Abstract
Ozonolysis of allyl-functional polycarbonates provides aldehyde-functional polycarbonates that have potential to be reactive platforms for transformation into diverse active materials.
Aldehydes are among the most reactive functional groups under mild reaction conditions, which can allow for entry to the construction of a diverse range of functional dynamic and/or static polymer materials. Aldehydes undergo a wide variety of chemical reactions with nucleophiles, resulting in both reversible and irreversible bonds.1 Among them, condensation reactions with nitrogen-containing nucleophiles such as hydrazines or alkoxyamines are classified as “click” reactions, due to the facile formation of stable hydrazones or oximes.2 Also, the condensation of aldehyde with primary amines yields imine derivatives (Schiff bases) and imine reduction provides stable amino bonds. Efforts to develop polymers bearing pendant aldehyde functionalities have been made since the 1950s. Conventional radical polymerization of aldehyde-bearing monomers yielded polymers with uncontrolled molecular weight and dispersity.3 Anionic polymerization was employed to produce well-defined aldehyde-functional polymers.4 However, the rigorous polymerization conditions required for controlled anionic polymerization limited their applications. Since the development of controlled radical polymerizations (CRPs), a few research groups have studied the synthesis and application of aldehyde-functional polymers. Maynard and coworkers prepared poly(3,3′-diethoxypropyl methacrylate) (pDEPMA) by atom transfer radical polymerization (ATRP) and reversible addition–fragmentation chain transfer (RAFT) polymerization.5 Aldehydes were produced by hydrolysis of the acetals and were then conjugated with aminooxy- or hydrazine-functionalized compounds including peptides and dyes via oxime or hydrazone linkages, respectively. Aldehyde-functional polymers were also synthesized from unprotected monomers by RAFT polymerization6 and ring-opening metathesis polymerization (ROMP).7
Although several examples of polymers having aldehyde side chain groups have been reported, most are comprised of non-degradable backbones. While this work was in progress, Hedrick, Yang and coworkers reported the synthesis of poly-(ethylene oxide)-b-polycarbonates (PEO-b-PCs) with pendant aldehyde groups via organocatalytic ring-opening polymerization (ROP) of aldehyde-functional cyclic carbonate monomers using PEO as a macroinitiator.8 Aliphatic PCs are remarkable candidates for biomedical applications on account of their biodegradability, biocompatibility, and low toxicity in vivo.9 In particular, various pendant functionalities can be introduced on the PC backbones by ROP of functionalized cyclic carbonate monomers.9b,10 These well-established synthetic methods for functional PCs expand potential applications of these types of materials.
We built a different strategy to prepare aldehyde-functional PCs. Our strategy involved the introduction of aldehyde functionalities via post-polymerization modification of functional PCs. Among many synthetic pathways to aldehydes, ozonolysis of alkenes is one of the most intriguing methods to attempt, due to: (1) aldehyde groups can be readily introduced by selective cleavage of alkenyl groups using ozone together with a reducing agent; (2) the initial alkenes can instead be utilized for other post-polymerization modifications, such as by thiol–ene reaction, epoxidation, halogenation, hydroboration, etc.; (3) the alkene precursors and resulting aldehydes are orthogonal functional groups for further conjugation reactions. In this study, we prepared allyl-functional PCs by ROP of a functionalized cyclic carbonate monomer using 1,8-diazabicyclo [5.4.0]-undec-7-ene (DBU) in order to develop a readily accessible synthetic method for versatile allyl-functional PCs. Aldehyde-functionalities were introduced by ozonolysis of the pendant alkene groups. The resulting aldehydes were conveniently functionalized with aminooxy-containing compounds via aldehyde–aminooxy “click” reactions. Furthermore, PC-based statistical copolymers bearing alkene and aldehyde functionalities were synthesized by partial ozonolysis and functionalized by stepwise aldehyde–aminooxy and thiol–ene “click” reactions in an orthogonal fashion.
