Abstract
The assembly of antigen receptor loci requires a developmentally regulated and lineage-specific recombination between variable (V), diversity (D), and joining (J) segments through V(D)J recombination. The process is regulated by accessibility control elements, including promoters, insulators, and enhancers. The IgH locus undergoes two recombination steps, D-JH and then VH-DJH, but it is unclear how the availability of the DJH substrate could influence the subsequent VH-DJH recombination step. The Eμ enhancer plays a critical role in V(D)J recombination and controls a set of sense and antisense transcripts. We epigenetically perturbed the early events at the IgH locus by inserting the imprinting control region (ICR) of the Igf2/H19 locus or a transcriptional insulator devoid of the imprinting function upstream of the Eμ enhancer. The insertions recapitulated the main epigenetic features of their endogenous counterparts, including differential DNA methylation and binding of CTCF/cohesins. Whereas the D-JH recombination step was unaffected, both the insulator insertions led to a severe impairment of VH-DJH recombination. Strikingly, the inhibition of VH-DJH recombination correlated consistently with a strong reduction of DJH transcription and incomplete demethylation. Thus, developmentally regulated transcription following D-JH recombination emerges as an important mechanism through which the Eμ enhancer controls VH-DJH recombination.
INTRODUCTION
Monoallelic gene expression in mammals is a hallmark of various complex processes, including X-chromosome inactivation in female cells, genomic imprinting, and allelic exclusion at antigen receptor loci (1, 2). In genomic imprinting, the allelic expression of a subset of genes depends on the parental origin of the allele and is controlled by specialized cis-regulatory elements called imprinting control regions (ICRs) (3). One of the best-studied imprinted domains is the Igf2/H19 locus, which is regulated by an ICR methylated on its paternally inherited copy. The unmethylated maternal copy is bound by the zinc finger protein CTCF and cohesins, leading to the insulation of the flanking Igf2 gene from enhancer sequences located downstream of the H19 gene (3).
Although X-chromosome inactivation, genomic imprinting, and allelic exclusion use common epigenetic mechanisms (1), they clearly display different features as well. Particularly, allelic exclusion additionally involves a unique developmentally regulated recombination process in B and T lymphocytes, called V(D)J recombination. This process is catalyzed by the lymphoid-specific recombinase complex RAG1/RAG2, which recognizes conserved recombination signal sequences (RSSs) flanking the variable (V), diversity (D), and joining (J) segments in the variable domain of antigen receptor (IgH, IgL, and TCR) loci (4–6).
The ∼3-Mb mouse IgH locus contains 195 VH genes spanning ∼2.5 Mb (7). The VH genes are subdivided into VH gene families, including the distal VH genes (VHJ558 and VH3609) and the proximal VH genes (VHQ52 and VH7183). The VH genes are followed by a dozen D segments (∼60 kb), 4 JH segments (∼2 kb), and 8 constant genes (∼200 kb) (7, 8).
The generation of the primary immunoglobulin repertoire involves developmentally ordered recombination steps. D-JH recombination occurs on both IgH alleles, before the initiation of VH-DJH joining on one of the two IgH alleles only. If productive, this produces a μ heavy chain that signals the arrest of the rearrangement on the opposite IgH allele (4, 6).
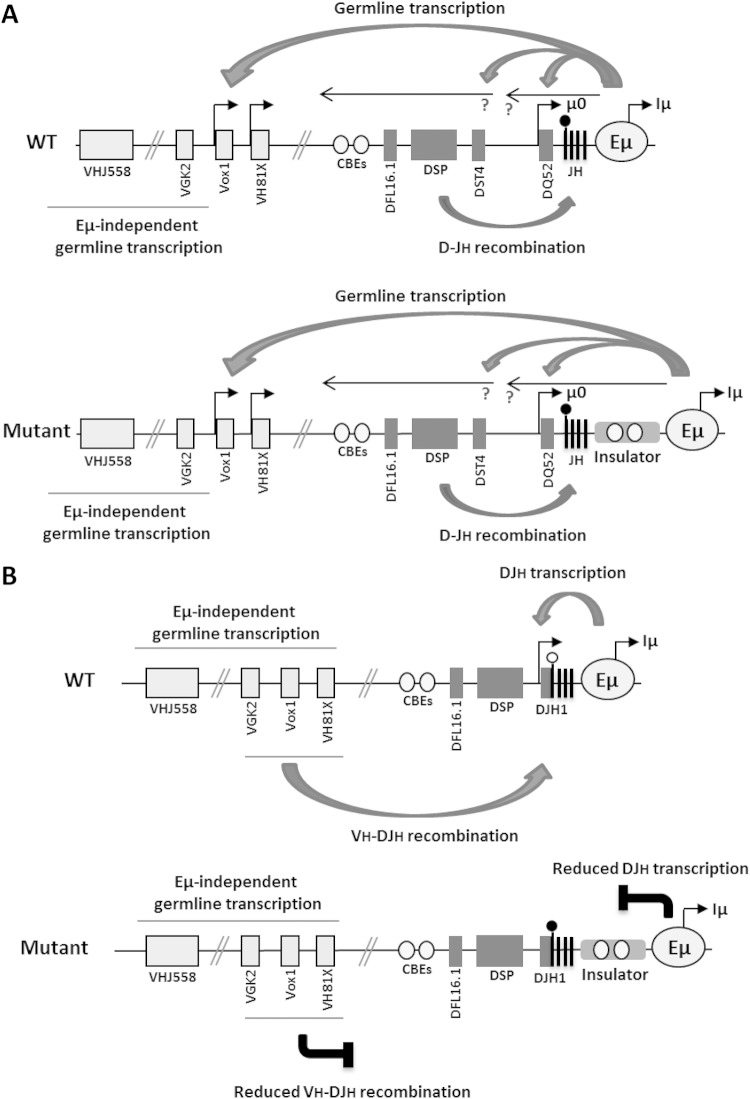
The ordered rearrangement of the IgH gene segments is associated with various transcriptional events and chromatin modifications and is controlled to a large extent by accessibility control elements, including enhancers, insulators, and promoters, in a cell type- and developmental stage-specific manner (4, 9). Two regulatory elements in the IgH locus were shown to control V(D)J recombination. The Eμ enhancer, located between the variable and the constant domains, plays a critical role in V(D)J recombination and associated germ line transcription. Deletion of Eμ affects D-JH recombination partially and VH-DJH recombination much more severely (10, 11). Eμ also controls the expression of a set of sense transcripts (STs) and antisense transcripts (ASTs) at specific sites of the IgH variable locus (10–14). The STs include Iμ transcripts derived from the Eμ enhancer and μ0 transcripts initiated from the DQ52 promoter upstream of the 3′-most D segment. ASTs are initiated within the JH-Eμ region (12, 14) and from an ill-defined antisense promoter within the D cluster (15). Deletion of Eμ also affects germ line transcription of the 3′-most VH segments of the proximal VH domain (13).
Additionally, CTCF-binding elements (CBEs) with insulator activity were identified between the VH and the D clusters (16, 17). Deletion of these CTCF sites within this intergenic control region (IGCR1) led to increased germ line transcription and recombination of the proximal VH genes and perturbed the order and the cell type specificity of V(D)J recombination, as well as feedback regulation of the proximal VH segments (18).
The IgH locus undergoes two recombination steps, D-JH and then VH-DJH recombination, and it remains unknown how the status of the DJH substrate influences the subsequent VH-DJH recombination step and what the role of the Eμ enhancer in this process could be. To address this key question, we inserted upstream of the Eμ enhancer either the ICR of the imprinted Igf2/H19 locus or the transcriptional insulator of the chicken β-globin locus (cHS4), devoid of imprinting function, and analyzed their effects on V(D)J recombination and associated germ line transcription. We found that both the insulators strongly affect VH-to-DJH recombination in developing B cells. This marked phenotype was linked to pronounced transcriptional changes and aberrant DNA methylation at the DJH segment following D-JH recombination. This main finding strongly suggests that local transcriptional and epigenetic changes following the first step of recombination modulate the occurrence of the subsequent VH-to-DJH recombination step.
MATERIALS AND METHODS
Mice.
The experiments on mice were carried out according to CNRS ethical guidelines and approved by the Midi-Pyrénées Regional Ethical Committee.
Gene targeting.
For the targeting constructs, a ∼2.4-kb BglII fragment containing the 4 CTCF sites of the ICR or a ∼2.6-kb XbaI fragment containing 2 cHS4 CTCF sites was first cloned into the BamHI site or the XbaI site of a modified pBluescript II KS(−) vector (Stratagene) containing an ∼1.3-kb floxed neomycin resistance [Neor] cassette. The inserts (ICR plus the Neor cassette or cHS4 plus the Neor cassette) were taken out as a blunt end or a ClaI fragment, respectively, and inserted into a blunt end or a ClaI site that replaced the unique NaeI site between Eμ and JH4 in the PA14 vector (kindly provided by M. Cogné, CNRS, Limoges, France) containing the sequence between the EcoRI site (upstream of DQ52) and AseI (in the 5′ part of Sμ). The AseI site was modified into the NotI site and subsequently used to insert the herpes simplex virus tk gene. The ES cell line CK35 (strain 129Sv; kindly provided by C. Kress, Institut Pasteur, Paris, France) was transfected by electroporation and selected using G418 (300 μg/ml) and ganciclovir (2 μM). Recombinant clones were identified by PCR and Southern blot analysis after BamHI (ICR construct) or KpnI (cHS4 construct) digestion with an external 3′ probe spanning the Cμ1 exon. Two ES clones showing homologous recombination were injected into C57BL/6 mouse blastocysts. The male chimera mice were then mated with C57BL/6 female mice in order to derive permanent mouse lines. Germ line transmission of the mutation was checked by PCR and Southern blotting after BamHI or KpnI digestion and by use of the same external probe. Homozygous N/N mutant mice were mated with EIIa-cre transgenic mice. The progeny were checked by PCR for Cre-mediated deletion of the Neor cassette. Additional checks were made by sequencing pertinent regions in the genomic DNA (see Fig. S1 in the supplemental material).
