Abstract
Background
For safe and efficacious treatment of hereditary angioedema, C1 esterase inhibitor (C1-INH) concentrates should have high purity and high amounts of functional protein. As no pharmacopoeia requirements exist for C1-INH concentrate lot release, biochemical characteristics as declared by the manufacturers may not be compared directly. This study compared the characteristics and purity profiles of four commercially available C1-INH concentrates.
Study Design and Methods
The analysis included one transgenic (Ruconest) and three plasma-derived (Berinert, Cetor, Cinryze) C1-INH concentrates. C1-INH antigen concentration was determined by nephelometry, total protein (specific activity) with a Bradford assay, purity by size-exclusion chromatography and gel electrophoresis, and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry was performed.
Results
Functionality (inversely proportional to antigen-to-activity ratio) was lowest for Ruconest (1.67), followed by Cetor (1.42), Berinert (1.24), and Cinryze (1.22). Specific activity (U/mg) and purity (%) were highest in Ruconest (12.13; 98.6) and Berinert (11.57; 97.0), followed by Cinryze (10.41; 89.5) and Cetor (9.01; 88.6). Main protein bands were found for all plasma-derived products at approximately 105 kDa, and for Ruconest, at approximately 98 kDa. Additional bands in the plasma-derived products were α1-antichymotrypsin, ceruloplasmin, Factor C3 (Cinryze/Cetor), and immunoglobulin heavy constant mu (Berinert).
Conclusion
Ruconest has a very high purity profile but is not identical to the human C1-INH protein. Of the plasma-derived products, Berinert has the highest purity profile. The impact of the nontherapeutic proteins identified has not yet been evaluated. For harmonization of the analysis for drug release, we recommend the establishment of regulatory requirements for purity determination and the implementation of threshold levels in C1-INH concentrates.
C1 esterase inhibitor (C1-INH) is a soluble single-chain glycoprotein with 478 amino acid residues and an apparent molecular weight of approximately 105 kDa. Approximately 50% of the total molecular mass results from posttranslational glycosylation of the protein.1 It is mainly produced in the parenchymal cells of the liver2 and is present in normal human plasma at concentrations of approximately 0.2 mg/mL, which is equivalent to 1 unit/mL plasma.3
Qualitative or quantitative deficiency of C1-INH is the fundamental cause of hereditary angioedema (HAE, Types I and II), a rare inherited disease that manifests as recurrent and potentially life-threatening episodes of swelling.2,3
Several treatment options for HAE, including attenuated androgens, kallikrein inhibitors, bradykinin receptor antagonists, and human C1-INH concentrates, have emerged over the past decades. Among these treatments, only C1-INH concentrates directly target the fundamental cause of HAE by replacing the missing or malfunctioning C1-INH protein. C1-INH products have been proven to effectively treat and prevent Type I and II HAE attacks4-8 and are recommended as a first-line treatment option for acute attacks or prophylactic therapy of HAE by several international guidelines.9-11
For the treatment of HAE attacks, four different C1-INH concentrates have been approved by European health authorities and/or the US Food and Drug Administration (FDA): the nanofiltered/virus filtered, pasteurized, human plasma–derived C1-INH concentrates Berinert (CSL Behring, Marburg, Germany), Cetor (Stichting Sanquin Bloedvoorziening, Amsterdam, the Netherlands), and Cinryze (ViroPharma Biologics, Inc., Exton, PA) and the transgenic C1-INH concentrate Ruconest (Pharming Group N.V., Leiden, the Netherlands).
To avoid adverse consequences for the recipients due to modification of the product during manufacturing, to minimize immunogenicity, and to provide rapid improvement of symptoms during an HAE attack, C1-INH concentrates should be native and have high purity profiles and high amounts of functional C1-INH protein. Current guidelines mandate thorough characterization of the final product.12 State-of-the-art methods should be used to ensure that the functional characteristics of the protein are maintained and that aggregated, degraded, or other modified forms are appropriately controlled.12 Due to a limited worldwide use of C1-INH products and the small number of manufacturers, currently no general pharmacopoeia requirements exist for C1-INH concentrates in Europe and the United States, and different manufacturers use a variety of different analytical methods for the biochemical characterization of purity of their products.
