Abstract
Background
At the turn of the 19th century the first observations of a female-biased sex ratio in broods and populations of the head louse, Pediculus humanus capitis, had been reported. A study by Buxton in 1940 on the sex ratio of lice on prisoners in Ceylon is still today the subject of reanalyses. This sex ratio distortion had been detected in ten different countries. In the last sixty years no new data have been collected, especially on scalp infestations under economically and socially more developed conditions.
Results
Here we report a female bias of head lice in a survey of 480 school children in Argentina. This bias is independent of the intensity of the pediculosis, which makes local mate competition highly unlikely as the source of the aberrant sex ratio; however, other possible adaptive mechanisms cannot be discounted. These lice as well as lice from pupils in Britain were carrying several strains of the endosymbiotic bacterium Wolbachia pipientis, one of the most wide spread intracellular sex ratio distorters. Similar Wolbachia strains are also present in the pig louse, Haematopinus suis, suggesting that this endosymbiont might have a marked influence on the biology of the whole order. The presence of a related obligate nutritional bacterium in lice prevents the investigation of a causal link between sex ratio and endosymbionts.
Conclusions
Regardless of its origin, this sex ratio distortion in head lice that has been reported world wide, is stable over time and is a remarkable deviation from the stability of frequency-dependent selection of Fisher's sex ratio. A female bias first reported in 1898 is still present over a hundred years and a thousand generations later.
Background
The early but comprehensive literature on human head lice, Pediculus humanus capitis, reveals a surprisingly homogeneous female bias of the sex ratio world wide [1-3]. The first reported observation of more female than male lice on human heads reaches back to Harding in 1898 in the USA [1]. Records of sex ratios in human head lice in Britain [2,3], Kenya [3,4], Tanzania, Colombia, Australia [3], Nigeria, Ceylon, Palestine [4], India [4,5], and North America [6] show ~65% of the adult lice to be female. Reanalyses of data collected in 1938 are still employed to deduce life history traits of human head lice [4,7,8]. The female bias of natural populations on heads is conserved in the sex ratio of individual broods of experimental infestations [2,6,7]. Many of the people sampled in these studies had been afflicted by poverty, confinement or war. However, in the last sixty years no data have been collected, especially on more normal levels of infestations. Local and temporary deviations from equality of the operational sex ratio should be expected as natural fluctuations. We wanted to find out whether this deviation is also maintained over time. Roughly one hundred years after the original surveys, we determined the sex ratio of adult head lice on pupils from 6 different schools in Argentina. This cohort is different from earlier studies in that it does no longer show any extremely high infestations due to an increase in general living standards. Nevertheless, a female bias is still preserved.
Sex ratio distortions in arthropods frequently originate from cytoplasmic or extrachromosomal factors and parasites of the host and are most often associated with the endosymbiont Wolbachia pipientis. Wolbachia has been shown to induce parthenogenesis in haplodiploid Hymenoptera and diplodiploid Collembola, feminisation in terrestrial isopods, and male killing in many insect species [9-13]. A new bacterium from the Bacteroidetes group (Cytophaga-Flavobacterium-Bacteroides, CFB) has been linked with feminisation and parthenogenesis in mites and insects [14-16]. We screened human lice from Argentina and Britain and pig lice form Poland for the presence of Wolbachia and CFB bacteria.
Results and discussion
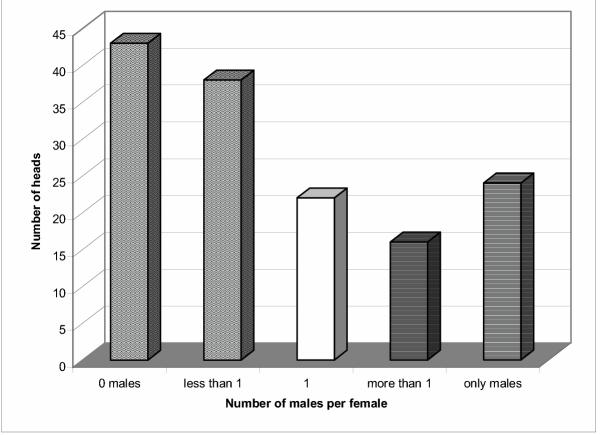
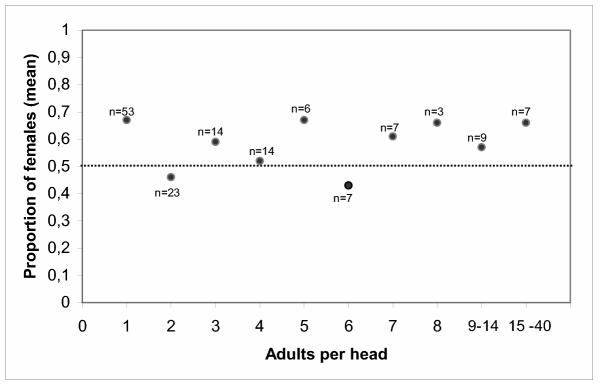
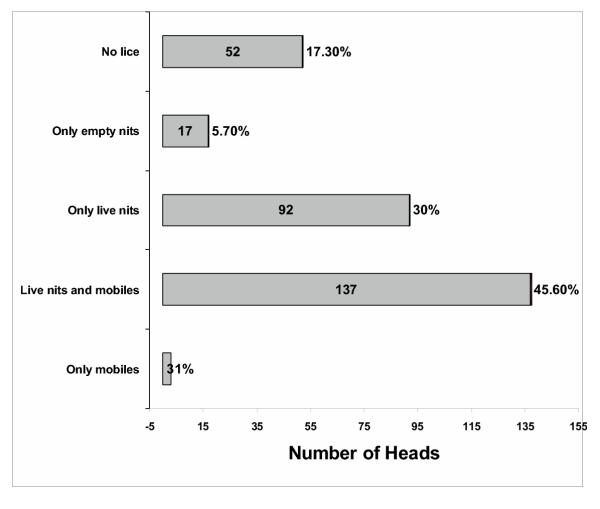