Antibodies.
Phycoerythrin (PE)-conjugated anti-IgMb, fluorescein isothiocyanate (FITC)-conjugated anti-IgMa, PE-conjugated anti-CD43, FITC-conjugated anti-κ, and FITC- and PE-conjugated anti-AA4.1 were purchased from BD Pharmingen. Allophycocyanin-conjugated anti-B220 and FITC- and PE-conjugated anti-IgM were from BioLegend. Anti-CTCF was purchased from Millipore, and anti-SMC1 was from Bethyl.
Fluorescence-activated cell sorting (FACS) analyses.
Single-cell suspensions from bone marrows and spleens from 6- to 8-week-old mice were prepared by standard techniques. Cells (5 × 105 cells/assay) were stained and gated as indicated in the appropriate figure legends. Data on 2.0 × 104 viable cells were obtained by using a BD FACSCalibur flow cytometer. Dead cells were excluded by labeling with propidium iodide.
DNA methylation analyses.
Splenic B cells were negatively sorted by using CD43 magnetic microbeads and LS columns (Miltenyi). Total bone marrow B cells were sorted by using CD19 magnetic microbeads and LS columns (Miltenyi). Genomic DNAs were extracted and subjected to methylation-sensitive restriction enzyme digestion (MSRED) followed by quantitative PCR (qPCR) or assayed by sodium bisulfite sequencing by using a bisulfite conversion kit (Active Motif). The primers used are listed in Table S1 in the supplemental material.
ChIP.
Chromatin was prepared from sorted splenic B cells, and chromatin immunoprecipitation (ChIP) was carried out as described previously (19).
V(D)J rearrangement assays.
Pro-B cells were sorted from bone marrow by using CD19 magnetic microbeads and LS columns (Miltenyi), labeled with anti-B220, anti-CD43, and anti-κ, and sorted as the B220-positive (B220+), κ-negative (κ−), and CD43high fraction. The purity of the sorted population was checked by FACS and by the rearrangement status of the κ locus. Splenic B cells were sorted by using CD43 magnetic microbeads and LS columns (Miltenyi). The CD4+ CD8+ thymocytes were sorted as described previously (18). Genomic DNAs from the sorted cell populations were prepared by standard techniques and diluted for the semiquantitative PCR or the qPCR assays. For quantification, agarose gels were dried for 1 h at 80°C using a gel dryer (Bio-Rad), stained with SYBR green I (Invitrogen) for 1 h, and scanned by using a PhosphorImager (Molecular Dynamics). After subtracting the background levels, the signals corresponding to the recombination products (DJH or VHDJH) in the diluted lanes were normalized against the HS4 signals. The histograms show the average for the four recombination products.
Reverse transcription-qPCR (RT-qPCR).
Pro-B cells from Rag2−/− mouse (strain 129Sv) bone marrow were sorted using CD19 magnetic microbeads (Miltenyi). Single IgMa- or IgMb-expressing B cells were sorted after staining with anti-B220+, anti-AA4.1-positive (anti-AA4.1+), and anti-IgMa (or anti-IgMb) antibodies. Total RNAs were reverse transcribed (Invitrogen) and subjected to semiquantitative PCR, using SYBR green I (Invitrogen) and ImageQuant software, or to qPCR, using Sso Fast Eva Green (Bio-Rad).
Statistical analysis.
Results are expressed as the mean ± standard error of the mean (GraphPad Prism), and overall differences between wild-type (WT) and mutant mice were evaluated by the analysis of variance parametric test with the Newman-Keuls posttest or the Kruskal-Wallis nonparametric test with Dunn's posttest. The difference between means was significant if the P value was <0.05, very significant if the P value was <0.01, and extremely significant if the P value was <0.001.
RESULTS
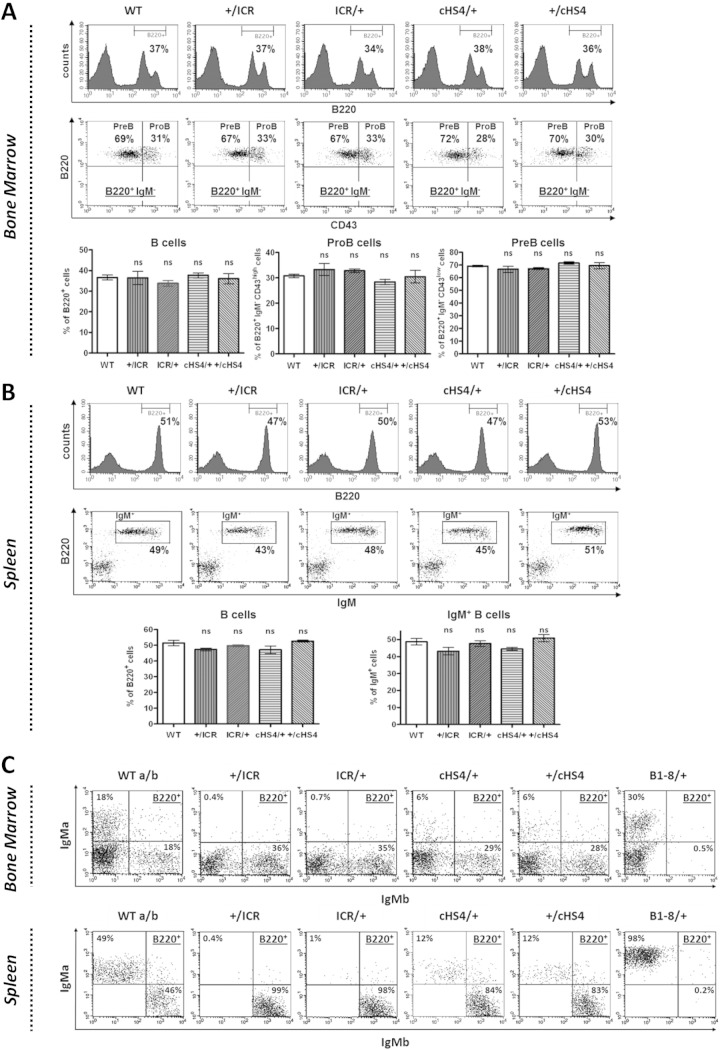
In the analyses described below, the homozygous mice are denoted ICR/ICR and cHS4/cHS4. In hemizygous mice, ICR/+ and +/ICR indicate the maternal and paternal origins of the mutant allele, respectively. Since no parent-of-origin effects on B cell development were found in cHS4 hemizygous mice (see Fig. 3; also see Fig. S3 in the supplemental material), analyses of these mice were performed regardless of the parental origin of the cHS4 insert.
FIG 3.
B cell development, allelic exclusion, and competition in hemizygous mice. (A) Single-cell suspensions from bone marrow with the indicated genotypes were stained with anti-B220 (top) or with anti-B220+, anti-CD43+, and anti-IgM (middle) and gated on the B220+ IgM-negative (IgM−) population. (Bottom) The histograms show the standard errors (n = 3). ns, not significant. (B) Single-cell suspensions from spleen with the indicated genotypes were stained with anti-B220 (top) or with anti-B220+ anti-IgM (middle). (Bottom) The histograms show the standard errors (n = 3). (C) Allelic exclusion. Single-cell suspensions from bone marrow (top) or spleen (bottom) of mice with the indicated genotypes were stained with anti-B220 and MAbs against the IgMa and IgMb allotypes and gated on the B220+ population (n = 6; for cHS4/+ cells, n = 2).
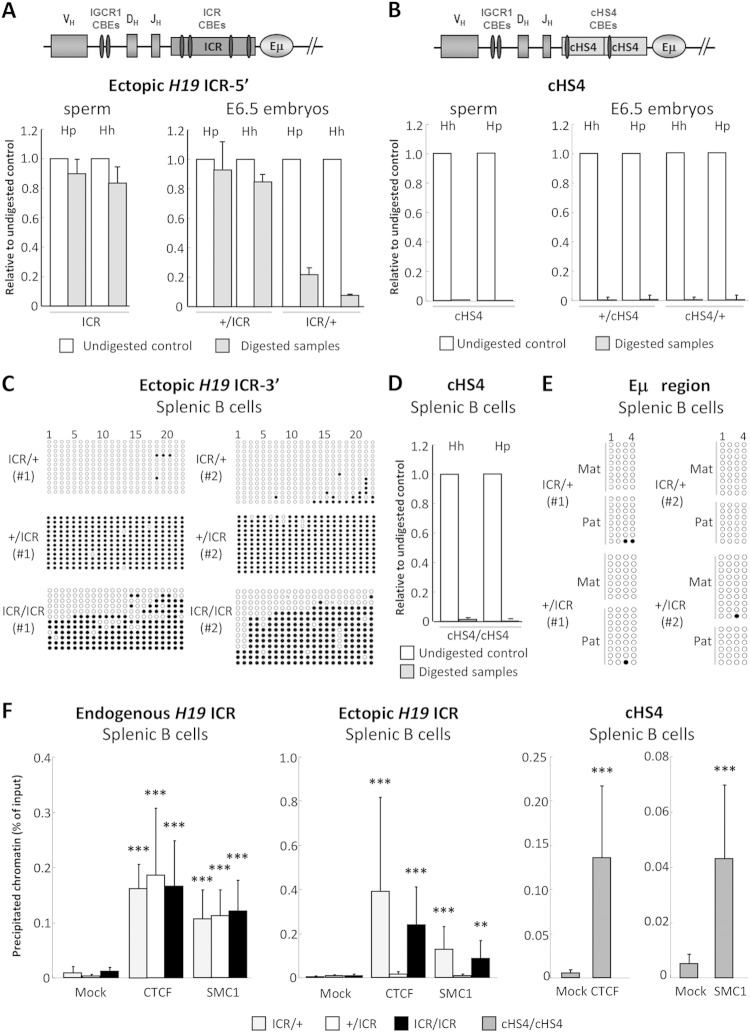
Acquisition of paternal allele-specific DNA methylation at the ectopic ICR.
