Abstract
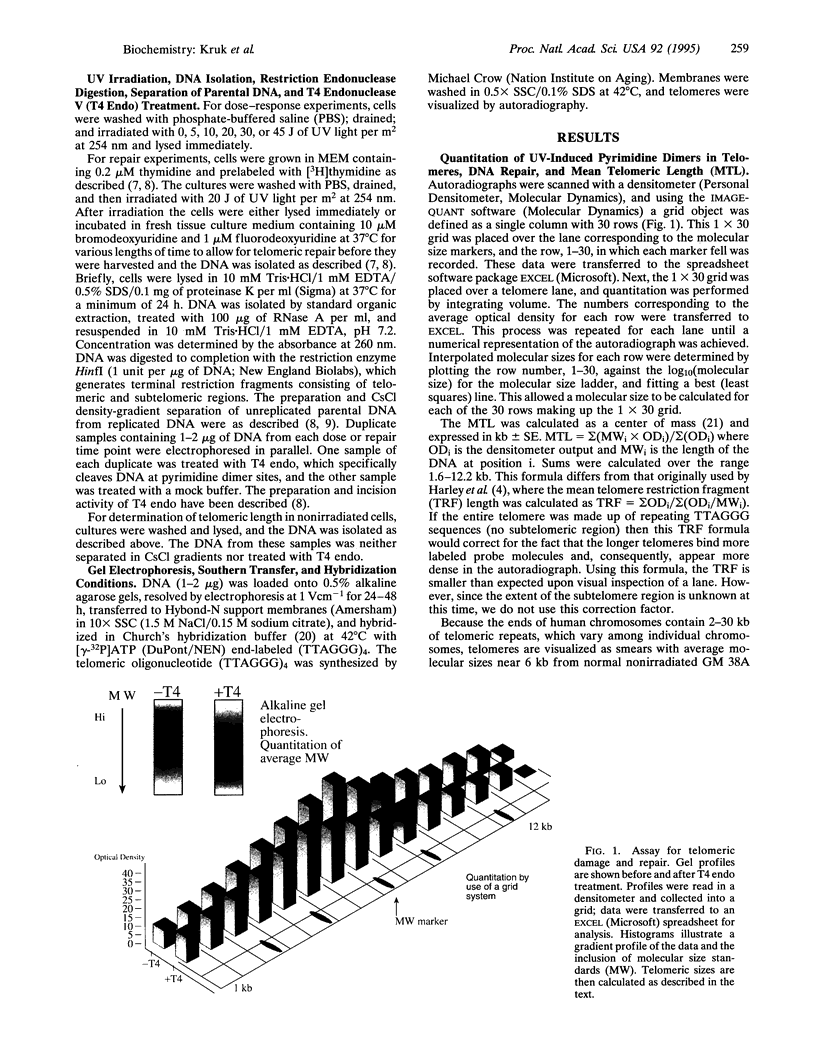
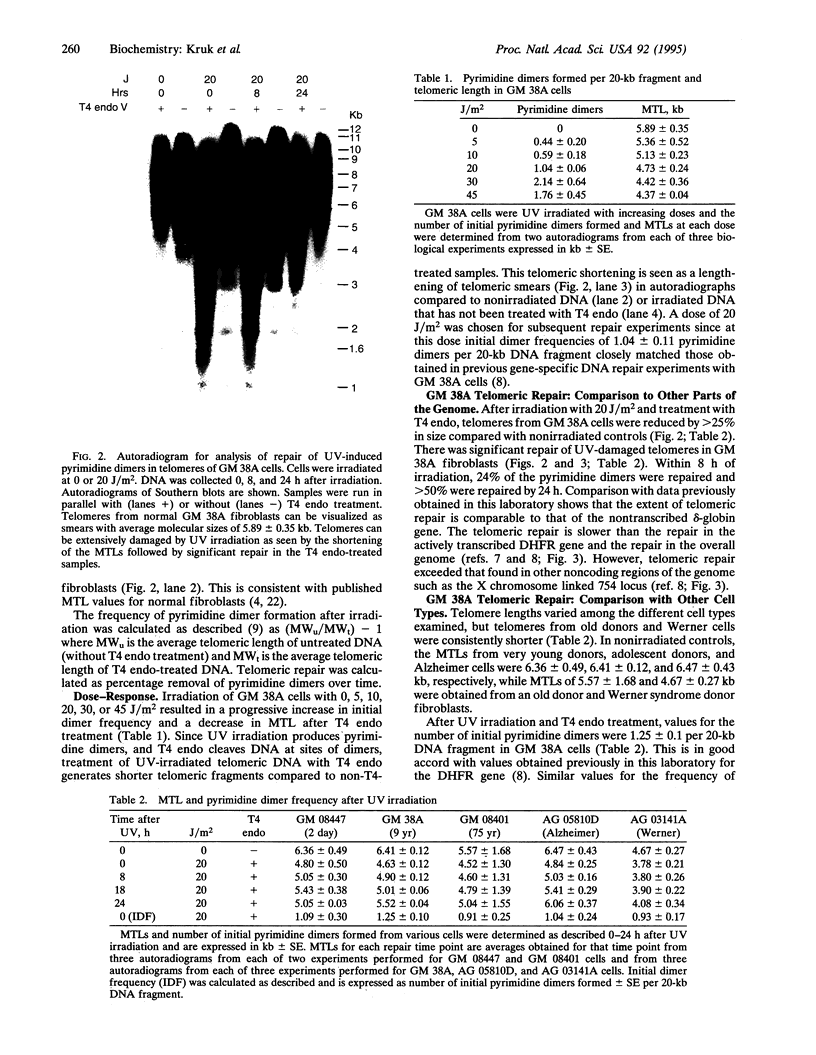
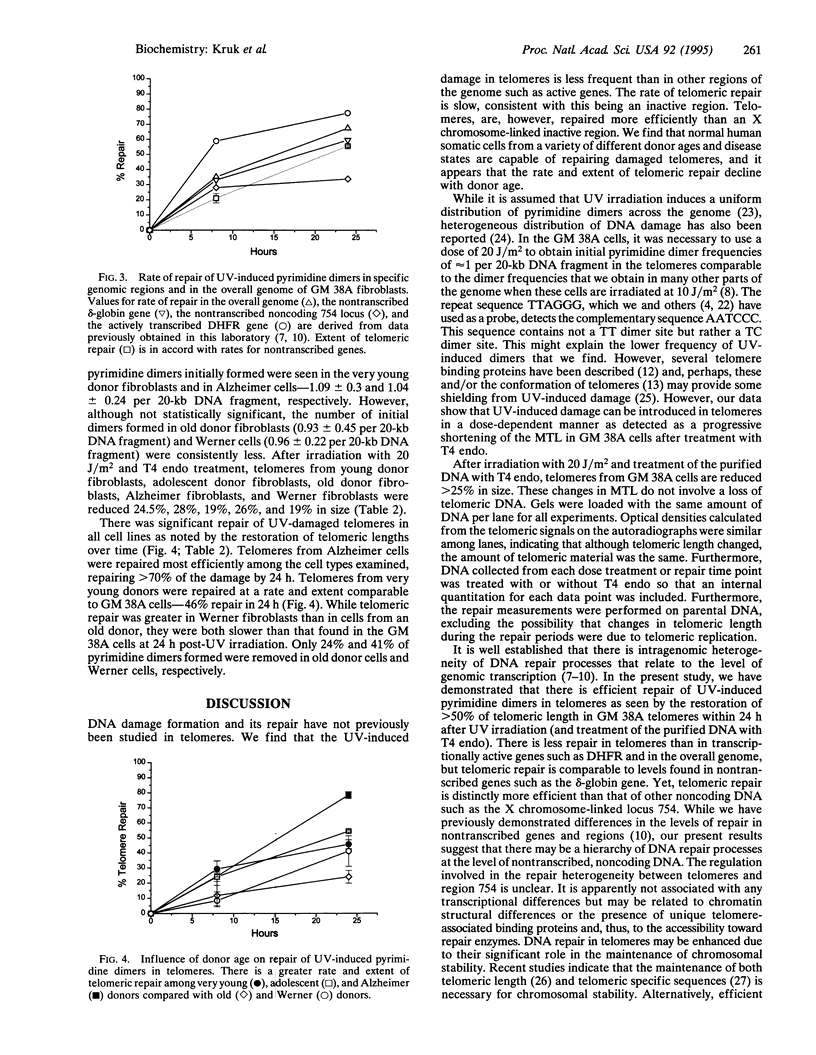
We have established a method for the detection of DNA damage and its repair in human telomeres, the natural ends of chromosomes which are necessary for replication and critical for chromosomal stability. We find that ultraviolet light-induced pyrimidine dimers in telomeric DNA are repaired less efficiently than endogenous genes but more efficiently than inactive, noncoding regions. We have also measured telomeric length, telomeric DNA damage, and its repair in relation to the progression of aging. Telomeres are shorter in fibroblasts from an old donor compared to fibroblasts from a young donor, shortest in cells from a patient with the progeroid disorder Werner syndrome, and relatively long in fibroblasts from a patient with Alzheimer disease. Telomeric DNA repair efficiency is lower in cells from an old donor than in cells from a young donor, normal in Alzheimer cells, and slightly lower in Werner cells. It is possible that this decline in telomeric repair with aging is of functional significance to an age-related decline in genomic stability.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman R., Saul R. L., Ames B. N. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham E. J., Jones G. M., Link C., Huppi K., Potter M., Mushinski J. F., Bohr V. A. DNA repair defects associated with chromosomal translocation breaksite regions. Mol Cell Biol. 1994 Feb;14(2):1204–1212. doi: 10.1128/mcb.14.2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerrigter M. E., Vijg J. Studies on DNA repair defects in degenerative brain disease. Age Ageing. 1993 Jan;22(1):S44–S52. doi: 10.1093/ageing/22.suppl_1.s44. [DOI] [PubMed] [Google Scholar]
