Abstract
Pulmonary hypertension (PHT) is associated with high mortality in sickle cell anemia (SCA). Previously, we showed that elevated levels of placenta growth factor (PlGF) in SCA patients correlate with increased levels of the potent vasoconstrictor endothelin-1 (ET-1) and PHT. Moreover, PlGF induced the expression of ET-1 via hypoxia-inducible factor 1α. Here, we show a novel example of ET-1 posttranscriptional regulation by PlGF via action of microRNA 648 (miR-648), which is subject to transcriptional coregulation with its host gene, MICAL3 (microtubule-associated monooxygenase, calponin, and LIM domain containing 3gene). PlGF repressed expression of miR-648 in endothelial cells. Luciferase reporter assays using wild-type and mutant ET-1 3′ untranslated region (UTR) constructs, and transfection of miR-648 mimics showed that miR-648 targets the 3′ UTR of ET-1 mRNA. Since miR-648 is located in a 5′-proximal intron of MICAL3, we examined which of three potential promoters was responsible for its expression. The MICAL3 distal promoter (P1) was the predominant promoter used for transcription of pre-miR-648, and it was under positive control by PAX5 (paired box protein 5) transcription factor, as demonstrated by the loss and gain of function of PAX5 activity, and chromatin immunoprecipitation analysis. These studies provide a novel link wherein PlGF-mediated downregulation of PAX5 attenuates miR-648 expression leading to increased ET-1 levels that are known to induce PHT in SCA.
INTRODUCTION
Endothelin-1 (ET-1), a 21-amino-acid peptide hormone primarily synthesized and secreted by endothelium in vivo, is involved in proliferation of smooth muscle cells and vascular tone (1–4). In the vasculature, the endothelin system, comprising endothelin ligands (ET-1, ET-2, and ET-3), endothelin receptors (ET-AR and ET-BR), and two activating peptidases, has a basal vasoconstricting role; dysfunction contributes to the development of diseases such as hypertension and other cardiovascular diseases (3). Hypoxia is a potent inducer of ET-1 gene expression in endothelial cells via activation of hypoxia-inducible factor 1α (HIF-1α) (5, 6).
Pulmonary hypertension (PHT), occurs in ca. 10% of adults with sickle cell anemia (SCA), and its diagnosis is associated with a 38 to 40% mortality at 2 to 6 years (7, 8). Sickle mice develop PHT with increasing age, manifested as high pulmonary artery pressures and right ventricular hypertrophy (9). Physiological factors implicated in PHT in SCA include endothelial dysfunction, pulmonary vasoconstriction, and vascular remodeling, all of which are associated with chronic hemolysis, hypoxia, hemostatic activation, and inflammation (8, 10–12). ET-1 and nitric oxide (NO) are mutually opposing pulmonary vasoactive factors that regulate pulmonary vascular tone. It is postulated that hemolysis leads to quenching of NO by extracellular/cell-free hemoglobin, thereby reducing NO bioavailability (11, 13), which in turn leads to the clinical manifestations of sickle PHT (13–15). Numerous studies in SCA and other hemolytic anemias have shown association of low NO bioavailability with PHT. In a related study, a clinical trial designed to increase bioavailability of NO, utilizing inhalation of nitric oxide, failed to improve time to crises resolution in SCA patients with vaso-occlusive crises (16, 17). Thus, in addition to NO, other factors may play a role in the etiology of PHT in SCA.
The levels of ET-1 in plasma are elevated in SCA patients with PHT (9, 18). The effects of ET-1 on vasoconstriction and regulation of blood pressure have been shown utilizing ET-1 knockout mice and ET-B receptor knockout mice (19). Furthermore, ET-1 receptor (ET-R) antagonists used for treatment of primary PHT (20) are found to be beneficial to sickle-Antilles-hemoglobin D mice in ameliorating symptoms of PHT (21), thus indicating a prominent role of ET-1/ET-receptor interaction in PHT in SCA. In contrast, the molecular mechanisms of ET-1 induction that result in sickle PHT are less well understood.
Our group previously reported that the high circulating levels of placenta growth factor (PlGF) in SCA, compared to healthy control subjects, correlate with an increased incidence of vaso-occlusive crises (22). We and others (22–24) have shown that PlGF is produced by erythroid cells and not by other hematopoietic cell types. We had initially hypothesized that PlGF was selectively activating cells expressing its cognate receptor (VEGFR1); therefore, it might be a key activator of endothelial cells and mononuclear cells for promoting inflammation and vasoconstriction in SCA (25, 26). Indeed, our studies showed that PlGF was involved in monocyte activation, coincident with increased inflammation seen in SCA (22, 25). We also observed that, similar to humans with SCA, humanized sickle (Berkeley-SS) mice also exhibit significantly elevated levels of PlGF and ET-1, resulting in right ventricular (RV) hypertrophy and increased RV pressures consistent with PHT (9, 11). We also demonstrated the specific contribution of elevated PlGF to PHT by increasing erythroid expression of PlGF, using a lentivirus vector in normal mice to levels seen in Berkeley-SS mice (9). Wild-type mice, overexpressing PlGF, consequently showed increased ET-1 production and correspondingly increased RV pressures, RV hypertrophy, and pulmonary fibrosis, all consistent with features of pulmonary hypertension (9). These animal findings were corroborated in 123 patients with SCA, whereupon increased plasma PlGF levels were associated with anemia, augmented ET-1, and increased tricuspid regurgitant velocity, the last feature being an indirect measure of peak pulmonary artery pressure (9). These studies in vivo showed that higher levels of PlGF in plasma were associated with anemia, higher levels of endothelin-1, and clinical features of pulmonary hypertension in SCA.
We have previously shown PlGF upregulates expression of ET-1, PAI-1, and lipoxygenase(s) in both human endothelial cells and monocytes by activation of HIF-1α, independent of hypoxia (26–28). In the present study, we examined the posttranscriptional mechanism of placenta growth factor mediated ET-1 expression. Here, we show that the level of microRNA 648 (miR-648), having a seed sequence complementary to the 3′ untranslated region (UTR) of ET-1 mRNA, was attenuated in response to treatment of cultured endothelial cells with PlGF. Moreover, we show that miR-648 located in a 5′-proximal intron of the MICAL3 gene (i.e., the microtubule-associated monooxygenase, calponin, and LIM domain containing 3 gene), a member of the MICAL family of flavoprotein monooxygenases (29), is cotranscribed with MICAL3 pre-mRNA and undergoes maturation following excision of the intron containing pre-miR-648. In addition, our studies show for the first time, to the best of our knowledge, that PAX5 (paired box protein 5) transcriptionally activates coexpression of MICAL3 and pre-miR-648 in human endothelial cells and that the 3′ UTR of ET-1 mRNA is indeed a target of miR-648. Since human miR-648 does not have a corresponding ortholog in mouse, this precluded study in animal models, e.g., Berkeley-SS or PlGF−/− mice. For this reason, a determination of plasma miR-648 levels in human SCA patients was undertaken in order to corroborate the in vitro findings.
MATERIALS AND METHODS
Reagents.
Human recombinant PlGF was purchased from R&D Systems (Minneapolis, MN); primary antibodies to endothelin-1, PAX5, and secondary antibodies conjugated to horseradish peroxidase (HRP) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies against β-actin were purchased from Sigma Chemical Company (St. Louis, MO). The PAX5 shRNA vector and corresponding control scrambled shRNA were from Open Biosystems as a gift from Jae Jung. Actinomycin D was purchased from Enzo Life Sciences (Plymouth Meeting, PA), and hsa-miR mimics and hsa-miR inhibitors were purchased from Shanghai Gene Pharma Co., Ltd. (Shanghai, China). Bacterial artificial chromosome (BAC) clones for ET-1 (EDN1) were obtained from Children's Hospital Oakland Research Institute (CHORI), BACPAC Resources (Oakland, CA). The primers used for PCR amplification of the ET-13 ′ UTR and mutagenesis were purchased from ValueGene (San Diego, CA). Plasmid pMI-PAX5 was a generous gift from Zhixin Zhang, University of Nebraska Medical Center, Omaha, NE. Unless otherwise specified, all other reagents were purchased from Sigma Chemical Company.
Endothelial cell culture.
Primary human pulmonary microvascular endothelial cells (HPMVEC) were obtained from Cell Applications, Inc. (San Diego, CA), and human umbilical vein endothelial cells (HUVEC) were from the American Type Culture Collection (ATCC) or Clonetics; cells were grown according to the vendor's protocols. These primary cells maintained characteristics of endothelial cell morphology and cell phenotype up to passages 7 and 8 and thus were not used beyond passage 8 (26). Human microvascular endothelial cell line 1 (HMEC-1) was obtained from the Centers for Disease Control and Prevention (Atlanta, GA) and cultured as described previously (30). Briefly, HMEC-1 cells were cultured in RPMI 1640 containing 10% fetal bovine serum, 1 mM sodium pyruvate, 1 mM glutamine, 5 mM HEPES, minimal essential medium vitamins and nonessential amino acids (1×), 50 μg/ml endothelial cell mitogen (Biomedical Technologies, Stoughton, MA), and heparin (20 U/ml). HMEC-1 cells were incubated overnight in RPMI 1640 complete medium containing 2% serum, followed by serum deprivation for 3 h and subsequent treatment with either PlGF (250 ng/ml) or other experimental conditions, as indicated.
Human subjects.
All blood samples were obtained from children with homozygous SCA at steady state during their elective clinic appointments with routine clinical draws provided through the Hematology Repository at Cincinnati Children's Hospital Medical Center, Cincinnati, OH. All samples were obtained with the informed consent of the patient/legal guardian using Institutional Review Board-approved protocols. The plasma samples were obtained from the SCA patients and unaffected sibling as controls (31).
Isolation of RNA and quantitative real-time-PCR (qRT-PCR).
Endothelial cells were treated with PlGF (250 ng/ml) for indicated time periods followed by total RNA extraction using TRIzol reagent (Invitrogen, Carlsbad, CA). Levels of mRNA and pre-miRNA were determined and quantified using specific primers (Table 1). Real-time quantitative PCR of mRNA and pre-miRNA templates was done using the iScript One-Step RT-PCR kit with SYBR green (Bio-Rad, Hercules, CA) and an ABI Prism 7900 HT sequence detection system (Applied Biosystems, Foster City, CA). PCR amplification of 100 ng of RNA was performed for 40 cycles under the following conditions: cDNA synthesis at 50°C for 10 min, iScript reverse transcriptase inactivation at 95°C for 5 min, and PCR cycling and detection at 95°C for 10 s, followed by 60°C for 45 s. Values are expressed as relative expression of mRNA and pre-miRNA normalized to endogenous GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA (27).