Since the H19 ICR acquires paternal allele-specific DNA methylation during spermatogenesis (20), we determined whether the ectopically inserted H19 ICR also acquired parent-of-origin-specific DNA methylation. Genomic DNA from sperm and day 6.5 (E6.5) embryos was analyzed by methylation-sensitive restriction enzyme digestion followed by qPCR (MSRED/qPCR). We assayed two CpG dinucleotides at the upstream part of the ICR insert and found that these are fully methylated in sperm (Fig. 1A). This paternal methylation was stably maintained in E6.5 embryos (Fig. 1A). Upon maternal inheritance, hardly any DNA methylation was detected at the ectopic ICR in E6.5 embryos (Fig. 1A).
FIG 1.
DNA methylation and CTCF/cohesin binding at the ectopic ICR and cHS4. (A, B) The schemes at the top show the CBEs within the ectopic inserts and the upstream endogenous IGCR1 CBEs. Not all CBEs within the IgH locus are shown. Genomic DNAs from sperm (left) and E6.5 embryos (right) of ICR (A) and cHS4 mice (B) were analyzed by MSRED/qPCR at the 5′ part of the inserts. Hh, HhaI digestion; Hp, HpyCH4IV digestion. Note that after enzymatic digestion, the intact methylated DNA was quantified by qPCR by comparison to the undigested samples (n = 3). (C) Genomic DNAs from sorted ICR splenic B cells with the indicated genotypes were analyzed by bisulfite sequencing. Methylated (filled circles) and unmethylated (open circles) cytosines within 22 CpGs at the 3′ part of the ectopic H19 ICR from two independent experiments are displayed. (D) Genomic DNA from sorted cHS4/cHS4 splenic B cells was analyzed as described in the legend to panel B (n = 3). (E) Bisulfite sequencing of 4 CpGs downstream of Eμ in genomic DNAs from sorted hemizygous ICR splenic B cells. Mat, maternal; Pat, paternal. (F) CTCF and cohesin binding to the ectopic ICR and cHS4 in B cells. Chromatin from purified splenic B cells with the indicated genotypes was immunoprecipitated with anti-CTCF or anti-SMC1 antibodies. ChIP-qPCR was performed on the endogenous H19 ICR (left), the ectopic ICR (middle), and the ectopic cHS4 (right). The histograms show the standard deviations (n = 4 for ICRs, n = 3 for cHS4). ***, P < 0.001; **, P < 0.01.
The cHS4 insulator binds CTCF (21) but has no imprinting function and was reported to remain hypomethylated at ectopic sites (22, 23). Concordantly, at four analyzed CpGs at the 5′ part of the ectopic cHS4, there was an absence of DNA methylation in sperm and E6.5 embryos, regardless of the parental origin of the cHS4 insert (Fig. 1B).
DNA methylation and CTCF/cohesin binding at the ectopic ICR in B cells.
Our finding of imprinted DNA methylation at the ectopic ICR in embryos did not preclude the possibility that these marks could be lost during B cell development, for instance, through a demethylating activity of the Eμ enhancer (24, 25).
Therefore, we analyzed by bisulfite sequencing and MSRED/qPCR the DNA methylation pattern of the ectopic ICR and cHS4 in purified splenic cells. In hemizygous B cells, the ectopic ICR DNA was highly methylated only when inherited from the father (+/ICR) (Fig. 1C). In homozygous ICR/ICR B cells, concordantly, about 50% methylation was detected (Fig. 1C). The ectopic cHS4, in contrast, was fully unmethylated in homozygous B cells (Fig. 1D).
To ascertain that DNA methylation at the ectopic ICR had not spread into the Eμ region, we assessed by bisulfite sequencing four CpGs located at the 3′ side of Eμ. This did not reveal any DNA methylation in hemizygous B cells, showing that this region had remained unmethylated (Fig. 1E).
To investigate whether the ectopic ICR and cHS4 were bound by CTCF and SMC1 (a subunit of the cohesin complex), we performed chromatin immunoprecipitation (ChIP)-qPCR. Chromatin was prepared from either hemizygous or homozygous splenic B cells. As an internal control, we studied the endogenous ICR. We focused on the ICR's fourth CTCF-binding site and used different backward primers which distinguish the ectopic and the endogenous ICR. CTCF and SMC1 were readily immunoprecipitated from the endogenous ICR (Fig. 1F, left). Also, the maternally inherited copy of the ectopic ICR bound CTCF and SMC1, whereas the paternal copy did not (Fig. 1F, middle). Concordantly, CTCF and SMC1 were immunoprecipitated in homozygous ICR/ICR B cells as well (Fig. 1F, middle).
ChIP-qPCR of homozygous B cells revealed binding of CTCF and SMC1 to the ectopic cHS4 as well (Fig. 1F, right). Although we did not perform the assay allele specifically, the ChIP data, together with those on DNA methylation (Fig. 1D) and B cell development in hemizygotes (see below), led us to infer that the ectopic cHS4 bound CTCF and SMC1 regardless of its parental origin.
Together, these data show parental allele-specific DNA methylation and CTCF/SMC1 binding at the ectopic ICR, which was stably maintained in B cells. Also, the ectopic cHS4 bound CTCF/SMC1 and remained fully unmethylated in B cells.
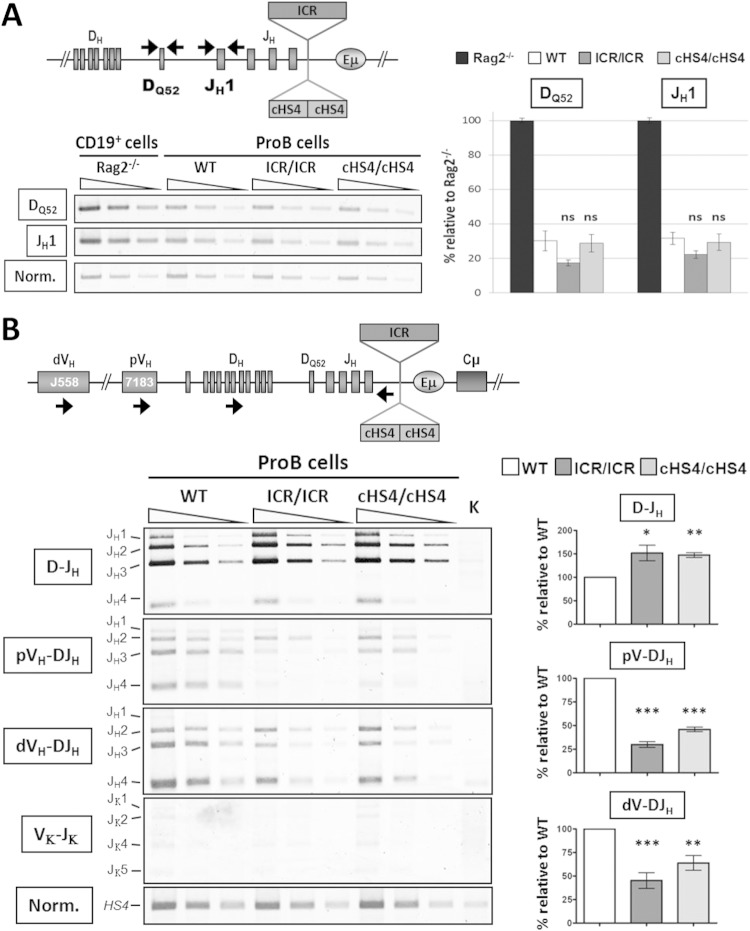
VH-DJH recombination, but not D-JH recombination, is altered in homozygous mutant mice.
Analysis of the bone marrow of homozygous mice revealed an accumulation of pro-B cells (see Fig. S3 in the supplemental material), suggesting that V(D)J recombination, normally completed at this developmental stage, was impaired. We therefore investigated if and at which step V(D)J recombination was compromised.
We quantified the proportion of the DQ52 and JH1 segments that had retained their unrearranged configuration (Fig. 2A, top). We found no difference in the total proportions of unrearranged DQ52 and JH1 segments between ICR, cHS4, and WT controls (Fig. 2A), suggesting that a similar number of alleles underwent D-JH recombination.
FIG 2.
V(D)J recombination is altered in homozygous mutant mice. (A) Genomic DNAs were prepared from sorted pro-B cells with the indicated genotypes and subjected to PCR to amplify unrearranged DQ52 and JH1 gene segments. The relative position of the primers is indicated in the scheme at the top. PCR products were detected and quantified using genomic DNA from Rag2−/− mice, which do not undergo V(D)J recombination, as a control (100% of the signal). PCR of the HS4 enhancer of the 3′ regulatory region was performed for normalization of the DNA input. (Left) Semiquantitative PCR was performed on serial 3-fold dilutions; (right) amplification of unrearranged DQ52 and JH1 gene segments by qPCR. The histograms show the standard errors (n = 4). ns, not significant. (B) Genomic DNAs were prepared from sorted pro-B cells and subjected to semiquantitative PCR to amplify D-JH, VH-DJH, and Vκ-Jκ rearrangements using primers that bind the indicated gene segments and primers that pair 3′ of JH4 for the IgH locus (scheme at the top) or 3′ of Jκ5 for the κ locus. dVH, distal VH; pVH, proximal VH. (Left) PCR was performed on serial 3-fold dilutions. Kidney (K) DNA was used as a negative control, and normalization (Norm.) was as described in the legend to panel A. (Right) Quantification of D-JH and VH-DJH rearrangements. The histograms show the standard errors (n = 6). ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Next, we performed a standard V(D)J recombination assay, which detects the recombined DJH segments. We used a forward primer that recognizes most D segments and a backward primer downstream of the JH4 segment (Fig. 2B, top). We found a slight accumulation of DJH segments in mutant pro-B cells (Fig. 2B, left and right), indicating that the D-JH recombination step was not impaired by the insertions.