A total of 480 heads were screened for adult lice along 6 schools exhibiting a prevalence of 29.8% (infested heads with at least 1 adult louse/head). 10.5% of the infested heads (15 heads, 94 lice) showed a male bias, 16.8% of the infested heads (24 heads, 32 lice) had only males, 14.7% of the infested heads (21 heads, 72 lice) had an equal sex ratio, 28.0% (40 heads, 344 lice) had a female bias and an additional 30.0% of the children (43 heads, 55 lice) did not carry any male lice, Fig. 1. This translates into an overall female bias of 59.6% (1.48) and a relative sex ratio of 0.40 (males/(males + females)) in all the populations based on 356 females and 241 males. The regression line for the distribution of female and male lice differs from an equal sex ratio distribution by a power of p < 0.01 using Fisher's exact test (Fig. 1). There is no difference between boys and girls. This is also the first survey of sex ratio in human head lice for South America. Applying the standard deviation of the data in Fig. 1, Fig. 2 shows the frequencies of heads that deviate from a normal distribution of sex ratio. Each of the six schools shows individually a female bias and replicate the bias of all the populations, Fig. 3. There is no difference between urban and rural schools. These results in female bias correspond with the data by Buxton and others [5-7]. The probability of a sampling error is p < 0.05; the difference of the actual percentages from 50 is 3.34 times the standard error of this difference [7,18]. Fig. 4 shows that this bias is independent of the intensity of infestation confirming earlier studies by Lang and others [6]. This suggests that the female bias is both conserved in space and time.
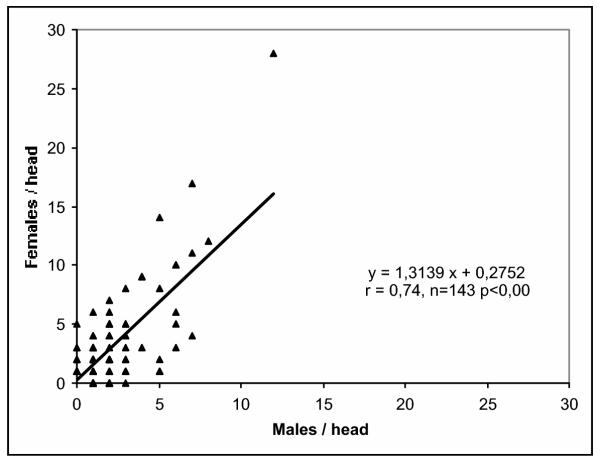
Figure 1.
Sex ratio of human head lice of school children in Argentina. The graph shows the distribution and regression of female and male lice per head with many data points overlapping.
Figure 2.
Number of male lice per female louse per head. The columns show the frequency with which on individual heads female lice were associated with no males, less than one male per female, equality, and more than one male per female. The boundaries of equality include one standard deviation (SD) on each side, the columns 'less than 1' and 'more than 1' are minus one SD.
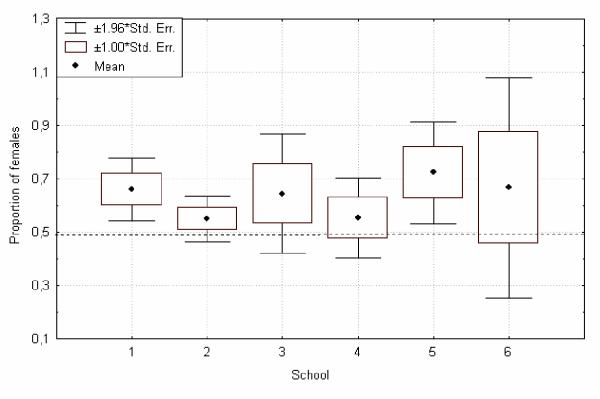
Figure 3.
Female bias of head lice per school. An infested head might be considered as a metapopulation, a school as a population, all six schools replicate the female bias; there is no significant difference between the schools, Chi square 2.44, p = 0.78, n = 6.
Figure 4.
The sex ratio of human head lice is independent of the infestation level. Number of means = 10, r = 0.07, p = 0.43; number of heads = 143, r = 0.00, p = 0.98. N: number of heads; the horizontal line indicates equality.
Pediculid lice are diploid amphimictic and exhibit an extra mitotic division following meiosis during spermatogenesis. Pediculus are sexually dimorphic with a small proportion of hermaphrodites. Siblings are not gregarious, males are abundant, both sexes eclose at similar times, mating starts within one day after eclosure or encounter, males are very promiscuous, females do not seem to store sperm, frequent copulation is a requirement for laying fertile eggs and both sexes show active migratory behaviour. At the moment it is difficult to determine the sex of immature stages and the sex-specific mortality of immature stages directly. However, single-pair matings sometimes produce all of one sex. Differential mortality of nymphs does not account for the unusual sex ratio under non-crowding conditions. P. capitis does not exhibit parthenogenesis or delayed fertilisation. The sex ratio does not change with increasing age of the mother. No evidence has been found that any environmental factor has any effect upon the sex ratio. Only in a single case under very crowded conditions, a male bias has been observed on some heads by Buxton [7,17]. He attributes this to a severely reduced life span of females [7]. The shortened life expectancy of females is ascribed to copulation prior to full sclerotisation leading to injury and death [6]. A selective reduction in life expectancy of females under crowded condition has also been observed in other insect taxa, e.g., tsetse flies. However, highly infested heads with a maximum of 1,434 lice in a study by Roy and Ghosh still showed a female bias [5].