The first steps toward the establishment of general guidelines for the release of C1-INH concentrates and other plasma-derived products have been taken: In 2010, the Expert Committee on Biological Standardization of the World Health Organization established the first international standard for C1-INH for the calibration of the measurement of functional C1-INH in concentrates (08/256 for plasma-derived concentrates).13,14 For further harmonization of the quality of different C1-INH concentrates, members of the European Pharmacopoeia Expert Group 6B are currently in the process of developing a European monograph for C1-INH products, which is expected to be published in Pharmeuropa15,16 in the first quarter of 2014. In the US pharmacopoeia, no such guideline exists, nor are we aware of any attempts to implement one.
Because of the current lack of general guidelines for purity determination, biochemical characteristics of C1-INH products as declared by the manufacturers may not be compared directly and to date, no investigations have been carried out to compare the biochemical characteristics and purity profiles of different C1-INH concentrates. We therefore performed a series of analytical studies and applied standardized methods to analyze and directly compare the biochemical characteristics of the four aforementioned commercially available C1-INH concentrates. Key components included the functionality and purity of the products.
Materials and Methods
C1-INH concentrates: starting material and manufacturing process
The analysis included commercially available batches of three plasma-derived C1-INH concentrates (two batches of Berinert, two batches of Cinryze, one batch of Cetor) and of one transgenic C1-INH concentrate (two batches of Ruconest).
The lyophilized vials of the products were reconstituted with sterile water according to the manufacturers' instructions, resulting in different activity levels per milliliter of solution as declared (Table 1). The solutions were then stored as 0.5-mL aliquots at −20°C. Before analysis, the samples were thawed in a water bath at 37°C for 5 minutes.
Table 1.
Content of one vial of C1-INH concentrate and C1bINH activity according to declaration
| Content of one vial | Berinert | Cetor | Cinryze | Ruconest |
|---|---|---|---|---|
| Therapeutic component | ||||
| C1-INH, U | 500 | 500 | 500 | 2100 |
| Sterile water, mL | 10 | 5 | 5 | 14 |
| C1-INH concentration (activity), U/mL | 50 | 100 | 100 | 150 |
| Excipients | Glycine, sodium citrate sodium chloride | Sucrose, sodium citrate, sodium chloride, l-valine, l-alanine, l-threonine | Sucrose, sodium citrate, sodium chloride, l-valine, l-alanine, l-threonine | Sucrose, sodium citrate, citric acid |
Berinert
The active drug substance for Berinert is C1-INH from human plasma provided by CSL Plasma (operated by CSL Behring). The manufacturing process of Berinert includes virus reduction steps, prion reduction steps, and precipitation and chromatographic steps for the reduction of impurities (Fig. 1).17 Postpasteurization purification steps include ammonium sulfate precipitation and hydrophobic interaction chromatography.
Figure 1.
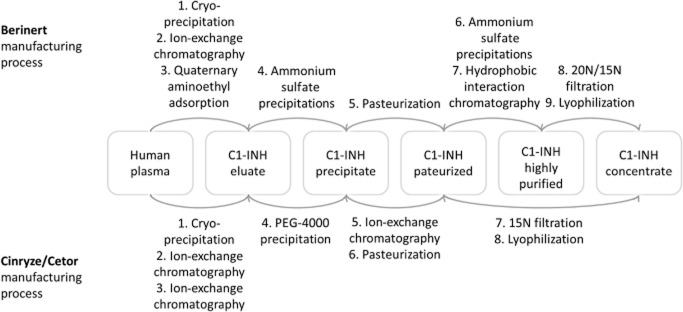
Purification schemes of human plasma-derived C1-INH concentrates.
Cetor and Cinryze
The active drug substance for Cetor and Cinryze is C1-INH from human plasma. Both products are manufactured by Sanquin Blood Supply Foundation. Sanquin also holds a marketing authorization for Cetor, whereas Cinryze is distributed by Viropharma. The manufacturing process is similar for Cetor and Cinryze and includes virus reduction steps, prion reduction steps, precipitation steps, and ion-exchange chromatography for the reduction of impurities (Fig. 1).18,19
Ruconest
The active substance of the transgenic product is an analogue of the human C1-INH protein (conestat alfa) that is purified from the milk of rabbits provided by Pharming. Ruconest is manufactured by Pharming and distributed by Swedish Orphan Biovitrum AB (SOBI, Stockholm, Sweden) in Europe. It is under review by the FDA for potential approval. The manufacturing process includes virus inactivation steps and chromatographic steps (cation-exchange, anion-exchange, and affinity chromatography) for reduction of impurities.20,21
Functionality of C1-INH protein
The functionality of C1-INH protein was determined by calculating the ratio of C1-INH antigen to C1-INH activity (according to declaration). The amount of C1-INH antigen was determined by nephelometry using a nephelometer (Beckman Coulter, Inc., Hialeah, FL) with a specific C1-INH antiserum (Siemens Healthcare Diagnostics GmbH, Marburg, Germany) and the antigen-antibody complex was optimized based on the Heidelberger-Kendall principle. The concentration evaluation was performed by using a calibration curve derived from N-Protein Standard PY (Siemens Healthcare Diagnostics GmbH).