TABLE 1.
Oligonucleotide primers used in this study
| Gene or target | Method | Primer sequence (5′–3′) |
|
|---|---|---|---|
| Forward | Reverse | ||
| ET-1 | qRT-PCR | GAAACCCACTCCCAGTCCAC | CGGGAGTGTTGACCCAAATG |
| GAPDH | qRT-PCR | AACCTGCCAAGTACGATGACATC | GTAGCCCAGGATGCCCTTGA |
| MICAL3 P1 | qRT-PCR | GCACCCACCCTGCCC | TGCTGCCTCACTCTCAG |
| pre-miR-934 | qRT-PCR | GAAATAAGGCTTCTGTCTAC | GAAATAAGGCTCCCGTCC |
| pre-miR-648 | qRT-PCR | CACAGACACCTCCAAGTG | CCCTCACTTCCGACTAAG |
| PAX5 | qRT-PCR | GACACCAACAAGCGCAAG | GTGCTGCCTCTCAAACAC |
| pLVXE-648 | Cloning PCR | CGGATCCATTTGCCAGCACAGTACTG | CGGATCCGGCAGCAAACCAGCTG |
| P2a | Cloning PCR | TCTTACGCGTGCTAGCTCCAGATGAGGAGCCGAG | CTTAGATCGCAGATCTCATCTGTCTATTGGTCCCC |
| ET-1 3′ UTR | Cloning PCR | CCTCTAGACCTTCGGGGCCTGTC | CCTCTAGATACACAGTAAGGAAAAAAATATTTATTTTCTAAAGTC |
| ET-1 3′ UTR (miR-648 mutant) | Cloning PCR | CTGACTCAGGCGCCTGGCACAAATCAGGGAGAAAC | GTTTCTCCCTGATTTGTGCCAGGCGCCTGAGTCAG |
| PAX5 binding site 1 | ChIP-PCR | ACTCCTATTGGGCATCTACCTG | CGATATTGCAAAACCAGGGGG |
| PAX5 binding sites 2 and 3 | ChIP-PCR | ATACCAGGTGAGGGCACAAC | GGGGATATCCAACTCACCCAG |
| P1 | RT-PCR | CGGCCTGAGAACCCCTG | GCTTCACAGCCGCATCAC |
| P2 | RT-PCR | CTTCTCCGGCTGCTACCTAC | CTCATACTGCGGTGTCCCTC |
| P2a | RT-PCR | AGTCTGTTAAGCATTTGCCAGC | CCTGCTCAACTGCACCCTC |
| GAPDH | RT-PCR | GGCCTCCAAGGAGTAAGACC | TGGTACATGACAAGGTGCGG |
| miR-648 | Northern blot | ACCAGTGCCCTGCACACTT | |
| 5S rRNA | Northern blot | CAGGCCCGACCCTGCTTAGCTTCCGAGATCAGACG | |
Isolation and quantification of microRNAs (miRNAs) and pre-miRNA.
Total RNA was isolated from endothelial cells using the mirVana miRNA isolation kit (Ambion-Applied Biosystems). Specific miRNA expression was determined using the TaqMan microRNA assay kits for indicated miRNAs (Applied Biosystems) according to the manufacturer's protocol. Briefly, 100 ng of small RNA was reverse transcribed at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min and then kept at 4°C. qRT-PCR (using 20-μl total reaction mixtures) was performed in a 384-well plate at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. All reactions were carried out in triplicate. MicroRNA expression was normalized to endogenous U6 snRNA (cell culture) or miR-16 (plasma). Pre-miRNA expression was detected and quantified, utilizing specific pre-miRNA primers (Table 1). Relative quantitative levels for pre-miRNA expression were calculated as 2–ΔΔCT by the comparative threshold cycle (CT) method, where ΔΔCT = (CT target pre-miRNA of treated sample − CT reference gene of treated sample) − (CT target pre-miRNA of control sample − CT reference gene of control sample) as previously described (31).
Northern blotting.
Briefly, 35 μg of total RNA was run on a 15% nondenaturing polyacrylamide gel. The RNA was transferred to a Biodyne B nylon membrane. The membrane was cross-linked under UV light and prehybridized for 30 min using the UltraHyb hybridization buffer (Ambion, Grand Island, NY) at 42°C. The membrane was then hybridized with biotinylated probes for miR-648 and 5S rRNA, synthesized at ValueGene, at 62°C overnight. The membrane was washed twice in washing buffer (Thermo Scientific, Rockford, IL), followed by blocking with 5% nonfat milk in 1× phosphate-buffered saline (PBS) at room temperature. Streptavidin-HRP (1:250 dilution) was added to the membrane, followed by incubation at room temperature for 3 h and then two washes with wash buffer according to the vendor's instructions. The membranes were developed utilizing Clarity Western ECL substrate (Bio-Rad, Richmond, CA), and the resulting images were quantified using ImageJ analysis software.
Transient transfections.
Endothelial cells (106 cells) were resuspended in 100 μl of serum-free RPMI 1640 medium containing indicated miRNA (60 to 90 pmol), anti-miRNA inhibitor (60 to 90 pmol), appropriate shRNA vector and expression constructs (0.5 μg), and luciferase reporter plasmids (1.0 μg), as indicated, followed by nucleofection utilizing appropriate programs in the Amaxa nucleofector device (Lonza, Basel, Switzerland), as previously described (32). The Renilla luciferase plasmid (pRLSV40, 1.0 μg) was cotransfected with firefly luciferase reporter constructs to monitor transfection efficiency. After nucleofection and after 24 h, the cells were incubated in growth medium overnight, followed by serum deprivation for 3 h and then treatment with PlGF (250 ng/ml) for the indicated time periods. The cell lysates were assayed for luciferase activity using a dual luciferase reagent kit (Promega, Madison, WI). Luciferase values were normalized to Renilla luciferase assay values and expressed relative to the activity of the pGL3 control vector, as appropriate.
Dox-inducible miR-648 expression plasmid.
A doxycycline (Dox)-inducible expression plasmid was generated by PCR amplification of a 271-bp region (chr22:18463549–18463819) of MICAL3 that included the pre-miR-648 region. The PCR fragment was inserted into the BamHI site in pLVX-EGFP vector (gift from Samad Amini-Bavil-Olyaee), using standard cloning techniques, and designated pLVXE-648. Correct insertion of the sequence was determined by DNA sequencing (Retrogen, San Diego, CA). HMECs were transfected with pLVXE-648 plasmid (1 μg/106 cells) by electroporation and transferred to complete medium. After 24 h, the medium was replaced with serum-free medium and kept for 3 h. Dox (100 ng/ml) was added for indicated time periods. For the washout experiment, cells treated with Dox for 4 h were washed twice in PBS, followed by PlGF treatment for 6 h. RNA was isolated and ET-1 mRNA was determined by qRT-PCR as described above.
ET-1 3′-UTR plasmid and mutagenesis.
Human BAC clone for ET-1 (EDN1; NCBI NM_001168319) was used as the template (CHORI, Oakland, CA). The 1,127-bp fragment spanning nucleotides (nt) +974 to +2100, inclusive, relative to the translation stop codon of ET-1 mRNA was PCR amplified using forward and reverse primers containing XbaI restriction enzyme sites, as listed in Table 1, and Deep Vent high-fidelity DNA polymerase (New England BioLabs, Ipswich, MA) according to standard methods. The PCR product was cloned downstream of the firefly luciferase gene in pGL3-Control (Promega, Madison, WI). The orientation and identity of the insert relative to the luciferase gene was confirmed by DNA sequencing, and the plasmid was purified by using the EndoFree Plasmid Maxi kit (Qiagen, Valencia, CA). The resulting plasmid is designated pGL3-ET-1-3′-UTR. Mutation of the miR-648 binding sites in the ET-1 3′ UTR was generated using pGL3-ET-1 3′ UTR as the template with the primers listed in Table 1 in accordance with the NEB Q5 site-directed mutagenesis protocol (New England BioLabs). The mutations were confirmed by DNA sequencing (Retrogen, La Jolla, CA).
Western blots.
Endothelial cells (106 cells) were incubated in serum-free media overnight, followed by treatment with PlGF for the indicated time periods. Protein lysates were prepared as previously described and subjected to electrophoresis. Samples were transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). Membranes were probed with antibodies (diluted as indicated) for PAX5 (1:250) and ET-1 (1:500). Membranes were stripped and reprobed with an antibody to β-actin (1:50,000) to normalize any loading differences. Protein bands were detected using the West Pico chemiluminescent substrate (Thermo Scientific), and sizes were compared to prestained molecular weight markers (New England BioLabs).
Quantitation of transcripts by semiquantitative reverse transcription-PCR (RT-PCR).
Total RNA was extracted from HMEC-1 cells using TRIzol reagent as previously described. RNA transcripts were reverse transcribed and amplified using specific primers for P1, P2, and P2a products and GAPDH (Table 1) utilizing the Access RT-PCR system (Qiagen, La Jolla, CA). Briefly, 200 ng (nonlimiting) or 25 ng (limiting) total RNA was reverse transcribed at 45°C for 45 min to generate cDNA products followed by heat inactivation at 94°C for 2 min. PCR amplification was performed as follows: 94°C for 30 s, 60°C for 1 min, and 68°C for 2 min for 40 cycles, followed by a final extension at 68°C for 7 min. The PCR products were run on a 2% agarose gel, visualized by ethidium bromide, and quantitated by ImageJ software analysis.
ChIP assays.
HMECs (107 cells) were serum starved prior to treatment with PlGF as described above. Chromatin immunoprecipitation (ChIP) was performed using PAX5 antibody as previously described (26). Immunoprecipitated DNA was subjected to PCR for 30 cycles as follows: 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min, using the primers specified in Table 1. PCR products were analyzed by agarose gel electrophoresis and compared to DNA size markers.
Statistical analysis.
Data are presented as means ± the standard errors of the mean (SEM). A Student t test was used to evaluate the significance of differences between paired samples. SCA versus control plasma samples for qRT-PCR were analyzed utilizing unpaired t test. P values of <0.05 were considered significant. Statistical significance is indicated in the figures.
RESULTS
Posttranscriptional regulation of PlGF-mediated ET-1 mRNA expression.