Focusing on the data which Buxton obtained in the Colombo prison in Sri Lanka from 1934 to 1936 that also showed a female bias, Rózsa suggests that the prison environment could increase inbreeding in lice. He argues that the transmission rate was likely to be low simply because the prison host population consisted of adult males only and because the prisoners couldn't get rid of their lice; these infrapopulations would subsist through several generations. These arguments were employed by Rózsa as indications for local mate competition leading to an adaptive sex-ratio manipulation by the lice [8]. This is neither supported by our data nor the data of Buxton, Roy and Ghosh, or Lang [5-7]. There is no evidence that female lice can manipulate the sex ratio of their offspring. Rózsa claims that both, individual heads of the men in jail and the prison itself constitute viscous populations in Hamilton's sense – that is to say, one where the individual louse can mate only with a rather permanent set of neighbours who tend also to be his relatives [19]. Whoever has or has had school children to look after can confirm that individual heads of pupils do not constitute a viscous population. Exchange of adult lice between heads is constantly ongoing and is a major reason for the continuous spread of lice in our society. Our data show that the female bias is present on individual heads as well as in all six schools surveyed. All the schools are coeducational.
Hamilton has proposed local mate competition as an explanation of female biased sex ratios in insect and mite populations. All the arthropod populations listed by Hamilton are characterised by inbreeding and haplodiploidy [19]. An ideal for an extreme biofacies may be described by eight properties. One, the primary sex ratio is spanandrous – that is, females greatly preponderate. In human lice, the operational sex ratio is close to equality. The sex ratio determined by dissecting second instar nymphs and the sex ratio at eclosion of imagines of experimental broods is not more spanandrous than the operational sex ratio. Broods of individual female lice exhibit the whole spectrum from male only offspring to female only offspring. Two, reproduction is arrhenotokous. Lice are diplodiploid and reproduction is amphimictic. Three, there is at least one male in every batch of offspring. Egg laying in the human head louse starts with the first blood meal regardless of insemination; it is more continuous given sufficient food than in batches. The complete brood of a female might contain only males, only females or any ratio in between. Four, there is gregarious development, as a group of siblings, from egg to adult. Unlike the body louse where the female tends to return to the same spot for oviposition and which will lead to a clustering of eggs, siblings of head lice are practically not gregarious. Five, adult males eclose first and can mate many times. Both sexes of head lice eclose at similar times but males are very promiscuous. Six, mating takes place immediately after (or even before) eclosure of adult females. Mating continues to take place at any time of the day or night and during the whole adult life span of lice. Mating happens as much before as after dispersal and both sexes show the same active migratory behaviour. Seven, males are disinclined, or unable, to emigrate from the batch. Male lice disperse as eagerly as females. This is very obvious in our data in the number of heads carrying male lice only (Fig. 2). Eight, females can store sperm; one insemination serves to fertilise the whole egg production. Female lice are stated to have no receptaculum seminiis (spermatheca) and therefore are considered not to be capable of storing sperm. Nevertheless, Bacot reported in 1916 a record observation for a female being able of laying fertile eggs twelve days after the removal of the male; however, the maximum ever observed by Nuttall in 1917 is only five days, which is in line with the assumption of limited or no sperm storage capability. These eight reproductive properties of lice make local mate competition as the cause of a female-biased sex ratio completely impossible. Further work on Hamilton's hypothesis has shown that within each local group, even if founded by a single female, the sex ratio favoured by individual selection is equality [20-23]. However, groups founded by female-biased genotypes contribute more individuals to the population as a whole than groups founded by unbiased genotypes [24]. This requires the ability of kin recognition and selection. However, in the female-biased parasitoid wasp Nasonia vitripennis, which is often used in textbooks as an example of local mate competition, mothers cannot discriminate between kin [25]. In many cases in which the female bias could not be attributed to local mate competition, local resource competition among females or female-biased dispersal have been put forward as alternative explanations [eg., [26,27]]. Local resource competition and differential dispersal can be ruled out in head lice for the same reasons discussed earlier for local mate competition. Why, for example, parasitoid wasps like Melittobia digitata that seemingly fit all the requirements set out by Hamilton fail to meet the sex ratio prediction of the local mate competition hypothesis remains unsolved [28]. Orzack emphasises that the occurrence of local mate competition is not documented for most species whose sex ratios have been explained by it [29]. He also documents that the original evidence presented by Hamilton in support of local mate competition is not as clear as originally assumed. This suggests that major adaptive and parasitic sex ratio effecting mechanisms still remain to be discovered.
Hamilton himself detected a maternally inherited microorganism, later identified as the endosymbiont Wolbachia, in four species of parasitoid wasps belonging to the genus Trichogramma, a genus originally listed as an example of an extreme biofacies leading to local mate competition. Removal of the bacterium changed reproduction from female only thelytoky to biparental arrhenotoky [30]. The possibility that maternally inherited bacteria might bias the sex ratio in favour of females has long been predicted [31]. Parthenogenesis-inducing Wolbachia are quite common in parasitoid wasp species [32]. However, in some species of female-biased parasitoid wasps Wolbachia does not influence the sex ratio directly. In double infected Nasonia for example, two strains of Wolbachia induce cytoplasmic incompatibility, in Asobara tabida, one of the three Wolbachia strains of the triple infection is essential for oocyte maturation, and in Melittobia australica, the effect of Wolbachia remains to be determined [33-35].
