-derived catalysts 1a–1h and catalyst 3.
Figure 8.

Optimized structure of a tartrate-derived diammonium catalyst (3)•enolate ion pair at the B3LYP/6-31G(d) level of theory.
2.2. Quaternary Phosphonium Cations
In recent years, chiral quaternary phosphonium salts have been identified as a new type of onium salt for asymmetric ion-pairing catalysis (Figure 9). Maruoka and co-workers have shown that C2-symmetric tetraalkyl phosphonium salt 6 is an effective phase-transfer catalyst for catalytic enantioselective amination,[30] Michael,[31] and Mannich reactions.[31] Ooi and co-workers accomplished the enantioselective alkylation of azlactones with N-benzylated D2-symmetric P-spiro tetraaminophosphonium salt 7 under phase-transfer conditions.[32]
Figure 9.

Representative chiral phosphonium phase-transfer catalyst structures.
Chiral phosphonium salts have been successfully employed not only as phase-transfer catalysts, but also as organic base catalysts.[33] In one of the most impressive examples, Ooi and co-workers designed P-spiro tetraaminophosphonium phenoxide salt 8 and demonstrated its utility in the enantioselective addition of azlactones to α,β-unsaturated acylbenzotriazoles (Scheme 3).[33d] This transformation is proposed to proceed via deprotonation of the azlactone by the phenoxide counterion of the catalyst, followed by ion-pairing of the resultant enolate nucleophile to a supramolecular assembly of the chiral, cationic catalyst and two phenol molecules (Scheme 3). An X-ray crystal structure analysis of the catalyst salt revealed that the supramolecular assembly of the aminophosphonium cation, two phenols, and a phenoxide ion is structured by a 10-membered, cyclic, network of hydrogen-bonding interactions, and strong experimental evidence was obtained that the same supramolecular assembly is generated under the conditions of reaction. Furthermore, the structure of the achiral phenolic component was shown to affect the enantioselectivity. The chiral ion pair 9, stabilized by a well-defined array of hydrogen bonds, was proposed to account for the observed highly enantioselective addition to α,β-unsaturated acylbenzotriazoles.[34]
Scheme 3.
Tetraaminophosphonium salt 8 as a supramolecular chiral organic base catalyst for conjugate additions to acylbenzotriazoles.
Asymmetric catalysis by onium salts has been explored for three decades. Yet, activity in this field is currently at its highest level with new discoveries being reported at a remarkably rapid pace. The intense interest in this research area can be attributed to the broad applicability of the approach, the discovery of new classes of chiral onium ions, and the identification of mechanistic principles that govern successful catalyst scaffolds.
3. Cation-Binding Catalysis
By capitalizing on the well-established cation-binding property of polyethers, researchers have long sought to devise effective enantioselective catalysts that operate through binding of a chiral polyether to the cationic counterion of the reacting anion (Figure 10). Phase-transfer catalysis with polyether compounds differs from catalysis with onium salts (Section 2) in that the entire reacting ion pair, not just the anion, is transferred into the organic phase.[35]
Figure 10.

Cation binding by chiral polyether catalysts.
To date, greatest success with this approach has been achieved using chiral crown ethers as asymmetric phase-transfer catalysts for Michael addition reactions. The first highly enantioselective Michael addition under phase-transfer catalysis was accomplished by Cram and Sogah in 1981. The potassium enolate of a β-keto ester was added to methyl vinyl ketone with very high enantioselectivity by employing a 1,1′-bi-2-naphthol (BINOL)-based chiral crown ether (Scheme 4a).[36] More recently, chiral crown ether catalyst structural motifs have been derived from naturally-abundant carbohydrates. For example, Akiyama and co-workers prepared a chiro-inositol-derived crown ether that enabled the highly enantioselective Michael addition of the potassium enolate of glycine Schiff base 4 to alkyl vinyl ketones (Scheme 4b).[37]
Scheme 4.
Chiral crown ethers as phase-transfer cation-binding catalysts for enantioselective Michael addition reactions.
Song and co-workers recently evaluated the reactivity and selectivity of a BINOL-based bis(hydroxy) polyether catalyst/KF complex in the desilylative kinetic resolution of silyl ethers (Scheme 5).[38] The polyether catalyst was designed such that simultaneous binding could take place to (i) the potassium cation, through chelation with the ether groups, and (ii) the fluoride anion and the silyl ether, through hydrogen bonding with the terminal hydroxy groups. Computational support was provided for the proposed cooperative mechanism.[39] An additional catalyst–ion-pair interaction was suggested by the observation that only catalysts having halogen substituents at the 3,3′-positions of the BINOL units exhibit catalytic activity, and that the reaction outcome is dramatically affected by the identity of the halogen atom. An X-ray crystal structure analysis of a bromo-substituted polyether catalyst/KF complex suggests that this effect is the result of a strong interaction between the halogen substituent and the potassium ion.
Scheme 5.

Bis(hydroxy) polyether-catalyzed desilylative kinetic resolution of silyl-protected secondary alcohols.
These reports and others[35] demonstrate the conceptual validity of cation-binding catalysis using chiral crown ethers. It should be noted, however, that the scope of this approach to catalysis has thus far been limited, perhaps because of the difficulty associated with creating a highly organized chiral environment around a reacting anion by simply binding a chiral catalyst to the associated cation. In more broadly successful asymmetric ion-pairing catalysis strategies such as those described in the other sections of this review, explicit secondary interactions have been shown to be essential for attaining high enantioselectivity. It may, therefore, prove useful to design cation-binding catalysts that bear ancillary components capable of creating specific steric and/or attractive secondary interactions. As discussed above, Song and co-workers nicely illustrate the potential validity of this hypothesis in their design of bis(hydroxy) polyether catalyst 11. It will be interesting to see if future work in this field follows along similar lines, or whether other concepts arise for achieving high enantioselectivity in cation-binding catalysis.
4. Chiral Anion-Directed Catalysis
While asymmetric catalysis of reactions proceeding via anionic intermediates through ion-pairing with chiral cationic catalysts has a relatively long history (Section 2), reports of analogous charge-inverted processes did not emerge until recently. The successful implementation of chiral anion-directed ion-pairing catalysis is potentially very powerful; this strategy provides a platform for absolute stereocontrol in reactions that proceed via cationic intermediates or that utilize cationic reagents or catalysts. The following section, organized by type of anionic counterion, details the impressive advances that have been reported to date.
Reactions catalyzed by strong Brønsted acids that proceed via ion pairs containing a hydrogen-bonding interaction directly involved in the activation of the reactive intermediate will not be included in this discussion (Figure 11). As an example, a recent NMR spectroscopy-based study established that significant hydrogen bonding still exists in the ion pair resulting from protonation of N-aryl imines by diphenyl phosphoric acid.[40] Such hydrogen bonds provide directionality to the electrostatic interaction between chiral catalysts and reactive electrophiles, thereby contributing significantly to the molecular organization necessary to favor one of the diastereomeric transition states. This type of asymmetric Brønsted acid catalysis has been particularly successful using chiral phosphoric acid derivatives, and has been discussed in several recent reviews on asymmetric catalysis with hydrogen-bond donors.[4b–d]
Figure 11.

Types of ion-pairing interactions.
The following discussion will focus on asymmetric catalytic systems operating through strict ion pair interactions of chiral anionic catalysts and cationic intermediates or reagents. When the information is available, attention will be drawn to the specific noncovalent interactions that serve to organize the contact ion pairs, enabling discrimination of the enantiotopic faces of the charged prochiral intermediates.
4.1. Borate Anions
The first report of asymmetric catalysis via ion pairing with a chiral anion was provided by Arndtsen and co-workers in 2000. Achiral cationic copper complexes with D2-symmetric BINOL-based chiral borate counterion 12 were applied as catalysts in a series of aziridination and cyclopropanation reactions (Scheme 6a).[41] Ion pairing between Cu(I)-intermediates and borate counterion 12 was proposed to be the source of enantioselectivity, rather than a covalent interaction with borate 1 acting as an anionic ligand. Support for such an electrostatic interaction was provided by the fact that the enantioselectivity was sensitive to changes in solvent in a manner consistent with the known inverse relationship between ion-pairing energy and solvent dielectric constant: benzene (ε = 2.3; 7% ee), methylene chloride (ε = 9.1; 4% ee), and acetonitrile (ε = 38.8; <1% ee). Analysis of a copper complex that was crystallized in the presence of 2,2′-bipyridine and styrene, to mimic the catalytic conditions, revealed a [(bipy)Cu(H2C=CHC6H5)+][12] structure. This result implies that borate anions do not interfere with the binding of olefin substrates and diimine ligands to the copper center, and strongly supports their role as counterions. While the initial experiments with borate 12 provided products with ≤ 10% ee, this work served, nevertheless, as an important proof-of-concept.
Scheme 6.
Proof-of-concept studies using chiral borate anions.
Subsequent studies on the cyclopropanation of styrene using copper catalysts with borate counterions prepared from tartaric acid and α-amino acid residues (13) demonstrated improved enantioselectivities of up to 34% ee (Scheme 6b).[42] When a borate anion derived from achiral glycine methyl esters (13 with R = H) was used, racemic product was obtained, indicating that the α-amino acid residues, and not the tartrate backbone, are critical for chiral induction in this case. 1H NMR and IR analyses of [Cu+][13] salts revealed only a single set of signals for the tartrate and amino acid portions of the catalyst, further bolstering the idea that the borate anions act as counterions and not as ligands. These studies with copper borate salts provided the earliest support for the concept of inducing asymmetry in metal-catalyzed reactions through ion-pairing cationic metal intermediates to chiral anions.
In the field of organocatalysis, the principle of stereochemical communication between chiral anions and prochiral cationic intermediates was first demonstrated by Nelson and co-workers in the context of the asymmetric ring-opening of meso-aziridinium cations (Scheme 7).[43] By using ammonium salts of borate 12, enantioselectivity of up to 15% was achieved. In line with an ion-pairing interaction, 1H NMR spectroscopy studies revealed proton and carbon shifts of the aziridinium to vary linearly with the amount of borate present. In this same study, the authors did not observe any splitting of the enantiotopic protons of the aziridinium salt, indicating that no specific interaction exists between the meso cation and the chiral anionic catalyst; this observation is consistent with the low levels of enantioselectivity attained. The aziridinium-opening reaction was later rendered highly enantioselective by employing a chiral phosphate-containing catalyst (vide infra).[44]
Scheme 7.