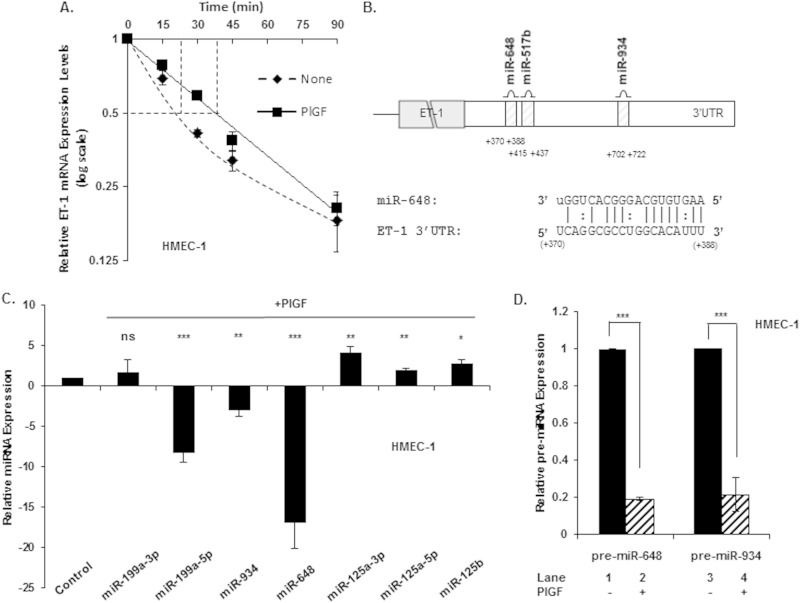
Our recent studies show PlGF-mediated ET-1 mRNA induction occurs in a time-dependent manner in endothelial cells, achieving a maximum 6 h postinduction, with declining levels persisting through 24 h (26). As shown in Fig. 1A, treatment of HMEC-1 (i.e., a human dermal microvascular endothelial cell line) in the presence of actinomycin D with PlGF resulted in significant stabilization of ET-1 mRNA, as evident in the increase of t1/2 of 22 ± 1 min to 36 ± 2 min. The decay kinetics of ET-1 mRNA in cells treated with actinomycin D in the absence of PlGF showed a biphasic decline consistent with a second-order degradation process, possibly a miRNA-dependent mechanism.
FIG 1.
PlGF-mediated expression of candidate miRNAs in a human endothelial cell line (HMEC-1) with a predicted binding site(s) within the 3′ UTR of ET-1 mRNA. (A) Determination of ET-1 mRNA half-life (t1/2) in response to PlGF treatment. Briefly, cells were treated with PlGF for 6 h, followed by actinomycin D (10 μg/ml) treatment for the indicated time period, and processed as previously described (31). (B) Schematic showing 3′ UTR of ET-1 mRNA with a binding site(s) for selected miRNAs. (C) HMEC-1 was treated with PlGF for 6 h, followed by isolation of miRNAs and quantitation by qRT-PCR. The relative expression levels of indicated miRNAs were normalized to U6 snRNA. (D) HMEC-1 cells were treated with PlGF (250 ng/ml) for 6 h, followed by mRNA isolation and quantitation of pre-miRs by qRT-PCR utilizing primers indicated in Table 1. The data represent means ± the SEM of four independent experiments. The results are expressed as the fold change between PlGF-treated versus untreated samples. ***, P < 0.001; **, P <0.01; *, P <0.05; ns, not significant.
Identification of miRNAs involved in destabilization of ET-1 mRNA in HMEC-1.
We used bioinformatic analysis (http://www.ebi.ac.uk/enright-srv/microcosm/cgi-bin/targets/v5/detail_view.pl?transcript_id=ENST00000379375) to identify miRNA candidates with specificity for ET-1 mRNA. Our analysis combined the degree of ET-1 mRNA complementarity to specific miRNAs, as judged by seed sequence stability (ΔG°) and the scope of evolutionary conservation among mammalian species of miRNA target sites within the ET-1 3′ UTR. Based on the criteria presented above the analysis yielded >7 miRNAs, from which we selected three candidates for further study, as depicted in the schematic shown in Fig. 1B (miR-648, miR-517B, and miR-934). In addition, we included miR-125a-3p, miR-125a-5p, and miR-125b, since these miRNAs are reported to be involved in oxidized low-density lipoprotein-mediated regulation of ET-1 mRNA in vascular endothelial cells (33). Lastly, we included miR-199a since the stability of HIF-1α mRNA is affected by miR-199a in response to ethanol (34).
The levels of the selected miRNAs were determined in HMEC-1 in the presence or absence of PlGF. As shown in Fig. 1C, levels of miR-934 and miR-648 decreased by ∼3-fold and ∼16-fold, respectively, after PlGF treatment; miR-199a-5p similarly decreased by ∼8-fold. In contrast, the level of miR-199a-3p, used as a control, did not change significantly in response to PlGF (Fig. 1C). Other miRNAs observed to increase after PlGF treatment were miR-125a-3p, miR-125a-5p, and miR-125b; these showed modest increases in the range of 2- to 4-fold (Fig. 1C). Since miRNAs are derived from pre-miRNAs, we examined whether a change in the expression of pre-miR-648 and pre-miR-934 correlated with the observed change in respective mature miRNAs. As shown in Fig. 1D, pre-miR-648 and pre-miR-934 levels decreased by ∼80 and ∼75%, respectively, in response to PlGF, in the HMEC-1 cell line. Taken together, these data showed that PlGF treatment was responsible for the decreased transcription of miR-648 and miR-934 in HMEC-1 cells.
Effect of miRs and anti-miRs on ET-1 mRNA level in the human endothelial cell line HMEC-1.
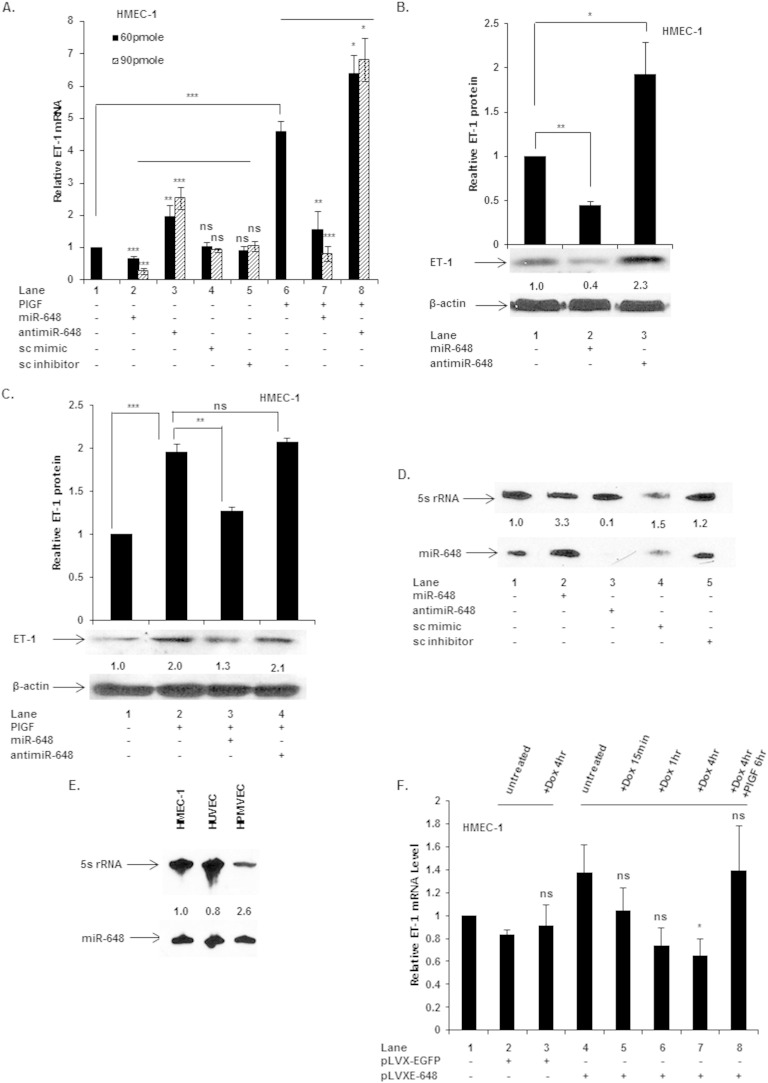
Since miR-648 levels decreased ∼16-fold in the PlGF-treated HMEC-1 cell line, we focused our studies on this miRNA. We examined whether mimics for miR-648 directly affected the level of ET-1 mRNA under basal and PlGF-treated conditions. As shown in Fig. 2A, transfection of HMEC-1 with 60 and 90 pmol of miR-648 mimic, dose dependently attenuated the basal level of ET-1 mRNA by ∼40 and ∼75%, respectively (Fig. 2A, lane 2 versus lane 1). Conversely, transfection of HMEC-1 cells with 60 and 90 pmol of anti-miR-648 antagonized endogenous miR-648 and augmented the levels of ET-1 mRNA by ∼2-fold and ∼2.5-fold, respectively (Fig. 2A, lane 3 versus lane 1). Transfection of HMEC-1 cells with scrambled (sc) miR siRNA (sc mimic) and anti-miR-siRNA (sc inhibitor) did not affect ET-1 mRNA expression, respectively (Fig. 2A, lanes 4 and 5 versus lane 1). The effects of exogenous miR-648 were also examined in HMEC-1 in the presence of PlGF. Transfection of these cells with miR-648 mimic at both 60 and 90 pmol inputs followed by PlGF treatment reduced ET-1 mRNA expression by ∼65 and ∼95%, respectively, relative to PlGF treatment alone (Fig. 2A, lane 7 versus lane 6). Conversely, transfection with 60 and 90 pmol of anti-miR-648, followed by PlGF treatment, antagonized endogenous miR-648, and resulted in increased levels of ET-1 mRNA by ∼38 and ∼45%, respectively, over and above PlGF treatment alone (Fig. 2A, lane 8 versus lane 6).
FIG 2.
Effect of exogenous miR-648 on ET-1 mRNA and protein expression in HMEC-1 cells. Cells were transfected with the indicated miRNA mimics, anti-miRNAs, and control scrambled miRNA (sc) or scrambled anti-miR (sc inhibitor). (A) ET-1 mRNA expression in cells transfected with miRs and anti-miR under basal and PlGF-treated conditions. (B) Western blot of HMEC-1 cell lysates, utilizing antibody to ET-1, following transfection of HMEC with either miR-648 mimic (90 pmol) or anti-miR (90 pmol). (C) Western blot of cell lysates obtained from HMEC-1 transfected with either miR-648 mimic or anti-miR after PlGF treatment for 24 h. ET-1 expression was normalized to β-actin in panels B and C. (D and E) Northern blot for relative amounts of miR-648 and 5S rRNA in endothelial cells. (F) ET-1 mRNA expression in cells transfected with pLVX-EGFP (control) and pLVXE-648 (miR-648 expression plasmid). All results are representative of three independent experiments and reflect the means ± the SEM. ***, P < 0.001; **, P <0.01; *, P <0.05; ns, not significant.