A sex ratio bias is often more likely related to the physiological state of its host and is consequently not directly controlled by the mother [36]. The physiological state includes the manipulation by symbiotic microorganisms. In the analysis and interpretation of sex ratio deviations, the discrimination between primary sex ratio and functional sex ratio is pivotal [37]. This allows the identification of symbiotic sex ratio distorters.
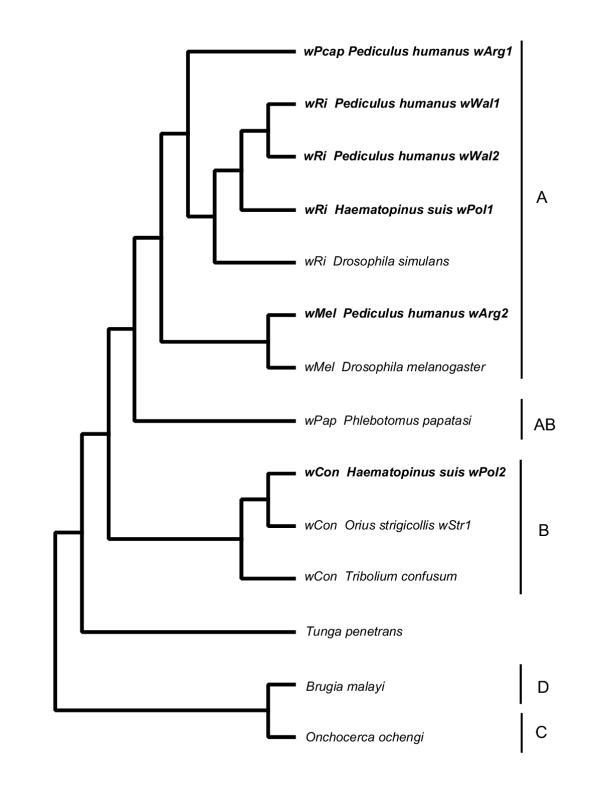
Using the polymerase chain reaction and specific primers for 16S rDNA and the Wolbachia outer surface protein gene wsp, we demonstrated the presence of Wolbachia pipientis in two blood-sucking Anoplura species, the human louse Pediculus humanus capitis, Pediculidae, and the pig louse, Haematopinus suis, Haematopinidae. Primers for insect-like CFB bacteria did not amplify any product; primers for mite-like CFB bacteria resulted only in unrelated lice sequences indicative for the absence of a specific target sequence. The highest amounts of Wolbachia were detected in thorax and head. All lice tested of both species harboured Wolbachia. The Wolbachia infection is likely close to fixation, which suggests either a relatively young infection showing no signs of host resistance or indicates an essential function for Wolbachia in the development and survival of its lice hosts. The fact that two phylogenetically very different lice species with two distinct hosts show similar high levels of penetrance of Wolbachia infection on two different continents leads us to expect Wolbachia infections to be widespread in many lice species. The wsp gene of Wolbachia is one of the fastest evolving. Wsp sequences of Wolbachia from human and animal lice overlap. No Wolbachia lineage specific to a louse species or to a louse host could be identified. Comparison of the wsp sequences revealed no special position of lice Wolbachia among other insect Wolbachia strains (Fig. 5). All lice showed double infections with two different Wolbachia strains based on wsp sequence. Human head lice from two continents have been analysed. Head lice from South America, Argentina, exhibit infections of two different strains of Wolbachia from supergroup A, wMel and a new group, which we propose to call wPcap, while from Europe, Wales, only two similar strains of supergroup A (wRi) have been detected. In contrast, pig lice harbour Wolbachia from supergroup A (wRi) and supergroup B (wCon closest to strain wStr1). While double infections with two A supergroup Wolbachia might reflect recombination events or in-host diversification of Wolbachia, double infections with A and B supergroup bacteria are likely to originate from repeated episodes of horizontal transmission. The diversity of A and B supergroup Wolbachia make it seem possible that lice have received recent horizontal transfer from a wide spectrum of Wolbachia donors. An infectious form of Wolbachia is not known. It is always vertically transmitted within a species. Lice are one of very few insect taxa that are not attacked by parasitoid wasps and endoparasitic Strepsiptera. Parasitoid wasps are the only so far confirmed vectors for horizontal Wolbachia transmission; the role of Strepsiptera in interspecies transmission is still controversal [38]. Interestingly, we were unable to find any report about lice-specific mites or nematodes, theorised vectors of Wolbachia. The literature is limited to the protozoan gut parasite of body lice, 'Crithidia' or Herpetomonas pediculi and omniphagous steinernematid and heterorhabditid nematodes, which do not carry Wolbachia [39-42]. This lack of parasites might be due in part to a deficiency of research; however, it requires at present to postulate a new route for the horizontal transmission of Wolbachia to and within lice species.
Figure 5.
Relationship of Wolbachia strains from lice to each other and to Wolbachia strains from other insects and filarial worms. Parsimony cladogram showing the relationship of Wolbachia strains detected in lice (bold) and their nearest known relatives based on the wsp gene and constructed with branch-and-bound. From right to left, the Wolbachia group is noted, where known, followed by the name of the host and the designation for the individual Wolbachia strain; after the bar the Wolbachia supergroup is indicated. Phlebotomus papatasi is a sand fly (Diptera), Orius strigicollis is a bug (Hemiptera), Tribolium confusum is a beetle (Coleoptera), Tunga penetrans is a flea (Siphonaptera), and Brugia malayi and Onchocerca ochengi are filarial nematodes.