Chiral borate anion-directed aziridinium opening reaction.
Even though only low levels of enantioselectivity were achieved, the pioneering studies with borate salts set the stage for further studies in the area of chiral anion-directed ion-pairing catalysis.
4.2. Phosphate Anions
Since their discovery by Akiyama[45] and Terada[46], BINOL-derived monophosphoric acids have proven to be an attractive and widely useful class of enantioselective Brønsted acid catalysts for hydrogen-bond catalysis.[4c] Furthermore, the conjugate bases of acids with this privileged structure have emerged as powerful counterions in the development of highly enantioselective reactions proceeding via strict ion pairs (Figure 12).
Figure 12.
BINOL-derived phosphate anions 14.
4.2.1. Reactions of Iminium Ions
In 2006, List and Mayer published a landmark paper describing the first highly enantioselective transformation that relies solely on the chirality of an anionic counterion to induce asymmetry.[47] The discovery was based on the biomimetic transfer hydrogenation of enals using a Hantzsch dihydropyridine as the reductant and a chiral secondary amine as the catalyst, which was reported independently the previous year by the List and MacMillan groups (Scheme 8a).[48] That reaction proceeds through a now-classical aminocatalytic mechanism, with LUMO-lowering activation of the α,β–unsaturated aldehyde via the reversible formation of iminium ions. Because the structure of the counterion of the iminium intermediate was found to have a strong effect on the yield and enantioselectivity of the reaction, List and Mayer hypothesized that the source of asymmetry in the catalyst system could be relocated to the counterion (Scheme 8b). The successful demonstration of this strategy was achieved with the morpholine salt of sterically hindered chiral phosphate 14a. Using this catalyst, β,β-aryl, methyl-disubstituted enals were reduced with up to 99% ee.[47, 49] It is particularly noteworthy that higher enantioselectivity was observed for all of the reported substrates with the ion-pairing approach compared to the prior covalent chiral iminium strategy. Studies on the basis for enantioselectivity were not reported. However, an organizational hydrogen-bond between the Hantzsch ester N-H and the Lewis basic oxygen of the phosphoryl group is likely based on detailed theoretical studies that support the presence of this interaction in the closely-related BINOL-phosphoric acid-catalyzed reductions of imines with Hantzsch dihydropyridines (Scheme 8b).[50]
Scheme 8.
Comparison of enantioselective transfer hydrogenations of enals via iminium catalysis by (a) chiral ammonium- or (b) chiral anion-based salts.
List and Wang have subsequently applied this new mode of chiral anion-directed asymmetric iminium activation to the epoxidation of α,β–unsaturated aldehydes (Scheme 9a).[51] The best catalyst for the conjugate reduction, the morpholine salt of 14a, was found to be only moderately enantioselective (54% ee) for the epoxidation reaction. By screening different amines, a new catalyst salt, derived from a trifluoromethyl-substituted dibenzylamine and phosphate 14a, was identified that catalyzes the epoxidation of β–aryl substituted enals in high yield (60–95%), diastereoselectivity (97:3 to >99:1), and enantioselectivity (84–96%). Notably, this secondary ammonium salt catalyst system is also effective for the epoxidation of β,β–disubstituted enals, a substrate class for which achieving high enantioselectivity has proven elusive with other asymmetric epoxidation methodologies.[52]
Scheme 9.
(a) Scope and (b) mechanism for the epoxidation of enals catalyzed by chiral secondary ammonium•phosphate salts.
The fact that excellent enantioselectivity was achieved with symmetric β,β–disubstituted enals provides valuable clues to the mechanism of stereoinduction in these epoxidation reactions. The intermediate (15) resulting from the conjugate addition of tert-butyl hydroperoxide to this class of substrates is achiral, and a stereogenic center is only formed in the subsequent cyclization step (Scheme 9b). As such, enantioselectivity is only possible if either 15 is generated in a chiral conformation through the influence of 14a and undergoes rapid cyclization before bond rotation to an achiral conformation is reached, or if the second cyclization step is enantioselectivity-determining. In the latter case, the chiral phosphate must be involved in this second C-O bond-forming event. Since the intermediate poised to undergo cyclization (15) is neutral, stereochemical communication with the catalyst cannot occur via ion-pairing, but must take place instead via hydrogen-bonding. The basis for stereoinduction with unsymmetric β,β–disubstituted enals or enals with a single β–substituent is also ambiguous, since the ion-pairing interaction of iminium ion intermediates with the chiral phosphates is only relevant to the observed asymmetric induction if the addition of tert-butyl hydroperoxide is irreversible. Thus, while this methodology represents an important synthetic advance by expanding the scope of asymmetric epoxidations, the implications it carries for ion-pairing catalysis must await further mechanistic analysis.
Both the transfer hydrogenation and epoxidation methodologies developed by List have been successfully extended to ketone substrates.[53] The epoxidation of α–branched enals has also been achieved.[54] Due to the increased steric requirements of the ketone and α–branched substrates, phosphoric acid salts of primary amines proved to be uniquely effective. While the reactions catalyzed by salts of secondary amines proceed via strict quaternary iminium-phosphate ion pair intermediates, salts of primary amines proceed via hydrogen-bonded protioiminium-phosphate ion pairs (Figure 11). For reasons discussed above, this reaction manifold is best described as hydrogen-bond catalysis and falls beyond the scope of the review.
N-Acyl iminium ions are highly reactive electrophiles that have been utilized extensively in the construction of various N-heterocyclic frameworks.[55] Due to the weak Lewis basicity of N-acyl iminium ions, the development of asymmetric transformations of this functional group by traditional Lewis acid catalysis approaches is not straightforward. In contrast, the advent of chiral anion-directed catalysis has enabled the discovery of several highly enantioselective counterion-controlled reactions of N-acyliminium ions promoted by either chiral phosphoric acids (vide infra) or thioureas (Section 5).
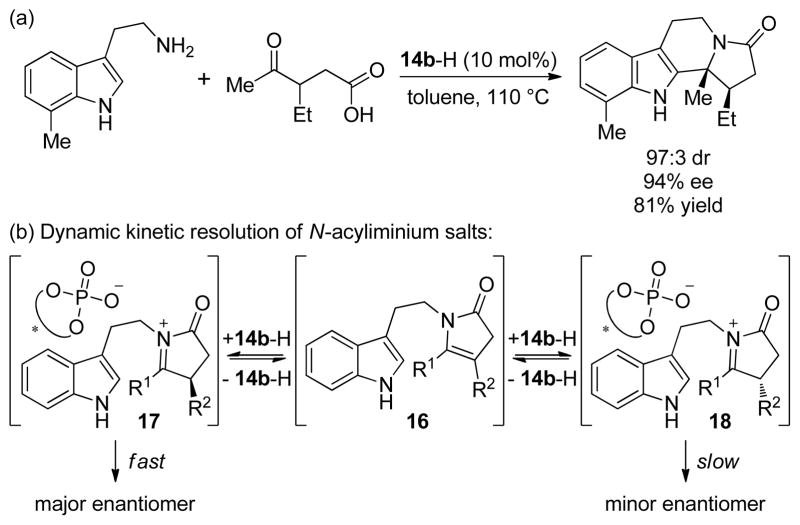
Dixon and co-workers reported an enantioselective N-acyliminium ion cyclization cascade that generated polycyclic tetrahydro-β-carboline products in 68–99% ee through a chiral phosphoric acid-catalyzed condensation of tryptamines and enol lactones[56] or keto acid derivatives[57] (Scheme 10a). The formation of cyclization products in high enantioselectivity and syn-diastereoselectivity was suggested to be the result of a dynamic kinetic resolution of the chiral iminium ion intermediates. The proposed mechanism involves fast and reversible formation of the diastereomeric N-acyliminium phosphate salts 17 and 18 via prochiral cyclic enamide intermediate 16, followed by rate-determining cyclization (Scheme 10b). In support of this mechanism, enamide intermediate 16 (R1, R2 = –(CH2)4–) could be isolated during the early stages of the reaction. Furthermore, subjecting enamide 16 to the reaction conditions afforded product in nearly identical yield and enantiomeric excess to that obtained from the reaction of the tryptamine and keto acid starting materials.
Scheme 10.
(a) Enantioselective phosphoric acid-catalyzed N-acyliminium ion cyclization cascade and (b) the proposed mechanism.
Huang and co-workers developed a (thio)phosphoric acid-catalyzed enantioselective N-alkylation of indoles with N-acyliminium ions.[58] Chiral N-acyliminium•phosphate ion pairs were proposed to form in situ upon treatment of α,β-unsaturated γ-lactams with (thio)phosphoric acid catalysts (Scheme 11). Subsequent addition of indole resulted in the enantioselective formation of N-pyrrolidine indole derivatives. Reactions catalyzed by thiophosphoric acid 19-H were shown to proceed with higher enantioselectivity, but lower yield, than those catalyzed by the analogous phosphoric acid. Substitution was tolerated at the C2 and C3 positions of the indole, and a range of 2,3-fused indoles reacted in high yield and enantioselectivity when using thiophosphoric acid 19-H.
Scheme 11.

(Thio)phosphoric acid-catalyzed enantioselective N-alkylation of indoles with cyclic N-acyliminium ion intermediates.
Huang and co-workers carried out preliminary mechanistic studies to better understand the reaction pathway and the role of the Brønsted acid. The observed absence of catalyst nonlinear effects provided evidence for a monomeric catalyst in both the resting and transition states. On the basis of deuterium labelling studies, it was found that: (i) formation of the N-acyliminium ion is reversible, (ii) the Brønsted acid is the proton source for generation of the N-acyliminium ion, (iii) N-acyliminium ion formation occurs prior to alkylation, and (iv) indole addition is not the rate-determining step. The stoichiometric reaction of phosphoric acid 14a-H and lactam 20 was followed by in situ FTIR and analyzed by HRMS. The mass spectrometry analysis clearly indicated that ion pair 21 is formed, and the FTIR data were most consistent with the enol tautomer dominating in the ground state. However, the identity of the reactive tautomer could not be established by this analysis.
Expanding upon the types of iminium ions that are amenable to asymmetric ion-pairing catalysis, Hiemstra and co-workers described phosphoric acid-catalyzed enantioselective Pictet-Spengler reactions that proceed through N-sulfenyliminium ions (Scheme 12a).[59] The highest levels of enantioselectivity were obtained with substrates bearing bulky substituents on the sulfenyl moiety, with trityl (Tr) substitution being optimal. The labile N-tritylsulfenyl group could be removed easily, and a one-pot cyclization/deprotection method provided access to tetrahydro-β-carboline products derived from both alkyl- and arylaldehydes in up to 87% ee. In later reports, Hiemstra and co-workers also expanded the Pictet-Spengler methodology to N,N-dialkylamine substrates (Scheme 12b).[60] Condensation and cyclization of N-benzyltryptamine with a series of aldehydes proceeded in good yield and up to 87% ee. The methodology was applied successfully to the concise synthesis of the tetracyclic indole alkaloid (−)-arboricine.[61]
Scheme 12.
Phosphoric acid-catalyzed Pictet-Spengler reaction proceeding via (a) sulfenyliminium ions or (b) dialkyl iminium ions.
4.2.2. Reactions of Carbocations
Several methods for the enantioselective synthesis of 3-substituted indoles, a structural motif prevalent in therapeutic agents and natural products, have been developed by applying asymmetric ion-pairing catalysis strategies. These methods generally rely on the formation of carbocationic intermediates through phosphoric acid-mediated dehydration of indolyl alcohols.[62] The resultant chiral indolyl carbocation•phosphate ion pairs can be intercepted by a variety of nucleophiles. Within this mechanistic framework, You and co-workers[63] discovered an intramolecular Friedel-Crafts reaction for the enantioselective synthesis of fluorene derivatives and Gong and co-workers[64] developed an intermolecular enantioselective enamide alkylation reaction. Another impressive application of this ion-pairing concept, developed by Antilla and coworkers, involves the first reported catalytic asymmetric pinacol rearrangement (Scheme 13).[65] Consistently high enantioselectivity was observed upon variation of the indole N-substituent or the identity of the migrating aryl group (91–96% ee, 14 examples). Lower enantioselectivity was observed, however, in reactions involving migration of non-aryl groups. The authors proposed that the chiral phosphate binds to the cationic intermediate through a combination of hydrogen-bonding and electrostatic interactions (Scheme 13).
Scheme 13.
Enantioselective phosphoric acid-catalyzed pinacol rearrangement.
By integrating dienamine and Brønsted acid catalysis, Melchiorre and co-workers developed a remarkable system for the enantioselective δ-alkylation of α,β-disubstituted enals (Scheme 14).[66] A quinidine-derived primary amine catalyst was employed for the activation of enals as the corresponding nucleophilic dienamines. These intermediates were shown to undergo δ-alkylation with the benzhydryl carbocation derived from acid-catalyzed ionization of bis(4-dimethylaminophenyl)methanol. In this dual-catalyst system, the enantioselectivity was found to be significantly higher when chiral phosphoric acid 14d-H (93% ee) was used in place of trifluoroacetic acid (60% ee). The matched and mismatched amine/acid catalyst combinations provided dramatically different results (93% yield, 95% ee vs. 30% yield, 21% ee), with the sense of absolute stereochemistry in the product determined by that of the phosphoric acid. The kinetic order in Brønsted acid was not established, however, a 1:2 amine/acid catalyst ratio was found to be optimal. Alcohols that are precursors to less stable carbocations were found not to be compatible substrates for the δ-alkylation, consistent with the proposed SN1-type pathway. Based on these experimental observations, a complex reaction assembly was proposed wherein phosphate 14d serves as the counterion for the basic quinuclidine moiety and another molecule of 14d serves as the counterion to the in situ-formed benzyhydryl cation. A stabilizing hydrogen-bond between the 6′-OH of the quinidine-derived catalyst and phosphate 14d was suggested based on the importance of this substituent in achieving high levels of reactivity and stereoselectivity.
Scheme 14.
Cooperative dienamine and ion-pairing catalysis for the δ-alkylation of α–branched enals.
4.2.3. Reactions of Oxocarbenium Ions
Several groups have reported efficient enantioselective transformations that are proposed to proceed via oxocarbenium•phosphate ion pairs. Based on the premise that Brønsted acids catalyze the breakdown of O,O-acetals to oxocarbenium intermediates, List and co-workers have developed a series of highly enantioselective intramolecular chiral phosphoric acid-catalyzed transacetalization reactions. Catalyst 14a-H was found to be optimal for the enantioselective transacetalization of achiral homoaldol starting materials (23, R1 = R2) (Scheme 15).[67] However, the highly selective kinetic resolution of chiral derivatives (23, R1 ≠ R2) required the development of a new 1,1′-spirobiindane phosphoric acid catalyst 24-H.[68] The kinetic resolution reactions were carried out with as little as 0.1 mol% 24-H. At this stage, it has not been established whether the cyclization proceeds through a discrete oxocarbenium ion by an SN1-type ion-pairing catalysis pathway or by a concerted SN2-type displacement of one of the diastereotopic ethoxy groups (Scheme 15).
Scheme 15.
Phosphoric acid-catalyzed enantioselective transacetalization reactions.
Oxocarbenium ion intermediates can also be generated through the protonation of vinyl ethers. Terada and co-workers utilized this strategy in the development of an enantioselective direct aldol-type reaction of azlactones with oxocarbenium ions. Activation of vinyl ether substrates was accomplished with phosphoric acid 14a-H to generate β-hydroxy-α-amino acid derivatives bearing a quaternary stereogenic center at the α-carbon atom in high enantio- and diastereoselectivity (Scheme 16a).[69] Experimental support for the intermediacy of an oxocarbenium ion was provided: (i) identical reaction outcomes were observed when using either (E)- or (Z)-vinyl ethers, and (ii) treatment of tert-butyl vinyl ether with a catalytic amount of phosphoric acid 14a-H in the presence of methanol resulted in the formation of tert-butyl methyl acetal.
Scheme 16.
a) Aldol-type reaction and (b) semipinacol rearrangement of oxocarbenium ions formed in situ by the protonation of vinyl ethers.
Terada and co-workers found that variation of the electronic properties of the C2-aryl substituent of the azlactone significantly affected the stereochemical outcome of the aldol-type reaction, with electron-donating methoxy substituents at the 3- and 5-positions providing the highest enantio- and diastereoselectivity. Interactions of the C2-aryl group with catalyst 14a were therefore suggested as an organizational element in the enantiodetermining transition structure. The authors further proposed that C–H•••O hydrogen-bonding interactions between the acidic protons of the oxocarbenium ion and the negative charge-bearing sites of the phosphate counterion restrict the conformational flexibility of the oxocarbenium•phosphate ion pair (Scheme 16a). DFT computational analysis of the oxocarbenium ion charge by a natural population analysis was shown to support the reasonable expectation that the oxocarbenium ion’s α-hydrogens are the most acidic. Lower enantioselectivity was observed with analogues in which the hydrogen atom directly attached to the carbenium carbon was replaced with a methyl group or a deuterium atom. These results suggest that direct interactions with this hydrogen atom may be important as well.
Tu and co-workers developed an enantioselective ring expansion-type semipinacol rearrangement that provides access to spiroethers in up to 98% ee (Scheme 16b).[70] The authors also designed this reaction on the premise that protonation of vinyl ether starting materials by a chiral phosphoric acid should result in the formation of chiral oxocarbenium•phosphate ion pair intermediates. By employing the silver salt of 14a as a precatalyst, mild conditions were identified that are optimal for most substrates. The authors propose that a phosphoric acid catalyst forms in situ by silver-proton exchange between the silver phosphate precatalyst and the alcohol moiety of the vinyl ether substrate.
4.2.4. Chiral Anion Phase-Transfer Catalysis
Although chiral cation phase-transfer catalysis (PTC) was discovered more than 25 years ago, the first example of chiral anion PTC was reported only in 2008 by Toste and co-workers.[44] In analogy to chiral cation PTC (Section 2), the authors envisioned that a lipophilic chiral anionic catalyst could extract a cationic reagent from an aqueous or solid phase into the organic phase. Ion-pairing of the cation with the chiral anionic catalyst would then provide a chiral environment for the desired enantioselective reaction with the substrate.
The first successful application of chiral anion PTC led to a new method for the asymmetric synthesis of β-alkoxy amines (Scheme 17a).[44] This reaction is proposed to involve extraction of Ag(I) from solid Ag2CO3 to the liquid phase by a chiral phosphoric acid catalyst, with the resulting silver phosphate salt abstracting chloride from a β-chloro tertiary amine substrate to generate a meso-aziridinium ion (Scheme 17b). Influenced by the chiral phosphate counterion, the aziridinium ion then undergoes ring opening by alcohols in an enantioselective manner. Indeed, under the optimized conditions, addition of sterically hindered alcohols proceeds with very high enantioselectivity (90–99% ee). Nucleophilic addition and proton transfer result in regeneration of the phosphoric acid catalyst. Control experiments established that both the phosphoric acid catalyst and Ag2CO3 are necessary for the reaction to proceed. The fact that Ag2CO3 does not promote the reaction by itself is consistent with the notion that the phosphate anion acts as a phase transfer agent. Furthermore, addition of more soluble silver salts such as AgOTs leads to significantly lower enantioselectivity. The observed diastereoselectivity strongly supports a double inversion mechanism, and rules out a pathway proceeding via direct SN2 substitution of the organochloride starting material.
Scheme 17.
(a) Desymmetrization of meso-aziridinium ions by chiral phosphate-directed PTC and (b) the proposed catalytic cycle for this application of chiral anion PTC compared to chiral cation PTC (X = halide, B = basic anion, A* = chiral anion, Q* = chiral cation, M = alkali metal).
More recently, a second application of chiral anion PTC was reported by Toste and co-workers. A strategy was described for asymmetric electrophilic fluorination that utilizes an achiral, insoluble cationic fluorinating agent and a chiral phosphate phase-transfer catalyst. The methodology was developed on the premise that ion exchange of lipophilic chiral phosphate anions with the tetrafluoroborate anions of Selectfluor would result in the formation of a more soluble, chiral electrophilic fluorinating agent (Scheme 18). Unlike the majority of previously reported enantioselective electrophilic fluorination methodologies,[71] this method allows for catalytic generation of the chiral fluorinating reagent.
Scheme 18.
Enantioselective fluorocyclization of olefins and fluorination of enamides by chiral anion PTC.
The enantioselective fluorocyclization of olefins[72] and fluorination of enamides[73] have been accomplished using the anion PTC approach (Scheme 18). In enamide fluorinations, the presence of an N-benzoyl group was shown to be important, as significantly lower enantioselectivity was observed with aliphatic acyl groups (e.g. acetyl, 3% ee). In agreement with the proposed role of the catalyst as a phase-transfer agent, the hydrophobic alkyl chains on the backbone of catalyst 14e-H were found to be beneficial for achieving high enantioselectivity. Additionally, the authors observed a nonlinear relationship between catalyst ee and product ee, consistent with the reaction proceeding via a pathway in which both tetrafluoroborate anions are exchanged for chiral phosphates. The discovery of two distinct applications of anion PTC within a short time frame suggests that this strategy may find broad utility in the development of enantioselective reactions that utilize positively charged reagents or proceed via cationic intermediates.
4.2.5. Desymmetrization Reactions of Episulfonium and Halonium Ions
Toste and co-workers have also extended the chiral anion concept to the desymmetrization of episulfonium ions (Scheme 19a).[44] To avoid potential issues arising from sequestration of Ag(I) by the sulfide products, an alternative to the silver-halide abstraction method that proved successful for the formation of aziridinium ions (vide supra) was devised. Trichloroacetimidate was chosen instead as the leaving group, allowing for generation of episulfonium•phosphate ion pair intermediates through direct activation by chiral phosphoric acid catalysts. This putative meso electrophilic intermediate can be intercepted by alcohol nucleophiles to provide β-alkoxy sulfide products in 90–98% yield and 87–92% ee. Enantioselectivity is likely achieved via ion-pairing between the meso episulfonium ion and the anionic catalyst in the stereoselectivity-determining ring-opening step.
Scheme 19.
Chiral phosphate-directed desymmetrization of (a) meso-episulfonium and (b) meso-halonium ions.
Frölich and co-workers have demonstrated that mesohaloniums represent another class of charged intermediates that are amenable to asymmetric ion-pairing catalysis. Using the sodium salt of phosphate catalyst 1b and N-haloamides as a halogen source, the enantioselective haloetherification of ene-diol substrates was achieved via the desymmetrization of meso-halonium ions (Scheme 19b).[74] The same enantioselectivity was observed regardless of whether the 1b•Na salt was preformed or generated in situ from the phosphoric acid catalyst and sodium carbonate. Small changes in enantioselectivity were observed with different metal counterions (Li, 56% ee; Na, 62% ee; K, 46% ee). Bromo- and iodo-etherification products were formed in up to 67% and 71% ee, respectively.[75] However, the iodolactonization of a diacid substrate resulted in only racemic product. The desymmetrization reactions developed by Toste and Frölich represent elegant applications of ion-pairing catalysis to the enantioselective desymmetrization of meso cationic reactive intermediates. Such transformations are not readily accomplished by traditional Lewis acid catalysis.
4.2.6. Transition-Metal Catalyzed Reactions
In 2007, Toste and co-workers reported the first highly enantioselective chiral anion-directed transition metal-catalyzed reaction.[76] It had long been recognized that the development of enantioselective Au(I)-catalyzed additions to π–systems using traditional chiral ligand approaches was rendered very difficult due to the fact that the linear coordination geometry of gold positions the chiral ligand far from the reaction site. In testing the hypothesis that asymmetric induction from a chiral counterion could provide a solution to this problem, the authors found that significantly higher levels of stereoinduction could be achieved in the cyclization of allenol substrates with cationic gold complexes bearing a chiral phosphate counterion than with neutral complexes bearing chiral phosphine ligands (Scheme 20). The chiral counterion strategy proved highly effective for an impressive array of electronically and sterically diverse nucleophiles, which is remarkable because of the likely presence of a secondary stabilizing H-bonding interaction between the phosphate anion and the nucleophile.[76–77] For particularly challenging substrates such as allene-carboxylates, high enantioinduction was achieved by the combination of chiral ligands and chiral counterions. The additive effect of the two chiral components was evidenced by the dramatically different enantioselectivity observed with the matched (82% ee) and mismatched (3% ee) combinations.
Scheme 20.
Chiral phosphate-directed Au(I)-catalyzed enantioselective hydrofunctionalization of allenes.
Consistent with an ion-pairing mechanism, a strong solvent effect was observed for the Au(I)-catalyzed transformations, with the highest enantioselectivity attained in nonpolar solvents such as benzene. Furthermore, because the only two available coordination sites on the Au(I) catalyst are occupied by the substrate and phosphine ligand, the role of the phosphate as a counterion is unambiguous. This work represented an important breakthrough in asymmetric ion-pairing catalysis, demonstrating that chiral anions could be applied to enantioselective transition-metal catalyzed transformations in a synthetically useful manner.
In view of the vast number of reactions catalyzed by ionic complexes of palladium, rhodium, iridium, and other metals, there is tremendous potential for the chiral counterion strategy in the field of asymmetric transition-metal catalysis.[78] Most transition metals, however, have more available coordination sites than Au(I). As a result, it is often difficult to distinguish whether chiral anions are functioning as counterions or as anionic ligands. Due to this ambiguity, the significant number of recent reports of asymmetric catalysis with transition metals associated with chiral phosphates are cited here, but will not be discussed further.[79] Cooperative catalysis by transition-metal complexes and chiral phosphoric acid Brønsted acids has served as the basis for significant recent contributions to the field of asymmetric catalysis.[80] This body of work, which is properly described as hydrogen-bonding catalysis, has recently been reviewed.[4c, 81] In particular, Rueping and co-workers have provided a detailed analysis of both chiral phosphate-transition metal complexes in catalysis and chiral phosphoric acid-transition metal co-catalysis.[82]
Ion pairing has also been applied successfully as a strategy for ligand design in asymmetric transition-metal catalysis.[83] Ooi and co-workers developed a new strategy for the supramolecular assembly of chiral ligands based on an electrostatic intramolecular interaction.[84] Achiral ammonium-phosphine ligands were paired with chiral binaphtholate anions for asymmetric palladium catalysis. This ion-pairing approach to ligand design allows for rapid access to a large number of chiral catalysts, and holds promise as an important tool in the development of metal-catalyzed stereoselective transformations.
4.3. N-Triflylphosphoramidate Anions
Motivated by the desire to develop more acidic catalysts than phosphoric acids for the activation of a wider range of substrates, Yamamoto and Nakashima introduced the strongly electron-withdrawing triflylamide group into the BINOL phosphate framework (Figure 13).[85] This strategy has proven successful, as the N-triflyl phosphoramide catalysts (14-H) have been applied successfully to the activation of weakly basic electrophiles that are unreactive towards phosphoric acids.[86]
Figure 13.