The effects of exogenous miR-648 and anti-miR-648 on ET-1 protein levels were examined in HMEC-1 cells. After transfection with miR-648 mimic there was ∼50% reduction of ET-1 protein (Fig. 2B, lane 2 versus lane 1); conversely, transfection with anti-miR-648 increased expression of ET-1 ∼2-fold (Fig. 2B, lane 3 versus lane 1) under basal conditions (n = 3). Thus, the effects of exogenous miR-648 correlated with both reduction of basal levels of ET-1 mRNA and concomitant decrease in ET-1 protein synthesis.
The effect of exogenous miR-648 treatment on ET-1 protein level was examined in PlGF-treated HMEC-1. As expected, PlGF induced ET-1 protein levels by 2-fold (Fig. 2C, lane 2 versus lane 1); however, transfection of HMEC-1 cells with miR-648, with PlGF treatment, abrogated induction of ET-1 protein by ∼70% (Fig. 2C, lane 3 versus lane 2). Conversely transfection with anti-miR-648 had no effect on ET-1 protein level (Fig. 2C, lane 4 versus lane 2). Next, we performed Northern blots to gauge the effectiveness of miR-648 mimic and anti-miR-648 on endogenous levels of miR-648, utilizing biotinylated miR-648 probe. As shown in Fig. 2D, transfection of miR-648 mimic in HMEC-1 cells augmented by ∼3-fold endogenous levels of miR-648. In contrast, transfection of HMEC-1 cells with anti-miR-648 completely antagonized endogenous levels of miR-648 (lane 3 versus lane 1). Since endogenous levels of miR-648 may vary between endothelial cells derived from primary culture versus established cell lines, we quantitated the expression of miR-648 in these cells by Northern blotting. As shown in Fig. 2E, both HUVEC and HMEC-1 showed almost equivalent amounts of miR-648, while HPMVEC showed ∼2.6-fold higher expression of miR-648 by Northern blotting. Thus, these results showed miR-648 is constitutively expressed in this cell type and would suggest it is responsible for maintenance of low ET-1 levels by posttranscriptional repression.
As a further demonstration that miR-648 is responsible for repressing ET-1 expression, we transfected a pre-miR-648 expression plasmid under the control of a doxycycline (Dox)-inducible promoter. As shown in Fig. 2F, Dox induction of these cells showed that time-dependent (0.25 to 4 h) miR-648 synthesis directly correlated with a decline in ET-1 mRNA expression levels in HMEC-1 cells (Fig. 2F, lanes 5, 6, and 7 versus lane 4). Dox induction of control vector-transfected cells showed no change in ET-1 mRNA level (Fig. 2F, lane 3 versus lane 1). Moreover, Dox washout, reversing miR-648 induction in these cells, followed by PlGF treatment, restored basal ET-1 mRNA expression (Fig. 2F, lane 8 versus lane 4). Taken together, these data confirmed that miR-648, expressed in situ, repressed the expression of ET-1 mRNA and protein in HMEC-1. Thus, physiological reduction of miR-648 levels in response to PlGF should positively contribute to expression of ET-1.
miR-648 regulates ET-1 mRNA and protein expression in primary human endothelial cells.
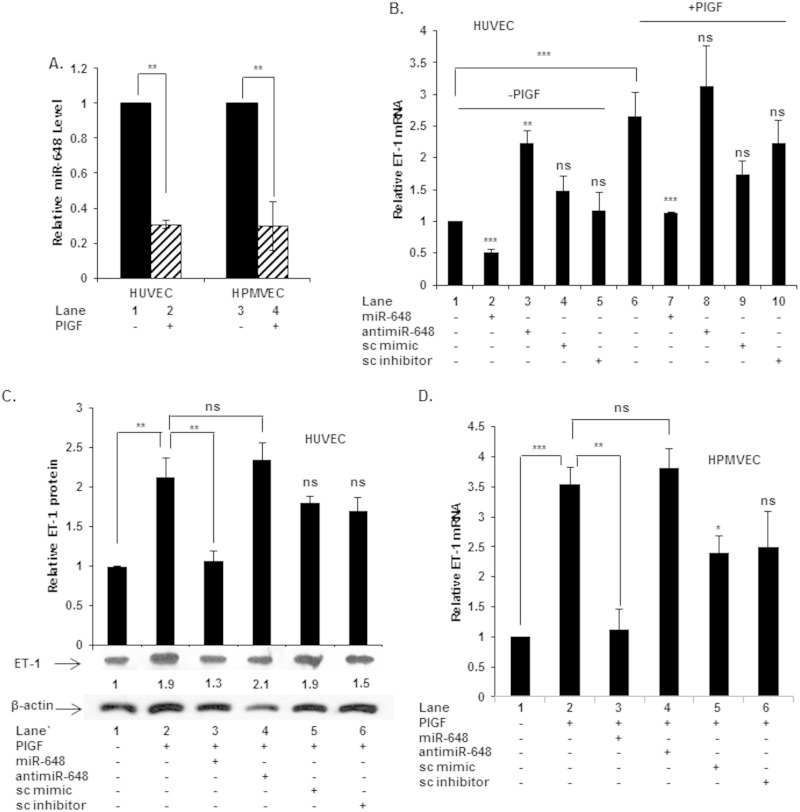
We examined whether miR-648 played a role in the regulation of ET-1 in primary human endothelial cells in vitro. Treatment of human umbilical vein endothelial cells (HUVEC) and human pulmonary microvascular endothelial cells (HPMVEC) with PlGF resulted in ∼70% reduction in miR-648 expression (Fig. 3A). Thus, the general reduction of miR-648 expression following PlGF treatment in these primary endothelial cells was identical to that observed in HMEC-1 cells.
FIG 3.
Endogenous miR-648 expression in primary endothelial cells and effects of exogenous miR-648 and anti-miR-648 on ET-1 mRNA and protein in cultured primary human endothelial cells. (A) miR-648 expression in PlGF-treated HUVEC and HPMVEC. (B) ET-1 mRNA expression in HUVEC transfected with miR and anti-miR under basal and PlGF-treated (6 h) conditions. (C) Western blot for expression of ET-1 protein in HUVEC transfected with indicated miR or anti-miR, followed by PlGF treatment for 12 h. (D) Effect of miR-648 and anti-miR-648 on ET-1 mRNA in HPMVEC. All data represent the means ± the SEM of three independent experiments. ***, P < 0.001; **, P <0.01; *, P <0.05; ns, not significant.
Next, the relationship between miR-648 levels to ET-1 induction was examined in these primary endothelial cells after treatment with PlGF. Transfection of HUVEC with miR-648 mimic reduced endogenous ET-1 mRNA levels by ∼50% (Fig. 3B, lane 2 versus lane 1). Conversely, transfection with anti-miR-648 augmented ET-1 mRNA by ∼2.2-fold compared to untreated cells (Fig. 3B, lane 3 versus lane 1). We then examined whether miR-648 modulated ET-1 expression after PlGF treatment. Incubation of HUVEC with PlGF for 6 h resulted in ∼2.5-fold induction of ET-1 mRNA level (Fig. 3B, lane 6 versus lane 1), as previously shown (26). Transfection of HUVEC with miR-648, followed by PlGF treatment, reduced ET-1 mRNA levels to the basal level (Fig. 3B, lane 7 versus lane 6). In comparison, transfection of HUVEC with anti-miR-648 followed by PlGF treatment did not change ET-1 mRNA expression beyond PlGF treatment alone (Fig. 3B, lane 8 versus lane 6). At the protein level, transfection of HUVEC with miR-648 followed by PlGF treatment attenuated ET-1 to the basal level (Fig. 3C, lane 3 versus lane 2). However, anti-miR-648 did not significantly increase ET-1 protein levels over and above that observed with PlGF plus sc anti-miR (Fig. 3C, lane 4 versus lane 6) and also not above PlGF-treatment alone (Fig. 3C, lane 4 versus lane 2). Similarly, in PlGF-treated HPMVEC miR-648 attenuated ET-1 mRNA expression (Fig. 3D, lane 3 versus lane 2), while anti-miR-648 did not significantly affect ET-1 mRNA levels over and above PlGF treatment alone (Fig. 3D, lane 4 versus lane 2). In summary, these data showed that miR-648 was responsible for modulating ET-1 mRNA and protein levels under basal and PlGF-treated conditions in primary human endothelial cells, as observed in the human endothelial cell line (HMEC-1). The data obtained from these primary cell lines further support the model whereby miR-648 levels are repressed during PlGF induction in endothelial cells, thus contributing to permissive conditions for ET-1 expression.
miR-648 interacts with predicted target site in the 3′ UTR of ET-1 mRNA.
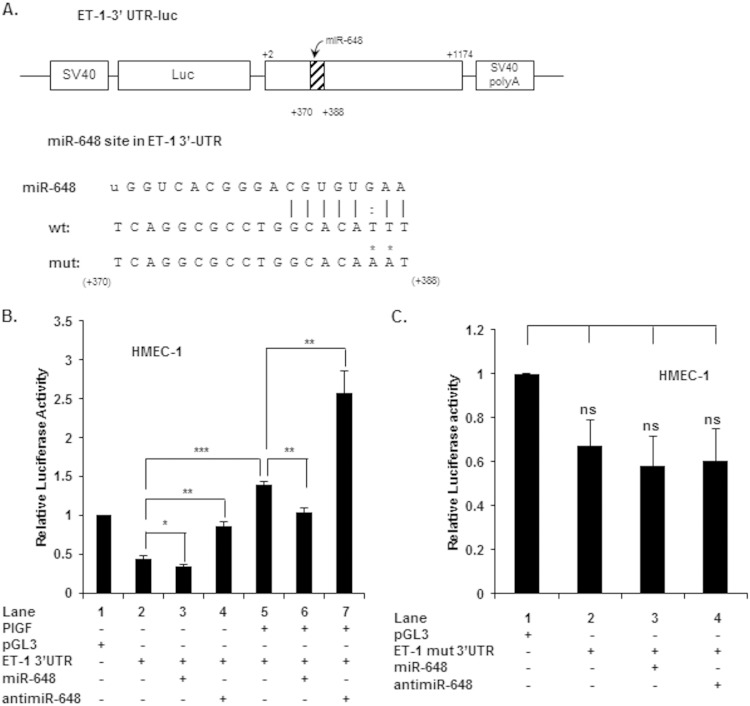
In order to establish whether miR-648 directly modulated the expression of ET-1 via interaction with the 3′ UTR of its mRNA, we generated a luciferase reporter construct in which the 3′ UTR of ET-1 mRNA (bases +2 to +1174, relative to the stop codon) was inserted downstream of the luciferase open reading frame. The resulting construct (pGL3-ET-1-3′UTR), as depicted in Fig. 4A, has the predicted binding site for miR-648. Transfection of this reporter plasmid into HMEC-1 demonstrated that the luciferase mRNA responded to PlGF treatment like native ET-1 mRNA (Fig. 4B, lane 5 versus lane 2); PlGF treatment resulted in an ∼3-fold increase in reporter activity. Cotransfection of pGL3-ET-1-3′UTR reporter with miR-648 (90 pmol) modestly reduced luciferase activity under basal conditions (Fig. 4B, lane 3 versus lane 2). However, miR-648 reduced reporter expression by ∼25% in cells treated with PlGF (Fig. 4B, lane 6 versus lane 5). The specificity of miR-648 on reporter mRNA activity was demonstrated by the use of anti-miR-648. The effects of endogenous miR-648 (previously demonstrated in Fig. 2A and B) were antagonized by transfecting cells with anti-miR-648 under both basal (Fig. 4B, lane 4 versus lane 2) and PlGF (Fig. 4B, lane 7 versus lane 5) treatments. In both conditions, anti-miR-648 transfection gave rise to an ∼2-fold increase in reporter activity. Transfection with sc miRNA mimics or sc miRNA inhibitors had no significant effect on luciferase activity (data not shown). Thus, these results clearly implicated miR-648 as a significant cytoplasmic modulator of ET-1 expression.