Overall composition and purity of C1-INH concentrate
Total protein
The total amount of protein per vial was determined for each product with the Bradford method,22 using a Bio-Rad protein assay (Bio-Rad Laboratories GmbH, Munich, Germany). Samples were dyed with Coomassie brilliant blue G-250 (Serva Electrophoresis GmbH, Heidelberg, Germany), and the measurements were performed at 595 nm with bovine serum albumin (BSA; Thermo Fisher Scientific, Inc., Waltham, MA) as a standard. Each sample was analyzed at two evaluable dilutions.
Specific activity
The specific activity, as a measure of protein purity, was calculated as the ratio of C1-INH activity (according to declaration) to total protein amount (as determined by the Bradford method).
C1-INH protein and residual plasma proteins
Size-exclusion chromatography (SEC) was used to separate the molecules in the final C1-INH products and to determine the amount of high- and low-molecular-weight proteins (including potential C1-INH protein aggregates and fragments). For SEC, 20 μL of C1-INH concentrate, irrespective of the concentration, was applied to SEC columns (TSK G3000SWXL, GE Healthcare GmbH, Munich, Germany) using a buffer that contained NaCl and orthophosphoric acid with pH 7.2 for isocratic elution. One percent BSA solution served as a control before and after each test run.
For each product, the elution times and the areas under the curve (AUCs) of the molecular size peaks were calculated and compared. Purity was evaluated for all C1-INH concentrates based on the AUC of C1-INH protein relative to the total AUC by two different methods: 1) A method for purity calculation, developed by CSL Behring and established for Berinert, was used for all C1-INH concentrates (“standard method”). The standard method defines C1-INH purity as the AUC of the main peak in the SEC (with an elution time of the maximum peak height of C1-INH protein between 13.5 and 15 min) in relation to the total AUC. 2) The second method (“adapted method”) was also applied to all C1-INH samples and used a wider range of elution times for calculating the AUCs (13.5 to 17 min) to cover splitting of the main peaks in Cetor and Cinryze.
The amount of potential C1-INH protein aggregates and other high-molecular-weight proteins was evaluated from the AUC at an elution time of less than 13.5 minutes. The amount of C1-INH protein fragments and low-molecular-weight proteins was evaluated from the AUC at an elution time of more than 17.0 minutes.
For further identification of therapeutic and nontherapeutic proteins, a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli,23 using 8% to 16% gradient precast polyacrylamide gels (Invitrogen, Life Technologies, Darmstadt, Germany) under reduced and nonreduced conditions. Each sample was diluted with 0.9% NaCl solution, adjusting the total amount of protein to a final concentration of 5 mOD/lane. A prestained molecular weight marker (Invitrogen) was run in parallel. After electrophoresis, the gel was stained with Coomassie brilliant blue G-250.
Protein mass
For determination of the absolute masses of therapeutic and nontherapeutic proteins with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF; 4800 Proteomics Analyzer, AB Sciex Pvt. Ltd., Concord, Ontario, Canada), the dried gels were rehydrated and the main bands were cut and reduced with dithiothreitol, alkylated with iodoacetamide, and digested with trypsin. N-terminal sequencing of the proteins was done by protein mass fingerprinting (PMF), comparing the peak list produced by MALDI-TOF to the ProFound database.24
Results
Functionality of C1-INH protein
The antigen-to-biologic activity ratio for C1-INH was similar for Berinert, Cinryze, and Cetor and slightly higher for Ruconest (Table 2), indicating a slightly higher amount of functional protein in the plasma-derived C1-INH concentrates than in the transgenic product.
Table 2.