- Boerrigter M. E., van Duijn C. M., Mullaart E., Eikelenboom P., van der Togt C. M., Knook D. L., Hofman A., Vijg J. Decreased DNA repair capacity in familial, but not in sporadic Alzheimer's disease. Neurobiol Aging. 1991 Jul-Aug;12(4):367–370. doi: 10.1016/0197-4580(91)90024-e. [DOI] [PubMed] [Google Scholar]
- Cardenas M. E., Bianchi A., de Lange T. A Xenopus egg factor with DNA-binding properties characteristic of terminus-specific telomeric proteins. Genes Dev. 1993 May;7(5):883–894. doi: 10.1101/gad.7.5.883. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. K., Bohr V. A. Gene-specific DNA repair of UV-induced cyclobutane pyrimidine dimers in some cancer-prone and premature-aging human syndromes. Mutat Res. 1994 May;314(3):221–231. doi: 10.1016/0921-8777(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Evans M. K., Robbins J. H., Ganges M. B., Tarone R. E., Nairn R. S., Bohr V. A. Gene-specific DNA repair in xeroderma pigmentosum complementation groups A, C, D, and F. Relation to cellular survival and clinical features. J Biol Chem. 1993 Mar 5;268(7):4839–4847. [PubMed] [Google Scholar]
- Evans M. K., Taffe B. G., Harris C. C., Bohr V. A. DNA strand bias in the repair of the p53 gene in normal human and xeroderma pigmentosum group C fibroblasts. Cancer Res. 1993 Nov 15;53(22):5377–5381. [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Lundblad V., Blackburn E. H. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993 Apr 23;73(2):347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. L., Jen J., Cleaver J. E. Sequence specificity of cyclobutane pyrimidine dimers in DNA treated with solar (ultraviolet B) radiation. Nucleic Acids Res. 1992 Jan 25;20(2):225–229. doi: 10.1093/nar/20.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., van Kesteren van Leeuwen A. C., van Zeeland A. A., Natarajan A. T. Nuclear matrix associated DNA is preferentially repaired in normal human fibroblasts, exposed to a low dose of ultraviolet light but not in Cockayne's syndrome fibroblasts. Nucleic Acids Res. 1988 Nov 25;16(22):10607–10622. doi: 10.1093/nar/16.22.