FIG 4.
Effects of miR-648 and anti-miR-648 oligonucleotides on ET-1 3′-UTR reporter luciferase activity. (A) Schematic representation of the ET-1 3′-UTR luciferase reporter construct. The region between nt +2 and +1174 of the ET-1 3′ UTR containing the predicted target site for miR-648 was cloned at the 3′ end of the luciferase reporter gene in pGL3. The nucleotide sequence of the predicted miR-648 binding site within the ET-1 3′ UTR is shown as the wild type (wt); point mutations in the miR-648 complementary sequence of the mutant (mut) are indicated by asterisks. (B) HMEC-1 were cotransfected with wild-type ET-1 3′-UTR plasmid and the indicated miR or anti-miR (90 pmol), followed by 6 h of PlGF treatment at 24 h posttransfection. The luciferase activity was normalized to the Renilla activity. (C) HMEC were transfected with mutant ET-1 3′-UTR reporter plasmid and the indicated miR-648 mimic (90 pmol) or anti-miR-648 (90 pmol). In panels B and C, each construct was used at 1.0 μg. In panel B, the comparison is between PlGF-treated cells versus cells transfected with either miR or anti-miR. In panel C, transfection with mutant plasmid is compared to cells cotransfected with either miR mimic or anti-miR-648. The data represent the means ± the SEM of three independent experiments. ***, P < 0.001; **, P <0.01; *, P <0.05; ns, not significant.
As a final demonstration that miR-648 was interacting with a target site residing in the 3′ UTR of ET-1 mRNA, we introduced base substitutions in the corresponding miR-648 seed sequence target of the pGL3-ET-1-3′-UTR reporter as depicted in Fig. 4A; this mutant plasmid is referred to as pGL3-ET-1(mut)-3′-UTR. As shown in Fig. 4C, cotransfection of miR-648 with the mutant reporter did not significantly affect basal luciferase expression (Fig. 4C, lane 3 versus lane 2). In addition, transfection of anti-miR-648 with pGL3-ET-1(mut)-3′-UTR also did not augment luciferase activity under basal conditions in HMEC-1 cells (Fig. 4C, lane 4 versus lane 2). Taken together, these data showed that miR-648 directly targeted the 3′ UTR of ET-1 mRNA for turnover and subsequently downregulated the expression of ET-1.
Transcription of miR-648, located in an intron of MICAL3, is coregulated with MICAL3.
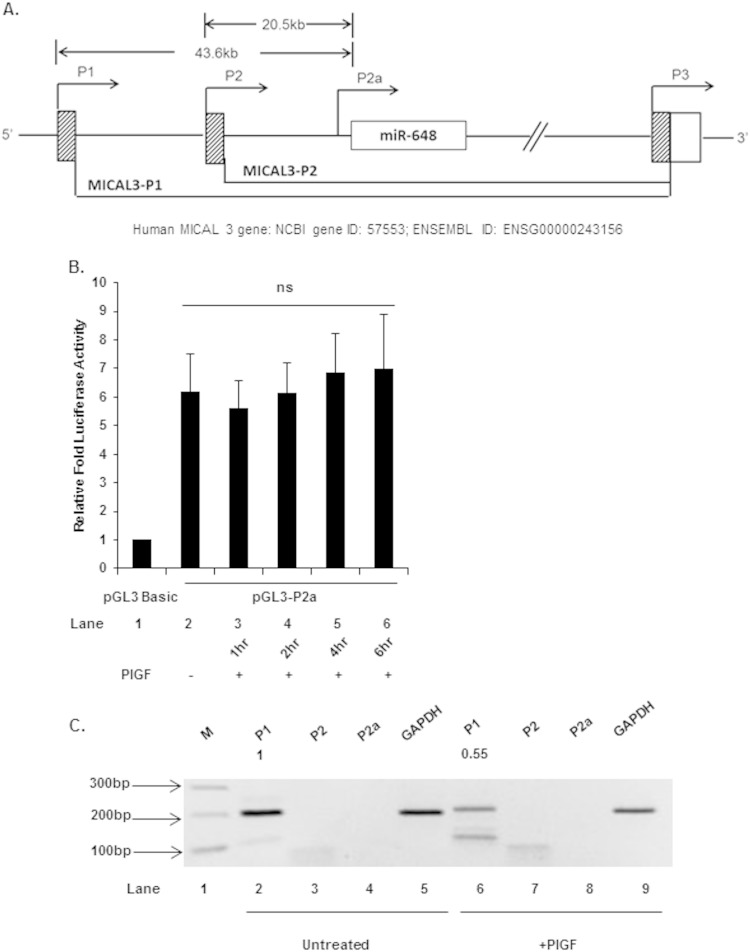
The gene for miR-648 is located in the first intron of MICAL3, encoding a member of the MICAL family of flavoprotein monooxygenases, implicated in axon guidance and actin remodeling (35, 36). More recently, MICAL3 has been shown to cooperate with Rab6 and Rab8 in exocytosis events (29). The orientation and location of the miR-648 gene indicates that it is transcribed from the same DNA template strand as MICAL3, thus it is likely that pre-miR-648 is within a MICAL3 transcription unit (Fig. 5A). There are at least three functional promoters (P1, P2, and P3) associated with the MICAL3 locus (Fig. 5A) and at least 19 possible primary transcripts (ENSEMBL). Not all of the primary transcripts encode a protein, suggesting that these may serve some other physiological function (37).
FIG 5.
Roles of P1, P2, and internal P2a promoter in transcription of MICAL3. (A) Schematic of 5′ region of MICAL3 showing promoters P1 and P2 and internal promoter P2a, the location of the 5′-proximal exons, and intronic miR-648. Lower lines indicate splicing patterns of transcripts initiated at either P1 (distal) or P2 (medial) promoters. (B) Effect of PlGF treatment on internal promoter P2a luciferase activity. Cells were transfected with pGL3-P2a luc plasmid (1 μg), followed by treatment with PlGF for the indicated time periods. (C) Quantitation of P1, P2, and P2a transcripts and GAPDH transcript in untreated and PlGF-treated cells, as analyzed by gel electrophoresis and ethidium bromide staining. The data represent means ± the SEM of three independent experiments.
The apparent functional role of miR-648 in destabilizing ET-1 mRNA suggested to us that a transcriptional mechanism was responsible for regulating miR-648 expression. A priori, constitutive basal expression of miR-648 leads to destabilization of ET-1 mRNA; PlGF signaling leads to decreased miR-648 synthesis by downregulating transcription factor activity or by repression of the transcription unit(s). Primary transcripts predicted to contain miR-648 initiate from distal (P1) and medial (P2) promoters. Thus, we examined whether PlGF treatment of endothelial cells affected these potential promoters for miR-648 transcription. Synthesis of miR-648 would, by default, follow transcription through the MICAL3 intronic region.
Given the large distance between the P1-TSS and miR-648, it was possible that a cryptic promoter proximal to miR-648 was actually responsible for its synthesis. In order to answer this question, we subcloned the ∼2-kb DNA fragment, 5′ adjacent to miR-648, into pGL3-Basic luciferase reporter (pGL3-P2a) to determine the presence of a potential internal promoter. As shown in Fig. 5B, this ∼2-kb DNA fragment in pGL3-Basic exhibited a strong, 6-fold expression of luciferase activity in HMEC-1 cells compared to the promoterless pGL3-Basic vector (Fig. 5B lane 2 versus lane 1). This indicated the ∼2-kb MICAL3 gene fragment has a functional DNA region with promoter activity. This promoter (provisionally named P2a) was tested for any change in activity in response to PlGF. HMEC-1 cells transfected with pGL3-P2a luciferase reporter, upon treatment with PlGF, showed no change in luciferase activity from 1 to 6 h post-PlGF addition, compared to the untreated control (Fig. 5B, lanes 3 to 6 versus lane 2). Since we observed a strong reduction of miR-648 in response to PlGF treatment, as previously described (Fig. 1C), it is unlikely that P2a is responsible for this reduction. Alternatively, it was also possible that a repressive element(s) was excluded from the ∼2-kb gene segment that was tested for promoter activity.
In order to determine the relative transcription activities of P1, P2, and P2a, a semiquantitative RT-PCR experiment of endogenous RNA was performed with primers targeting the first exons of P1, P2 and P2a RNA products. Using limiting RT-cDNA substrate, P1 RNA product showed the strongest signal (product size, 183 bp), while primers for detection of P2 showed modest signal (product size, 102 bp), and P2a RNA did not yield amplimer products (product size, 173 bp) (Fig. 5C). These results indicated that P1 promoter was ∼12-fold more active than P2 promoter under basal conditions. Moreover, the P1 RNA product showed an ∼45% reduction after PlGF treatment compared to the untreated control (Fig. 5C, lane 6 versus lane 2). Primers detecting GAPDH, used as an internal control (Fig. 5C, lanes 5 and 9) showed any changes were not the result of uneven sample handling or PCR analysis. Taken together, these results indicate that P1 is likely to be the promoter controlling transcription of miR-648. Using nonlimiting RT-cDNA substrate to detect alternative promoter usage, 102- and 173-bp PCR products were detectable for P2 and P2a transcripts, respectively, with the appropriate primers (data not shown). Since neither product was observed under limiting substrate conditions, we concluded that although minimally functional, P2 and P2a were not responsible for the bulk of miR-648 synthesis compared to P1 in HMEC-1 cells.