Functionality and purity of C1-INH protein concentrates
| Content | Berinert | Cetor | Cinryze | Ruconest |
|---|---|---|---|---|
| Total protein, mean (mg/500 U) | 43.2 | 55.5 | 48.1 | 41.2 |
| Batch 1 | 44.6 | 55.5 | 49.3 | 40.2 |
| Batch 2 | 41.8 | NA | 46.8 | 42.2 |
| Specific activity, mean (U/mg) | 11.6 | 9.0 | 10.4 | 12.1 |
| Batch 1 | 11.2 | 9.0 | 10.2 | 12.4 |
| Batch 2 | 12.0 | NA | 10.7 | 11.8 |
| C1-INH antigen, mean (mg/mL) | 13.6 | 31.3 | 26.9 | 55.0 |
| Batch 1 | 13.1 | 31.3 | 25.9 | 54.3 |
| Batch 2 | 14.1 | NA | 27.8 | 55.8 |
| Ratio antigen to activity,* mean | 1.24 | 1.42 | 1.22 | 1.67 |
| Batch 1 | 1.19 | 1.42 | 1.18 | 1.64 |
| Batch 2 | 1.28 | NA | 1.26 | 1.69 |
C1-INH activity converted from U/mL to mg/mL, 1 U corresponding to 220 μg.
Overall composition and purity of C1-INH concentrate
Total protein and specific activity
The mean amount of total protein in 500 U was lowest for Ruconest and Berinert, followed by Cinryze and Cetor (Table 2). The specific activity, as an indicator of purity, was highest for Ruconest and Berinert followed by Cinryze and Cetor (Table 2).
C1-INH protein and residual plasma proteins
Regardless of what method (standard method or adapted method) was applied, purity determined with SEC was highest for Ruconest and Berinert, followed by Cinryze and Cetor (Fig. 2, Table 3).
Figure 2.
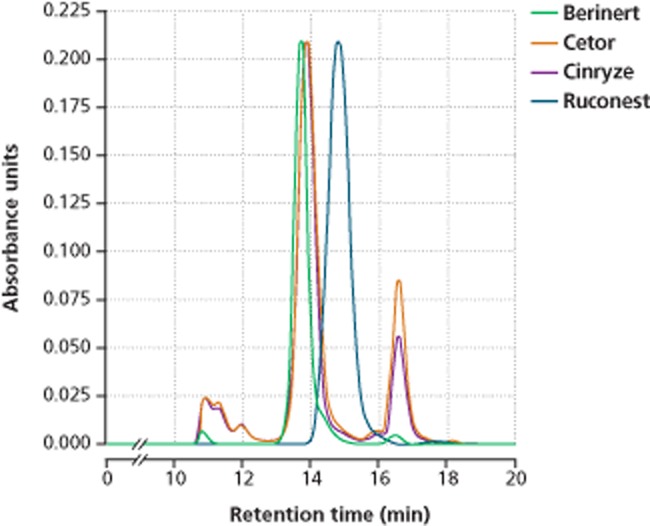
SEC determination of purity of C1-INH concentrates. Results for one batch of each product are shown.
Table 3.
Purity evaluation of C1-INH concentrates
| Content | Purity defined as AUC (% of total AUC) | |||
|---|---|---|---|---|
| Berinert | Cetor | Cinryze | Ruconest | |
| High-molecular-weight proteins, mean | 2.8 | 11.0 | 10.2 | 0.6 |
| Batch 1 | 2.8 | 11.0 | 12.2 | 0.6 |
| Batch 2 | 2.8 | NA | 8.2 | 0.6 |
| C1-INH protein, mean (standard method)* | 94.7 | 67.4 | 69.6 | 98.6 |
| Batch 1 | 94.6 | 67.4 | 71.3 | 98.6 |
| Batch 2 | 94.9 | NA | 68.0 | 98.6 |
| C1-INH protein, mean (adapted method)† | 97.0 | 88.6 | 89.5 | 98.6 |
| Batch 1 | 97.0 | 88.6 | 87.6 | 98.6 |
| Batch 2 | 97.0 | NA | 91.5 | 98.6 |
| Low-molecular-weight proteins, mean | 0.2 | 0.3 | 0.3 | 0.8 |
| Batch 1 | 0.2 | 0.3 | 0.2 | 0.8 |
| Batch 2 | 0.2 | NA | 0.3 | 0.8 |
Amount of C1-INH protein evaluated with the standard Berinert method at an elution time of 13.5 to 15.0 minutes.