Allyl-functional PCs [poly(5-methyl-5-allyloxycarbonyl-1,3-dioxan-2-one), PMAC] were synthesized by organocatalytic ROP of the functionalized cyclic carbonate monomer, 5-methyl-5-allyloxycarbonyl-1,3-dioxan-2-one (MAC). MAC was synthesized by a simple two-step procedure, as reported previously.11 Dove reported the ROP of MAC using the dual catalyst system of (−)-sparteine in combination with a thiourea.12 However, the limited availability of (−)-sparteine could hinder the further application of these conditions. Thus, our focus turned to DBU as a catalyst (Scheme S1†). In Dove's report, polymerizations using DBU as a catalyst were retarded beyond ca. 70% monomer conversion, and led to isolated polymers having broad molecular weight distributions and bimodal GPC traces. In this study, polymerizations were carried out under more dilute conditions ([MAC]0 = 0.5 M in this study vs. 2 M as reported), in order to provide enhanced control, which was expected to occur by maintaining uniform solubility throughout the polymerization while also inhibiting transesterification reactions. Investigation of the living characteristics of the polymerization was performed in dichloromethane (DCM) at ambient temperature in a glovebox (29 °C) with [M]0/[I]0 = 50. Application of DBU revealed a linear correlation between the number-average molecular weight (Mn) against monomer conversion until ca. 80% monomer conversion, at which point the ROPs became sluggish. However, minimal transesterification was observed until 2 h after polymerization was suspended (Fig. S1†). After the polymerizations were quenched by addition of benzoic acid in DCM, residual monomer and catalyst were removed by column chromatography using silica gel to yield the purified polymers (Fig. S2†). Both the molecular weight and molecular weight distribution could be manipulated by polymer fractionation using column chromatography (Fig. S3†). Furthermore, chain extension experiments after complete monomer conversion or retardation of polymerization showed that the catalyst and chain end remained active for ring opening of monomers for growth of a second block (Table S1†).