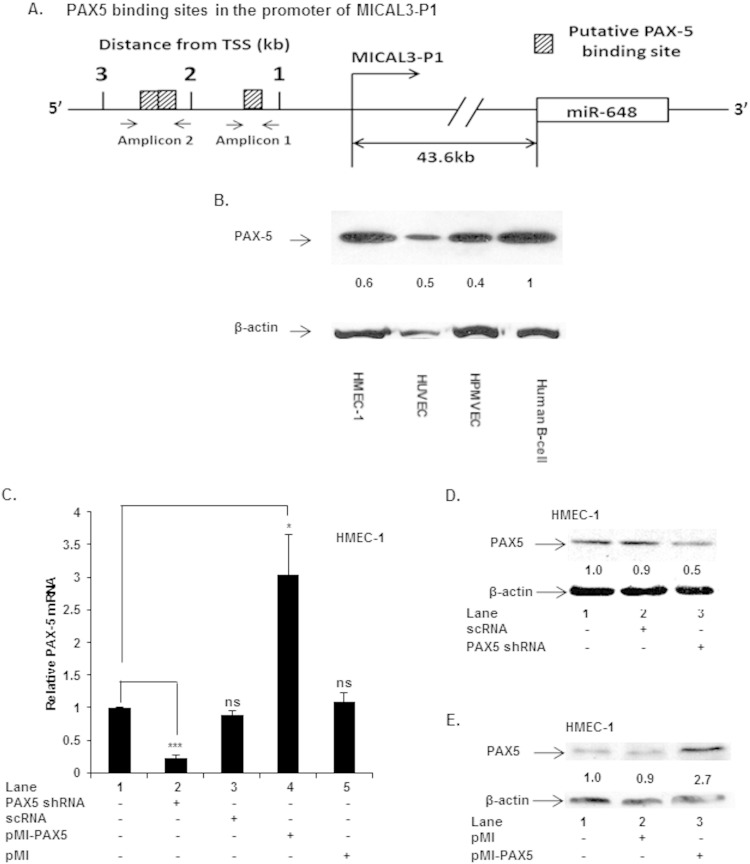
A bioinformatics analysis (TRANSFAC) of the MICAL3 locus revealed the presence of several transcription factor binding motifs, e.g., sites for PAX5, SMAD3, HNF3A, and HNF4A, upstream of the P1 promoter. Since one or more of these transcription factors could potentially regulate transcription of the MICAL3 locus, we focused on PAX5, since in silico analysis revealed the presence of three putative binding sites for PAX5 (Fig. 6A) nearest the P1-TSS of MICAL3.
FIG 6.
Expression of PAX5 in endothelial cells. (A) Schematic showing PAX5 binding sites in the 5′ region of MICAL3 P1 promoter. (B) Western blots of cell lysates from indicated cells, showing expression of PAX5 protein. (C to E) Effects of silencing and gain of function of PAX5 on PAX5 mRNA and protein. HMEC-1 was transfected with PAX5 shRNA or expression plasmid pMI-PAX5. ***, P < 0.001; *, P <0.05; ns, not significant.
We first determined whether PAX5 was present in cultured endothelial cells. Western blot analysis of cell lysates obtained from HMEC-1, HUVEC, and HPMVEC were all positive for expression of PAX5 protein; a B cell lysate was used as a positive control for this factor (Fig. 6B). Previous reports indicate PAX5 is predominantly involved in B cell lineage differentiation (38, 39), although its expression is observed in other hematopoietic cells, cancers, and neural cell types (40–42).
In order to establish a possible role of PAX5 in MICAL3 transcription, we performed loss and gain of PAX5 function experiments in HMEC-1 cells. PAX5 was knocked down by transfection of shRNA expression vector specific for PAX5 (PAX5 shRNA), while overexpression of PAX5 was accomplished by transfection of an exogenous gene copy (pMI-PAX5). First, we determined whether these plasmids were functional in modulating PAX5 at both mRNA and protein levels. Transfection with PAX5 shRNA reduced endogenous PAX5 mRNA levels (Fig. 6C, lane 2 versus lane 1), while ectopic PAX5 expression in HMEC-1 increased intracellular levels of PAX5 mRNA (Fig. 6C, lane 4 versus lane 1). Moreover, PAX5 shRNA attenuated PAX5 protein levels by ∼50% (Fig. 6D, lane 3 versus lane 1), while PAX5 expression plasmid augmented PAX5 protein levels by ∼170% compared to untreated cells (Fig. 6E, lane 3 versus lane 1). These results clearly showed PAX5 is present in an endothelial cell line (HMEC-1) under basal conditions.
miR-648 is cotranscribed with MICAL3 P1 transcript and regulated by PAX5 transcription factor.
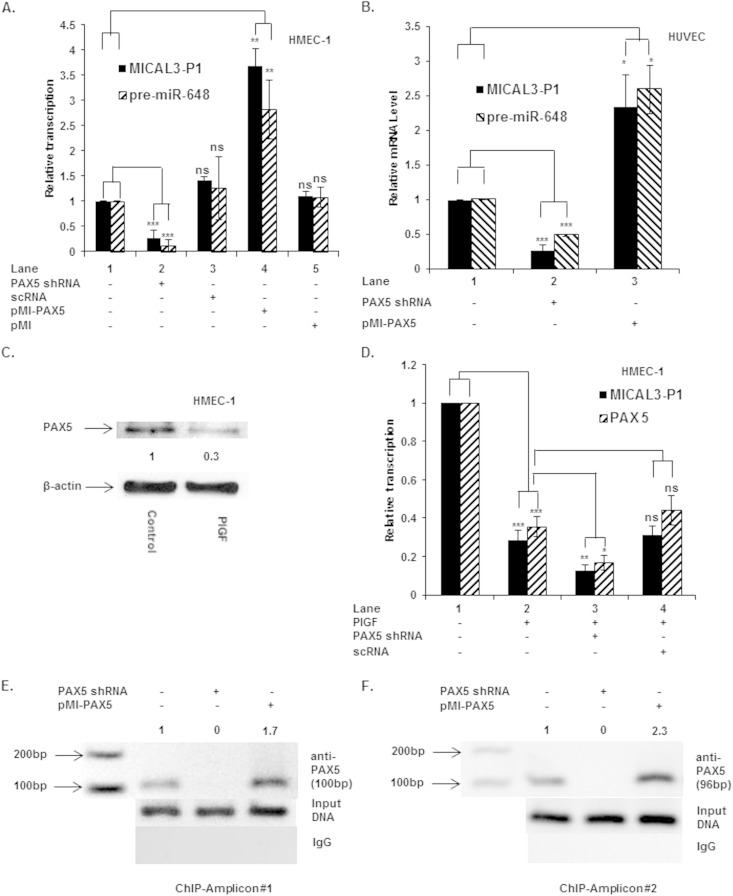
We examined the effect of PAX5 knockdown and its overexpression on transcription from the MICAL3 P1 promoter. As discussed above, transcription from P1 was responsible for miR-648 synthesis. Under basal conditions, the P1 transcript(s) was significantly reduced (∼60%) by PAX5 shRNA (Fig. 7A, lane 2 versus lane 1), while overexpression of PAX5, utilizing pMI-PAX5 plasmid, increased P1 utilization by ∼3.5-fold relative to control (Fig. 7A, lane 4 versus lane 1). Transfection with either control scRNA or pMI empty vector did not significantly affect levels of MICAL3 P1 promoter transcript(s) (Fig. 7A, lanes 3 and 5 versus lane 1). Taken together, these data indicated PAX5 was required for initiating transcription from the distal (P1) promoter of MICAL3.
FIG 7.
PAX5 regulates transcription from MICAL3 P1 promoter and expression of pre-miR-648. (A and B) Cells were transfected with PAX5 shRNA and pMI-PAX5 expression plasmid or scrambled RNA. RNA was isolated, and transcripts were quantified by using qRT-PCR in HMEC (A) and HUVEC (B). (C) PlGF treatment of HMEC reduced the PAX5 protein level. (D) Treatment of HMEC with PlGF reduced the expression of PAX5 and MICAL3 P1 promoter transcripts, which was further reduced by PAX5 shRNA. The data represent results from three independent experiments. (E and F) In vivo association of PAX5 with PAX5 sites proximal to P1 (as previously shown in Fig. 6A). Amplicons 1 (expected PCR product of 100 bp) and 2 (expected PCR product of 94 bp) were detected, indicating PAX5 occupancy. PCR primers used to detect and amplify immunoprecipitated DNA containing a PAX5 site(s) in the MICAL3 promoter are indicated in Table 1. The data are representative of two independent experiments. ***, P < 0.001; **, P <0.01; *, P <0.05; ns, not significant.
Furthermore, we examined whether PAX5 transcriptionally regulated expression of pre-miR-648 in cultured human endothelial cells. We assumed that splicing of MICAL3 pre-mRNAs was an obligatory event leading to pri-miR-648 and pre-miR-648 synthesis, barring any regulation of posttranscriptional processing or nuclear export of the pre-miRNA. Transfection of HMEC-1 with PAX5 shRNA reduced pre-miR-648 expression by >90% (Fig. 7A, lane 2 versus lane 1), while overexpression of PAX5 (pMI-PAX5) augmented pre-miR-648 by >2.5-fold relative to control (Fig. 7A, lane 4 versus lane 1). Transfection of HMEC-1 with either sc-RNA or empty vector (pMI) had no significant effect on pre-miR-648 expression (Fig. 7A, lanes 3 and 5 versus lane 1).
Next, we extended these observations to confirm whether PAX5 was involved in MICAL3 and pre-miR-648 transcription in primary human endothelial cells. Transfection of HUVEC with PAX5 shRNA as expected attenuated pre-miR-648 synthesis and MICAL3 P1 promoter transcripts (Fig. 7B). Conversely, transfection with PAX5 expression plasmid augmented pre-miR-648 synthesis and MICAL3 P1 promoter activity (Fig. 7B). Taken together, these data clearly showed that PAX5 was present in primary human endothelial cells (i.e., HUVEC) and was functional in regulating the expression of both MICAL3 and pre-miR-648 in primary human endothelial cells.
PlGF attenuates PAX5 levels and associated transcription from the MICAL3 P1 promoter.