We then asked whether the mutations affected VH-DJH recombination. For this, genomic DNA from sorted pro-B cells was subjected to semiquantitative PCR amplification of recombined VHDJH segments. A reduction of VH-DJH recombination was detected in ICR/ICR and cHS4/cHS4 pro-B cells at both proximal and distal VH genes. This reduction was most pronounced in ICR/ICR pro-B cells (Fig. 2B, left and right).
Thus, homozygous insertion of the ICR or the cHS4 upstream of Eμ was not by itself incompatible with V(D)J recombination. The slight accumulation of D-JH recombination events correlated with a reduction of VH-DJH recombination, with no evidence for a differential effect on proximal versus distal VH gene recombination. Moreover, insertion of the ICR or cHS4 upstream of Eμ did not perturb the order of rearrangements or the strict B cell type specificity of IgH VH-DJH recombination (see Fig. S4 in the supplemental material).
Allelic exclusion and competition in hemizygous ICR and cHS4 mice.
While the analyses on homozygous mice indicated an inefficiency of the mutant alleles to drive normal B cell development (see Fig. S3 in the supplemental material), the findings could have been blurred by cellular selection (26). In addition, this analysis was not informative with regard to the actual contribution of the mutant allele to B cell development, when put in competition with the WT allele.
In hemizygous animals, no significant difference from the WT controls was observed regardless of the parental origin of the ectopic ICR or cHS4 (Fig. 3A and B). Still, this normal development could have masked an altered allelic exclusion or an impaired expression of the mutant allele, which could have been compensated for by the WT allele.
To analyze more closely allelic exclusion and competition between WT and mutant alleles, we bred the mutant mice, which express an IgMa allotype (they are derived from strain 129Sv), with WT C57BL/6 mice (which express an IgMb allotype). As an additional control, we used B1-8 mice, in which the DQ52-JH region is replaced by a prerearranged V(D)J exon expressing IgMa, leading to a complete exclusion of the WT allele (27). FACS analyses were performed by using monoclonal antibodies (MAbs) which readily distinguished between the IgM products of the two parental alleles.
Strikingly, the mutant allele from either ICR/+ or +/ICR mice was totally excluded, and this exclusion pattern was stably maintained in the spleen (Fig. 3C). In cHS4/+ mice, IgMb-expressing B cells clearly outnumbered IgMa-expressing B cells, with no evidence for allelic inclusion, as no cells double expressing IgMa and IgMb at levels above the background levels were detected. Also here, a similar pattern was found in the spleen (Fig. 3C).
Thus, when put in competition with the WT allele, the mutant ICR allele was impotent in driving B cell development and was totally excluded, regardless of its parental origin. The defect was clearly less severe in the case of the mutant cHS4 allele, with no evidence for a breakdown of allelic exclusion.
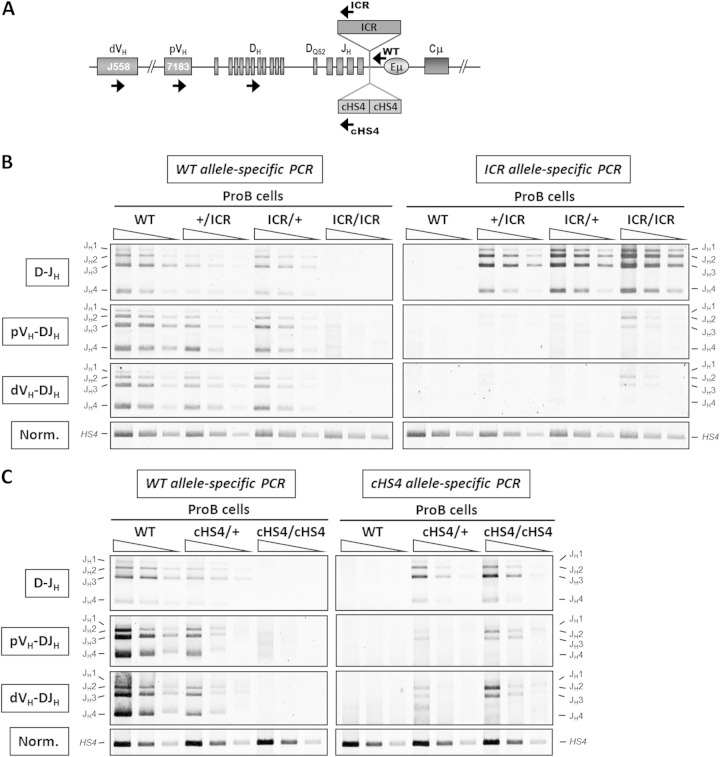
The hemizygous ICR and cHS4 insertions specifically affect VH-DJH recombination.
Next, we analyzed V(D)J recombination in an allele-specific manner in the hemizygous ICR mice, where selection pressure on the mutant allele is less stringent than that in the homozygous mice, as B cell development can be driven by the WT allele.
Genomic DNA was prepared from sorted pro-B cells and subjected to PCR using the same forward primers for all the genotypes. Backward primers were designed so that they preferentially amplified the WT allele or specifically amplified the mutant allele (Fig. 4A). Interestingly, D-JH recombination was readily detected on the mutant ICR allele, regardless of its parental origin, and on the mutant cHS4 allele (Fig. 4B and C). In contrast, only low levels of VH-DJH recombination were detectable for both proximal and distal VH genes on the mutant ICR and cHS4 alleles (Fig. 4B and C).
FIG 4.
The ICR and cHS4 insertions into the IgH locus specifically impair VH-to-DJH recombination. (A) A scheme of the IgH locus indicating the relative position of the primers used in PCR. (B) Genomic DNAs were prepared from sorted ICR pro-B cells with the indicated genotypes and were subjected to a semiquantitative PCR to amplify D-JH and VH-DJH rearrangements. The PCR was performed on serial 3-fold dilutions. A PCR of the HS4 enhancer from the 3′ regulatory region was used for normalization of the DNA input (n = 3). (C) Genomic DNAs were prepared from sorted cHS4 pro-B cells and assayed as described in the legend to panel B (n = 3).
Our allelic analysis of V(D)J recombination established that the ICR and cHS4 insertions had no significant effect on D-JH recombination but severely reduced VH-DJH recombination of both the proximal and the distal VH genes.
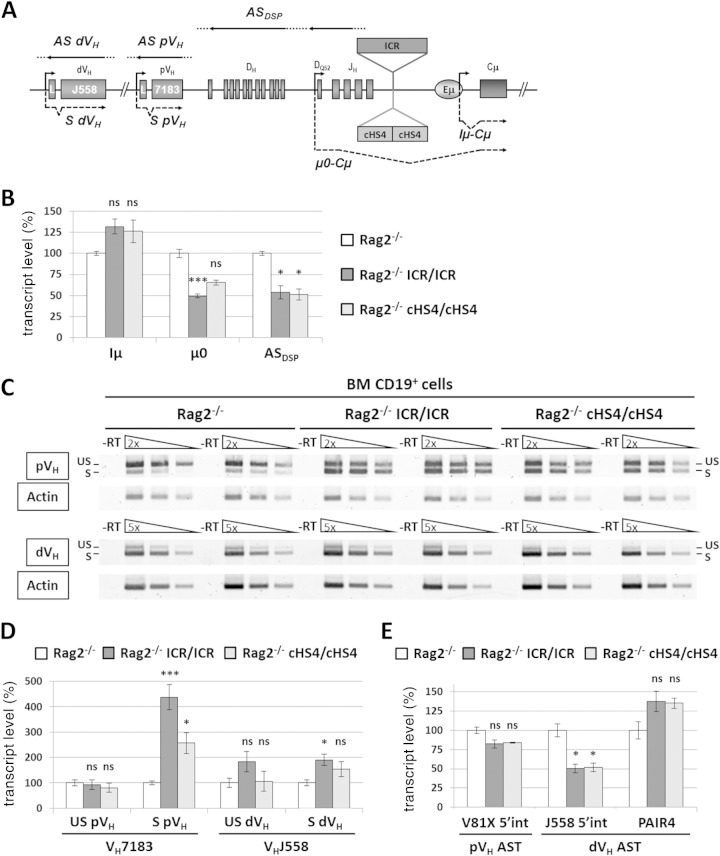
Increased sense and decreased antisense germ line transcription at distal VH genes on the mutant alleles.
Previous studies showed that deletion of the Eμ enhancer drastically affected μ0 and Iμ STs, derived from the DQ52 and Iμ germ line promoters, respectively; ASTs across the D-JH domain; and a set of proximal VH germ line transcripts (10–14) (Fig. 5A). To investigate the effect of the ICR and cHS4 insertions on germ line transcription, homozygous ICR and cHS4 mice were brought into a Rag2-deficient background. While Iμ ST levels did not markedly vary regardless of the genotype (Fig. 5B), μ0 ST and DSP AST levels were mildly reduced (∼2-fold) in ICR/Rag2 and cHS4/Rag2 mutants (Fig. 5B).