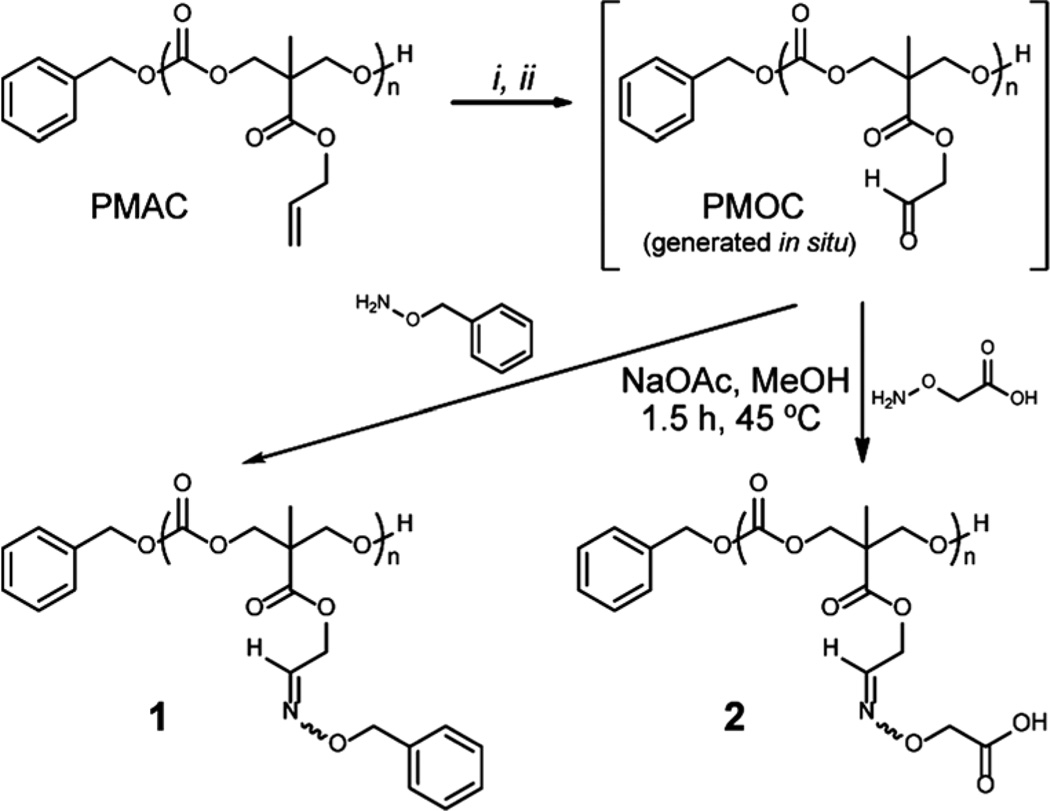
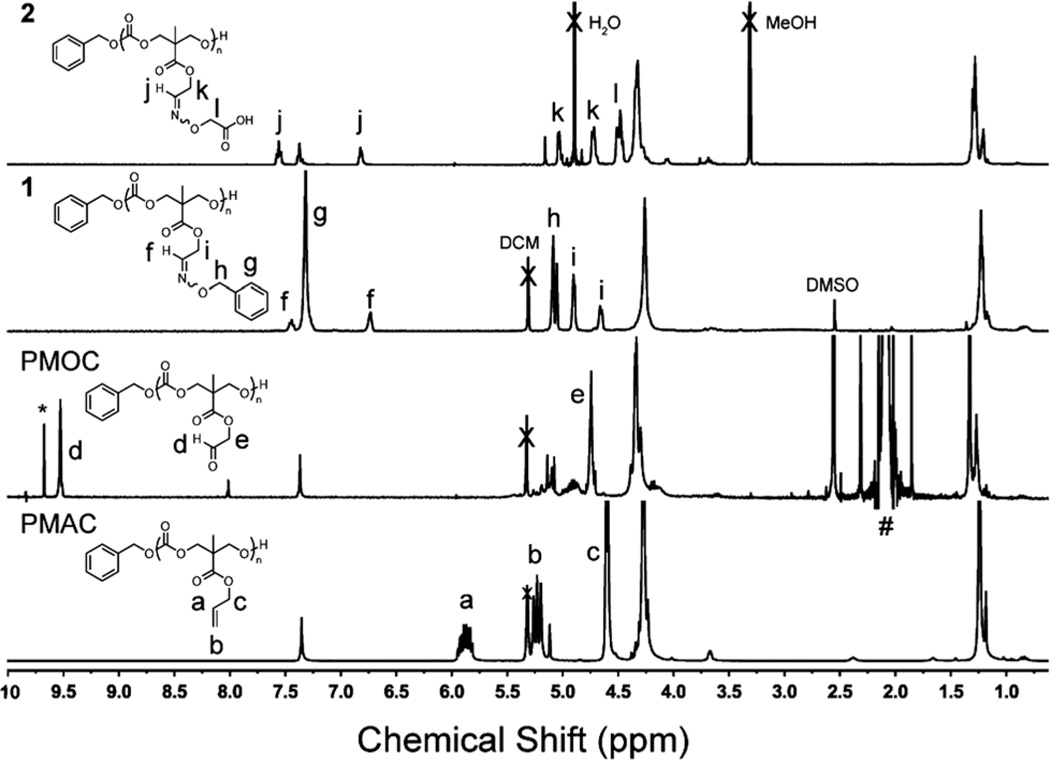
Aldehyde-functional PCs [poly(5-methyl-5-oxoethyloxycarbonyl-1,3-dioxan-2-one), PMOC] were prepared by ozonolysis of allyl-functional polymer precursors and their reductive work-up (Scheme 1). Ozone serves as “molecular scissors”, cutting double bonds in molecules,13 at specific sites to produce new functional groups. We hypothesized that the PC backbones would remain intact under ozonolysis conditions. Initial ozonolysis studies were conducted in CD2Cl2 (20 mg of PMAC15 in 2 mL of CD2Cl2) at −78 °C. Ozone was delivered at a rate of 100 mg h−1 as a mixture of column-dried compressed air (2 L min−1) from a Red Sea AquaZone 100 ozone generator. For unknown reasons, the characteristic blue color of the unreacted ozone in both DCM and methanol (MeOH) was difficult to be recognized, even in the solvents themselves. In order to determine the time to halt the exposure to ozone, aliquots of the reaction mixture were collected, dried in vacuo, and analyzed by 1H NMR spectroscopy to monitor the disappearance of alkene proton resonances (a and b in Fig. 1) after the allotted period of time (1, 3, 5, 7, 9 or 11 min). The cleavage of alkene double bonds was complete within 9 min (Fig. S4†) and reductive workup provided aldehyde groups (d in Fig. 1). The resulting polymer was isolated by precipitation into cold diethyl ether. Unfortunately, after full conversion to PMOC, the isolated polymers had limited solubility in common organic solvents, except tri-fluoroacetic acid (TFA). Inter- and intra-molecular hydrogen bonding and dipole–dipole interactions of pendant aldehydes might contribute to the observed solubility challenges.5b,7 As a result, further reactions with polymers bearing aldehyde groups were performed by the in situ generation of the aldehyde functionalities and reaction directly without an initial purification. Under ideal conditions, ozonolysis and reductive work-up with dimethyl sulfide (Me2S) give only dimethyl sulfoxide (DMSO) and volatile formaldehyde as the by-products, neither of which would interfere with the subsequent reactions with the aldehydes.