Microscopic analysis shows that intracellular Wolbachia-like bacteria are not associated with the well described but yet unidentified nutritional lice endosymbionts found in mycetomes (Fig. 6). While the bacteria originating from the stomach disc mycetome migrate to the ovaries and enter the developing oocyte at a specialised invagination forming the future mycetome [43-45], intracellular Wolbachia-like bacteria are already present in the egg cell and are dispersed in the cytoplasm of the egg. Due to the difficulty of cutting thin sections of the yolk part of eggs, Wolbachia infections are easily missed in ultrastructural investigations.
Figure 6.
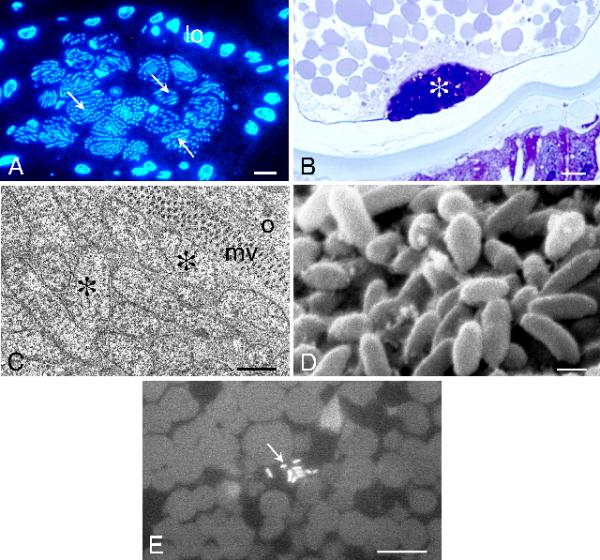
Localization of microorganisms within reproductive organs of the piglouse, Haematopinus suis. A, Intraovarian mycetome consists of several mycetocytes containing rod-shaped microorganisms (arrows). The mycetome is located in contact with lateral oviduct cells (lo). Fluorescence microscope, DAPI; scale bar = 10 μm. B, Posterior pole of the oocyte, mycetome located in a depression of the posterior pole of the oocyte (asterisk). Light microscope, methylene blue; scale bar = 10 μm. C, Mycetome microorganisms (asterisks) are located extracellularly, in contact with oocyte microvilli (mv); oocyte (o). TEM; scale bar = 1 μm. D, Mycetome microorganisms visualized in SEM; scale bar = 1 μm. E, Intracellular microorganisms (arrow) within the oocyte cytoplasm. Fluorescence microscope, DAPI; scale bar = 10 μm.
The sex ratio bias reported here for the human head louse does not seem to be an isolated phenomenon. Unusual sex ratios have also been reported for the human body louse, Pediculus humanus humanus (P. corporis, P. vestimenti), the human pubic louse, Pthirus pubis, the cattle biting louse Bovicola bovis, the shortnosed cattle louse, Haematopinus eurysternus, the longnosed cattle louse, Linognathus vituli, and the little blue cattle louse, Solenopotes capillatus [3,46-49]. The biting louse B. bovis also exhibits parthenogenetic reproduction. The search for Wolbachia in many biting and sucking lice (Anoplura, Mallophaga) is ongoing [50].
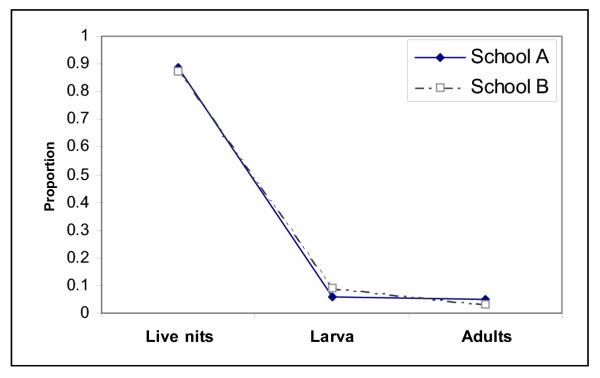
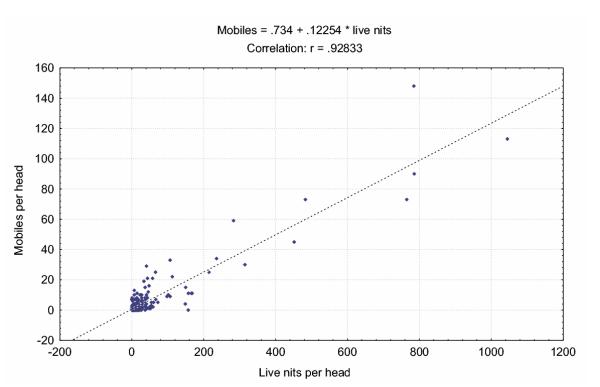
Human head lice show a female biased sex ratio that has been conserved for over 100 years with a global distribution which represents a remarkable deviation from the stability of frequency-dependent selection of Fisher's sex ratio [1,51]. The association of this sex ratio distortion with a sex-ratio distorting endosymbiont is intriguing. A direct treatment of lice with antibiotics to cure them of Wolbachia is not feasible at the moment because of the lethal effect on mobile stages of lice, as it eliminates the Gram-negative obligate lice endosymbionts as well [52]. Male killing is one of several possible mechanisms leading to distorted sex ratios. In other insects, male-killing Wolbachia act at the earliest stages of development and manifest as eggs that fail to hatch [53]. Indeed, Buxton identified the failure of eggs to hatch as an important cause of mortality in Pediculus humanus corresponding to what would be expected with low-level male killing as it is well known, for example in lady bird beetles [54,55]. Mortality data have not been reported for head lice. In a new study we determined the number of empty eggs, alive eggs, nymphs, and adults of 300 children of two coeducational schools at an age between 6 and 8 years, Fig. 7. Per head, alive eggs represent 90% of the accountable metapopulation, nymphs and adults, which can be combined as mobiles, account for the further 10%. This relationship is not affected by the social status of the pupils. The living standard of the children might be used as a proxy of environmental influences on the lice. In Fig. 8, we compared the data from a school (A, 161 children) in a deprived neighbourhood with the data from a school in an affluent surrounding (B, 139 children). The pattern is identical. The correlation between living eggs and mobiles is significant (p < 0.01) and suggests a high mortality at the egg stage, Fig. 9, the origin of which remains to be determined.