FIG 5.
Sense and antisense germ line transcription at VH genes on the mutant allele. (A) The scheme shows some of the germ line transcripts analyzed at the mutant IgH locus. Dots indicate that the initiation and termination sites of the indicated transcripts have not been mapped yet. L, leader; S, sense; AS, antisense. (B) Total RNA from sorted CD19+ cells from the bone marrow of Rag2−/−, Rag2−/− ICR/ICR, and Rag2−/− cHS4/cHS4 mice was assayed by RT-qPCR for the indicated transcripts. The corresponding transcript levels in the Rag2−/− controls were set at 100% of the signal. Gapdh (glyceraldehyde-3-phosphate dehydrogenase gene) and Ywhaz (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta gene) expression was used for normalization. The histograms show the standard errors (n ≥ 4). ***, P < 0.001; *, P < 0.05; ns, not significant. (C) Analysis of proximal VH (pVH; top) and distal VH (dVH; bottom) germ line transcripts by semiquantitative RT-PCR. Two independent samples from each genotype are shown. −RT, no reverse transcription; S, spliced transcripts; US, unspliced (antisense/primary sense) transcripts. (D) Quantification of germ line transcripts of proximal and distal VH genes by semiquantitative PCR. The signals of the corresponding transcripts in the Rag2−/− controls were set at 100%. The histograms show the standard errors (n ≥ 4). ***, P < 0.001; *, P < 0.05. (E) Quantification by qPCR of ASTs within the indicated regions of the IgH variable locus. The histograms show the standard errors (n ≥ 4). *, P < 0.05. int, intergenic.
For proximal VH germ line transcripts, we found an increase in spliced ST levels in ICR/Rag2 and cHS4/Rag2 mice (∼4-fold and ∼3-fold, respectively) (Fig. 5C and D). In contrast, the levels of unspliced transcripts (covering both ASTs and primary STs) (Fig. 5C and D) and the exclusively antisense VH81X transcripts (the most proximal, functional VH gene segment) (Fig. 5E) did not vary significantly. For the distal VH gene transcripts, ST and unspliced transcript levels did not vary markedly under semiquantitative conditions, regardless of the genotype (Fig. 5C and D). By using RT-qPCR to quantify exclusively intergenic ASTs in the distal VH region, we detected at best a 2-fold decrease within the large J558 family region and no significant variation for the Pax5-activated intergenic repeat 4 (PAIR4) germ line transcripts (28) (Fig. 5E).
Combined, these data establish that insertion of ICR or cHS4 upstream of Eμ has no major effect on the sense and antisense germ line transcripts produced within the D-Cμ domain or within the distal VH domain but affects the proximal VH genes, where sense transcripts were upregulated (see Fig. 8).
FIG 8.
An Eμ bimodal activity model. This working model stipulates that Eμ activity shifts during early B cell development. (A) Prior to D-JH recombination, the Eμ enhancer controls sense and antisense transcription within the D-Cμ chromatin domain and sense transcription of a set of proximal VH gene segments that are more than 400 kb away. Eμ also controls D-JH recombination. Neither of the activities of the Eμ enhancer is hampered by either the IGCR1 CTCF/cohesin sites or the ectopic insulators. (B) After D-JH recombination, Eμ activity is focused on the nearby DJH promoter and becomes sensitive to the insulators' blocker effect. The control of DJH transcription is crucial for VH-DJH recombination and allelic exclusion. The arrows indicate only the sense and antisense transcripts which were shown to be controlled by the Eμ enhancer. The question marks mean that initiation and termination of the indicated antisense transcripts have not been unambiguously defined. The CpG of the JH1 segment is shown as a filled (methylated) or empty (unmethylated) lollipop. CBEs denote CTCF-binding elements within the intergenic control region (IGCR1) upstream of DFL16.1. The figure was compiled from information presented in references 12 to 18 and the present study.
DJH transcription is strongly decreased on the ICR and cHS4 mutant alleles.
Taking germ line transcription as a measure of the chromatin accessibility of unrearranged gene segments poised for recombination, the increase of the proximal VH-specific germ line STs and the relatively normal distal VH-specific germ line transcripts in ICR and cHS4 mice indicated that it was unlikely that a reduced accessibility of the VH RSSs was the cause of the impairment of VH-DJH recombination in these mice. This led us to explore the possibility that the ectopic inserts had interfered with DJH transcription, potentially rendering the RSSs of the DJH segments inaccessible for VH-DJH recombination.
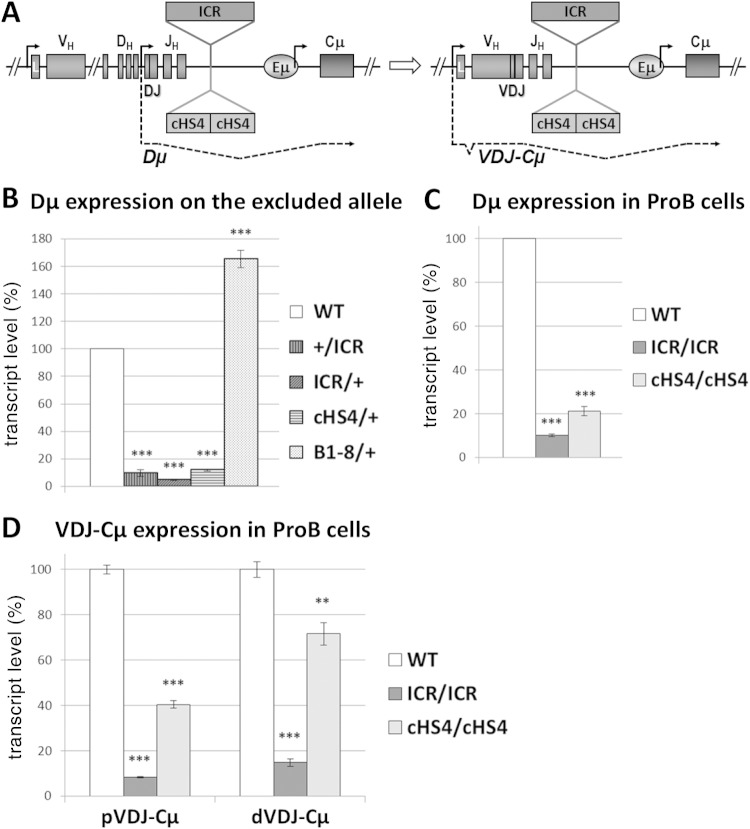
Following D-JH recombination, the D promoters upstream of assembled DJH segments are activated and generate the so-called Dμ transcripts (29, 30). To investigate whether the Dμ transcripts were produced from the excluded allele, we purified B cell populations from ICR × C57BL/6 or cHS4 × C57BL/6 hemizygous mice in which B cell development was driven by the WT IgMb-expressing allele (the IgMa-expressing allele in B1-8 × C57BL/6 hemizygous mice) (Fig. 3C).
Since the IgMb allotype is derived from a productively rearranged WT allele in WT, ICR, and cHS4 hemizygotes (IgMa from the VHDJH2 gene in B1-8 hemizygotes), any detectable Dμ transcripts can originate only from the excluded alleles, which can be either in the DJH configuration or in a nonproductive VHDJH configuration. Only the former fraction would produce Dμ transcripts, whose spliced forms can be detected by using a degenerate forward primer that pairs with the majority of D-segment RSSs and a Cμ reverse primer (Fig. 6A).
FIG 6.
DJH transcription is strongly decreased on the excluded alleles. (A) A scheme showing a partially (DJH2) rearranged IgH locus and the resulting DJH transcript (Dμ) and a fully (VHDJH2) rearranged gene and the resulting VDJ-Cμ transcript. (B) B220+, AA4.1-positive (AA4.1+), and IgMb-positive (IgMb+) B cells from the bone marrow of hemizygous mice with the indicated genotypes were sorted (B220+, AA4.1+, and IgMa-positive [IgMa+] B cells were used for B1-8/+ mice). The WT allele is derived from C57BL/6 mice. Total RNA was extracted and reverse transcribed. Spliced DJH transcripts were quantified by qPCR. WT transcript levels were set as 100% of the signal. Gapdh and Ywhaz transcripts were used for normalization. The histograms show the standard errors (n = 3). ***, P < 0.001. (C) RNA from sorted homozygous pro-B cells was assayed as described in the legend to panel B (n = 3). ***, P < 0.001. (D) RNA from sorted homozygous pro-B cells was assayed as described in the legend to panel B (n = 3). ***, P < 0.001; **, P < 0.01.
Remarkably, Dμ transcripts were readily detected in WT controls, indicating that expression of the productive allele did not inhibit Dμ transcription on the excluded allele. Dμ transcript levels in B1-8 hemizygotes were slightly superior to those in the WT controls (Fig. 6B), likely reflecting a lower proportion of nonproductive rearrangements on the excluded allele. In stark contrast, the levels of the Dμ transcripts were decreased at least 10 times in ICR and cHS4 hemizygotes (Fig. 6B). The drop of Dμ transcript levels was also evident in ICR and cHS4 homozygous pro-B cells, excluding the possibility of any selection bias as a reason for this drop (Fig. 6C; see Fig. 8). We also quantified mature μ (VHDJHCμ) transcripts (derived from the PVH promoter following VH-DJH recombination) in sorted pro-B cells and found a severe reduction in ICR cells and a milder decrease in cHS4 pro-B cells for both the proximal and the distal VH genes (Fig. 6D).
The data reveal a strong inhibition of DJH transcription on the excluded ICR and cHS4 mutant alleles.
The ICR affects DNA methylation at DJH recombination intermediates in a parent-of-origin manner.