Scheme 1.
Ozonolysis of PMAC and functionalization by aldehyde–aminooxy “click” reaction. Conditions: (i) O3, DCM, −78 °C; (ii) Me2S, −78 °C to RT.
Fig. 1.
1H NMR spectra (300 MHz) of PMAC (in DCM), PMOC (generated in situ, in DCM), and conjugates after functionalization via aldehyde–aminooxy “click” reactions with O-benzylhydroxylamine (conjugate 1, in DCM) and O-(carboxymethyl)hydroxylamine (conjugate 2, in MeOD). *: formaldehyde, #: Me2S.
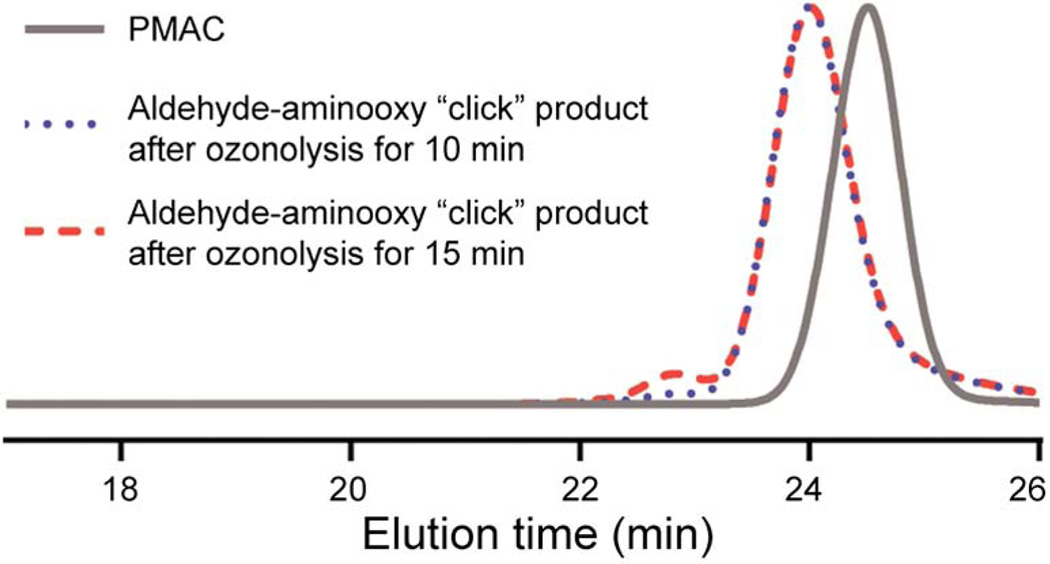
The availability of in situ-generated aldehyde groups for further functionalization was confirmed by aldehyde–aminooxy “click” reactions. We chose two model compounds to study formation of oxime bonds: O-benzylhydroxylamine as a model hydrophobic compound and O-(carboxymethyl)hydroxylamine as a model hydrophilic compound (Scheme 1). In particular, the latter can be useful for applications, in terms of conjugation with various compounds having alcohol or amine functionalities, such as peptides, drugs, etc. The desired aminooxy compound and sodium acetate (NaOAc) in MeOH were added to the in situ-generated PMOC in DCM and stirred at 45 °C for 1.5 h. The resulting polymers were purified by precipitation into water for hydrophobic conjugate 1 and dialysis against water (or centrifugal filtration) for hydrophilic conjugate 2. Formation of conjugates was verified by monitoring disappearance of aldehyde resonances (d) of PMOC and appearance of the trans and cis protons (f and j) of the oxime bonds using 1H NMR spectroscopy (Fig. 1). Also, the chemical shifts of the methylene proton resonances of the side chain (c, e, i, and k) were changed. The 1H NMR integrations indicated that conjugation yields were quantitative. GPC profiles verified that transesterification and/or degradation of the PC backbone linkages by ozonolysis and/or over-oxidation were not significant up to the time of completion of the reaction (Fig. 2). Moreover, we found that insoluble PMOCs became soluble during conjugation reactions. Therefore, isolated PMOCs, despite their limited solubility, are applicable to further reactions, without the requirement of in situ generation and reaction of the aldehyde functionalities.
Fig. 2.
GPC profiles (THF as eluent, 1 mL min−1) of BnO-PMAC15-OH and isolated products of aldehyde–aminooxy “click” reactions with O-benzylhydroxylamine after ozonolysis of PMAC, with application of ozone for 10 or 15 min, each followed by reduction with Me2S overnight.