Amount of C1-INH protein evaluated with the adapted Berinert method to cover peak split in Cetor and Cinryze at an elution time of 13.5 to 17.0 minutes.
SEC revealed the lowest amount of C1-INH protein aggregates and other high-molecular-weight proteins for Ruconest and fewer high-molecular-weight proteins for Berinert than for Cetor and Cinryze (Table 3). The amount of fragments and other low-molecular-weight proteins was similar for the plasma-derived products but slightly elevated for Ruconest (Table 3). While the main peak for C1-INH protein in Berinert, Cetor, and Cinryze had an elution time of approximately 13.5 to 14.0 minutes, for Ruconest, a shift in elution time to approximately 15.0 minutes was observed. In Cinryze and Cetor, peak splitting occurred with an elution time of approximately 17.0 minutes for the second peak (Fig. 2).
Protein mass
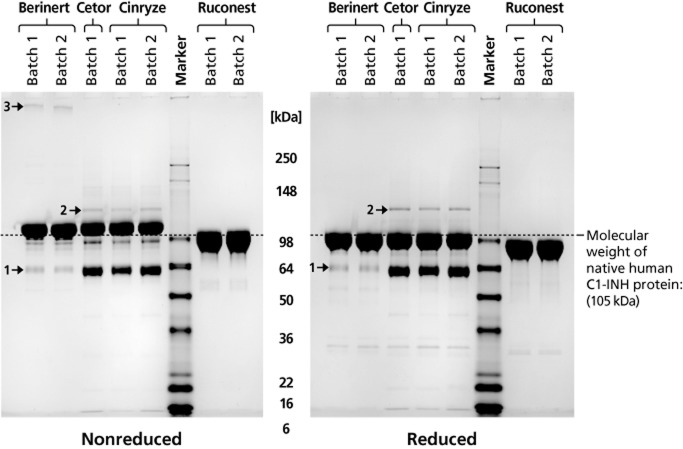
SDS-PAGE revealed main protein bands for all plasma-derived C1-INH concentrates at approximately 105 kDa and for the transgenic product at approximately 98 kDa (Fig. 3). Additional band patterns were identical within the batches of a single product but differed among products. Among the plasma-derived C1-INH preparations, a band was found at approximately 64 kDa, which was less prominent for Berinert than for Cinryze and Cetor and was identified as α1-antichymotrypsin (Fig. 3, 1) by PMF analysis. Other nontherapeutic proteins included complement factor C3 in Cinryze and Cetor, immunoglobulin heavy constant mu in Berinert, and ceruloplasmin in all plasma-derived C1-INH concentrate samples (Fig. 3, 2). No coagulation factors were detected in any of the samples. The PMF confirmed that all bands detected in Ruconest were C1-INH protein or fragments of C1-INH.
Figure 3.
Composition and purity of C1-INH concentrates determined by protein gel electrophoresis (8%-16% Tris-glycine, 5 mOD/lane). OD = optical density. 1 → α1-Antichymotrypsin; 2 → ceruloplasmin; 3 → immunoglobulin heavy constant mu.
Discussion
To address the lack of comparative analyses of the biochemical characteristics of different C1-INH concentrates, we have analyzed the purity profiles of three plasma-derived and one transgenic C1-INH preparation with two different methods, standardized for all four products. Additionally, the amount of functional protein, the amount of protein aggregation and fragmentation, and the specific activity of C1-INH proteins were determined.
While the three plasma-derived C1-INH concentrates Berinert, Cinryze, and Cetor have a higher amount of functional protein (indicated by a low antigen-to-activity ratio) than the transgenic product, the three-step purification process of Ruconest, nevertheless, yields a highly functional protein preparation with very high purity. Yet, it must be borne in mind that the product is not identical to the human C1-INH protein. SDS-PAGE and SEC revealed a lower molecular weight and a higher elution time for the C1-INH protein in Ruconest compared with the plasma-derived products, which are likely to result from different glycosylation patterns.25 The decreased half-life of Ruconest resulting from lower glycosylation might raise concern for relapses of acute HAE attacks.3,25 This study did not involve the analysis of carbohydrate side chains and glycosylation and their potential effect on the biologic half-life of the proteins was not evaluated.
