Abstract
Development of an in vivo gene reporter assay to assess interactions among the components of the transcription machinery in Mycobacterium tuberculosis remains a challenge to scientists due to the tediousness of generation of mutant strains of the extremely slow-growing bacterium. We have developed a recombinant mCherry reporter assay that enables us to monitor the interactions of Mycobacterium tuberculosis transcriptional regulators with its promoters in vivo in Escherichia coli. The assay involves a three-plasmid expression system in E. coli wherein two plasmids are responsible for M. tuberculosis RNA polymerase (RNAP) production and the third plasmid harbors the mCherry reporter gene expression cassette under the control of either a σ factor or a transcriptional regulator-dependent promoter. We observed that the endogenous E. coli RNAP and σ factor do not interfere with the assay. By using the reporter assay, we found that the functional interaction of M. tuberculosis cyclic AMP receptor protein (CRP) occurs with its own RNA polymerase, not with the E. coli polymerase. Performing the recombinant reporter assay in E. coli is much faster than if performed in M. tuberculosis and avoids the hazard of handling the pathogenic bacterium. The approach could be expanded to develop reporter assays for other pathogenic and slow-growing bacterial systems.
INTRODUCTION
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is the second most frequent cause of death worldwide from a single infectious disease (1–3). The pathogen remains deadly because of its ability to adapt to the harsh environmental conditions it encounters in the course of a successful infection. The bacterium can survive the unfavorable environmental conditions by adopting alternative gene expression systems and employing important players from the pool of 13 σ factors and 180 transcriptional regulators (4). It is believed that a set of genes are expressed under a particular environmental cue to maintain the minimum cellular activities required for its survival under stress (5, 6). One or more σ factors along with transcriptional regulators are thought to be responsible for gene regulation under the particular stress conditions. Upon encountering a different stress condition, the bacterium may switch gene expression from one set of genes to another by altering the σ factors in the transcriptional machinery.
In order to understand M. tuberculosis regulation under different stress conditions, many researchers have performed chromatin immunoprecipitation (ChIP) assays, microarray analysis, or quantitative reverse transcription-PCR (7–10) to identify the regulons for several σ factors and transcriptional regulators. However, there is a need to develop rapid assays to validate the above-described findings by assessing the in vivo interactions of a transcriptional factor with its cognate promoters.
One of the ways to validate gene regulation by a σ factor and transcriptional regulator is to develop a reporter gene assay in M. tuberculosis. For such an assay, the first step would be to prepare a mutant strain either by removing the gene encoding the regulator protein to ensure loss of expression of the regulator (knockout strain) (11–14) or by replacing the endogenous promoter element of the regulator gene via an inducible exogenous promoter, to ensure a minimum level of endogenous protein in the absence of any inducer molecule (knockdown strain) (15, 16). However, the broad manifestations of reporter gene assays in M. tuberculosis would be extremely tedious and time-consuming because of the slow-growing nature of mycobacteria (17). As a consequence, very few successful endeavors involving reporter gene assays have been made with M. tuberculosis to study the interactions of its promoters with regulatory proteins. Assuming that the transcriptional apparatus of Mycobacterium smegmatis is similar to that of M. tuberculosis, attempts were made to develop recombinant reporter gene assays to study the functions of M. tuberculosis promoters in M. smegmatis (18–22). However, functional orthologs of M. tuberculosis transcriptional regulators are often present in M. smegmatis (23–25). Therefore, study of the interactions of these regulators in M. smegmatis demands the generation of knockout strains for the regulators. This approach would be comparatively faster than performing the reporter assay in M. tuberculosis, but it would still be a time-consuming assay, as generation of knockout strains is involved. In this report, we present an alternative recombinant in vivo reporter assay which is less time-consuming and is devoid of the generation of a knockout strain. We describe an mCherry reporter assay in E. coli that has enabled us to monitor the interactions of M. tuberculosis σ factors and transcriptional regulators with its promoters in vivo. The assay employs recombinant M. tuberculosis RNA polymerase (RNAP) holo enzyme and a plasmid that harbors an mCherry reporter gene expression cassette under the control of either a σ factor or a transcriptional regulator-dependent promoter element.
MATERIALS AND METHODS
Cloning strategies.
Cloning of the genes encoding different RNAP subunits in different Duet vectors, with use of appropriate enzymes, has been discussed by Banerjee et al. (26). sigA and sigE were amplified from genomic DNA and cloned in pAcYc Duet (see Table S2 in the supplemental material). sinP3 promoter DNA was amplified from synthetic oligonucleotide template and cloned in pFPVmCherry (see Tables S1 and S2 in the supplemental material). sigBpr and WhiB1were amplified from genomic DNA of M. tuberculosis H37Rv (see Tables S1 and S2) and cloned in pBluescript II SK(+) by blunt end ligation and subsequently in pFPVmCherry (see Table S2). An rrnA DNA template, a kind gift from Jaya Tyagi (AIIMS, India) (21) was amplified and cloned in pFPVmCherry. The M. tuberculosis cyclic AMP (cAMP) receptor protein (CRP; Rv3676) was amplified from genomic DNA of M. tuberculosis cloned first in pET28a and subsequently in pFPVmCherry (see Table S2).
Purification of proteins.