The properties of the resulting polymer conjugates were readily modified by varying the aminooxy compounds (Table S2†). Introduction of aldehydes to the PMAC, which was a viscous liquid with a low Tg of −16.0 °C, led to an obvious morphology change, as PMOC was a white solid (Tg = 49.0 °C). After aldehyde–aminooxy “click” reactions, conjugate 1 was a viscous liquid (Tg = 0.0 °C), while 2 was a white solid (Tg = 76.0 °C). Thus, the polymer bulk properties could be readily modified by alteration of the aminooxy compounds. Also, as expected, the solubilities of the conjugates were distinctive. Conjugate 1 was soluble in most organic solvents, while conjugate 2 was soluble in water and MeOH.
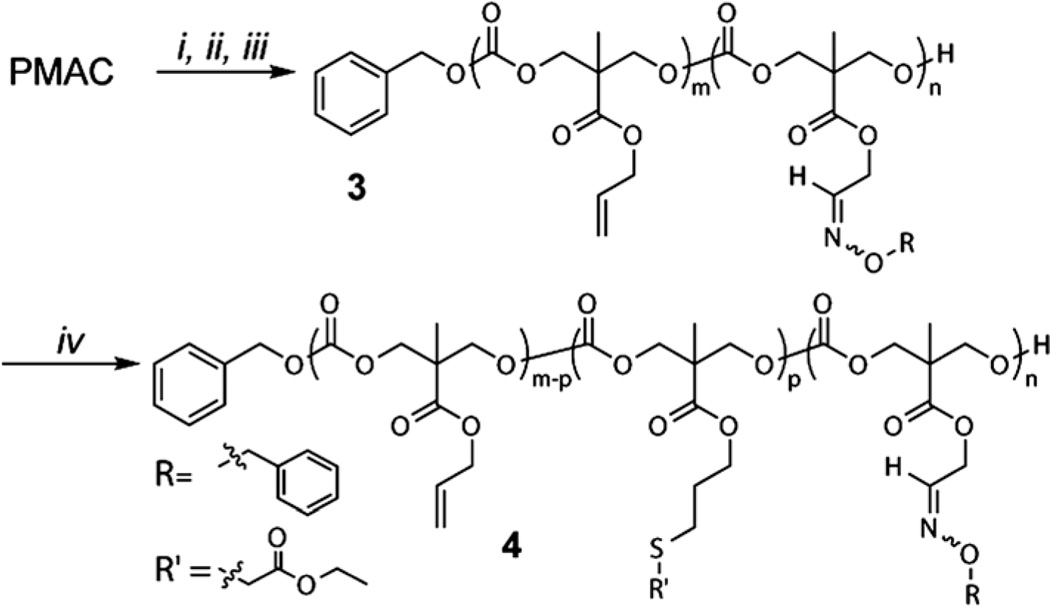
Orthogonal functional groups, alkenes and aldehydes, were easily introduced into the well-defined polymer scaffold. Partial conversions of alkenes to aldehydes were controlled simply by adjusting the times of exposure to ozone. After 3 and 5 min of bubbling ozone and reductive work-up, ca. 33% and 60% of alkenes were converted to aldehydes, respectively. The kinetics of ozonolysis made conversions predictable (Fig. S4†). Aldehyde–aminooxy “click” reaction of the resulting alkeneand aldehyde-functional PC (PMAC-co-PMOC) with O-benzyl-hydroxylamine and consecutive thiol–ene reaction with ethyl 2-mercaptoacetate demonstrated orthogonal functionalization of the resulting statistical copolymer (Scheme 2 and Fig. S5†).
Scheme 2.
Synthesis of PMAC-co-PMOC by partial ozonolysis of PMAC (i and ii), followed by in situ functionalization via consecutive aldehyde–aminooxy (iii) and thiol–ene “click” (iv) reactions. Conditions: (i) O3, DCM, −78 °C, 3 min; (ii) Me2S, −78 °C to RT; (iii) O-benzylhydroxylamine, NaOAc, MeOH, 45 °C, 1.5 h; (iv) ethyl 2-mercaptoacetate, DMPA, DMSO, UV365 nm, 2 h.