During the manufacturing process of the plasma-derived products, elaborate techniques are applied to reduce impurities to a very low level of no concern.17,18 We have, however, identified small amounts of nontherapeutic proteins in the analyzed samples. The main sources of contaminating plasma protein in all plasma-derived C1-INH concentrates were α1-antichymotrypsin, an α-globulin glycoprotein that, like C1-INH, belongs to the serpin family and ceruloplasmin. Both are, just like C1-INH, acute-phase proteins that are produced in the liver. The potential impact of these and other nontherapeutic proteins on the efficacy and safety of the products has not been evaluated. Since it is known from other plasma-derived products that protein aggregation and degradation are often accompanied by reduced activity and can induce immunogenicity, the native conformation of the C1-INH protein in therapeutic preparations should be ensured.3 Additionally, high product purity can help avoid side effects that can be caused by copurifying plasma proteins.3
Variations in purity among the three different plasma products are attributed to the application of different purification methods. As shown in Fig. 1, the manufacturing process of Berinert, unlike that of Cinryze and Cetor, includes two additional purification steps subsequent to pasteurization: ammonium sulfate precipitation and hydrophobic interaction chromatography. These steps allow depletion of aggregates and fragments that might develop during pasteurization.
A potential limitation of this study is the small number of batches that were analyzed for each product. Given that before marketing authorization of plasma-derived products, it is a legal requirement for manufacturers to ensure and demonstrate batch-to-batch consistency12 and provided that manufacturers comply with this regulation, the interbatch variation of a given product can be assumed to be smaller compared with the interproduct differences observed in this study. We therefore believe that, despite the limited number of batches analyzed, our data provide valuable insight in the biochemical properties of C1-INH preparations and give evidence on differences between the products in terms of purity.
In conclusion, Ruconest, as a transgenic preparation, and Berinert, which is plasma derived, have the highest purity profile of the available products, containing fewer nontherapeutic proteins than Cetor and Cinryze. In general, the amount of nontherapeutic proteins and C1-INH protein aggregates and fragments should be minimized in a C1-INH preparation to reduce immunogenicity and avoid possible side effects.
Recommendation
To date, no published pharmacopoeia requirements exist for the release of C1-INH concentrates and other plasma-derived products. To harmonize the analysis for drug release testing, we recommend the strict adherence to existing international standards and the establishment of regulatory requirements for the determination of purity of C1-INH products by SEC. Additionally, we recommend the implementation of purity threshold levels for the amount of nontherapeutic protein and C1-INH protein aggregation in C1-INH concentrates. It is expected that our recommendation will be taken into consideration during the development of the European C1-INH monograph that is currently in progress. A similar guideline as part of the US pharmacopoeia could help to further harmonize the quality of different C1-INH products.
Acknowledgments
We thank Sven Karschnia, Volker Blanke, Erwin Boland, and Johanna Ochs (CSL Behring GmbH, Germany) for their technical assistance and support in the conduct of the study. Furthermore, we thank TOPLAB GmbH for MALDI-TOF analysis and Eva Kestner (Trilogy Writing & Consulting GmbH, Germany) for medical writing services on behalf of CSL Behring GmbH.
Glossary
- AUC
area under the curve
- C1-INH
C1 esterase inhibitor
- HAE
hereditary angioedema
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- PMF
protein mass fingerprinting
- SEC
size-exclusion chromatography
Conflict of Interest
All authors are employees of CSL Behring, Marburg, Germany.