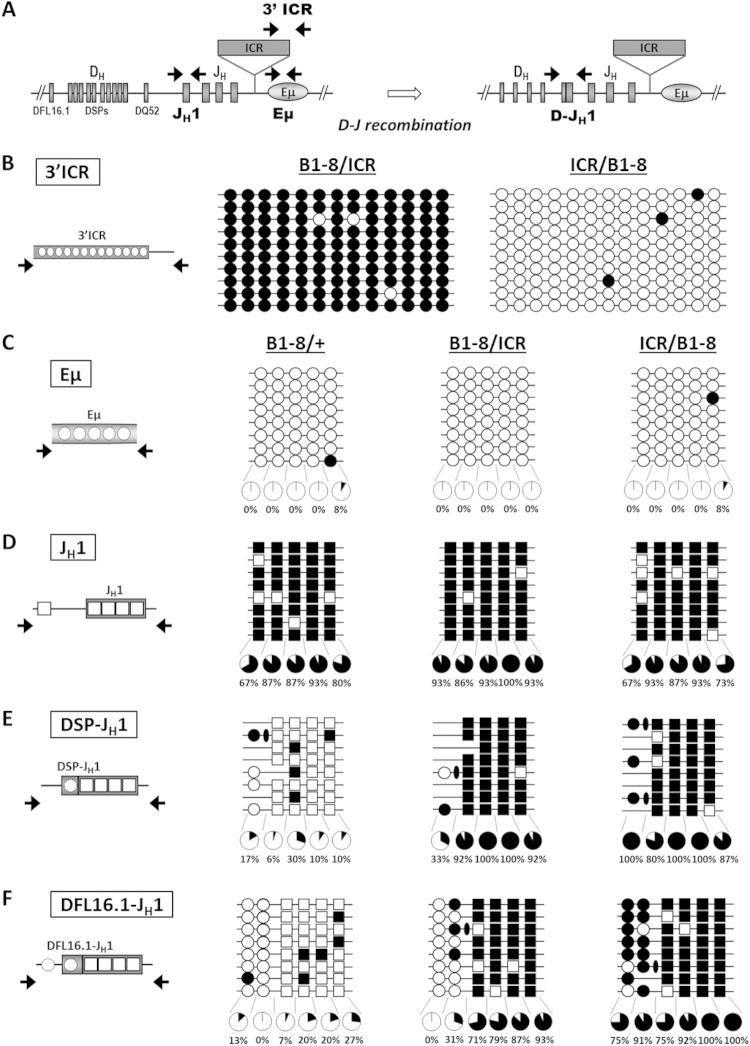
The marked decrease of DJH transcription on the ICR mutant alleles regardless of their parental origin (Fig. 6B and C) led us to investigate the methylation state of DJH segments on the excluded alleles. To this end, ICR/ICR homozygous mice were crossed with B1-8/B1-8 mice. In the resulting progeny, the ICR mutant allele was totally excluded independently of its parental origin (Fig. 3C and D). To analyze the methylation pattern of the excluded alleles, genomic DNAs were extracted from bone marrow B cells of B1-8/+, B1-8/ICR, and ICR/B1-8 mice and assayed by bisulfite sequencing. We first ascertained that the paternal but not the maternal ectopic ICR was heavily methylated (Fig. 7A and B). We also verified that DNA methylation at the ectopic ICR on the excluded, paternal allele had not spread into the Eμ enhancer. Indeed, the Eμ enhancer was unmethylated regardless of the genotype (Fig. 7C). Interestingly, while the unrearranged JH1 region was fully methylated (Fig. 7D), the rearranged JH1 segment on the excluded allele displayed different methylation patterns depending on the genotype. In B1-8/+ mice, it was demethylated, suggesting that allelic exclusion in this system did not correlate with methylation of the excluded DJH allele. In contrast, in both ICR/B1-8 and B1-8/ICR mice, the rearranged JH1 segment remained heavily methylated (Fig. 7E and F). Strikingly, the rearranged D segments on the excluded alleles were unmethylated in B1-8/ICR mice but were heavily methylated in ICR/B1-8 mice. This pattern was more obvious for the rearranged DFL16.1 segment, which retained its upstream CpG, as it is the 5′-most functional D segment (Fig. 7E and F). The untemplated CpGs, added by the terminal deoxynucleotidyl transferase during V(D)J recombination, were consistently methylated (Fig. 7E and F) (see Discussion).
FIG 7.
DNA methylation patterns of DJH segments are differentially affected by the paternal and maternal ectopic ICR. Genomic DNAs from bone marrow B cells of B1-8/+, B1-8/ICR, and ICR/B1-8 mice were assayed by bisulfite sequencing. (A) The scheme shows the localization of the primer pairs used prior to and after D-JH recombination. (B to F) The analyzed CpGs are displayed in the left panel. Methylated CpGs are indicated by filled circles or squares. Unmethylated CpGs are indicated by open circles or squares. The percentages are based on 15 individual sequences for each region derived from two independent pools of mice. Representative sets of sequences are shown. (B) Methylation status of 13 CpGs at the 3′ part of the ectopic H19 ICR is shown. (C) Results of bisulfite sequencing of the Eμ region from mice of the indicated genotypes. (D) Methylation of CpGs of the unrearranged JH1 region. Note that 4 CpGs lie within and 1 CpG lies right upstream of the JH1 segment. The latter is deleted upon any D-JH1 recombination event. (E and F) DNA methylation of DJH recombination intermediates involving DSP segments (E) or the DFL16.1 segment (F). Circles, CpGs of the D segments; squares, CpGs of the JH1 segment; ovals, the nontemplated CpGs added during D-JH1 recombination. Note that the 5′-most CpG of the DFL16.1 segment lies within the promoter region and is not lost upon DFL16.1-JH1 recombination. Throughout, the clonality of the sequences was established on the basis of sequence diversity at D-JH1 junctions. Sequences with identical junctions were counted as one.
Thus, in contrast to the excluded wild-type allele, the rearranged JH1 segment remains fully methylated on the excluded ICR allele. Moreover, when the ectopic ICR binds CTCF, the rearranged DJH segment on the excluded allele is methylated. In contrast, when the ectopic ICR is methylated, the aberrant DNA methylation is highly confined to the rearranged JH1 segment.
DISCUSSION
Our main finding is that ectopic insertion of either the imprinted H19 ICR or the cHS4 insulator upstream of the Eμ enhancer leads to a severe impairment of VH-DJH recombination and an almost complete ablation of DJH transcription. For the first time, our experimental in vivo system reveals a strong correlation between reduced DJH transcription and impairment of VH-DJH recombination. We do not infer that this is the sole mechanism explaining the reduced VH-DJH recombination in our mutants, as other mechanisms underlying, for instance, the architecture of the IgH locus (31, 32) could also be involved.
Another key finding of this study is that the H19 ICR faithfully recapitulates the main epigenetic features of its endogenous counterpart. On the maternal allele, the ectopic ICR binds CTCF/cohesins. During spermatogenesis, just like the endogenous ICR, the ectopic ICR acquires DNA methylation. This paternal methylation is stably maintained in the embryo and during development into B cells. In the context of the IgH locus, therefore, the ∼2.4-kb ICR fragment seems to have all the requirements for the recruitment of the de novo methyltransferase Dnmt3a and its cofactor, Dnmt3L, which mediate establishment of imprinted DNA methylation at ICRs (20, 33). Our findings differ from previous reports, where insertions of the H19 ICR into other ectopic loci or in transgenic systems were found to acquire DNA methylation after fertilization only (34–37). How the ectopic ICR recruits the DNA methyltransferase complex in developing male germ cells remains unclear. At the endogenous locus, this process is associated with transcription through the ICR (19), but there is no evidence that the IgH locus is transcriptionally active in fetal male germ cells. It seems unlikely that the insertion site at the IgH locus dictates DNA methylation because the cHS4 insulator remained completely unmethylated at the same site. Rather, our observations suggest that DNA methylation acquisition at the ectopic ICR is an active process that is specifically targeted by the ICR sequence itself.
The inserted ICR exerted its phenotypic effects regardless of its parental origin. This unexpected finding was not related to DNA insertion per se, since insertion of cHS4, which has a similar size, did not yield the same phenotype. Moreover, insertion of the Neor gene at different sites upstream of Eμ led to qualitatively different effects on germ line transcription and V(D)J recombination (38, 39).
Whereas potential CTCF-mediated looping from the maternal ICR to other CTCF-binding sites at the IgH locus may be envisaged on the maternal chromosome, the long- and short-range effects of the methylated, paternal ICR were unexpected. These effects were clearly not due to an altered Eμ configuration, since this enhancer was still transcribed and unmethylated. One plausible explanation for the phenotypic effect of the paternal inheritance of the inserted ICR comes from a recent targeting study in the mouse (40), which shows that the methylated paternal allele of the H19 ICR exerts a repressive effect in cis on transcription. It remains to be discovered what precisely mediates the repressive cis effects of the methylated H19 ICR after D-JH recombination and whether this could be related to the density of CpGs and/or the binding of methyl CpG binding domain (MBD) proteins.
Not only in the ICR/+ hemizygotes but also in the +/ICR hemizygotes, DJH transcription was severely reduced on the mutated allele. Significantly, a recent comprehensive analysis of DNA methylation revealed widespread CpG methylation at the D-Eμ domain prior to V(D)J recombination, but the Eμ and the DQ52 promoter remained unmethylated at this developmental stage (25). After D-JH recombination, however, Eμ-mediated demethylation occurred specifically at the recombined DJH segments (25). We find that this demethylation process was affected in both +/ICR and ICR/+ hemizygous mice.