In order to establish the role of PlGF and PAX5 in regulation of MICAL3 P1 transcription, HMEC-1 cells were analyzed for the expression of PAX5 in response to PlGF. PlGF treatment repressed levels of PAX5 protein ∼70% as shown by Western blotting of cell lysates (Fig. 7C), which correlated with an ∼65% decrease in levels of PAX5 mRNA, as indicated by qRT-PCR (Fig. 7D, lane 2 versus lane 1). The decreased expression of PAX5, following PlGF treatment, correlated with ∼70% reduction of transcription activity from MICAL3 P1 (Fig. 7D, lane 2 versus lane 1); as shown above, under these conditions, transcription of pre-miR-648 was reduced ∼80% (Fig. 1D). Furthermore, transfection of HMEC-1 with PAX5 shRNA, followed by PlGF treatment, further reduced PAX5 mRNA levels (Fig. 7D, lane 3 versus lane 2), whereas scRNA (nonspecific shRNA) had no such effect (Fig. 7D, lane 4 versus lane 2). Taken together, these results showed that PAX5 levels were reduced significantly by PlGF, which is consistent with the reduced levels of MICAL3 P1 transcript(s) and pre-miR-648, thus indicating that PAX5 is an important positive regulator of MICAL3 and miR-648 transcription and needed for basal level expression.
PAX5 binds to native chromatin for transcription of MICAL3 as demonstrated by ChIP analysis.
We began studies to establish the role of PAX5 in basal MICAL3 transcription by using ChIP analysis of this locus in uninduced HMEC-1 cells. These cells were also transfected with either PAX5 shRNA vector or PAX5 expression plasmid pMI-PAX5 and subjected to the same ChIP analysis. The predicted PAX5 binding sites are located at bases −1327 to −1323 (site 1), bases –2247 to −2243 (site 2), and bases −2312 to −2308 (site 3). Site 1 can be detected independently (as amplicon 1), whereas sites 2 and 3 are too close together to be resolved by PCR amplification (Fig. 6A). For this reason, amplicon 2 (bases −2331 to −2238) will detect occupancy of either sites 2 or 3 or both. As shown in Fig. 7E and F, this analysis showed that PAX5 was present on the MICAL3 promoter under basal conditions, based on recovery of amplicon 1 (100-bp product) and amplicon 2 (94-bp product) (Fig. 7E, lane 1, and Fig. 7F, lane 1, respectively). Knockdown of PAX5 by PAX5 shRNA vector completely abrogated recovery of amplicon 1 and 2 (Fig. 7E, lane 2, and Fig. 7F, lane 2, respectively), whereas cells overexpressing PAX5 showed ∼70 and ∼125% increases in amplicon 1 and 2 PCR products (Fig. 7E, lane 3, and Fig. 7F, lane 3, respectively). Chromatin immunoprecipitated with nonspecific IgG did not yield a MICAL3-specific PCR product (Fig. 7E and F, bottom panels). Furthermore, the input DNA was equivalent in control-, PAX5 shRNA-, and pMI-PAX5-transfected chromatin samples (see the middle panels of Fig. 7E and F). Together, these data showed that PAX5 binding, proximal to P1, is correlated with basal MICAL3 and pre-miR-648 transcription in endothelial cells.
miR-648 levels in plasma from SCA patients.
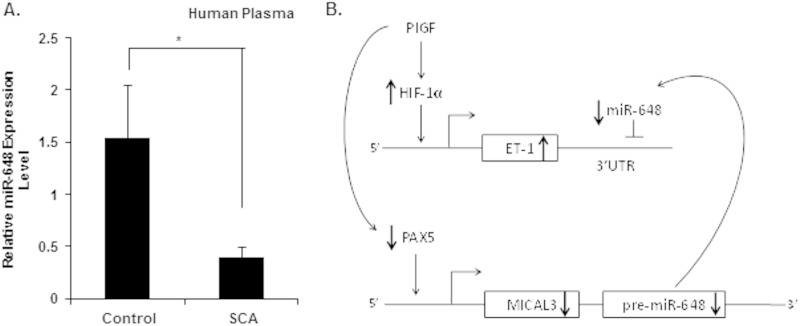
Our previous studies showed that PlGF levels are elevated in SCA patients and significantly correlated with increased plasma ET-1 levels and tricuspid regurgitant velocity; the latter is reflective of peak pulmonary artery pressure or an indicator of PHT. It is pertinent to mention that human miR-648 does not have a corresponding ortholog in mice; this precluded studies in animal models, e.g., Berkeley sickle mice or PlGF−/− mice. Since miR-648, in the present study, posttranscriptionally modulated ET-1 expression, we measured plasma levels of miR-648 in SCA patients, along with their unaffected sibling controls. The results showed circulating levels of miR-648 were detectable in samples from both SCA patients (n = 13) and controls (n = 13). However, plasma miR-648 levels were statistically different between SCA patients compared to unaffected controls (mean ± the SEM of 0.39 ± 0.10 in SCA patients versus 1.54 ± 0.50 in unaffected controls; P = 0.034) as indicated in Fig. 8A. The significantly lower plasma miR-648 levels in SCA patients compared to their controls are in agreement with the relationship between miR-648 and ET-1 observed in vitro. However, further studies are warranted in a larger population of SCA patients with various degrees of PHT to ascertain whether miR-648 is a valid biomarker of PHT in SCA patients.
FIG 8.
Plasma miR-648 levels in SCA and control subjects and working model of miR-648-mediated regulation of ET-1 expression. (A) The concentrations of miR-648 in plasma were quantified from SCA patients (n = 13) and healthy matched controls (n = 13), using specific TaqMan microRNA assay kit. The relative expression levels of miRNAs in these samples were normalized to endogenous miR-16. Means ± the SEM are indicated. (B) Working model of miR-648 mediated regulation of ET-1 expression via its binding to the 3′ UTR of ET-1 mRNA. miR-648 is colocated in the first intron of MICAL3, and the transcription of both MICAL3 and pre-miR-648 is regulated by PAX5, which binds and transactivates MICAL3 P1 promoter. In response to high circulating levels of PlGF in SCA, reduced PAX5 levels result in a decline of pre-miR-648 transcription and subsequent reduction of ET-1 mRNA turnover. As a consequence of these events, higher circulating levels of ET-1 will ensue in SCA. *, P <0.05.
DISCUSSION
This study extends our understanding of the regulatory mechanisms underlying expression of endothelin-1 and its association with pulmonary hypertension in sickle cell disease. PlGF treatment of endothelial cells resulted in a net stabilization of ET-1 mRNA from the basal state, and the mechanism for this change exhibited characteristics of an miRNA-based process. For this reason we examined whether expression of specific miRNAs predicted to bind to the 3′ UTR of ET-1 mRNA changed in endothelial cells upon PlGF treatment. From seven candidate miRNAs identified by bioinformatics analysis as potential effectors of ET-1 mRNA stability, we found that PlGF treatment significantly reduced the expression of miR-648 and miR-934. Of these, miR-648 was further analyzed based on the experimentally observed large reduction in response to PlGF. Thus, we reasoned that changes in endogenous miR-648 levels would be expected to have a significant effect on cytoplasmic ET-1 mRNA levels.
We tested the posttranscriptional regulation of ET-1 by miR-648. The specific downregulation of miR-648 in response to PlGF was linked to increased stabilization of ET-1 mRNA. This link was confirmed and extended using miR-648 mimic to decrease endogenous ET-1 under basal conditions and during PlGF induction. The anti-miR-648 abrogated basal turnover of ET-1 mRNA, demonstrating the importance of miR-648 in suppressing activity of this potent vasoconstrictor under these conditions. Finally, we demonstrated using a luciferase translation reporter that the primary sequence required for regulating ET-1 mRNA stability did indeed reside in the 3′ UTR of this mRNA. The synthetic luciferase reporter fused to the wt ET-1 3′ UTR behaved exactly like native ET-1 mRNA under basal and PlGF induction in endothelial cells. In contrast, mutation of the miR-648 recognition element in the 3′ UTR of the reporter completely nullified any effect of endogenous miR-648 or the synthetic mimic.
The synthesis of miR-648 afforded new insights into how this regulatory RNA is itself regulated at the level of transcription. The miR-648 gene is present in the first intron of MICAL3, encoding a member of the microtubule associated monooxygenase, calponin, and LIM domain-containing (MICAL) family of flavoprotein monooxygenases, which participate in axon guidance, actin remodeling (36, 43), and redox activity in promoting vesicle-docking complexes in the process of exocytosis (29). We sought to determine whether the precursor of this miRNA was derived from MICAL3 transcripts or arose from an independent transcription unit driven by an independent promoter. Although the MICAL3 locus is regulated by at least three promoters, giving rise to a total of 19 alternatively spliced transcripts (ENSEMBL), our results showed that the P1 (distal) promoter of MICAL3 was regulated and involved in the expression of the RNA precursor of miR-648.
We identified PAX5 as a transcription factor involved in the cotranscriptional regulation of MICAL3 and miR-648 utilizing loss and gain of PAX5 function approaches in endothelial cell line HMEC-1. The requirement for PAX5 in basal MICAL3 transcription was demonstrated by transfection of HMEC-1 and HUVEC with PAX5 shRNA, wherein the latter attenuated MICAL3 mRNA and miR-648 expression. Conversely, constitutive expression of PAX5 with exogenous PAX5 augmented MICAL3 transcription, indicating that PAX5 was indeed functional in regulating the transcription of this gene in endothelial cells.
These studies described above were extended by demonstrating enrichment of PAX5 binding to the proximal 2.5-kb segment upstream of the MICAL3 P1 promoter transcription start site (TSS), as shown by ChIP analysis. We actually identified seven potential PAX5 sites based on the consensus PAX5 binding motif, upstream of the MICAL3 P1 promoter. However, as the present study demonstrated, the three PAX5 sites nearest the TSS were sufficient to retain regulation by PlGF. Further studies will be needed to establish how PAX5 activity is downregulated in the context of PlGF induction and identification of additional bona fide PAX5 binding sites proximal to P1.
It was also of interest to learn that PAX5 protein is expressed in HUVEC, HMEC-1, and HPMVEC. Although PAX5 is well known as a B cell lineage-specific activator protein and required in B cell lineage development (38, 39), it is in fact expressed in almost all cell types (44). Thus, it would be of great interest to determine how PAX5 expression is regulated in endothelial cells in response to PlGF.
Our studies on ET-1 offer a second example of a bipartite mechanism for gene induction, analogous to one we showed for PAI-1 (42). In the ET-1 system, downregulation of miR-648 apparently increased steady-state levels of ET-1 mRNA, prior to the major increase in cytoplasmic levels of this mRNA, resulting from HIF-1α induction of the ET-1 gene. It remains to be seen whether this is a general cytoplasmic mechanism for rapid induction of physiological modifiers preceding de novo gene transcription needed for sustained expression.