Acidity of phosphoric acid versus N-triflylphosphoramide catalysts.[86–87]
Rueping and co-workers reported the asymmetric addition of indoles to N-methylindolium[88] and N-acyliminium ions[89] generated in situ using N-triflylphosphoramide catalysts 25-H (Scheme 21). In both cases, no product formation was observed with weaker phosphoric acid catalysts 14-H. Addition to an indolium•N-triflylphosphoramidate ion pair, formed upon 25a-H–catalyzed elimination of a tertiary indolyl alcohol, resulted in the formation of a bisindole with axial chirality in 56% ee (Scheme 21a). This serendipitous discovery inspired the development of an alkylation reaction of γ-hydroxy lactams (Scheme 21b). Highly electrophilic N-acyl iminium ion intermediates, generated by N-triflylphosphoramide-catalyzed ionization of the γ-hydroxy lactams, underwent nucleophilic addition by indole to provide disubstituted lactams in good yields and moderate enantioselectivity (up to 84% ee).
Scheme 21.
N-triflylphosphoramide-catalyzed nucleophilic addition to (a) N-alkylindolium and (b) N-acyliminium ions.
More recently, Rueping and co-workers developed an organocatalytic asymmetric allylic alkylation reaction that provides access to biologically-relevant chromenes (Scheme 22).[90] Again, N-triflylphosphoramide catalysts were found to be more reactive and enantioselective than phosphoric acid catalysts. The transformation was proposed to proceed via chiral contact ion-pair catalysis: protonolysis of the allylic alcohol by the Brønsted acid catalyst followed by intramolecular attack on the resulting chiral allyl cation•phosphoramidate ion pair. Evidence for this SN1-type mechanism was provided by the observation that racemic product is formed in the reaction of optically pure allylic alcohol with an unspecified achiral catalyst. However, an alternative dynamic kinetic resolution mechanism consisting of an enantioselective SN2′ substitution pathway coupled to rapid racemization of the allylic alcohol cannot be ruled out on the basis of this experiment. In addition to hydrogen-bonding with the phenol, a cation-π interaction between the allylic cation intermediate and the catalyst was proposed to play a role in organizing the ion pair.
Scheme 22.

Organocatalytic enantioselective allylic alkylation reaction.
Enantioselective protonation of prochiral enol silanes represents an attractive method for the synthesis of chiral, α-branched ketones. In an effort to develop an organocatalytic process, Yamamoto and Cheon focused on the design of new, highly acidic chiral Brønsted acids (Scheme 23).[91] In the presence of the appropriate achiral, stoichiometric proton source (phenol), N-triflylphosphoramide and N-triflylthiophosphoramide derivatives were found to catalyze the transformation efficiently and with high enantioselectivity, whereas phosphoric and thiophosphoric acids were shown to be unreactive. The highest enantioselectivity was achieved with the more acidic N-triflyl thiophosphoramide catalysts. Catalyst loadings as low as 0.1 mol% were used without deleterious effects on yield or enantioselectivity.
Scheme 23.
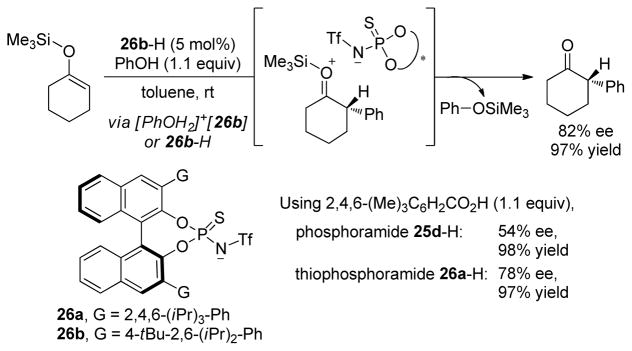
N-triflyl thiophosphoramide-catalyzed enantioselective protonation of silyl enol ethers.
On the basis of preliminary mechanistic studies, Yamamoto and Cheon suggests that the protonation reaction proceeds through a two-step process, with initial protonation of the silyl enol ether by catalyst 26b-H followed by desilylation of the intermediary chiral ion pair by phenol (Scheme 23). No reaction was observed in the absence of the phenol, even when a stoichiometric amount of the N-triflyl thiophosphoramide catalyst was used. Additionally, the structure of both the phenol and the silyl group were found to affect the enantioselectivity. These results do not distinguish between two possible mechanistic scenarios: (i) the protonation is rate- and ee-determining and takes place via a [PhOH2]+•26b complex that is generated by rapid proton transfer between 26b-H and PhOH, or (ii) the protonation is reversible and the desilylation is rate- and ee-determining. The latter scenario, which would represent a clear example of asymmetric ion-pairing catalysis, appears more likely.[92]
4.4. Noncovalent Interactions between Cationic Intermediates and BINOL-derived Phosphates and Phosphoramidates
The transformations described in section 4.2 and 4.3 demonstrate the ability of chiral BINOL-derived phosphate and related anions to promote highly enantioselective reactions of positively charged intermediates or reagents. A common theme among the most enantioselective anions of this type is the requirement for bulky 3,3′-substituents on the BINOL framework. Subtle variations of the electronic and steric properties of these groups often have pronounced effects on the enantioselectivity. On the basis of computational studies[93] and crystal structures of hydrogen-bonded protioiminium•BINOL-derived phosphate ion pairs[93–94], it has been suggested that the importance of the 3,3′-substituents stems from their role in creating a specific substrate recognition site. In these systems, hydrogen-bonding interactions[95] with the bifunctional phosphoric acid moiety, as well as steric shielding[96] and stabilizing π-π interactions[97] with the 3,3′-substituents of the catalyst are proposed to provide the conformational constraints required for high stereoinduction. In the strict ion pair systems described in sections 4.2 and 4.3, the hydrogen-bond interaction between the phosphate and the cationic electrophile is replaced by an electrostatic interaction, and the same secondary noncovalent interactions are likely also important for organizing the substrate-catalyst interaction in the enantiodetermining transition structures (Figure 14). Detailed studies aimed at further elucidating the interactions that enable high levels of asymmetric induction in these systems are warranted and should enable the design of new catalysts and transformations.
Figure 14.