Figure 7.
Occurrence of eggs, larvae and adults of human head lice. Distribution of various stages on heads; larvae and adults are combined as mobiles. Numbers inside the columns denote children's heads followed by the corresponding percentage.
Figure 8.
Influence of social environment on sex ratio of lice. Comparison of a school in a deprived neighbourhood (A) with a school in an affluent neighbourhood (B).
Figure 9.
The highest mortality of head lice is at the egg stage. Correlation between live eggs and mobiles (nymphs and adults) per head. Pearson, n = 229, p < 0.001.
Conclusions
Adaptive sex ratio distortions fluctuate over time and are often specific to local habitats and environmental conditions. Mechanisms such as local mate competition, local resource competition or differential dispersal can lead to female bias populations. The female bias in the human head louse cannot be ascribed to any of these three mechanisms. This bias is found on 4 continents. It is stable over more than one century. This makes it one of the best documented deviations from the stability of frequency-dependent selection of Fisher's equal sex ratio.
Methods
Lice
The surveys and collections of head lice have been conducted between October 2000 and August 2003. In Argentina, the schools are situated in urban and rural parts of the provinces of La Rioja and Córdoba. The lice were collected by the authors [AVO and SSC]. The hair was wetted and treated with conditioner. With the help of a metal comb, eggs, nits and adults were collected under water and immediately transferred into 95% alcohol. This method of collecting lice preludes any sampling bias favouring larger females over smaller males as suggested by Nuttall and Marshall [37]. Eggs and empty egg cases were distinguished and the sexes of imagines were determined using a light microscope. The lice samples from Britain were collected by their parents in Llandudno, Conwy, and Bangor, Gwynedd, both in northern Wales, United Kingdom. The pig lice were collected from domestic pigs kept on a small farm near Kraków, Poland.
Wolbachia
Total genomic DNA was extracted from single lice. Males and females were sectioned in two parts: head-thorax and abdomen and DNA was extracted separately for both sections. QIAGEN DNAeasy™ Tissue Kit was used for DNA extraction following the DNeasy Protocol for Animal Tissues. The DNA obtained was purified with 10% CHELEX® 100 at 60°C for 2 h. The samples were centrifuged at 8,000 rpm for 5 min and the supernatant was stored at -20°C. PCR was performed with specific primers for Wolbachia 16S rDNA, 99F and 994R [56], Wolbachia outer surface protein, wsp81F and wsp691R [57] and universal primers for 28S rDNA, 28Sa and 28Sb [58] as control. CFB bacteria were detected with primers for 16S rDNA. Primers for insect-like CFB bacteria were based on sequences derived from the parasitoid wasps of the genus Encarsia, EPS-f and EPS-r [15], mite-like CFB bacteria were designed with the help of the sequence from the feminised mite Brevipalpus phoenicis, GenBank accession AF350221, CFBmite16S-F 5'-CCT GCG GGG GCT CTT GA-3' and CFBmite16S-R 5'-GGG TTT CGC TCG TTA TAG GAC TTA-3' amplifying a 644 bp fragment in B. phoenicis. Relative amounts were estimated with serial dilutions of the template before amplifications. The products were ligated into QUIAGEN pDrive Cloning Vectors and 8 positive colonies per ligation were sequenced in both directions. The sequences are deposited in GenBank with accession numbers AY596781-AY596786.
Authors' contributions
MAP carried out the molecular work. AO collected the human lice in Argentina. MZ and SMB collected the pig lice and performed the microscopic work. SSC, MAP and HRB analysed the data. MAP and HRB wrote a first draft of the manuscript. All authors contributed to the final analyses and writing of the manuscript.
Acknowledgments
Acknowledgements
Field and laboratory work in Argentina (SSC and AO) have been supported by Fundación Barceló. MAP and HRB are supported by funding from the Natural Environment Research Council (GR3/13199) and HRB acknowledges funding by the European Commission (EUWOL, QLRT-2000-01079).
Contributor Information
M Alejandra Perotti, Email: bssc13@bangor.ac.uk.
Silvia S Catalá, Email: scatala@crilar-conicet.com.ar.
Analía del V Ormeño, Email: lia414ville@yahoo.com.
Monika Żelazowska, Email: zawadz@zuk.iz.uj.edu.pl.
Szczepan M Biliński, Email: sbili@zuk.iz.uj.edu.pl.
Henk R Braig, Email: h.braig@bangor.ac.uk.