Particularly, on the excluded paternal ICR allele, there was highly confined DNA methylation at the rearranged JH1 segment. The single CpG dinucleotide in the promoter region of the DFL16.1 segment was consistently unmethylated. This suggests that the promoter is, at least with regard to DNA methylation, in an open configuration. On the excluded maternal ICR allele, in ICR/+ mice, DNA methylation spanning both rearranged D and JH1 segments occurred, a situation that is reminiscent of the reported Eμ deletion effect (25). Regardless of the parental origin of the ectopic ICR, however, the aberrant DNA methylation was clearly polarized toward upstream sequences. The Eμ enhancer itself remained fully unmethylated on the excluded alleles. Together, these data indicate that the ectopic ICR differentially affects the demethylation of recombined DJH segments and, as a corollary, that the demethylating activity of the Eμ enhancer was differentially impaired by the ectopic ICR insertion.
The observed aberrant methylation states of DJH recombination intermediates correlated with a dramatic reduction of DJH transcription. Despite this clear correlation, we think that reduced DJH transcription, rather than the aberrant DJH methylation, accounts best for the observed reduced VH-DJH recombination. The RAG complex is able to efficiently catalyze D-JH recombination, despite extensive methylation of germ line D segments (with the exception of DQ52) and JH segments (25; this study). Therefore, it is difficult to envisage that methylation of DJH segments per se would hamper the action of RAG complexes. Additionally, the lack of a correlation between DNA methylation, on the one hand, and germ line transcription, active histone marks, and the recombination potential of germ line D and JH segments (25, 41), on the other hand, leads us to suggest that Eμ controls DJH transcription and demethylation by distinct mechanisms. This should be relevant for further exploration in future studies.
We found in our study that in a Rag2-deficient background, there was only a mild decrease of germ line transcription within the D-Cμ domain and, in Rag2-proficient pro-B cells, D-JH recombination was unaffected. These findings indicate that neither the ICR nor the cHS4 insertion interfered in a major way with the accessibility control function of Eμ within the D-Cμ domain or with the recruitment of the RAG complex for the first, D-JH, recombination step. Indeed, this process was unaffected.
Previous studies have shown that deletion of the Eμ enhancer impaired D-JH recombination and more severely VH-DJH recombination (10, 11). These findings led to the proposal that impaired VH-DJH recombination was a downstream consequence of the primary block in D-JH recombination (10). We found that it was transcription of DJH segments and not D-JH recombination itself which was severely reduced in the ICR and cHS4 mice. This key finding highlights the importance of DJH transcription in VH-DJH recombination.
Our combined data favor the view that Eμ is developmentally bimodal in its action (Fig. 8). Prior to D-JH recombination, Eμ controls germ line transcription within the D-Cμ domain and of a set of proximal VH genes as far away as 400 kb (13), despite the presence of the insulators (our study) and CTCF/cohesin binding at IGCR1 CBEs (16–18). Moreover, while deletion of Eμ induced a drastic reduction of μ0 ST and DSP ASTs (11, 12), we found only a mild reduction of these transcripts. After D-JH recombination, however, Eμ activity was focused on the nearby DJH promoter and was particularly sensitive to the insulators' blocker effect (Fig. 8). It should be noted that D-JH recombination brings the DJH promoter close to the inserted insulators (at ∼1.8 kb for DJH4 and ∼3.2 kb for DJH1), whereas the DQ52 promoter is ∼3.6 kb from the ICR and cHS4. The fact that μ0 transcripts were only modestly affected, whereas Dμ transcripts were severely reduced, further suggests that the Eμ enhancer is developmentally programmed to shift its activity after DJH rearrangement. Additionally, any D-JH recombination not involving DQ52 brings closer the heterochromatic marks associated with the far upstream DSP segments (42), which may spread to the DJH promoter. In the absence of counteracting active chromatin marks normally promoted by Eμ (13), the DJH promoter may become silenced.
Our Eμ bimodal activity model predicts that after VH-DJH recombination, Eμ-mediated effects shift to the PVH promoter of the rearranged V(D)J exon. In support of this prediction, μ gene transcript levels were reported to be reduced in the bone marrow in the absence of Eμ or when this element is insulated (43; this study).
The correlation between VH sense and antisense germ line transcription and VH recombination is still unclear (10–15, 18, 44–49). Deletion of the whole VH-D intergenic region led to an increase of proximal VH-DJH recombination which correlated with increased antisense germ line transcription originating from the D cluster and spanning the now close by proximal VH genes (15). In contrast, the increased proximal VH-DJH recombination in mice devoid of the IGCR1 CTCF sites was associated with increased sense germ line transcription of proximal VH genes (18). Interestingly, in both ICR and cHS4 mice, an increased sense germ line transcription of proximal VH genes was seen. This hints to the possibility that the ectopic sequences are engaged in a CTCF-mediated interaction with the IGCR1. However, this hypothesis cannot explain the similar effect conveyed by the paternal ectopic ICR, which rather points to a distinct, CTCF-independent mechanism. Notwithstanding, in our study, the reduction of proximal VH-DJH recombination was seen, despite increased sense and normal antisense germ line transcription. This suggests that the accessibility of proximal VH genes, at least as measured by transcription, was not the limiting factor in the ICR and cHS4 mice, and this emphasizes again the likely importance of the defect in DJH transcription.
Within the distal VH domain, overall normal ST and AST levels did not correlate with an efficient distal VH-DJH recombination, despite the accessibility of the VH RSSs. Again, this clearly shows that VH transcription per se is not sufficient for V(D)J recombination. This is in agreement with the results of previous studies, which did not find a causal relationship between germ line transcription of distal VH genes and their recombination. Indeed, distal VH-DJH recombination was reduced in different mutants (including mutants with the deletion of Eμ, IGCR1 CTCF sites, Pax5, EZH2, Ikaros, and YY1) that displayed apparently unchanged (11–13, 18, 45, 46), seemingly increased (48), or reduced (47, 49) distal VH transcription.
In the context of allelic competition, the reduced efficiency with which the ICR and, to a lesser extent, the cHS4 mutant alleles undergo VH-DJH recombination may be explained by reduced DJH transcription. This may indicate that the latter is a mark of the excluded allele. However, the high levels of DJH transcripts from the excluded alleles in WT and B1-8 hemizygotes showed the opposite. Thus, the complete exclusion of the ICR mutant allele may actually result from two additive yet distinct mechanisms: a transcriptional silencing upon D-JH recombination, leading to a defective allele, followed by enforcement by allelic exclusion. In support of this notion, the cHS4 allele could sustain some allelic competition, and the levels of DJH transcripts on the excluded cHS4 allele were relatively higher than those on the ICR allele.
Our findings strongly suggest that genuine allelic exclusion requires efficient DJH transcription. Functionally, this would ensure that the DJH RSSs of the second allele will be available if VH-DJH recombination on the first allele is not productive. We propose that one mechanism by which the Eμ enhancer controls allelic exclusion could be through the control of DJH transcription levels, which should be interesting to explore further in future studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank F. W. Alt for providing genomic DNA from IGCR1-deficient mice and K. Rajewsky for the B1-8 mouse line. We also thank P. Mercier and M. Philippe at the IPBS transgenesis platform and F. L'Faqihi and V. Duplan-Eche at the Purpan CPTP platform for their excellent work and J. Tessier for his help with embryonic stem cell transfections. We thank M. Moutahir for handling the mouse lines.
This work was supported by grants from the INCa, ANR, the Fondation ARC, the Ligue Contre le Cancer-Comité de Haute-Garonne, and the Cancéropôle GSO. N.-S.N.H. was supported by a fellowship from INCa, and R.H. was supported by a fellowship from the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad. R.F. is associated with the Labex EpiGenMed and the European NoE EpiGeneSys.
We declare that we have no conflict of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00235-14.