Substrate recognition site created by the 3,3′-substituents (G) of chiral BINOL-derived anions (Y = O, S; X = O, NTf).
4.5. Disulfonimide Anion
List and co-workers discovered the binaphthyl-derived disulfonimide catalyst 27-H while exploring new chiral Brønsted acid motifs for the activation of simple aldehydes. The authors were initially drawn to this C2-symmetric class of catalysts because the acidic proton appears to reside more deeply within the asymmetric environment than in the corresponding BINOL-derived phosphoric acid and phosphoramide catalysts. In fact, catalysis of the Mukaiyama aldol reaction of 2-naphthaldehyde with an isobutyrate-derived ketene acetal was found to proceed only with disulfonimide catalyst 27-H among several chiral Brønsted acid catalysts examined. The catalyst was shown to be quite broad in scope, providing aldol products from isobutyrate- and acetate-derived ketene acetals in high yields and enantioselectivity (Scheme 24a).[98]
Scheme 24.
(a) Enantioselective disulfonimide-catalyzed Mukaiyama aldol reaction and (b) the proposed in situ silylation of the catalyst.
Analysis of these reactions by NMR spectroscopy revealed that catalyst 27-H undergoes rapid silylation by ketene acetals (Scheme 24b). The resulting 27-SiMe3 species was shown to be the active catalyst in the aldol reaction, with the reaction proceeding even in the presence of a hindered base known to suppress Brønsted acid catalysis. Aldehyde activation is therefore proposed to take place through O-silylation, with reactions occurring via disulfonimide•oxocarbenium ion pair 28. Catalyst 27-SiMe3 shows extraordinary promise for inducing enantioselectivity in the wide range of reactions susceptible to catalysis by SiR3NTf2-type Lewis acids.
4.6. Imidodiphosphate Anion
Despite the impressive successes achieved with chiral anionic catalysts, the scope of many of the enantioselective transformations described in the preceding sections is limited by the presence of sterically demanding substituents or specific ancillary functionality on the substrate required to achieve the requisite organization in the enantiodetermining transition structure. For example, many of the cationic intermediates subjected successfully to chiral anion-directed catalysis bear aromatic groups that may be engaged in crucial π-π interactions with the catalysts. In an important recent study, List and Coric reported a highly enantioselective spiroacetalization of simple hydroxyenol ethers (Scheme 25).[99] The new, C2-symmetric imidodiphosphate catalyst 29 was proposed to confine the reaction within a rigid, highly restricted environment. Interestingly, 29 has the same binaphthyl-based dimer backbone as quaternary ammonium phase-transfer catalyst 2, and these two catalysts thus can be viewed as charge inverted analogs of each other.
Scheme 25.

Enantioselective spiroacetalization catalyzed by an exceptionally bulky imidodiphosphoric acid.
Broad scope was observed in enantioselective spiroacetalizations to form 5- and 6-membered rings from 5-, 6-, and 7-membered cyclic enol ethers. An X-ray crystal structure of 29-H confirmed that the imidodiphosphate moiety resides in a confined space created by the interlocking BINOL subunits. It remains to be seen whether the proposed confined-space catalyst concept will prove useful for other catalytic asymmetric reactions of small, structurally and functionally-unbiased substrates.[100]
5. Anion-Binding Catalysis
Asymmetric catalysis by chiral dual hydrogen-bond donors has enabled the development of a remarkable number of highly enantioselective transformations.[4b] Discoveries in this field were initially centered on the direct activation of neutral electrophiles by hydrogen bonding (Figure 15). In recent years, the chemistry has been expanded to reactions proceeding via ion-pair intermediates by taking advantage of the well-established anion-binding properties of ureas and thioureas.[7, 101] This approach to asymmetric ion-pairing catalysis relies on the binding of neutral hydrogen-bond donor catalysts to the unreactive or reactive counterion of cationic intermediates in the enantiodetermining transition-structures (Figure 15). To date, successful implementations of the anion-binding approach have been documented only with (thio)urea catalysts. However, it is very likely that other hydrogen-bond donor catalysts that are proficient anion-binders will soon find application in this arena.[102] Two distinct types of anion-binding catalysis have been uncovered thus far: (i) (thio)urea-assisted ionization of ion-pair precursors through anion-abstraction and (ii) (thio)urea-controlled reactivity of ion-pair intermediates through anion-binding. The work covered in this section, organized by type of (thio)urea-bound anion, demonstrates the generality of these concepts.
Figure 15.

Modes of electrophile activation by dual hydrogen-bond donors.
5.1. Alkoxide Anions
In 2006, Schreiner and Kotke discovered a highly efficient achiral thiourea-catalyzed acetalization of aliphatic and aromatic carbonyl compounds (Scheme 26).[103] Broad scope was observed in the addition of ethanol and 1,2-ethanediol to a variety of aldehydes and ketones. The reactions reached completion at room temperature with catalyst loadings as low as 0.01–1 mol%. The rate accelerations provided by the thiourea were significant, as the uncatalyzed reactions generally provided <1% product under otherwise identical conditions. Mechanistic insight was gleaned from attempted thioacetalization reactions, which resulted in the formation of diethyl acetals as opposed to thioacetals in the presence of the HC(OEt)3 orthoester. If the reaction were to proceed by direct carbonyl electrophile activation (Scheme 26), thioacetal formation would be expected even in the presence of the orthoester because of the greater nucleophilicity of thiols compared to alcohols. Thus, an alternative mechanism was proposed that involves generation of an alkoxide nucleophile by thiourea-assisted heterolysis of the orthoester through stabilization of the incipient anion. This is akin to well-established enzymatic reaction mechanisms in which stabilization of high-energy alkoxide intermediates is achieved through hydrogen-bonding.[104]
Scheme 26.

Acetalization by thiourea-assisted orthoester hydrolysis through anion-binding.
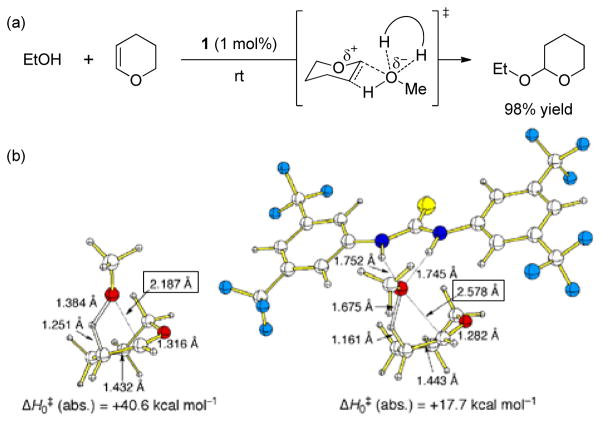
The anion-binding concept was subsequently applied by Schreiner and Kotke to the development of an acid-free, thiourea-catalyzed tetrahydropyranylation of alcohols.[105] Thiourea 30 was found to catalyze the acetalization of primary, secondary, and tertiary alcohols, as well as many acid-sensitive substrates such as cyanohydrins and oximes, with remarkable efficiency (Scheme 27a). A mechanism was proposed wherein protonation of the dihydropyran olefin is followed by alkoxide addition in a formally concerted, but highly asynchronous manner. DFT computation analyses predict that thiourea 30 lowers the activation barrier for the addition step by about 20 kcal mol−1 (Scheme 27b). The observed increase in length of the newly-forming C–O bond in the transition structure of the thiourea-catalyzed compared to the uncatalyzed reaction (2.587 Ǻ versus 2.187 Ǻ) supports the notion that the thiourea assists in generation of the alkoxide nucleophile by stabilizing the developing negative charge. While the acetalization reactions reported by Schreiner involved only achiral catalysts and were therefore not enantioselective, they provided key proof-of-concept for the applicability of the anion-binding properties of thioureas in catalysis.
Scheme 27.
(a) Thiourea-catalyzed tetrahydropyranylation of alcohols by anion-binding and (b) a comparison of transition structures for the uncatalyzed and thiourea-catalyzed addition of methanol to dihydropyran at the B3LYP/6-31G(d,p) level of theory (adapted from reference 105).
5.2. Cyanide Anion
α-Amino acid derivatives are important building blocks for the synthesis of complex, biologically active molecules and chiral catalysts. The addition of hydrogen cyanide to imines, also known as the Strecker reaction, is one of the most valuable and widely used approaches to the α-amino acid motif. As such, the development of asymmetric variants of this transformation has received much attention. In the earliest applications of urea and thiourea derivatives in asymmetric catalysis, Jacobsen and co-workers discovered that these hydrogen-bond donors can promote the hydrocyanation of a variety of imine substrates with high enantioselectivity.[106] After several iterations of catalyst refinement and optimization, thiourea 31 was identified as a practical and broadly applicable catalyst for the hydrocyanation of imines using trimethylsilyl cyanide or potassium cyanide as the cyanide source (Scheme 28a).[107]
Scheme 28.
(a) Thiourea-catalyzed enantioselective Strecker reaction, (b) potential activation mechanisms, and (c) correlation of selected, calculated transition structure hydrogen-bond lengths with enantioselectivity for the hydrocyanation of N-benzhydryl pivaldimine. for eight structurally distinct thiourea catalysts.
A detailed investigation into the mechanism of hydrocyanations catalyzed by 31 established that this transformation proceeds via anion-binding catalysis, and also elucidated the noncovalent interactions that are the basis for enantioselectivity.[108] Hammett, catalyst structure/activity relationships, isotope labelling, and computational studies pointed to a reaction mechanism involving a catalyst-bound cyanide•iminium ion pair (Scheme 28b). In the proposed pathway, proton transfer from thiourea-bound HCN (or HNC) to imine generates a catalyst-bound cyanide•iminium ion pair. Rate- and enantio-determining rearrangement of this ion pair leads to separation of the charged species, and results in a subsequent stereospecific collapse to the α-aminonitrile product. The key ion pair rearrangement is proposed to occur through transferring the hydrogen-bonding interaction of the protioiminium ion N-H from the bound cyanide to the carbonyl of the catalyst amide. Additional support for the accuracy of the proposed mechanism was the strong correlation (P ≪ 0.01) between experimental and calculated enantioselectivity for eight different thiourea catalysts.
To elucidate the basis for enantioselectivity, the relationship between enantioselectivity and various calculated transition structure hydrogen-bond lengths was evaluated. No trend was observed when plotting the sum of the thiourea-cyanide bond lengths (d1 + d2) versus enantioselectivity (Scheme 28c). Thus, differences in the observed enantioselectivity cannot be traced to the degree of stabilization of the cyanide nucleophile. There was, however, a positive correlation between enantioselectivity and the calculated lengths of the stabilizing hydrogen bonds to the protioiminium ion N-H from the cyanide anion and amide carbonyl (d3 + d4). This observation indicates that the enantioselectivity can be ascribed to the differential stabilization of the iminium cation in the diastereomeric transition states of the ion pair rearrangement.
List and Pan developed an enantioselective thiourea-catalyzed Strecker reaction using acetyl cyanide as the cyanide source; this reagent offers practical advantages over HCN or TMSCN (Scheme 29).[109] Two mechanisms were proposed for the acylcyanation: (i) formation of an N-acyliminium intermediate, followed by the addition of cyanide or (ii) cyanation with traces of HCN formed in the reaction mixture, followed by acetylation. In an experiment in which acetyl cyanide was added after hydrocyanation of the imine with HCN, the acylated product was obtained in nearly identical enantioselectivity as in the one-pot procedure. This result provides support for the reaction proceeding through addition of HCN, as established in the closely-related Strecker reactions developed by Jacobsen and co-workers (vide supra).[108]
Scheme 29.
Thiourea-catalyzed acylcyanation of imines.
5.3. Halide Anions
Ureas and thioureas are potent halide-anion binders.[110] Over the past few years, a wide variety of enantioselective reactions proceeding via reactive cationic intermediates have been developed by halide-binding thiourea catalysis.
The Pictet-Spengler reaction, the cyclization of electron-rich aryl groups onto iminium electrophiles, is a powerful method for the synthesis of alkaloids.[111] The first catalytic asymmetric variant of this reaction was developed by Jacobsen and Taylor with the discovery that chiral thioureas catalyze the cyclization of indoles onto N-acyliminium ions generated in situ by the acylation of imines (Scheme 30a).[112] The efficient total synthesis of (+)-yohimbine was subsequently achieved by employing this thiourea-catalyzed acyl-Pictet-Spengler reaction.[113] The enantiodifferentiation of prochiral N-acyliminium ion intermediates by chiral thioureas was also applied successfully in acyl-Mannich reactions of isoquinolines (Scheme 30b).[114] The enantioselectivity of both of these reactions was shown to depend strongly on the polarity of the reaction solvent and structure of the acylating agent.
Scheme 30.
Thiourea-catalyzed (a) acyl-Pictet-Spengler and (b) acyl-Mannich reactions proceeding via N-acyliminium ions.
In an effort to further broaden the scope of thiourea-catalyzed reactions of N-acyliminium ions and obtain insight into the nature of the substrate-catalyst interactions, an acyl-Pictet-Spengler-type cyclization of tryptamine derivatives was developed wherein the N-acyl iminium ions are generated in situ by the dehydration of hydroxylactams (Scheme 31).[115] 1H-NMR studies of a hydroxylactam substrate in the presence of the TMSCl dehydrating reagent indicated that formation of the corresponding chlorolactam is fast and irreversible. The experimentally observed increase in reactivity with hydroxylactam derivatives generated by imide alkylation (R = Me) versus by imide reduction (R = H) pointed to an SN1-type mechanism. While these experiments established that the reaction proceeds via an N-acyliminium ion, the mode of the catalyst interaction in the enantiodetermining transition state was not yet apparent.
Scheme 31.
Pictet-Spengler-type cyclization of hydroxylactams and the proposed anion-binding mechanism.
DFT computational analyses failed to converge for all structures of the thiourea bound to the N-acyliminium ion carbonyl, but a productive interaction was identified between the thiourea and the α-chloroamide via the α–chloro substituent. Enantioselective cyclization of the indole was therefore proposed to occur via a chiral thiourea-bound N-acyliminium•chloride ion pair resulting from thiourea-induced dissociation of the chloride. Further support for the anion-binding model includes the pronounced halide counterion effects (Cl, 97% ee; Br, 68% ee; I, <5% ee) and solvent effects (TBME, 97% ee; CH2Cl2, <5% ee) on enantioselectivity. Additionally, treatment of the thiourea catalyst with a chloride source resulted in a 0.56 ppm downfield shift of the thiourea N-H protons, consistent with strong binding to the chloride anion.
The hydroxylactam-based acyl-Pictet-Spengler methodology has been extended successfully to the regio- and enantioselective cyclization of pyrrole nucleophiles using the same thiourea catalyst 32.[116] In agreement with the proposed SN1-type mechanism, rate acceleration due to increased substitution at the electrophilic center was observed again. An intermolecular addition of indoles to N-acyl iminium ions derived from hydroxylactams was also achieved upon fine tuning the catalyst structure.[117] In this case, replacing the pyrrole moiety on the thiourea catalyst with a Schiff base was necessary for achieving high enantioselectivity. These variants of the hydroxylactam-based acyl-Pictet-Spengler methodology,[116–117] in addition to the acylative acyl-Pictet-Spengler[112] and acyl-Mannich[114] reactions described above, were proposed to proceed via an anion-binding mechanism by analogy.
The first catalytic asymmetric Petasis reaction was reported by Takemoto and co-workers in 2007.[118] The authors designed a chiral bifunctional thiourea that promotes the enantioselective addition of vinyl boronic acids to in situ generated N-acylquinolinium salts (Scheme 32). The 1,2-amino alcohol and thiourea moieties of the catalyst were both shown to be important for achieving high reactivity and enantioselectivity. The 1,2-amino alcohol was proposed to chelate the boronic acid and form a reactive “ate” complex, whereas the thiourea was presumed to control the cis/trans isomer distribution of the N-acylquinolinium amide bond by hydrogen bonding to the carbonyl. However, in light of the mechanistic studies outlined above,[115] it is worth considering whether the thiourea moiety is in fact interacting with the N-acylquinolinium salt via the chloride anion in an ion-pairing catalysis mechanism (Scheme 32).
Scheme 32.