References
- Harding GF. Pediculosis. Boston Med Surg J. 1898;138:95. [Google Scholar]
- Bacot A. A contribution to the bionomics of Pediculus humanus (vestimenti) and Pediculus capitis. Parasitolol. 1917;9:228–258. [Google Scholar]
- Nutall GHF. The biology of Pediculus humanus. Supplementary notes. Parasitol. 1918;11:201–220. [Google Scholar]
- Buxton PA. Studies on populations of head lice (Pediculus humanus capitis: Anoplura). II. Parasitol. 1938;30:85–110. [Google Scholar]
- Roy DN, Ghosh SM. Studies on the population of the head lice, Pediculus humanus var. capitis De G. Parasitol. 1944;36:69–71. [Google Scholar]
- Lang JD. Sex ratio of adult head lice under crowded conditions. J New York Entomol Soc. 1976;84:243–245. [Google Scholar]
- Buxton PA. Studies on populations of head lice (Pediculus humanus capitis: Anoplura). IV. The composition of populations. Parasitol. 1940;33:224–242. [Google Scholar]
- Rózsa L. Adaptive sex-ratio manipulation in Pediculus humanus capitis: Possible interpretations of Buxton's data. J Parasitol. 1997;83:543–544. [PubMed] [Google Scholar]
- Hurst LD. The incidences, mechanisms and evolution of cytoplasmic sex ratio distorters in animals. Biol Rev. 1993;68:121–193. doi: 10.1086/417973. [DOI] [Google Scholar]
- O'Neill SL, Hoffmann AA, Werren JH. Influential Passengers. Oxford: Oxford University Press; 1997. [Google Scholar]
- Braig HR, Turner BD, Normark BB, Stouthamer R. Microorganism-induced parthenogenesis. In: Hughes RN, editor. In Reproductive Biology of Invertebrates. Chichester: John Wiley & Sons; 2002. pp. 1–62. [Adiyodi KG, Adiyodi RG (Series Editors): Progress in Asexual Reproduction, vol XI] [Google Scholar]
- Bourtzis K, Miller TA. Insect Symbiosis. Boca Raton: CRC Press; 2003. [Google Scholar]
- Ebbert MA. Endosymbiontic sex ratio distorters. In: Wrensch DL, Ebbert MA, editor. In Evolution and Diversity of Sex Ratio in Insects and Mites. New York: Chapman & Hall; 1993. pp. 150–191. [Google Scholar]
- Weeks AR, Marec F, Breeuwer JAJ. A mite species that consists entirely of haploid females. Science. 2001;292:2479–2482. doi: 10.1126/science.1060411. [DOI] [PubMed] [Google Scholar]
- Zchori-Fein E, Gottlieb Y, Kelly SE, Brown JK, Wilson JM, Karr TL, Hunter MS. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc Natl Acad Sci USA. 2001;98:12555–12560. doi: 10.1073/pnas.221467498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AR, Breeuwer JAJ. A new bacterium from the Cytophaga-Flavobacterium-Bacteroides phylum that causes sex ratio distortion. In: Bourtzis K, Miller TA, editor. In Insect Symbiosis. Boca Raton: CRC Press; 2003. pp. 165–176. [Google Scholar]
- Buxton PA. The numbers of males and females in natural populations of head lice (Pediculus: Anoplura) Proc Roy Ent Soc Lond A. 1937;12:12–14. [Google Scholar]
- Hardy ICW. Sex Ratios, Concepts and Research Methods. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Hamilton WD. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- Wilson DS, Colwell RK. Evolution of sex-ratio in structured demes. Evolution. 1981;35:882–897. doi: 10.1111/j.1558-5646.1981.tb04952.x. [DOI] [PubMed] [Google Scholar]
- Colwell RK. Group selection is implicated in the evolution of female-biased sex-ratios. Nature. 1981;290:401–404. [Google Scholar]
- Frank SA. Hierarchical selection theory and sex ratios. 1. General solutions for structured populations. Theor Popul Biol. 1986;29:312–342. doi: 10.1016/0040-5809(86)90013-4. [DOI] [PubMed] [Google Scholar]
- Antolin MF. Genetics of biased sex ratios in subdivided populations: models, assumptions, and evidence. Oxford Surv Evol Biol. 1993;9:239–281. [Google Scholar]
- Futuyma DJ. Evolutionary Biology. 3. Sunderland: Sinauer Associates; 1998. [Google Scholar]
- Reece SE, Shuker DM, Pen I, Duncan AB, Choudhary A, Batchelor CM, West SA. Kin discrimination and sex ratios in a parasitoid wasp. J Evol Biol. 2004;17:208–216. doi: 10.1046/j.1420-9101.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Santolamazza-Carbone S, Rivera AC. Superparasitism and sex ratio adjustment in a wasp parasitoid: results at variance with Local Mate Competition? Oecologia. 2003;136:365–373. doi: 10.1007/s00442-003-1269-5. [DOI] [PubMed] [Google Scholar]
- Dagg JL, Vidal S. Sex ratio adjustment and maternal condition in two aphid species. Behav Ecol Sociobiol. 2004;55:231–235. doi: 10.1007/s00265-003-0702-4. [DOI] [Google Scholar]
- Cooperband MF, Matthews RW, Vinson SB. Factors affecting the reproductive biology of Melittobia digitata and failure to meet the sex ratio predictions of Hamilton's local mate competition theory. Entomol Exp Appl. 2003;109:1–12. doi: 10.1046/j.1570-7458.2003.00084.x. [DOI] [Google Scholar]
- Orzack SH. Using sex ratios: the past and the future. In: Hardy ICW, editor. In Sex Ratios, Concepts and Research Methods. Cambridge: Cambridge University Press; 2002. pp. 383–398. [Google Scholar]