References
- 1.Bock SC, Skriver K, Nielsen E, et al. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry. 1986;25:4292–4301. doi: 10.1021/bi00363a018. [DOI] [PubMed] [Google Scholar]
- 2.Weiler CR, Van Dellen RG. Genetic test indications and interpretations in patients with hereditary angioedema. Mayo Clin Proc. 2006;81:958–972. doi: 10.4065/81.7.958. [DOI] [PubMed] [Google Scholar]
- 3.Over J, Kramer C, Koenderman A. C1-inhibitor. In: Bertolini J, Goss N, Curling J, et al., editors. Production of plasma proteins for therapeutic use. Hoboken (NJ): John Wiley & Sons; 2013. pp. 241–258. [Google Scholar]
- 4.Craig TJ, Levy RJ, Wasserman RL, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009;124:801–808. doi: 10.1016/j.jaci.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Craig TJ, Bewtra AK, Bahna SL, et al. C1 esterase inhibitor concentrate in 1085 hereditary angioedema attacks—final results of the I.M.P.A.C.T.2 study. Allergy. 2011;66:1604–1611. doi: 10.1111/j.1398-9995.2011.02702.x. [DOI] [PubMed] [Google Scholar]
- 6.Zuraw B, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363:513–522. doi: 10.1056/NEJMoa0805538. [DOI] [PubMed] [Google Scholar]
- 7.Riedl MA, Hurewitz DS, Levy R, et al. Nanofiltered C1 esterase inhibitor (human) for the treatment of acute attacks of hereditary angioedema: an open-label trial. Ann Allergy Asthma Immunol. 2012;108:49–53. doi: 10.1016/j.anai.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Zuraw B, Cicardi M, Levy RJ, et al. Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol. 2010;126:821–827. doi: 10.1016/j.jaci.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Bowen T, Cicardi M, Farkas H, et al. 2010 International consensus algorithm for the diagnosis, therapy, and management of hereditary angioedema. Allergy Asthma Clin Immunol. 2010;6:24–36. doi: 10.1186/1710-1492-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig T, Ayören-Pürsün EA, Bork K, et al. WAO guideline for the management of hereditary angioedema. World Allergy Organ J. 2012;5:182–199. doi: 10.1097/WOX.0b013e318279affa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahn V, Aberer W, Eberl W, et al. Hereditary angioedema (HAE) in children and adolescents—a consensus on therapeutic strategies. Eur J Pediatr. 2012;171:1339–1348. doi: 10.1007/s00431-012-1726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Medicines Agency. Guideline on plasma-derived medicinal products. 2011. 21 July [cited 2104 Apr 16]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/07/WC500109627.pdf.
- 13.World Health Organization. WHO 1st International Standard for C1-inhibitor, concentrate. 2010. NIBSC code: 08/256. [cited 2014 Feb 18]. Available from: http://www.nibsc.org/documents/ifu/08-256.pdf.
- 14.Thelwell C, Rigsby P, Longstaff C. An international collaborative study to establish the WHO 1st international standards for C1 inhibitor, plasma and concentrates. J Thromb Haemost. 2011;9:2097–2099. doi: 10.1111/j.1538-7836.2011.04455.x. [DOI] [PubMed] [Google Scholar]
- 15.European Directorate for the Quality of Medicines & Health Cares. European Pharmacopoia. Technical guide for the elaboration and use of monographs on human plasma-derived products [cited 2013 Nov 25] 2011. Available from: https://www.edqm.eu/site/NEW_Technical_Guide_for_the_elaboration_and_use_ofpdf-en-30577-2.html.
- 16.European Directorate for the Quality of Medicines & Health Cares. Pharmeuropa, Pharmeuropa Bio, and Scientific notes. 2014. 8th [cited 2014 Apr 16]. Available from: http://online.edqm.eu/EN/entry.htm.
- 17.Gröner A, Nowak T, Schäfer W. Pathogen safety of human C1 esterase inhibitor concentrate. Transfusion. 2012;52:2104–2112. doi: 10.1111/j.1537-2995.2012.03590.x. [DOI] [PubMed] [Google Scholar]
- 18.Terpstra FG, Kleijn M, Koenderman AH, et al. Viral safety of C1-inhibitor NF. Biologicals. 2007;35:173–181. doi: 10.1016/j.biologicals.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Cinryze CHMP assessment report. 2011. July [cited 2013 Nov 25]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001207/WC500108898.pdf.
- 20.European Medicines Agency. Ruconest CHMP assessment report. 2010. 24 June [cited 2013 Nov 25]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001223/WC500098546.pdf.
- 21.van Veen HA, Koiter J, Vogelezang CJ, et al. Characterization of recombinant human C1 inhibitor secreted in milk of transgenic rabbits. J Biotechnol. 2012;162:319–326. doi: 10.1016/j.jbiotec.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 23.Lämmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Chait BT. ProFound: an expert system for protein identification using mass spectrometric peptide mapping information. Anal Chem. 72:2482–2489. doi: 10.1021/ac991363o. [DOI] [PubMed] [Google Scholar]
- van Doorn MB, Burggraaf J, van Dam T, et al. A phase I study of recombinant human C1 inhibitor in asymptomatic patients with hereditary angioedema. J Allergy Clin Immunol. 2005;116:876–883. doi: 10.1016/j.jaci.2005.05.019. [DOI] [PubMed] [Google Scholar]