Thiourea-catalyzed enantioselective Petasis-type reaction of quinolines proceeding via N-acylquinolinium ions.
Jacobsen and co-workers recently reported the development of a new thiourea catalyst for the enantioselective bicyclization of hydroxylactams. A marked correlation between the size of the aromatic group of 2-arylpyrrolidine catalysts 33–36 and catalytic performance was observed, with larger arenes providing improved reactivity and selectivity (Scheme 33a). Pyrenyl-substituted thiourea derivative 36 was found to catalyze the bicyclization of substrates with various aromatic terminating nucleophiles in 89–95% ee.[119] Given the cationic nature of the substrate and the dependence of enantioselectivity on the expanse of the catalyst arene, experiments were undertaken to establish whether stabilizing cation-π interactions are directing the stereochemical course of the reaction in a manner akin to the biosynthetic proposal for cyclase enzymes.[120]
Scheme 33.
(a) Effect of thiourea catalyst aromatic group on the efficiency and enantioselectivity of the bicyclization of hydroxylactam 37 and (b) the proposed stabilizing cation–π interaction in the dominant transition state.
It has been proposed that the energetic benefit of noncovalent binding interactions are generally manifested enthalpically.[121] An Eyring analysis revealed that enantioselectivity is indeed enthalpically controlled for catalysts 34–36, and that the degree of the differential enthalpy increases for the more extended arenes. Additionally, there is a positive linear free energy relationship between the quadrupole moment and polarizability of the arenes and the degree of asymmetric induction. Taken together, these data provide strong support for a cation-π interaction being responsible for the differential stabilization of the diastereomeric transition states (Scheme 33b). This work provides a clear illustration of how asymmetric induction can be achieved through stabilization of the major transition state through attractive interactions, as opposed to destabilization of the minor transition state through steric repulsion.
The principle of asymmetric hydrogen-bond catalysis by anion binding has been applied successfully to transformations involving various cationic species aside from iminium ions. For example, the asymmetric thiourea-catalyzed addition of silyl ketene acetals to 1-chloroisochromans was achieved in 70–95% yield and 74–95% enantioselectivity (Scheme 34).[122] The addition reaction proceeds by a dynamic kinetic resolution because the products are isolated in >50% yield and the enantioselectivity remains constant throughout the reaction. Racemization of the chloroether starting materials is believed to occur via the in situ generation of oxocarbenium ions by thiourea-assisted chloride dissociation. Support for chloride-binding catalysis is provided by the fact that addition of 10 mol% n-Bu4NCl resulted in complete inhibition of the reaction. The presence and configuration of an aryl group in the amide component of the catalyst had a large impact on the reaction rate and enantioselectivity, which could be indicative of this group’s involvement in stabilizing interactions in the rate-determining transition state (Scheme 34).
Scheme 34.
Enantioselective thiourea-catalyzed addition of silyl ketene acetals to oxocarbenium ions.
Asymmetric anion-binding catalysis has also been applied to reactions proceeding via carbocations that are not stabilized by a heteroatom. A primary aminothiourea was found to catalyze the enantioselective α-alkylation of α-branched aldehydes with symmetrical diarylbromomethanes (Scheme 35).[123] Several experiments were performed to elucidate the mechanism of this reaction. A normal secondary kinetic isotope effect was observed for the benzhydryl proton, indicating that the electrophilic carbon undergoes rehybridization from sp3 to sp2 in the rate-determining transition state. A Hammett study was also consistent with the development of positive charge at the benzhydryl carbon in the transition state (ρ = −1.95). Additionally, a competition experiment established that, under the catalytic conditions, only alkylation of bromodiphenylmethane was observed in the presence of an equimolar amount of benzyl bromide. The results of these three experiments suggest strongly that the alkylation proceeds through a discrete, catalyst-associated benzhydryl cation by an SN1-like substitution mechanism (Scheme 35). Interestingly, the reaction of enantioenriched diarylchloromethanes was found to be 95% stereospecific. When considered along with the previous experiments, this finding implies that addition of the catalyst-associated enamine to the ion-pair intermediate is faster than ion-pair racemization.
Scheme 35.
Enantioselective, catalytic SN1-type alkylation of aldehydes with benzhydryl cations.
Asymmetric chloride-binding hydrogen-bond catalysis has been applied successfully to several different classes of electrophiles. Combined with recent advances with bromide-[123] and fluoride-binding systems[124] (vide infra), there is clearly broad potential in asymmetric ion-pairing catalysis using halide counterions.
5.4. Carboxylate Anions
Urea- and thiourea-based compounds are excellent synthetic receptors for Y-shaped, coplanar anions such as carboxylates.[125] The topology of this class of anions allows for hydrogen-bonding in a bidentate fashion. It is perhaps because of this constrained geometry that carboxylate-binding by chiral thiourea catalysts has been applied with particular success in asymmetric catalysis.
Using a chiral thiourea/benzoic acid dual catalyst system,[126] Jacobsen and co-workers developed mild and operationally simple protocols for enantioselective Pictet-Spengler[127] and iso-Pictet-Spengler[128] reactions (Scheme 36). The one-pot method affords unprotected tetrahydro-β-carboline and tetrahydro-γ-carboline products directly from tryptamine and aldehyde derivatives. Formation of a benzoate•iminium ion salt was proposed to be facilitated by the thiourea catalyst through hydrogen-bonding (Scheme 36). Cyclization followed by rearomatization of the thiourea-bound benzoate•indolyl cation ion pair then affords the tetrahydrocarboline products. Notably, rearomatization was found to be rate-determining based on the observed primary kinetic isotope effect (KIE = 2.9–3.3) with a 2-deuterotryptamine derivative.[129] As such, catalysis by the thiourea was proposed to involve stabilization of the key ion-pair intermediates and association to the benzoate in the enantioselectivity-determining final deprotonation step. This appears to represent a new manifold for asymmetric ion-pairing catalysis, wherein the chiral catalyst-anion complex acts as a Brønsted base in the key step. Other recently-developed enantioselective catalytic Pictet-Spengler reactions,[130] currently proposed to proceed via H-bonding catalysis mechanisms, might also proceed via rate-limiting rearomatization, in which case ion-pairing interactions would play a key role in achieving enantioselectivity in those systems as well.
Scheme 36.
Thiourea-catalyzed enantioselective protio-Pictet-Spengler reaction with rate-determining rearomatization.
A dual catalyst system consisting of a chiral primary aminothiourea and achiral thiourea 30 was found to promote enantioselective intramolecular oxidopyrylium-based [5+2] cycloadditions, thereby providing access to valuable chiral 8-oxabicyclo[3.2.1]octane architectures (Scheme 37).[131] A remarkable cooperative effect was observed between the thiourea catalysts, with significant improvement in both reactivity and enantioselectivity in the presence of achiral thiourea 30 (32% yield, 72% ee vs. 72% yield, 91% ee). To elucidate the roles of the different components in the thiourea catalyst system, a series of catalyst structure–activity–relationship studies were carried out. A chiral amino catalyst lacking the thiourea moiety could effect the reaction in good yield and up to 87% enantioselectivity, but only in the presence of thiourea 30. A tertiary aminothiourea, in contrast, was unreactive regardless of whether thiourea 30 was present. Based on these experiments, an enamine catalysis mechanism was proposed. Additional support for an aminopyrilium, and not an oxido- or amido-pyrilium, intermediate was provided by the correlation of computational frontier molecular orbital predictions with observed experimental trends. Thus, the suggested mechanism involves condensation of the primary aminothiourea with the ketone of the pyranone substrate to form a dienamine after tautomerization, followed by benzoate abstraction by achiral thiourea 30 to generate the reactive aminopyrylium•benzoate ion pair 39 that is poised to undergo the cycloaddition.
Scheme 37.
Enantioselective oxidopyrilium-based [5+2] cycloaddition via anion-binding/enamine co-catalysis.
Catalytic asymmetric acyl transfer reactions generally rely on the use of chiral variants of nucleophilic catalysts such as derivatives of 4-(dimethylamino)pyridine (DMAP).[132] Seidel and co-workers have designed an alternative approach by taking advantage of the anion-binding properties of thioureas. They reasoned that an achiral acylpyridinium salt, formed in situ from a dialkylaminopyridine catalyst and an acylating agent, could be rendered chiral upon binding of a chiral thiourea catalyst to the associated anion (Scheme 38). The authors anticipated that catalysis could be achieved due to the higher reactivity and/or higher concentration of the catalyst-bound ion pair relative to the unbound ion pair.
Scheme 38.
Acylative kinetic resolution of primary amines by an anion-binding approach to nucleophilic catalysis.
Amines are notoriously challenging substrates for asymmetric nucleophilic catalysis due to their inherent nucleophilicity. Seidel’s chiral thiourea–dialkylaminopyridine co-catalysis approach, however, enabled the acylative kinetic resolution of benzylic,[133] propargylic,[133b] and allylic[134] amines with selectivity factors of up to 67 (Scheme 38). The desymmetrization of meso-diamines by enantioselective monoacylation was also achieved.[135] Remarkably, all of these variations can be accomplished under nearly identical reaction conditions with the same thiourea catalyst. As might be anticipated, the nature of the carboxylate anion was found to be important, with benzoate giving the best results. Control experiments established that both the dialkylaminopyridine nucleophilic catalyst and the thiourea anion-binding co-catalyst were required for good conversion and enantioselectivity. Under the standard reaction conditions, no conversion was observed with the dialkylaminopyridine alone. However, some acylation was observed with only the thiourea catalyst. It is interesting to note that the reaction promoted by the thiourea alone, which presumably occurs via direct activation of the anhydride acylating reagent through hydrogen-bonding, was found to afford only racemic products.
5.5. Enolate Anions
Asymmetric anion-binding/nucleophilic co-catalysis has been applied successfully by Jacobsen and co-workers to the C-acylation of enolate equivalents. By using chiral arylpyrrolidine-based thiourea catalyst 41 in combination with 4-pyrrolidinopyridine, the enantioselective acylation of silyl ketene acetals with acyl fluorides was developed to generate valuable α,α-disubstituted butyrolactone products (Scheme 39).[124] A marked dependency on the nature of the acylating agent was observed, with benzoyl fluoride being significantly more reactive and selective than benzoic anhydride. This reaction represents the first example of anion-binding catalysis with fluoride. The excellent hydrogen-bond acceptor properties and silicon affinity of fluoride likely play important roles in enabling this transformation. Key insight into the mechanism was gained from experiments that varied the silyl group of the silyl ketene acetal. The size of the silyl group considerably affected the rate of the reaction, but did not influence the enantioselectivity. A mechanism consistent with this observation was proposed involving formation of a thiourea-bound acylpyridinium•fluoride ion pair, followed by rate-determining desilylation of the silyl ketene acetal, and then enantio-determining acylation via a thiourea-bound acylpyridinium•enolate ion pair (Scheme 39).
Scheme 39.
Enantioselective acylation of silyl ketene acetals by an anion-binding approach to nucleophilic catalysis.
The dual catalysis approach was also extended by Seidel and coworkers to reactions proceeding via thiourea-bound acylpyridinium•enolate ion pair intermediates (Scheme 40).[136] A chiral thiourea–DMAP co-catalyzed asymmetric Steglich reaction, the rearrangement of O-acylated azlactones to the corresponding C-acylated products, affords α,α-disubstituted amino acid derivatives in 49–65% yield and 87–91% ee. By replacing the DMAP nucleophilic co-catalyst with isoquinoline, Seidel and co-workers achieved incorporation of the nucleophile framework into the product; the azlactone-derived enolates were found to add to the 1-position of the isoquinoline ring as opposed to the acyl group of the acylisoquinolinium ion. This transformation resulted in the efficient synthesis of α,β–diamino acid derivatives in 81–95% yield with high levels of enantio- and diastereocontrol (Scheme 40). The optimal thiourea catalyst (42) for both the azlactone addition and rearrangement reactions, is structurally similar to the optimal catalyst (41) identified by Jacobsen and co-workers for a reaction that is also proposed to proceed via a thiourea-bound enolate. It is worth noting that the facial selectivity of the azlactone addition to isoquinolines is opposite to that of the azlactone rearrangement reaction, suggesting that significantly different secondary interactions must be at play in the corresponding thiourea-bound ion pair intermediates. The reports by the Seidel[133–136] and Jacobsen[124] groups demonstrate the power of anion-binding in asymmetric nucleophilic catalysis, and suggest that this strategy may be broadly applicable.
Scheme 40.
Dual catalysis approach to the enantioselective Steglich rearrangement and addition of O-acylated azlactones to isoquinolines.
5.6. Imidate Anions
The development of catalytic, enantioselective iodolactonizations has proven challenging due to the inherent difficulty in controlling the stereoselectivity in the formation and reaction of iodonium ions. Inspired by the success of (thio)urea catalysts in effecting enantioselective reactions of a diverse array of cationic intermediates, Jacobsen and Veitch developed a tertiary aminourea-catalyzed enantioselective iodolactonization (Scheme 41).[137] 1H-NMR studies of the aminourea catalyst in the presence of the iodinating agents suggested an N-iodo derivative of the catalyst is formed in situ. This species was proposed to undergo reaction with the hexanoic acid substrate to form thiourea-bound imidate•iodonium ion pair intermediate 43. Based on the difference in reactivity observed with substrates forming 5-, 6-, or 7-membered lactone products (5 > 6 ≫ 7), the subsequent cyclization step was proposed to be rate- and enantio-determining. The level of enantioinduction was shown to be sensitive to the structure of the imidate, indicating that it is directly involved in the enantiodetermining step. Preliminary computational studies of putative intermediate 43 support a tertiary amino-iodonium interaction and deprotonation of the carboxylic acid by the urea-bound phthalimide in the enantiodetermining cyclization event.
Scheme 41.