In conclusion, our studies showed PlGF treatment of endothelial cells reduced levels of miR-648, which binds the 3′ UTR of ET-1 mRNA as a regulatory target. This posttranscriptional mechanism affecting turnover of ET-1 mRNA, as illustrated in the regulatory schematic (Fig. 8B), is proposed to be a means to negatively modulate ET-1 expression. The miR-648 gene is located in the first intron of MICAL3, and our studies showed its expression was cotranscriptionally regulated with MICAL3 by PAX5 from the distal promoter (P1; Fig. 6A). Under basal conditions, the levels of miR-648 are high, which maintain low levels of ET-1 mRNA and protein; conversely, in response to PlGF, the levels of miR-648 are reduced, leading to increased ET-1. The high levels of PlGF seen in SCD patients would have an expected outcome of low PAX5 activity, as observed in vitro. This would result in reduced expression of miR-648 concomitantly leading to higher ET-1 levels (Fig. 8B). Ideally, further studies are warranted in an animal model; however, human miR-648 does not have a corresponding ortholog in mouse; thus, this precludes studies in Berkeley sickle mice or PlGF−/− mice (9). Our limited observations in SCA patients showed significant differences in levels of miR-648 in plasma compared to unaffected siblings as controls (Fig. 8A). We feel further studies are needed in a larger SCA population to test the hypothesis that miRNAs can be used as prognostic biomarkers for pulmonary hypertension in SCA patients.
ACKNOWLEDGMENTS
This study was supported by grant R01-HL111372 from the National Heart, Lung, and Blood Institute (NHLBI) and by grant P30-DK048522 from the Analytical-Metabolic Instrumentation Core, University of Southern California Research Center for Liver Disease.
The content is solely the responsibility of authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health.
We thank Zhixin Zhang, University of Nebraska Medical Center, Omaha, NE, for generously providing the PAX5 expression plasmid. We thank Alan Epstein, University of Southern California, for providing the human B-cell line.
REFERENCES
- 1.Rubanyi GM, Polokoff MA. 1994. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 46:325–415. [PubMed] [Google Scholar]
- 2.Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, Duriez P, Staels B. 1999. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res 85:394–402. doi: 10.1161/01.RES.85.5.394. [DOI] [PubMed] [Google Scholar]
- 3.Kedzierski RM, Yanagisawa M. 2001. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol 41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 4.Barton M, Yanagisawa M. 2008. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol 86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- 5.Hu J, Discher DJ, Bishopric NH, Webster KA. 1998. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun 245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. 2001. Molecular regulation of the endothelin-1 gene by hypoxia: contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, and p300/CBP. J Biol Chem 276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- 7.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. 2012. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA 307:1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca GHH, Souza R, Salemi VMC, Jardim CVP, Gualandro SFM. 2012. Pulmonary hypertension diagnosed by right heart catheterization in sickle cell disease. Eur Respir J 39:112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 9.Sundaram N, Tailor A, Mendelsohn L, Wansapura J, Wang X, Higashimoto T, Pauciulo MW, Gottliebson W, Kalra VK, Nichols WC, Kato GJ, Malik P. 2010. High levels of placenta growth factor in sickle cell disease promote pulmonary hypertension. Blood 116:109–112. doi: 10.1182/blood-2009-09-244830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro O, Gladwin MT. 2005. Pulmonary hypertension in sickle cell disease: mechanisms, diagnosis, and management. Hematol Oncol Clin N Am 19:881–896. doi: 10.1016/j.hoc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. 2007. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 109:3088–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, Sohier C, Hinderliter A, Parise LV, Orringer EP. 2008. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica 93:20–26. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 13.Morris CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, Vichinsky EP, Shigenaga M, Ames B, Kuypers FA, Klings ES. 2008. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood 111:402–410. doi: 10.1182/blood-2007-04-081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladwin MT, Vichinsky E. 2008. Pulmonary complications of sickle cell disease. N Engl J Med 359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 15.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. 2009. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol 84:618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE. 2010. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 116:687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 17.Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, Hagar RW, Howard T, Nuss R, Okam MM, Tremonti CK, Berman B, Villella A, Krishnamurti L, Lanzkron S, Castro O, Gordeuk VR, Coles WA, Peters-Lawrence M, Nichols J, Hall MK, Hildesheim M, Blackwelder WC, Baldassarre J, Casella JF, De NI. 2011. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 305:893–902. doi: 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybicki AC, Benjamin LJ. 1998. Increased levels of endothelin-1 in plasma of sickle cell anemia patients. Blood 92:2594–2596. [PubMed] [Google Scholar]
- 19.Kisanuki YY, Emoto N, Ohuchi T, Widyantoro B, Yagi K, Nakayama K, Kedzierski RM, Hammer RE, Yanagisawa H, Williams SC, Richardson JA, Suzuki T, Yanagisawa M. 2010. Low blood pressure in endothelial cell-specific endothelin 1 knockout mice. Hypertension 56:121–128. doi: 10.1161/HYPERTENSIONAHA.109.138701. [DOI] [PubMed] [Google Scholar]
- 20.Benza RL. 2008. Pulmonary hypertension associated with sickle cell disease: pathophysiology and rationale for treatment. Lung 186:247–254. doi: 10.1007/s00408-008-9092-8. [DOI] [PubMed] [Google Scholar]
- 21.Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbabi H, Galaup A, Maier-Redelsperger M, Vandermeersch S, Scarpa A, Janin A, Levy B, Girot R, Beuzard Y, Leboeuf C, Henri A, Germain S, Dussaule JC, Tharaux PL. 2008. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest 118:1924–1933. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perelman N, Selvaraj SK, Batra S, Luck LR, Erdreich-Epstein A, Coates TD, Kalra VK, Malik P. 2003. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood 102:1506–1514. doi: 10.1182/blood-2002-11-3422. [DOI] [PubMed] [Google Scholar]
- 23.Tordjman R, Delaire S, Plouet J, Ting S, Gaulard P, Fichelson S, Romeo PH, Lemarchandel V. 2001. Erythroblasts are a source of angiogenic factors. Blood 97:1968–1974. doi: 10.1182/blood.V97.7.1968. [DOI] [PubMed] [Google Scholar]
- 24.Tordjman R, Delaire S, Plouët J, Ting S, Gaulard P, Fichelson S, Roméo P-H, Lemarchandel V. 2001. Erythroblasts are a source of angiogenic factors. Blood 97:1968–1974. doi: 10.1182/blood.V97.7.1968. [DOI] [PubMed] [Google Scholar]
- 25.Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. 2003. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood 102:1515–1524. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- 26.Patel N, Gonsalves CS, Malik P, Kalra VK. 2008. Placenta growth factor augments endothelin-1 and endothelin-B receptor expression via hypoxia-inducible factor-1α. Blood 112:856–865. doi: 10.1182/blood-2007-12-130567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel N, Gonsalves CS, Yang M, Malik P, Kalra VK. 2009. Placenta growth factor induces 5-lipoxygenase-activating protein to increase leukotriene formation in sickle cell disease. Blood 113:1129–1138. doi: 10.1182/blood-2008-07-169821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel N, Sundaram N, Yang M, Madigan C, Kalra VK, Malik P. 2010. Placenta growth factor (PlGF), a novel inducer of plasminogen activator inhibitor-1 (PAI-1) in sickle cell disease (SCD). J Biol Chem 285:16713–16722. doi: 10.1074/jbc.M110.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grigoriev I, Yu KL, Martinez-Sanchez E, Serra-Marques A, Smal I, Meijering E, Demmers J, Peranen J, Pasterkamp RJ, van der Sluijs P, Hoogenraad CC, Akhmanova A. 2011. Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr Biol 21:967–974. doi: 10.1016/j.cub.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. 2006. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J Immunol 177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- 31.Patel N, Tahara SM, Malik P, Kalra VK. 2011. Involvement of miR-30c and miR-301a in immediate induction of plasminogen activator inhibitor-1 by placental growth factor in human pulmonary endothelial cells. Biochem J 434:473–482. doi: 10.1042/BJ20101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang J, Ramu S, Lee S, Aguilar B, Ganesan SK, Yoo J, Kalra VK, Koh CJ, Hong YK. 2009. Phosphate-buffered saline-based nucleofection of primary endothelial cells. Anal Biochem 386:251–255. doi: 10.1016/j.ab.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Yang P, Xiong Q, Song X, Yang X, Liu L, Yuan W, Rui YC. 2010. MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J Hypertens 28:1646–1654. doi: 10.1097/HJH.0b013e32833a4922. [DOI] [PubMed] [Google Scholar]
- 34.Yeligar SM, Machida K, Kalra VK. 2010. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. J Biol Chem 285:35359–35373. doi: 10.1074/jbc.M110.138636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolk SM, Pasterkamp RJ. 2007. MICAL flavoprotein monooxygenases: structure, function, and role in semaphorin signaling. Adv Exp Med Biol 600:38–51. doi: 10.1007/978-0-387-70956-7_4. [DOI] [PubMed] [Google Scholar]
- 36.Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJ, Terman JR. 2010. Mical links semaphorins to F-actin disassembly. Nature 463:823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JT. 2012. Epigenetic regulation by long noncoding RNAs. Science 338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- 38.He T, Hong SY, Huang L, Xue W, Yu Z, Kwon H, Kirk M, Ding SJ, Su K, Zhang Z. 2011. Histone acetyltransferase p300 acetylates Pax5 and strongly enhances Pax5-mediated transcriptional activity. J Biol Chem 286:14137–14145. doi: 10.1074/jbc.M110.176289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cobaleda C, Schebesta A, Delogu A, Busslinger M. 2007. Pax5: the guardian of B cell identity and function. Nat Immunol 8:463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- 40.Gerard M, Abitbol M, Delezoide AL, Dufier JL, Mallet J, Vekemans M. 1995. PAX-genes expression during human embryonic development, a preliminary report. C R Hebd Seances Acad Sci III 318:57–66. [PubMed] [Google Scholar]
- 41.O'Brien P, Morin P Jr, Ouellette RJ, Robichaud GA. 2011. The Pax-5 gene: a pluripotent regulator of B-cell differentiation and cancer. Dis Cancer Res 71:7345–7350. doi: 10.1158/0008-5472.CAN-11-1874. [DOI] [PubMed] [Google Scholar]
- 42.Zhao GJ, Xu LX, Chu ESH, Zhang N, Shen JY, Damirin A, Li XX. 2012. Establishment of an orthotopic transplantation tumor model of hepatocellular carcinoma in mice. World J Gastroenterol 18:7087–7092. doi: 10.3748/wjg.v18.i47.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. 2002. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 109:887–900. doi: 10.1016/S0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 44.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G. 2004. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]