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olovnikov A. M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973 Sep 14;41(1):181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- Poot M., Hoehn H., Rünger T. M., Martin G. M. Impaired S-phase transit of Werner syndrome cells expressed in lymphoblastoid cell lines. Exp Cell Res. 1992 Oct;202(2):267–273. doi: 10.1016/0014-4827(92)90074-i. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Brumback R. A., Polinsky R. J., Wirtschafter J. D., Tarone R. E., Scudiero D. A., Otsuka F. Hypersensitivity to DNA-damaging agents in abiotrophies: a new explanation for degeneration of neurons, photoreceptors, and muscle in Alzheimer, Parkinson and Huntington diseases, retinitis pigmentosa, and Duchenne muscular dystrophy. Basic Life Sci. 1985;35:315–344. doi: 10.1007/978-1-4899-2218-2_20. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Polinsky R. J., Moshell A. N. Evidence that lack of deoxyribonucleic acid repair causes death of neurons in xeroderma pigmentosum. Ann Neurol. 1983 Jun;13(6):682–684. doi: 10.1002/ana.410130621. [DOI] [PubMed] [Google Scholar]
- Sandell L. L., Zakian V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993 Nov 19;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Schwaiger H., Weirich H. G., Brunner P., Rass C., Hirsch-Kauffmann M., Groner Y., Schweiger M. Radiation sensitivity of Down's syndrome fibroblasts might be due to overexpressed Cu/Zn-superoxide dismutase (EC 1.15.1.1). Eur J Cell Biol. 1989 Feb;48(1):79–87. [PubMed] [Google Scholar]