Urea-catalyzed enantioselective iodolactonization.
5.7. Sulfonate Anions
Jacobsen and co-workers demonstrated that anion-binding catalysis can be used as a strategy for inducing enantioselectivity in reactions proceeding via specific acid mechanisms.[138] The combination of an achiral sulfonic acid and chiral bifunctional sulfinamidourea 44 was found to be optimal for promoting a highly enantioselective [4+2] cycloaddition of N-aryl imines and electron-rich olefins, also known as the Povarov reaction (Scheme 42a). Structure–reactivity/enantioselectivity studies established the importance of both the urea and sulfinamide groups of the catalyst. Kinetic analysis of the reaction revealed a first-order dependence on triflic acid (TfOH) and a zeroth-order dependence on imine 45, consistent with quantitative protonation of imine 45 by triflic acid (TfOH) under the reaction conditions and protioiminium triflate 45•HOTf as the resting state of the sulfonic acid catalyst. A large binding constant (K = 9 x 103 M–1) was determined for the interaction of the urea catalyst 44 with salt 45•HOTf. Computational and 1H NMR spectroscopic analysis of the ternary complex (44•45•HOTf) shed light on the nature of the binding interaction. A dynamic structure was elucidated, consisting of the urea-sulfonate anion complex associated to the protioiminium ion through a combination of electrostatic and hydrogen-bonding interactions (Scheme 42b).
Scheme 42.
(a) Urea/strong Brønsted acid co-catalyzed enantioselective Povarov reaction (NBSA = o-nitrobenzenesulfonic acid), and energy and geometry-minimized structures of (b) the ground state catalyst-substrate interactions (Ar = 3,5-(CF3)2C6H3) and (c) the cycloaddition transition structure leading to the major enantiomer of product at the B3LYP/6-31G(d) level of theory.
A comparison of the kinetics of the HOTf/urea 44 co-catalyzed reaction relative to the reaction catalyzed by HOTf alone revealed that the urea induces a several-fold decrease in the reaction rate. It was concluded that 44 serves to stabilize the protoiminium ion triflate ground state more than the cycloaddition transition state. However, the racemic pathway was shown to be suppressed, and high enantioselectivity was still achieved because the high association equilibrium of the urea catalyst and the protioiminium intermediate ensures that the iminium ion undergoes cycloaddition only in association with the chiral urea. Additional investigations led to the proposed basis for enantioselectivity: a stabilizing π-π interaction between the (bis)trifluoromethylphenyl group of the catalyst and the aniline arene of the imine. This attractive interaction is apparent in the computed transition structure leading to the major enantiomer, but absent in the competing diastereomeric transition state (Scheme 42c). Careful study of the urea-catalyzed Povarov reaction thus revealed that a network of weak, noncovalent interactions can function cooperatively to attenuate the reactivity of a highly reactive intermediate as well as effect a highly enantioselective transformation.
Detailed mechanistic studies such as this one have provided valuable insight into how the stereochemical outcome of reactions can be controlled solely through noncovalent interactions between a neutral catalyst and an ion-pair intermediate. In several cases of anion-binding catalysis mechanisms, it has been established that high levels of enantioselectivity are attained via stabilization of the dominant transition state through attractive noncovalent interactions.[139] The small molecules that achieve this mode of catalysis have been compared to enzymes because this type of activation is ubiquitously observed in Nature’s catalysts.[140] This ‘enzyme-like’ basis for catalysis stands in contrast to the majority of stereochemical models for small molecule catalysts, including the BINOL-derived phosphate anions in section 4.2–4.4, which most often invoke destabilization of competing transition structures by repulsive steric interactions.
6. Summary and Outlook
By taking advantage of ion-pairing interactions, several powerful approaches have been developed for asymmetric catalysis of transformations that involve charged intermediates or reagents. We have sought to demonstrate in this review that even though the electrostatic attraction of two oppositely charged species is only weakly directional, the conformational constraint required for high stereoinduction can be attained through secondary noncovalent interactions operating in concert. In addition to destabilizing steric interactions, attractive secondary interactions such as hydrogen-bonding, π-π, and cation-π interactions have been demonstrated to play important roles in organizing the enantiodetermining transition states. We anticipate that future catalyst designs will also capitalize on other known attractive noncovalent interactions, such as halogen-bonding or anion-π interactions.[141]
Highly enantioselective phase-transfer catalysis with chiral cationic and cation-binding catalysts was first demonstrated in the 1980’s, and important advances in this area continue to be made at an impressive pace. Much more recently, charge-inverted strategies involving anionic and anion-binding catalysts have emerged, and these areas are the focus of an explosion of current research activity. At this stage, enantioselective catalytic reactions that engage a wide variety of cationic intermediates have been developed primarily using catalysts from one of only two structural classes: binaphthol-derived phosphates and related PV-based anions in chiral anion-directed catalysis, and chiral urea and thiourea catalysts in anion-binding catalysis. Continued exploration of these and closely related systems will undoubtedly lead to the discovery of interesting and useful new enantioselective transformations and concepts in stereoselective catalysis, especially if novel catalyst structures are identified.
The different classes of asymmetric ion-pairing catalysts described in this review all rely on networks of noncovalent interactions to achieve energetic differentiation of selectivity-determining transition structures. Due to the often weak and non-directional nature of such noncovalent interactions, determining the basis for catalysis and enantioselectivity is generally extremely challenging. The increasing power of computational methods is enabling the study of noncovalent interactions in such catalytic reactions with increasing accuracy.[142] In combination with experimental investigations, such analyses will facilitate elucidation of the factors controlling enantioselectivity. Increased understanding of asymmetric ion-pairing mechanisms will open up new opportunities for this important mode of catalysis.
Acknowledgments
This work was supported by the NIH (GM43214) and through a postdoctoral fellowship to K.B. (GM090477).
Biographies

Katrien Brak was born in Leuven, Belgium in 1983. She obtained her B.S. degree in chemistry in 2000 from MIT, and completed her Ph.D. studies at UC Berkeley working under the supervision of Prof. Jonathan A. Ellman. Her graduate work focused on the design of nonpeptidic cruzain inhibitors and the development of methods for the asymmetric synthesis of α-branched allylic amines. She is currently an NIH postdoctoral fellow in the laboratories of Prof. Eric N. Jacobsen. Her research involves the development of enantioselective catalytic reactions of cationic intermediates by anion-binding catalysis.