- Stouthamer R, Luck RF, Hamilton WD. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera/Trichogrammatidae) to revert to sex. Proc Natl Acad Sci USA. 1990;87:2424–2427. doi: 10.1073/pnas.87.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmides LM, Tooby J. Cytoplasmic inheritance and intragenomic conflict. J Theor Biol. 1981;89:83–129. doi: 10.1016/0022-5193(81)90181-8. [DOI] [PubMed] [Google Scholar]
- Koivisto RKK, Braig HR. Microorganisms and parthenogenesis. Biol J Linn Soc. 2003;79:43–58. doi: 10.1046/j.1095-8312.2003.00185.x. [DOI] [Google Scholar]
- Bordenstein SR, Uy JJ, Werren JH. Host genotype determines cytoplasmic incompatibility type in the haplodiploid genus Nasonia. Genetics. 2003;164:223–233. doi: 10.1093/genetics/164.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Boulétreau M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA. 2001;98:6247–6252. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe J, Kamimura Y, Kondo N, Shimada M. Extremely female-biased sex ratio and lethal male-male combat in a parasitoid wasp, Melittobia australica (Eulophidae) Behav Ecol. 2003;14:34–39. doi: 10.1093/beheco/14.1.34. [DOI] [Google Scholar]
- Orzack SH. Sex ratio evolution in parasitic wasps. In: Wrensch DL, Ebbert MA, editor. In Evolution and Diversity of Sex Ratio in Insects and Mites. New York: Chapman & Hall; 1993. pp. 477–511. [Google Scholar]
- Marshall AG. The sex ratio in ectoparasitic insects. Ecol Entomol. 1981;6:155–174. [Google Scholar]
- Hughes DP, Pamilo P, Kathirithamby J. Horizontal transmission of Wolbachia by strepsipteran endoparasites? A response to Noda et al., 2001. Mol Ecol. 2004;13:507–509. doi: 10.1046/j.1365-294X.2003.02083.x. [DOI] [PubMed] [Google Scholar]
- Mackie FP. The part played by Pediculus corporis in the transmission of relapsing fever. Brit Med J. 1907;2:1706–1709. doi: 10.1136/bmj.2.2450.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantham HB. Herpetomonas pediculi nov. spec., parasite in the alimentary tract of Pediculus vestimenti, the human body louse. Proc Roy Soc B. 1912;84:505–517. [Google Scholar]
- Weiss M, Glazer I, Mumcuoglu KY, Elking Y, Galun R. Infectivity of steinernematid and heterorhabditid nematodes for the human body louse Pediculus humanus humanus (Anoplura: Pediculidae) Fund Appl Nematol. 1993;16:489–493. [Google Scholar]
- De Doucet MMA, Miranda MB, Bertolotti MA. Infectivity of entomogenous nematodes (Steinernematidae and Heterorhabditidae) to Pediculus humanus capitis De Geer (Anoplura: Pediculidae) Fund Appl Entomol. 1998;21:13–16. [Google Scholar]
- Eberle MW, McLean DL. Initiation and orientation of the symbiote migration in the human body louse Pediculus humanus L. J Insect Physiol. 1982;28:417–422. doi: 10.1016/0022-1910(82)90068-3. [DOI] [Google Scholar]
- Eberle MW, McLean DL. Observation of symbiotic migration in human body lice with scaning and transmission electron microscopy. Can J Microbiol. 1983;29:755–762. doi: 10.1139/m83-123. [DOI] [PubMed] [Google Scholar]
- Zelazowska M, Bilinski SM. Distribution and transmission of endosymbiotic microorganisms in the oocytes of the pig louse, Haematopinus suis (L.) (Insecta: Phthiraptera) Protoplasma. 1999;209:207–213. [Google Scholar]
- Hindle E. Notes on the biology of Pediculus humanus. Parasitol. 1917;9:259–265. [Google Scholar]
- Hindle E. Sex inheritance in Pediculus humanus var. corporis. J Genet. 1919;8:267–277. [Google Scholar]
- Nuttall GHF. The pathological effects of Phthirus pubis. Parasitol. 1918;10:375–405. [Google Scholar]
- Watson DW, Lloyd JE, Kumar R. Density and distribution of cattle lice (Phthiraptera: Haematopinidae, Linognathidae, Trichodectidae) on six steers. Vet Parasitol. 1997;69:283–296. doi: 10.1016/S0304-4017(96)01122-3. [DOI] [PubMed] [Google Scholar]
- Kyei-Poku GK, Colwell DD, Coghlin P, Floate KD. On the ubiquity and phylogeny of Wolbachia in lice. J Mol Ecol. [DOI] [PubMed]
- Fisher RA. The General Theory of Natural Selection. Oxford: Oxford University Press; 1930. [Google Scholar]
- Burgess IF. Human Lice and Their Control. Annu Rev Entomol. 2004;49:457–481. doi: 10.1146/annurev.ento.49.061802.123253. [DOI] [PubMed] [Google Scholar]
- Hurst GDD, Jiggins FM. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg Infect Dis. 2000;6:329–336. doi: 10.3201/eid0604.000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton PA. The biology of the body louse (Pediculus humanus corporis: Anoplura) under experimental conditions. Parasitol. 1940;32:303–312. [Google Scholar]
- Majerus MEN, Schulenburg HJGvd, Zakharov IA. Multiple causes of male-killing in a single sample of the two-spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae) from Moscow. Heredity. 2000;84:605–609. doi: 10.1046/j.1365-2540.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- O'Neill SL, Giordano R, Colbert AM, Karr T, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig HR, Zhou W, Dobson S, O'Neill SL. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia. J Bacteriol. 1998;180:2373–2378. doi: 10.1128/jb.180.9.2373-2378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting MF, Carpenter JC, Wheeler QD, Wheeler WC. The strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Syst Biol. 1997;46:1–68. doi: 10.1093/sysbio/46.1.1. [DOI] [PubMed] [Google Scholar]