In summary, we have developed aldehyde-functional PCs via ozonolysis and reductive work-up of allyl-functional PCs. Ozone efficiently cleaves the double bonds without damage to the PC backbone. The resulting aldehydes were functionalized with aminooxy compounds under mild conditions. Furthermore, alkene- and aldehyde-bearing PCs were prepared by partial ozonolysis and functionalized by consecutive aldehyde–aminooxy and thiol–ene “click” reactions in an orthogonal fashion. Although we demonstrated a few examples, aldehyde-functional PCs are potentially excellent platforms for divergent research directions, due to their capability to form various conjugates via combinations of oxime, hydrazone, and/or Schiff base linkages with aldehydes and also thioether or other linkages to the alkenes.
Supplementary Material
Acknowledgements
We gratefully acknowledge financial support from the National Heart Lung and Blood Institute as a Program of Excellence in Nanotechnology (HHSN268201000046C) and from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK082546). The Welch Foundation is gratefully acknowledged for support through the W. T. Doherty-Welch Chair in Chemistry, Grant no. A-0001.
Footnotes
Electronic supplementary information (ESI) available: General considerations, synthesis and characterization; NMR, IR, GPC profiles, TGA, DSC, and kinetic studies data. See DOI: 10.1039/c4py00456f
Notes and references
- 1.(a) Carey FA, Sundberg RJ. Advanced Organic Chemistry: Part B: Reaction and Synthesis. Springer; 2007. [Google Scholar]; (b) Smith MB, March J. March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. Wiley; 2007. [Google Scholar]
- 2.(a) Moses JE, Moorhouse AD. Chem. Soc. Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]; (b) Iha RK, Wooley KL, Nyström AM, Burke DJ, Kade MJ, Hawker CJ. Chem. Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Wiley RH, Hobson PH. J. Polym. Sci. 1950;5:483–486. [Google Scholar]; (b) Mulvaney JE, Chang DM. Macromolecules. 1980;13:240–243. [Google Scholar]
- 4.(a) Hirao A, Ishino Y, Nakahama S. Makromol. Chem. 1986;187:141–147. [Google Scholar]; (b) Hirao A, Nakahama S. Macromolecules. 1987;20:2968–2972. [Google Scholar]
- 5.(a) Hwang J, Li RC, Maynard HD. J. Controlled Release. 2007;122:279–286. doi: 10.1016/j.jconrel.2007.04.010. [DOI] [PubMed] [Google Scholar]; (b) Li RC, Broyer RM, Maynard HD. J. Polym. Sci., Part A: Polym. Chem. 2006;44:5004–5013. [Google Scholar]; (c) Li RC, Hwang J, Maynard HD. Chem. Commun. 2007:3631–3633. doi: 10.1039/b709304g. [DOI] [PubMed] [Google Scholar]
- 6.(a) Sun G, Cheng C, Wooley KL. Macromolecules. 2007;40:793–795. doi: 10.1021/ma062592x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Alconcel SN, Kim SH, Tao L, Maynard HD. Macromol. Rapid Commun. 2013;34:983–989. doi: 10.1002/marc.201300205. [DOI] [PubMed] [Google Scholar]
- 7.Yang SK, Weck M. Macromolecules. 2007;41:346–351. [Google Scholar]
- 8.Ke X, Coady DJ, Yang C, Engler AC, Hedrick JL, Yang YY. Polym. Chem. 2014;5:2621–2628. [Google Scholar]
- 9.(a) Suriano F, Coulembier O, Hedrick JL, Dubois P. Polym. Chem. 2011;2:528–533. [Google Scholar]; (b) Tempelaar S, Mespouille L, Coulembier O, Dubois P, Dove AP. Chem. Soc. Rev. 2013;42:1312–1336. doi: 10.1039/c2cs35268k. [DOI] [PubMed] [Google Scholar]
- 10.Sanders DP, Fukushima K, Coady DJ, Nelson A, Fujiwara M, Yasumoto M, Hedrick JL. J. Am. Chem. Soc. 2010;132:14724–14726. doi: 10.1021/ja105332k. [DOI] [PubMed] [Google Scholar]
- 11.Hu X, Chen X, Xie Z, Liu S, Jing X. J. Polym. Sci., Part A: Polym. Chem. 2007;45:5518–5528. [Google Scholar]
- 12.Tempelaar S, Mespouille L, Dubois P, Dove AP. Macromolecules. 2011;44:2084–2091. [Google Scholar]
- 13.Reingold ID. Organic Chemistry: An Introduction Emphasizing Biological Connections REVISED Edition. Indo American Books; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.