Eric N. Jacobsen was born and raised in New York City. He earned his B.S. degree from New York University in 1982 and his Ph.D. degree at the UC Berkeley in 1986, working under the direction of Robert Bergman. He carried out postdoctoral studies with Barry Sharpless at MIT. In 1988, he began his independent career at the University of Illinois at Urbana–Champaign. He moved to Harvard University in 1993, where he is currently the Sheldon Emory Professor of Organic Chemistry and Department Chair. His research interests lie in the discovery, mechanistic elucidation, and application of new catalytic processes.
References
- 1.a) Bjerrum N. K Dan Vldesk Selesk Math Phys Medd. 1926;7:3. [Google Scholar]; b) Szwarc M. Acc Chem Res. 1969;2:87–96. [Google Scholar]
- 2.Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; Sausalito: 2006. [Google Scholar]
- 3.a) Winstein S, Clippinger E, Fainberg AH, Robinson GC. J Am Chem Soc. 1954;76:2597–2598. [Google Scholar]; b) Sadek H, Fuoss RM. J Am Chem Soc. 1954;76:5897–5901. [Google Scholar]
- 4.a) Taylor MS, Jacobsen EN. Angew Chem. 2006;118:1550–1573. [Google Scholar]; Angew Chem Int Ed. 2006;45:1520–1543. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]; b) Doyle AG, Jacobsen EN. Chem Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; c) Terada M. Synthesis. 2010;12:1929–1982. [Google Scholar]; d) Akiyama T. Chem Rev. 2007;107:5744–5758. doi: 10.1021/cr068374j. [DOI] [PubMed] [Google Scholar]
- 5.a) O’Donnell MJ. Acc Chem Res. 2004;37:506–517. doi: 10.1021/ar0300625. [DOI] [PubMed] [Google Scholar]; b) Lygo B, Andrews BI. Acc Chem Res. 2004;37:518–525. doi: 10.1021/ar030058t. [DOI] [PubMed] [Google Scholar]; c) Hashimoto T, Maruoka K. Chem Rev. 2007;107:5656–5682. doi: 10.1021/cr068368n. [DOI] [PubMed] [Google Scholar]; d) Ooi T, Maruoka K. Angew Chem. 2007;119:4300–4345. [Google Scholar]; Angew Chem Int Ed. 2007;46:4222–4266. doi: 10.1002/anie.200601737. [DOI] [PubMed] [Google Scholar]; e) Jew S-s, Park H-g. Chem Commun. 2009:7090–7103. doi: 10.1039/b914028j. [DOI] [PubMed] [Google Scholar]; f) Maruoka K. Asymmetric Phase Transfer Catalysis. Wiley-VCH; New York: 2008. [Google Scholar]; g) Ojima I. Catalytic Asymmetric Synthesis. 3. Wiley-VCH; New York: 2010. [Google Scholar]; h) Ooi T, Maruoka K. Acc Chem Res. 2004;37:526–533. doi: 10.1021/ar030060k. [DOI] [PubMed] [Google Scholar]; i) Dolling UH, Davis P, Grabowski EJJ. J Am Chem Soc. 1984;106:446–447. [Google Scholar]
- 6.a) Lacour J, Moraleda D. Chem Commun. 2009:7073–7089. doi: 10.1039/b912530b. [DOI] [PubMed] [Google Scholar]; b) Adair G, Mukherjee S, List B. Aldrichimica Acta. 2008;41:31–39. [Google Scholar]
- 7.Zhang Z, Schreiner PR. Chem Soc Rev. 2009;38:1187–1198. doi: 10.1039/b801793j. [DOI] [PubMed] [Google Scholar]
- 8.For a recent review on chiral anions in asymmetric catalysis, see: Phipps RJ, Hamilton GL, Toste FD. Nature Chem. 2012;4:603–614. doi: 10.1038/nchem.1405.
- 9.O’Donnell MJ, Bennett WD, Wu S. J Am Chem Soc. 1989;111:2353–2355. [Google Scholar]
- 10.Rabinovitz M, Cohen Y, Halpern M. Angew Chem. 1986;98:958–968. [Google Scholar]; Angew Chem Int Ed. 1986;25:960–970. [Google Scholar]
- 11.Hughes DL, Dolling UH, Ryan KM, Schoenwaldt EF, Grabowski EJJ. J Org Chem. 1987;52:4745–4752. [Google Scholar]
- 12.Denmark SE, Weintraub RC. Heterocycles. 2011;82:1527–1540. [Google Scholar]
- 13.Corey EJ, Xu F, Noe MC. J Am Chem Soc. 1997;119:12414–12415. [Google Scholar]
- 14.Lygo B, Wainwright PG. Tetrahedron Lett. 1997;38:8595–8598. [Google Scholar]
- 15.Hofstetter C, Wilkinson PS, Pochapsky TC. J Org Chem. 1999;64:8794–8800. doi: 10.1021/jo990530h. [DOI] [PubMed] [Google Scholar]
- 16.Reetz MT. Angew Chem. 1988;100:1026–1030. [Google Scholar]; Angew Chem Int Ed. 1988;27:994–998. [Google Scholar]
- 17.a) Reetz MT, Hutte S, Goddard R. J Am Chem Soc. 1993;115:9339–9340. [Google Scholar]; b) Reetz MT, Hutte S, Goddard R, Robyr C. Chem Eur J. 1996;2:382–384. [Google Scholar]
- 18.Cannizzaro CE, Houk KN. J Am Chem Soc. 2002;124:7163–7169. doi: 10.1021/ja012417q. [DOI] [PubMed] [Google Scholar]
- 19.Lipkowitz KB, Cavanaugh MW, Baker B, O’Donnell MJ. J Org Chem. 1991;56:5181–5192. [Google Scholar]
- 20.For an alternative, qualitative model that invokes a perpendicular association of the enolate to the tetrahedron face, see ref. 13.
- 21.Jew S-s, Yoo M-S, Jeong B-S, Park YI, Park H-g. Org Lett. 2002;4:4245–4248. doi: 10.1021/ol0267679. [DOI] [PubMed] [Google Scholar]
- 22.Yoo M-S, Jeong B-S, Lee J-H, Park H-g, Jew S-s. Org Lett. 2005;7:1129–1131. doi: 10.1021/ol050123u. [DOI] [PubMed] [Google Scholar]
- 23.Thalladi VR, Weiss H, Blaeser D, Boese R, Nangia A, Desiraju GR. J Am Chem Soc. 1998;129:8702–8710. [Google Scholar]
- 24.Maruoka K, Ooi T, Kano T. Chem Commun. 2007:1487–1495. doi: 10.1039/b613049f. [DOI] [PubMed] [Google Scholar]
- 25.a) Ohshima T, Shibuguchi T, Fukuta Y, Shibasaki M. Tetrahedron. 2004;60:7743–7754. [Google Scholar]; b) Okada A, Shibuguchi T, Ohshima T, Masu H, Yamaguchi K, Shibasaki M. Angew Chem. 2005;117:4640–4643. doi: 10.1002/anie.200500927. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:4564–4567. doi: 10.1002/anie.200500927. [DOI] [PubMed] [Google Scholar]
- 26.Other binaphthyl-based chiral quaternary ammonium catalysts have also been developed, see: Ooi T, Uematsu Y, Kameda M, Maruoka K. Angew Chem. 2002;114:1621–1624. doi: 10.1002/1521-3773(20020503)41:9<1551::aid-anie1551>3.0.co;2-l.Angew Chem Int Ed. 2002;41:1551–1554. doi: 10.1002/1521-3773(20020503)41:9<1551::aid-anie1551>3.0.co;2-l.Kitamura M, Shirakawa S, Maruoka K. Angew Chem. 2005;117:1573–1575. doi: 10.1002/anie.200462257.Angew Chem Int Ed. 2005;44:1549–1551. doi: 10.1002/anie.200462257.
- 27.a) Shibuguchi T, Fukuta Y, Akachi Y, Sekine A, Ohshima T, Shibasaki M. Tetrahedron Lett. 2002;43:9539–9543. [Google Scholar]; b) Ooi T, Kameda M, Maruoka K. J Am Chem Soc. 1999;121:6519–6520. [Google Scholar]
- 28.Ooi T, Kameda M, Maruoka K. J Am Chem Soc. 2003;125:5139–5151. doi: 10.1021/ja021244h. [DOI] [PubMed] [Google Scholar]
- 29.Halpern J. Science. 1982;217:401–407. doi: 10.1126/science.217.4558.401. [DOI] [PubMed] [Google Scholar]
- 30.He R, Wang X, Hashimoto T, Maruoka K. Angew Chem. 2008;120:9608–9610. doi: 10.1002/anie.200804140. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:9466–9468. doi: 10.1002/anie.200804140. [DOI] [PubMed] [Google Scholar]
- 31.He R, Ding C, Maruoka K. Angew Chem. 2009;121:4629–4631. [Google Scholar]; Angew Chem Int Ed. 2009;48:4559–4561. doi: 10.1002/anie.200901277. [DOI] [PubMed] [Google Scholar]
- 32.Uraguchi D, Asai Y, Ooi T. Angew Chem. 2009;121:747–751. doi: 10.1002/anie.200803661. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:733–737. doi: 10.1002/anie.200803661. [DOI] [PubMed] [Google Scholar]
- 33.a) Uraguchi D, Ito T, Ooi T. J Am Chem Soc. 2009;131:3836–3837. doi: 10.1021/ja810043d. [DOI] [PubMed] [Google Scholar]; b) Uraguchi D, Sakaki S, Ooi T. J Am Chem Soc. 2007;129:12392–12393. doi: 10.1021/ja075152+. [DOI] [PubMed] [Google Scholar]; c) Uraguchi D, Ueki Y, Ooi T. J Am Chem Soc. 2008;130:14088–14089. doi: 10.1021/ja806311e. [DOI] [PubMed] [Google Scholar]; d) Uraguchi D, Ueki Y, Ooi T. Science. 2009;326:120–123. doi: 10.1126/science.1176758. [DOI] [PubMed] [Google Scholar]
- 34.For examples of other cationic phase-transfer catalysts that interact with anions primarily via H-bonding, see: Kita T, Georgieva A, Hashimoto Y, Nakata T, Nagasawa K. Angew Chem. 2002;114:2956–2958. doi: 10.1002/1521-3773(20020802)41:15<2832::AID-ANIE2832>3.0.CO;2-Q.Angew Chem Int Ed. 2002;41:2832–2834. doi: 10.1002/1521-3773(20020802)41:15<2832::AID-ANIE2832>3.0.CO;2-Q.Ohmatsu K, Kiyokawa M, Ooi T. J Am Chem Soc. 2011;133:1307–1309. doi: 10.1021/ja1102844.
- 35.For a comprehensive review of PTC by polyether catalysts, as well as other metal cation complexing agents such as TADDOL, NOBIN, and metal(salen) complexes, see ref. 5d and 5f
- 36.Cram DJ, Sogah GDY. J Chem Soc Chem Commun. 1981:625–628. [Google Scholar]
- 37.Akiyama T, Hara M, Fuchibe K, Sakamoto S, Yamaguchi K. Chem Commun. 2003:1734–1735. doi: 10.1039/b304649d. [DOI] [PubMed] [Google Scholar]
- 38.Yan H, Jang HB, Lee JW, Kim HK, Lee SW, Yang JW, Song CE. Angew Chem. 2010;122:9099–9101. doi: 10.1002/anie.201004777. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:8915–8917. doi: 10.1002/anie.201004777. [DOI] [PubMed] [Google Scholar]
- 39.Lee WJ, Yan H, Jang HB, Kim HK, Park SW, Lee S, Chi DY, Song CE. Angew Chem. 2009;121:7819–7822. doi: 10.1002/anie.200903903. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:7683–7686. doi: 10.1002/anie.200903903. [DOI] [PubMed] [Google Scholar]
- 40.Fleischmann M, Drettwan D, Sugiono E, Rueping M, Gschwind RM. Angew Chem. 2011;123:6488–6493. doi: 10.1002/anie.201101385. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:6364–6369. doi: 10.1002/anie.201101385. [DOI] [PubMed] [Google Scholar]
- 41.Llewellyn DB, Adamson D, Arndtsen BA. Org Lett. 2000;2:4165–4168. doi: 10.1021/ol000303y. [DOI] [PubMed] [Google Scholar]
- 42.Llewellyn DB, Arndtsen BA. Tetrahedron: Asymmetry. 2005;16:1789–1799. [Google Scholar]
- 43.Carter C, Fletcher S, Nelson A. Tetrahedron: Asymmetry. 2003;14:1995–2004. [Google Scholar]
- 44.Hamilton GL, Kanai T, Toste FD. J Am Chem Soc. 2008;130:14984–14986. doi: 10.1021/ja806431d. [DOI] [PubMed] [Google Scholar]
- 45.Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew Chem. 2004;116:1592–1594. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]
- 46.Uraguchi D, Terada M. J Am Chem Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- 47.Mayer S, List B. Angew Chem. 2006;118:4299–4301. [Google Scholar]; Angew Chem Int Ed. 2006;45:4193–4195. doi: 10.1002/anie.200600512. [DOI] [PubMed] [Google Scholar]
- 48.a) Yang JW, Fonseca MTH, Vignola N, List B. Angew Chem. 2005;117:110–112. [Google Scholar]; Angew Chem Int Ed. 2005;44:108–110. [Google Scholar]; b) Ouellet SG, Tuttle JB, MacMillan DWC. J Am Chem Soc. 2005;127:32–33. doi: 10.1021/ja043834g. [DOI] [PubMed] [Google Scholar]
- 49.Stadler M, List B. Synlett. 2008;4:597–599. [Google Scholar]
- 50.Simon L, Goodman JM. J Am Chem Soc. 2008;130:8741–8747. doi: 10.1021/ja800793t. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, List B. Angew Chem. 2008;120:1135–1138. [Google Scholar]; Angew Chem Int Ed. 2008;47:1119–1122. doi: 10.1002/anie.200704185. [DOI] [PubMed] [Google Scholar]
- 52.a) Marigo M, Franzen J, Poulsen TB, Zhuang W, Jorgensen KA. J Am Chem Soc. 2005;127:6964–6965. doi: 10.1021/ja051808s. [DOI] [PubMed] [Google Scholar]; b) Sparr C, Schweizer WB, Senn HM, Gilmour R. Angew Chem Int Ed. 2009;121:3111–3114. doi: 10.1002/anie.200900405. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:3065–3068. doi: 10.1002/anie.200900405. [DOI] [PubMed] [Google Scholar]
- 53.a) Martin NJA, List B. J Am Chem Soc. 2006;128:13368–13369. doi: 10.1021/ja065708d. [DOI] [PubMed] [Google Scholar]; b) Zhou J, List B. J Am Chem Soc. 2007;129:7498–7499. doi: 10.1021/ja072134j. [DOI] [PubMed] [Google Scholar]; c) Wang X, Reisinger CM, List B. J Am Chem Soc. 2008;130:6070–6071. doi: 10.1021/ja801181u. [DOI] [PubMed] [Google Scholar]
- 54.Lifchits O, Reisinger CM, List B. J Am Chem Soc. 2010;132:10227–10229. doi: 10.1021/ja1037935. [DOI] [PubMed] [Google Scholar]
- 55.Maryanoff BE, Zhang HC, Cohen JH, Turchi IJ, Maryanoff CA. Chem Rev. 2004;104:1431–1628. doi: 10.1021/cr0306182. [DOI] [PubMed] [Google Scholar]
- 56.Muratore ME, Holloway CA, Pilling AW, Storer RI, Trevitt G, Dixon DJ. J Am Chem Soc. 2009;131:10796–10797. doi: 10.1021/ja9024885. [DOI] [PubMed] [Google Scholar]
- 57.Holloway CA, Muratore ME, Storer RI, Dixon DJ. Org Lett. 2010;12:4720–4723. doi: 10.1021/ol101651t. [DOI] [PubMed] [Google Scholar]
- 58.Xie Y, Zhao Y, Qian B, Yang L, Xia C, Huang H. Angew Chem. 2011;123:5800–5804. doi: 10.1002/anie.201102046. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:5682–5686. doi: 10.1002/anie.201102046. [DOI] [PubMed] [Google Scholar]
- 59.Wanner MJ, van der Haas RNS, de Cuba KR, van Maarseveen JH, Hiemstra H. Angew Chem. 2007;119:7629–7631. doi: 10.1002/anie.200701808. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:7485–7487. doi: 10.1002/anie.200701808. [DOI] [PubMed] [Google Scholar]
- 60.Sewgobind NV, Wanner MJ, Ingemann S, de Gelder R, van Maarseveen JH, Hiemstra H. J Org Chem. 2008;73:6405–6408. doi: 10.1021/jo8010478. [DOI] [PubMed] [Google Scholar]
- 61.Wanner MJ, Boots RNA, Eradus B, de Gelder R, van Maarseveen JH, Hiemstra H. Org Lett. 2009;11:2579–2581. doi: 10.1021/ol900888e. [DOI] [PubMed] [Google Scholar]
- 62.Indolyl N-tosyl amines have also been used as carbocation precursors: Sun F-L, Zheng X-J, Gu Q, He Q-L, You S-L. Eur J Org Chem. 2010:47–50.
- 63.Sun FL, Zeng M, Gu Q, You SL. Chem Eur J. 2009;15:8709–8712. doi: 10.1002/chem.200901369. [DOI] [PubMed] [Google Scholar]
- 64.Guo QX, Peng YG, Zhang JW, Song L, Feng Z, Gong LZ. Org Lett. 2009;11:4620–4623. doi: 10.1021/ol901892s. [DOI] [PubMed] [Google Scholar]
- 65.Liang T, Zhang Z, Antilla JC. Angew Chem. 2010;122:9928–9930. doi: 10.1002/anie.201004778. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:9734–9736. doi: 10.1002/anie.201004778. [DOI] [PubMed] [Google Scholar]
- 66.Bergonzini G, Vera S, Melchiorre P. Angew Chem. 2010;122:9879–9882. doi: 10.1002/anie.201004761. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:9685–9688. doi: 10.1002/anie.201004761. [DOI] [PubMed] [Google Scholar]
- 67.Coric I, Vellalath S, List B. J Am Chem Soc. 2010;132:8536–8637. doi: 10.1021/ja102753d. [DOI] [PubMed] [Google Scholar]
- 68.Coric I, Muller S, List B. J Am Chem Soc. 2010;132:17370–17373. doi: 10.1021/ja108642s. [DOI] [PubMed] [Google Scholar]
- 69.Terada M, Tanaka H, Sorimachi K. J Am Chem Soc. 2009;131:3430–3431. doi: 10.1021/ja8090643. [DOI] [PubMed] [Google Scholar]
- 70.Zhang QW, Fan CA, Zhang HJ, Tu YQ, Zhao YM, Gu P, Chen ZM. Angew Chem. 2009;121:8724–8726. doi: 10.1002/anie.200904565. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:8572–8574. doi: 10.1002/anie.200904565. [DOI] [PubMed] [Google Scholar]