REFERENCES
- 1.Goldmit M, Bergman Y. 2004. Monoallelic gene expression: a repertoire of recurrent themes. Immunol Rev 200:197–214. doi: 10.1111/j.0105-2896.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 2.Yang PK, Kuroda MI. 2007. Noncoding RNAs and intranuclear positioning in monoallelic gene expression. Cell 128:777–786. doi: 10.1016/j.cell.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Delaval K, Feil R. 2004. Epigenetic regulation of mammalian genomic imprinting. Curr Opin Genet Dev 14:188–195. doi: 10.1016/j.gde.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. 2006. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol 24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 5.Schatz DG, Ji Y. 2011. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 11:251–263. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 6.Vettermann C, Schlissel MS. 2010. Allelic exclusion of immunoglobulin genes: models and mechanisms. Immunol Rev 237:22–42. doi: 10.1111/j.1600-065X.2010.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston CM, Wood AL, Bolland DJ, Corcoran AE. 2006. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J Immunol 176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 8.Retter I, Chevillard C, Scharfe M, Conrad A, Hafner M, Im TH, Ludewig M, Nordsiek G, Severitt S, Thies S, Mauhar A, Blocker H, Muller W, Riblet R. 2007. Sequence and characterization of the Ig heavy chain constant and partial variable region of the mouse strain 129S1. J Immunol 179:2419–2427. doi: 10.4049/jimmunol.179.4.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobb RM, Oestreich KJ, Osipovich OA, Oltz EM. 2006. Accessibility control of V(D)J recombination. Adv Immunol 91:45–109. doi: 10.1016/S0065-2776(06)91002-5. [DOI] [PubMed] [Google Scholar]
- 10.Afshar R, Pierce S, Bolland DJ, Corcoran A, Oltz EM. 2006. Regulation of IgH gene assembly: role of the intronic enhancer and 5′DQ52 region in targeting DHJH recombination. J Immunol 176:2439–2447. doi: 10.4049/jimmunol.176.4.2439. [DOI] [PubMed] [Google Scholar]
- 11.Perlot T, Alt FW, Bassing CH, Suh H, Pinaud E. 2005. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc Natl Acad Sci U S A 102:14362–14367. doi: 10.1073/pnas.0507090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolland DJ, Wood AL, Afshar R, Featherstone K, Oltz EM, Corcoran AE. 2007. Antisense intergenic transcription precedes IgH D-to-J recombination and is controlled by the intronic enhancer Emu. Mol Cell Biol 27:5523–5533. doi: 10.1128/MCB.02407-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty T, Perlot T, Subrahmanyam R, Jani A, Goff PH, Zhang Y, Ivanova I, Alt FW, Sen R. 2009. A 220-nucleotide deletion of the intronic enhancer reveals an epigenetic hierarchy in immunoglobulin heavy chain locus activation. J Exp Med 206:1019–1027. doi: 10.1084/jem.20081621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlot T, Li G, Alt FW. 2008. Antisense transcripts from immunoglobulin heavy-chain locus V(D)J and switch regions. Proc Natl Acad Sci U S A 105:3843–3848. doi: 10.1073/pnas.0712291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giallourakis CC, Franklin A, Guo C, Cheng HL, Yoon HS, Gallagher M, Perlot T, Andzelm M, Murphy AJ, Macdonald LE, Yancopoulos GD, Alt FW. 2010. Elements between the IgH variable (V) and diversity (D) clusters influence antisense transcription and lineage-specific V(D)J recombination. Proc Natl Acad Sci U S A 107:22207–22212. doi: 10.1073/pnas.1015954107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degner SC, Wong TP, Jankevicius G, Feeney AJ. 2009. Cutting edge: developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol 182:44–48. doi: 10.4049/jimmunol.182.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Featherstone K, Wood AL, Bowen AJ, Corcoran AE. 2010. The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J Biol Chem 285:9327–9338. doi: 10.1074/jbc.M109.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo C, Yoon HS, Franklin A, Jain S, Ebert A, Cheng HL, Hansen E, Despo O, Bossen C, Vettermann C, Bates JG, Richards N, Myers D, Patel H, Gallagher M, Schlissel MS, Murre C, Busslinger M, Giallourakis CC, Alt FW. 2011. CTCF-binding elements mediate control of V(D)J recombination. Nature 477:424–430. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henckel A, Chebli K, Kota SK, Arnaud P, Feil R. 2012. Transcription and histone methylation changes correlate with imprint acquisition in male germ cells. EMBO J 31:606–615. doi: 10.1038/emboj.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kota SK, Feil R. 2010. Epigenetic transitions in germ cell development and meiosis. Dev Cell 19:675–686. doi: 10.1016/j.devcel.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Bell AC, West AG, Felsenfeld G. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98:387–396. doi: 10.1016/S0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- 22.Szabo PE, Tang SH, Reed MR, Silva FJ, Tsark WM, Mann JR. 2002. The chicken beta-globin insulator element conveys chromatin boundary activity but not imprinting at the mouse Igf2/H19 domain. Development 129:897–904. [DOI] [PubMed] [Google Scholar]
- 23.Dickson J, Gowher H, Strogantsev R, Gaszner M, Hair A, Felsenfeld G, West AG. 2010. VEZF1 elements mediate protection from DNA methylation. PLoS Genet 6:e1000804. doi: 10.1371/journal.pgen.1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirillov A, Kistler B, Mostoslavsky R, Cedar H, Wirth T, Bergman Y. 1996. A role for nuclear NF-kappaB in B-cell-specific demethylation of the Igkappa locus. Nat Genet 13:435–441. doi: 10.1038/ng0895-435. [DOI] [PubMed] [Google Scholar]
- 25.Selimyan R, Gerstein RM, Ivanova I, Precht P, Subrahmanyam R, Perlot T, Alt FW, Sen R. 2013. Localized DNA demethylation at recombination intermediates during immunoglobulin heavy chain gene assembly. PLoS Biol 11:e1001475. doi: 10.1371/journal.pbio.1001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Boehmer H, Melchers F. 2010. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol 11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 27.Sonoda E, Pewzner-Jung Y, Schwers S, Taki S, Jung S, Eilat D, Rajewsky K. 1997. B cell development under the condition of allelic inclusion. Immunity 6:225–233. doi: 10.1016/S1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- 28.Ebert A, McManus S, Tagoh H, Medvedovic J, Salvagiotto G, Novatchkova M, Tamir I, Sommer A, Jaritz M, Busslinger M. 2011. The distal V(H) gene cluster of the IgH locus contains distinct regulatory elements with Pax5 transcription factor-dependent activity in pro-B cells. Immunity 34:175–187. doi: 10.1016/j.immuni.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Reth MG, Alt FW. 1984. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. Nature 312:418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- 30.Alessandrini A, Desiderio SV. 1991. Coordination of immunoglobulin DJH transcription and D-to-JH rearrangement by promoter-enhancer approximation. Mol Cell Biol 11:2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo C, Gerasimova T, Hao H, Ivanova I, Chakraborty T, Selimyan R, Oltz EM, Sen R. 2011. Two forms of loops generate the chromatin conformation of the immunoglobulin heavy-chain gene locus. Cell 147:332–343. doi: 10.1016/j.cell.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medvedovic J, Ebert A, Tagoh H, Tamir IM, Schwickert TA, Novatchkova M, Sun Q, Huis In't Veld PJ, Guo C, Yoon HS, Denizot Y, Holwerda SJ, de Laat W, Cogne M, Shi Y, Alt FW, Busslinger M. 2013. Flexible long-range loops in the VH gene region of the IgH locus facilitate the generation of a diverse antibody repertoire. Immunity 39:229–244. doi: 10.1016/j.immuni.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki H, Matsui Y. 2008. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet 9:129–140. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- 34.Gebert C, Kunkel D, Grinberg A, Pfeifer K. 2010. H19 imprinting control region methylation requires an imprinted environment only in the male germ line. Mol Cell Biol 30:1108–1115. doi: 10.1128/MCB.00575-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuzaki H, Okamura E, Shimotsuma M, Fukamizu A, Tanimoto K. 2009. A randomly integrated transgenic H19 imprinting control region acquires methylation imprinting independently of its establishment in germ cells. Mol Cell Biol 29:4595–4603. doi: 10.1128/MCB.00275-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park KY, Sellars EA, Grinberg A, Huang SP, Pfeifer K. 2004. The H19 differentially methylated region marks the parental origin of a heterologous locus without gametic DNA methylation. Mol Cell Biol 24:3588–3595. doi: 10.1128/MCB.24.9.3588-3595.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanimoto K, Shimotsuma M, Matsuzaki H, Omori A, Bungert J, Engel JD, Fukamizu A. 2005. Genomic imprinting recapitulated in the human beta-globin locus. Proc Natl Acad Sci U S A 102:10250–10255. doi: 10.1073/pnas.0409541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Young F, Bottaro A, Stewart V, Smith RK, Alt FW. 1993. Mutations of the intronic IgH enhancer and its flanking sequences differentially affect accessibility of the JH locus. EMBO J 12:4635–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delpy L, Decourt C, Le Bert M, Cogne M. 2002. B cell development arrest upon insertion of a neo gene between JH and Emu: promoter competition results in transcriptional silencing of germline JH and complete VDJ rearrangements. J Immunol 169:6875–6882. doi: 10.4049/jimmunol.169.12.6875. [DOI] [PubMed] [Google Scholar]
- 40.Ideraabdullah FY, Abramowitz LK, Thorvaldsen JL, Krapp C, Wen SC, Engel N, Bartolomei MS. 2011. Novel cis-regulatory function in ICR-mediated imprinted repression of H19. Dev Biol 355:349–357. doi: 10.1016/j.ydbio.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subrahmanyam R, Du H, Ivanova I, Chakraborty T, Ji Y, Zhang Y, Alt FW, Schatz DG, Sen R. 2012. Localized epigenetic changes induced by DH recombination restricts recombinase to DJH junctions. Nat Immunol 13:1205–1212. doi: 10.1038/ni.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chakraborty T, Chowdhury D, Keyes A, Jani A, Subrahmanyam R, Ivanova I, Sen R. 2007. Repeat organization and epigenetic regulation of the DH-Cmu domain of the immunoglobulin heavy-chain gene locus. Mol Cell 27:842–850. doi: 10.1016/j.molcel.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Li F, Eckhardt LA. 2009. A role for the IgH intronic enhancer E mu in enforcing allelic exclusion. J Exp Med 206:153–167. doi: 10.1084/jem.20081202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuxa M, Skok J, Souabni A, Salvagiotto G, Roldan E, Busslinger M. 2004. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev 18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hesslein DG, Pflugh DL, Chowdhury D, Bothwell AL, Sen R, Schatz DG. 2003. Pax5 is required for recombination of transcribed, acetylated, 5′ IgH V gene segments. Genes Dev 17:37–42. doi: 10.1101/gad.1031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K. 2007. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev 21:1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynaud D, Demarco IA, Reddy KL, Schjerven H, Bertolino E, Chen Z, Smale ST, Winandy S, Singh H. 2008. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol 9:927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su IH, Basavaraj A, Krutchinsky AN, Hobert O, Ullrich A, Chait BT, Tarakhovsky A. 2003. Ezh2 controls B cell development through histone H3 methylation and IgH rearrangement. Nat Immunol 4:124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 49.Verma-Gaur J, Torkamani A, Schaffer L, Head SR, Schork NJ, Feeney AJ. 2012. Noncoding transcription within the IgH distal V(H) region at PAIR elements affects the 3D structure of the IgH locus in pro-B cells. Proc Natl Acad Sci U S A 109:17004–17009. doi: 10.1073/pnas.1208398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.