- Thompson J. S., Johnson L. M., Grunstein M. Specific repression of the yeast silent mating locus HMR by an adjacent telomere. Mol Cell Biol. 1994 Jan;14(1):446–455. doi: 10.1128/mcb.14.1.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Zakian V. A. Telomere-telomere recombination provides an express pathway for telomere acquisition. Nature. 1990 May 31;345(6274):456–458. doi: 10.1038/345456a0. [DOI] [PubMed] [Google Scholar]
- Wassermann K., Kohn K. W., Bohr V. A. Heterogeneity of nitrogen mustard-induced DNA damage and repair at the level of the gene in Chinese hamster ovary cells. J Biol Chem. 1990 Aug 15;265(23):13906–13913. [PubMed] [Google Scholar]
- Wei Q., Matanoski G. M., Farmer E. R., Hedayati M. A., Grossman L. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1614–1618. doi: 10.1073/pnas.90.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirich-Schwaiger H., Weirich H. G., Gruber B., Schweiger M., Hirsch-Kauffmann M. Correlation between senescence and DNA repair in cells from young and old individuals and in premature aging syndromes. Mutat Res. 1994 Feb;316(1):37–48. doi: 10.1016/0921-8734(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Williams J. I., Friedberg E. C. Deoxyribonucleic acid excision repair in chromatin after ultraviolet irradiation of human fibroblasts in culture. Biochemistry. 1979 Sep 4;18(18):3965–3972. doi: 10.1021/bi00585a019. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991 Nov 15;67(4):823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- de Lange T. Human telomeres are attached to the nuclear matrix. EMBO J. 1992 Feb;11(2):717–724. doi: 10.1002/j.1460-2075.1992.tb05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeeland A. A., Smith C. A., Hanawalt P. C. Sensitive determination of pyrimidine dimers in DNA of UV-irradiated mammalian cells. Introduction of T4 endonuclease V into frozen and thawed cells. Mutat Res. 1981 Jun;82(1):173–189. doi: 10.1016/0027-5107(81)90148-2. [DOI] [PubMed] [Google Scholar]