- 71.a) Takeuchi Y, Suzuki E, Shibata N. J Am Chem Soc. 2000;122:10728–10729. [Google Scholar]; b) Cahard D, Audouard C, Plaquevent JC, Roques N. Org Lett. 2000;2:3699–3701. doi: 10.1021/ol006610l. [DOI] [PubMed] [Google Scholar]; c) Ishimaru T, Shibata N, Horikawa T, Yasuda N, Nakamura S, Toru T, Shiro M. Angew Chem. 2008;120:4225–4229. doi: 10.1002/anie.200800717. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:4157–4161. doi: 10.1002/anie.200800717. [DOI] [PubMed] [Google Scholar]; d) Lozano O, Blessley G, del Campo TM, Thompson AL, Giuffredi GT, Bettati M, Walker M, Borman R, Gouverneur V. Angew Chem. 2011;123:8255–8259. doi: 10.1002/anie.201103151. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:8105–8109. doi: 10.1002/anie.201103151. [DOI] [PubMed] [Google Scholar]
- 72.Rauniyar V, Lackner AD, Hamilton GL, Toste FD. Science. 2011;334:1681–1684. doi: 10.1126/science.1213918. [DOI] [PubMed] [Google Scholar]
- 73.Phipps RJ, Hiramatsu K, Toste FD. J Am Chem Soc. 2012;134:8376–8379. doi: 10.1021/ja303959p.A phosphoric acid-catalyzed bromination of enecarbamates that may proceed via asymmetric ion-pairing catalysis was also reported: Alix A, Lalli C, Retailleau P, Masson G. J Am Chem Soc. 2012;134:10389–10392. doi: 10.1021/ja304095z.
- 74.Hennecke U, Muller CH, Frolich R. Org Lett. 2011;13:860–863. doi: 10.1021/ol1028805. [DOI] [PubMed] [Google Scholar]
- 75.Additional reports of chiral phosphoric acid-catalyzed bromocycloetherifications that may proceed via ion-pairing catalysis: Huang D, Wang H, Xue F, Guan H, Li L, Peng X, Shi Y. Org Lett. 2011;13:6350–6353. doi: 10.1021/ol202527g.Denmark SE, Burk MT. Org Lett. 2012;14:256–259. doi: 10.1021/ol203033k.
- 76.Hamilton GL, Kang EJ, Mba M, Toste FD. Science. 2007;317:496–499. doi: 10.1126/science.1145229. [DOI] [PubMed] [Google Scholar]
- 77.LaLonde RL, Wang ZJ, Mba M, Lackner AD, Toste FD. Angew Chem. 2010;122:608–611. doi: 10.1002/anie.200905000. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:596–601. [Google Scholar]
- 78.For a review on the effects of achiral counterions on chiral ionic transition-metal complexes, see: Macchioni A. Chem Rev. 2005;105:2039–2073. doi: 10.1021/cr0300439.
- 79.Examples of transition-metal catalysis with chiral phosphates: Alper H, Hamel N. J Am Chem Soc. 1990;112:2803–2804.Mukherjee S, List B. J Am Chem Soc. 2007;129:11336–11337. doi: 10.1021/ja074678r.Zhao B, Du H, Shi Y. J Org Chem. 2009;74:8392–8395. doi: 10.1021/jo901685c.Yazaki R, Kumagai N, Shibasaki M. J Am Chem Soc. 2010;132:10275–10277. doi: 10.1021/ja105141x.Liao S, List B. Angew Chem. 2010;122:638–641.Angew Chem Int Ed. 2010;49:628–631. doi: 10.1002/anie.200905332.Rauniyar V, Wang ZJ, Burks H, Toste EFD. J Am Chem Soc. 2011;133:8486–8489. doi: 10.1021/ja202959n.Jiang G, Halder R, Fang Y, List B. Angew Chem. 2011;123:9926–9929. doi: 10.1002/anie.201103843.Angew Chem Int Ed. 2011;50:9752–9755.Jiang G, List B. Angew Chem. 2011;123:9643–9646.Angew Chem Int Ed. 2011;50:9471–9474. doi: 10.1002/anie.201103263.Jiang G, List B. Chem Commun. 2011;47:10022–10024. doi: 10.1039/c1cc12499d.Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Science. 2012;336:324–327. doi: 10.1126/science.1219274.
- 80.Examples of cooperative transition metal and Bronsted-acid catalysis: Komanduri V, Krische MJ. J Am Chem Soc. 2006;128:16448–16449. doi: 10.1021/ja0673027.Rueping M, Antonchick AP, Brinkmann C. Angew Chem. 2007;119:7027–7030. doi: 10.1002/anie.200702439.Angew Chem Int Ed. 2007;46:6903–6906. doi: 10.1002/anie.200702439.Hu W, Xu X, Zhou J, Liu W-J, Huang H, Hu J, Yang L, Gong L-Z. J Am Chem Soc. 2008:130. doi: 10.1021/ja801755z.Li C, Wang C, Villa-Marcos B, Xiao J. J Am Chem Soc. 2008;130:14450–14451. doi: 10.1021/ja807188s.Li C, Villa-Marcos B, Xiao J. J Am Chem Soc. 2009;131:6967–6969. doi: 10.1021/ja9021683.
- 81.Yu Z, Jin W, Jiang Q. Angew Chem Int Ed. 2012;51:6060–6072. doi: 10.1002/anie.201200963. [DOI] [PubMed] [Google Scholar]
- 82.Rueping M, Koenigs RM, Atodiresei I. Chem Eur J. 2010;16:9350–9365. doi: 10.1002/chem.201001140. [DOI] [PubMed] [Google Scholar]
- 83.Meeuwissen J, Reek JNH. Nature Chem. 2010;2:615–621. doi: 10.1038/nchem.744. [DOI] [PubMed] [Google Scholar]
- 84.Ohmatsu K, Ito M, Kunieda T, Ooi T. Nature Chem. 2012;4:473–477. doi: 10.1038/nchem.1311. [DOI] [PubMed] [Google Scholar]
- 85.Nakashima D, Yamamoto H. J Am Chem Soc. 2006;128:9626–9627. doi: 10.1021/ja062508t. [DOI] [PubMed] [Google Scholar]
- 86.Rueping M, Nachtsheim BJ, Ieawsuwan W, Atodiresei I. Angew Chem. 2011;123:6838–6853. doi: 10.1002/anie.201100169. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:6706–6720. doi: 10.1002/anie.201100169. [DOI] [PubMed] [Google Scholar]
- 87.Christ P, Lindsay AG, Vormittag SS, Neudorfl JM, Berkessel A, O’Donoghue AC. Chem Eur J. 2011;17:8524–8528. doi: 10.1002/chem.201101157. [DOI] [PubMed] [Google Scholar]
- 88.Rueping M, Nachtsheim BJ, Moreth SA, Bolte M. Angew Chem. 2008;120:603–606. doi: 10.1002/anie.200703668. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:593–596. doi: 10.1002/anie.200703668. [DOI] [PubMed] [Google Scholar]
- 89.Rueping M, Nachtsheim BJ. Synlett. 2010:119–122. [Google Scholar]
- 90.Rueping M, Uria U, Lin MY, Atodiresei I. J Am Chem Soc. 2011;133:3732–3735. doi: 10.1021/ja110213t. [DOI] [PubMed] [Google Scholar]
- 91.Cheon CH, Yamamoto H. J Am Chem Soc. 2008;130:9246–9247. doi: 10.1021/ja8041542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Another example of asymmetric ion-pairing catalysis if desilylation is rate and ee-determining: Sickert M, Schneider C. Angew Chem. 2008;120:3687–3680. doi: 10.1002/anie.200800103.Angew Chem Int Ed. 2008;47:3631–3634. doi: 10.1002/anie.200800103.
- 93.Storer RI, Carrera DE, Ni Y, MacMillan DWC. J Am Chem Soc. 2006;128:84–86. doi: 10.1021/ja057222n. [DOI] [PubMed] [Google Scholar]
- 94.Sickert M, Abels F, Lang M, Sieler J, Birkemeyer C, Schneider C. Chem Eur J. 2010;16:2806–2818. doi: 10.1002/chem.200902537. [DOI] [PubMed] [Google Scholar]
- 95.Terada M. Chem Commun. 2008:4097–4112. doi: 10.1039/b807577h. [DOI] [PubMed] [Google Scholar]
- 96.a) Gridnev ID, Kouchi M, Sorimachi K, Terada M. Tetrahedron Lett. 2007;48:497–500. [Google Scholar]; b) Simon L, Goodman JM. J Org Chem. 2011;76:1775–1788. doi: 10.1021/jo102410r. [DOI] [PubMed] [Google Scholar]
- 97.Yamanaka M, Itoh J, Fuchibe K, Akiyama T. J Am Chem Soc. 2007;129:6756–6764. doi: 10.1021/ja0684803. [DOI] [PubMed] [Google Scholar]
- 98.a) Garcia-Garcia P, Lay F, Garcia-Garcia P, Rabalakos C, List B. Angew Chem. 2009;121:4427–4430. [Google Scholar]; Angew Chem Int Ed. 2009;48:4363–4366. doi: 10.1002/anie.200901768. [DOI] [PubMed] [Google Scholar]; b) Ratjen L, Garcia-Garcia P, Lay F, Beck ME, List B. Angew Chem. 2011;123:780–784. doi: 10.1002/anie.201005954. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:754–758. doi: 10.1002/anie.201005954. [DOI] [PubMed] [Google Scholar]
- 99.Coric I, List B. Nature. 2012;483:315–319. doi: 10.1038/nature10932. [DOI] [PubMed] [Google Scholar]
- 100.Liao S, Corey EJ, Qinggang W, List B. J Am Chem Soc. 2012;134:10765–10768. doi: 10.1021/ja3035637. [DOI] [PubMed] [Google Scholar]
- 101.a) Schmidtchen FP, Berger M. Chem Rev. 1997;97:1609–1646. doi: 10.1021/cr9603845. [DOI] [PubMed] [Google Scholar]; b) Bates GW, Light ME, Albrecht M, Gale PA. J Org Chem. 2007;72:8921–8927. doi: 10.1021/jo701702p. [DOI] [PubMed] [Google Scholar]
- 102.a) Rodriguez AA, Yoo H, Ziller JW, Shea KJ. Tetrahedron Lett. 2009;50:6830–6833. doi: 10.1016/j.tetlet.2009.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hay BP. Chem Soc Rev. 2010;39:3700–3708. doi: 10.1039/c0cs00075b. [DOI] [PubMed] [Google Scholar]
- 103.Kotke M, Schreiner PR. Tetrahedron. 2006;62:434–439. [Google Scholar]
- 104.Tantillo DJ, Houk KN. J Comput Chem. 2002;23:84–95. doi: 10.1002/jcc.10019. [DOI] [PubMed] [Google Scholar]
- 105.Kotke M, Schreiner PR. Synthesis. 2007:779–790. [Google Scholar]
- 106.a) Sigman MS, Jacobsen EN. J Am Chem Soc. 1998;120:4901–4902. [Google Scholar]; J Am Chem Soc [Google Scholar]; b) Sigman MS, Vachal P, Jacobsen EN. Angew Chem Int Ed. 2000;39:1279–1281. doi: 10.1002/(sici)1521-3773(20000403)39:7<1279::aid-anie1279>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed [Google Scholar]; c) Vachal P, Jacobsen EN. Org Lett. 2000;2:867–870. doi: 10.1021/ol005636+. [DOI] [PubMed] [Google Scholar]; Org Lett [Google Scholar]
- 107.Zuend SJ, Coughlin MP, Lalonde M, Jacobsen EN. Nature. 2008;461:968–971. doi: 10.1038/nature08484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zuend SJ, Jacobsen EN. J Am Chem Soc. 2009;131:15358–15374. doi: 10.1021/ja9058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan CS, List B. Chem Asian J. 2008;3:430–437. doi: 10.1002/asia.200700327. [DOI] [PubMed] [Google Scholar]
- 110.a) Amilan Jose D, Singh A, Das A, Ganguly B. Tetrahedron Lett. 2007;48:3695–3698. [Google Scholar]; b) Nishizawa S, Buhlmann P, Iwao M, Umezawa Y. Tetrahedron Lett. 1995;36:6483–6486. [Google Scholar]; c) Allerhand A, Schleyer PvR. J Am Chem Soc. 1963;85:1233–1237. [Google Scholar]
- 111.Stockigt J, Antonchick AP, Wu F, Waldmann H. Angew Chem. 2011;123:8692–8719. doi: 10.1002/anie.201008071. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:8538–8564. doi: 10.1002/anie.201008071. [DOI] [PubMed] [Google Scholar]
- 112.Taylor MS, Jacobsen EN. J Am Chem Soc. 2004;126:10558–10559. doi: 10.1021/ja046259p. [DOI] [PubMed] [Google Scholar]
- 113.Mergott DJ, Zuend SJ, Jacobsen EN. Org Lett. 2008;10:745–748. doi: 10.1021/ol702781q. [DOI] [PubMed] [Google Scholar]
- 114.Taylor MS, Tokunaga N, Jacobsen EN. Angew Chem. 2005;117:6858–6862. doi: 10.1002/anie.200502277. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:6700–6704. doi: 10.1002/anie.200502277. [DOI] [PubMed] [Google Scholar]
- 115.Raheem IT, Thiara PS, Peterson EA, Jacobsen EN. J Am Chem Soc. 2007;129:13404–13405. doi: 10.1021/ja076179w. [DOI] [PubMed] [Google Scholar]
- 116.Raheem IT, Thiara PS, Jacobsen EN. Org Lett. 2008;10:1577–1580. doi: 10.1021/ol800256j. [DOI] [PubMed] [Google Scholar]
- 117.Peterson EA, Jacobsen EN. Angew Chem. 2009;121:6446–6449. [Google Scholar]; Angew Chem Int Ed. 2009;48:6328–6331. doi: 10.1002/anie.200902420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamaoka Y, Miyabe H, Takemoto Y. J Am Chem Soc. 2007;129:6686–6687. doi: 10.1021/ja071470x. [DOI] [PubMed] [Google Scholar]
- 119.Knowles RR, Lin S, Jacobsen EN. J Am Chem Soc. 2010;132:5030–5032. doi: 10.1021/ja101256v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thoma R, Schulz-Gasch T, D’Arcy B, Benz J, Aebi J, Dehmlow H, Hennig M, Stihle M, Ruf A. Nature. 2004;432:118–122. doi: 10.1038/nature02993. [DOI] [PubMed] [Google Scholar]
- 121.Calderone CT, Williams DH. J Am Chem Soc. 2001;123:6262–6267. doi: 10.1021/ja003016y. [DOI] [PubMed] [Google Scholar]
- 122.Reisman SE, Doyle AG, Jacobsen EN. J Am Chem Soc. 2008;130:7198–7199. doi: 10.1021/ja801514m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brown AR, Kuo WH, Jacobsen EN. J Am Chem Soc. 2010;132:9286–9288. doi: 10.1021/ja103618r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Birrell JA, Desrosiers JN, Jacobsen EN. J Am Chem Soc. 2011;133:13872–13875. doi: 10.1021/ja205602j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kelly TR, Kim MH. J Am Chem Soc. 1994;116:7072–7080. [Google Scholar]
- 126.A nonstereoselective application of this principle: Weil T, Kotke M, Kleiner CM, Schreiner PR. Org Lett. 2008;10:1513–1516. doi: 10.1021/ol800149y.
- 127.Klausen RS, Jacobsen EN. Org Lett. 2009;11:887–890. doi: 10.1021/ol802887h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee Y, Klausen RS, Jacobsen EN. Org Lett. 2011;13:5564–5567. doi: 10.1021/ol202300t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klausen RS, Hyde AM, Jacobsen EN. unpublished results. [Google Scholar]
- 130.Seayad J, Seayad AM, List B. J Am Chem Soc. 2006;128:1086–1087. doi: 10.1021/ja057444l. [DOI] [PubMed] [Google Scholar]
- 131.Burns NZ, Witten MR, Jacobsen EN. J Am Chem Soc. 2011;133:14578–14581. doi: 10.1021/ja206997e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fu GC. Acc Chem Res. 2004;37:542–547. doi: 10.1021/ar030051b. [DOI] [PubMed] [Google Scholar]
- 133.a) De KC, Klauber EG, Seidel D. J Am Chem Soc. 2009;131:17060–17061. doi: 10.1021/ja9079435. [DOI] [PubMed] [Google Scholar]; b) Klauber EG, De KC, Shah TK, Seidel D. J Am Chem Soc. 2010;132:13624–13626. doi: 10.1021/ja105337h. [DOI] [PubMed] [Google Scholar]; c) Mittal N, Sun DX, Seidel D. Org Lett. 2012;14:3084–3087. doi: 10.1021/ol301155b. [DOI] [PubMed] [Google Scholar]
- 134.Klauber EG, Mittal N, Shah TK, Seidel D. Org Lett. 2011;13:2464–2467. doi: 10.1021/ol200712b. [DOI] [PubMed] [Google Scholar]
- 135.De KC, Seidel D. J Am Chem Soc. 2011;133:14538–14541. doi: 10.1021/ja2060462. [DOI] [PubMed] [Google Scholar]
- 136.De KC, Mittal N, Seidel D. J Am Chem Soc. 2011;133:16802–16805. doi: 10.1021/ja208156z. [DOI] [PubMed] [Google Scholar]
- 137.Veitch GE, Jacobsen EN. Angew Chem. 2010;122:7490–7493. doi: 10.1002/anie.201003681. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:7332–7335. doi: 10.1002/anie.201003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu H, Zuend SJ, Woll MG, Tao Y, Jacobsen EN. Science. 2010;327:986–990. doi: 10.1126/science.1182826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Knowles RR, Jacobsen EN. Proc Natl Acad Sci U S A. 2010;107:20678–20685. doi: 10.1073/pnas.1006402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.a) Pauling L. Chem Eng News. 1946;24:1377. [Google Scholar]; b) Jencks WP. Catalysis in Chemistry and Enzymology. McGraw Hill; New York: 1989. [Google Scholar]
- 141.Chudzinski MG, McClary CA, Taylor MS. J Am Chem Soc. 2012;133:10559–10567. doi: 10.1021/ja202096f. [DOI] [PubMed] [Google Scholar]
- 142.Houk KN, Cheong PHY. Nature. 2008;455:309–313. doi: 10.1038/nature07368. [DOI] [PMC free article] [PubMed] [Google Scholar]