In vivo-assembled M. tuberculosis RNAP core and M. tuberculosis RNAP-σA holo were purified as described by Banerjee et al. (26). The E. coli RNAP core was purified as described by Mukhopadhyay et al. (27). M. tuberculosis CRP was purified essentially as described by Bai et al. (28), except that a different resuspension buffer (50 mM Tris-HCl [pH 7.9], 200 mM NaCl, 10% glycerol, and 1 mM phenylmethylsulfonyl fluoride) was used instead of sodium phosphate buffer.
In vitro transcription assays.
To perform the transcription assay with M. tuberculosis RNAP holo with or without the ω-subunit, increasing concentrations (50, 100, and 200 nM) of RNAP holo were incubated with 40 nM DNA template (sinP3 or rrnA) in 18 μl transcription reaction buffer (45 mM Tris-HCl [pH 8.0], 70 mM KCl, 1 mM dithiothreitol, 10% glycerol, 5 mM MgCl2, and 1.5 mM MnCl2) at 37°C for 20 min. One microliters of heparin (0.5 mg/ml) was added to the reaction mixture to inhibit nonspecific RNAP-DNA complex formation. RNA synthesis was initiated by the addition of 1 μl nucleoside triphosphate mix (final concentrations, 125 μM ATP, GTP, and UTP and 50 μM CTP containing 0.4 μCi [α-32P]CTP) (26). Following incubation at 37°C for 30 min, the reactions were terminated by adding 5 μl FLB dye (80% formamide, 10 mM EDTA, 0.01% bromophenol blue, 0.01% xylene cyanol). Products were heated at 95°C for 5 min, chilled on ice, and resolved by urea-PAGE (29), analyzed with a storage phosphor scanner (Typhoon Trio+; GE Healthcare), and quantified using ImageQuant TL software.
The in vitro transcription assay with the M. tuberculosis σE-dependent promoter sigBpr was conducted following the same protocol described above, except that M. tuberculosis RNAP (σE) holo was used instead of RNAP (σA) holo.
To perform the in vitro transcription assay with the M. tuberculosis CRP-dependent promoter WhiB1, 400 nM M. tuberculosis CRP was incubated with increasing concentrations of RNAP holo (with or without the ω-subunit) for 20 min at 37°C in transcription buffer. RNA synthesis was initiated following the same protocol described above.
In vitro transcription assays using M. tuberculosis RNAP and σA, E. coli RNAP and σ70, or hybrid RNAP holo enzymes formed by interchanging the sigma factors were conducted following the above-described protocol. Native or hybrid RNAP holo enzymes were formed by incubating RNAP and sigma factor for 20 min at 37°C. For conducting the in vitro transcription assay using the WhiB1 promoter, M. tuberculosis RNAP and E. coli RNAP were incubated with either E. coli CRP or M. tuberculosis CRP for 20 min at 37°C prior to addition of DNA template.
In vivo recombinant reporter assays. (i) Reporter assay with σA-RNAP holo.
E. coli BL21(DE3) was transformed with the three plasmids indicated in the figures (see Fig. 1A; see also Fig. S2 in the supplemental material). Cotransformed cells were grown in 50 ml LB broth at 37°C with appropriate antibiotics (chloramphenicol at 35 μg/ml, ampicillin at 100 μg/ml, kanamycin at 50 μg/ml) to an optical density at 595 nm (OD595) of 0.4, induced by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and were further grown at 37°C for 6 h or at 16°C for 16 h (until the OD595 reached 1.5).
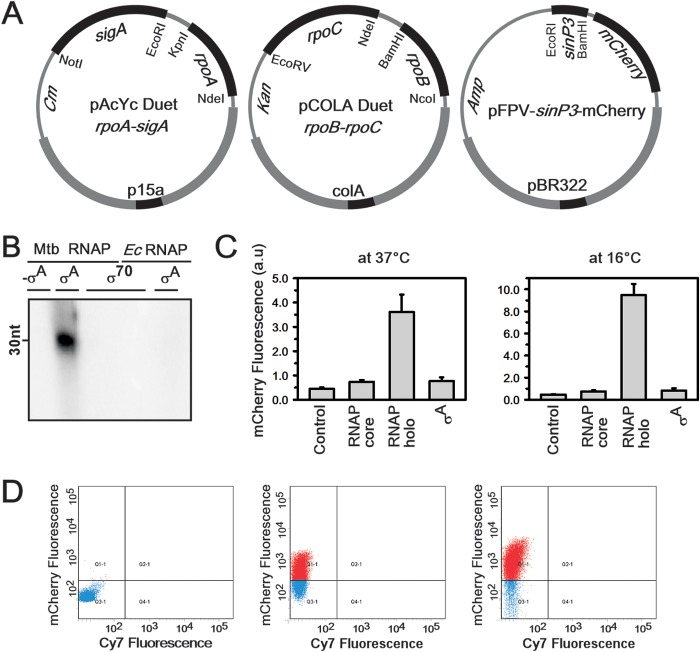
FIG 1.
Recombinant in vivo M. tuberculosis reporter assay using M. tuberculosis σA and its specific promoter, sinP3. (A) Strategy for use of the three-plasmid expression system, with dual plasmids (first two plasmids) for expression of M. tuberculosis RNAP holo and the third plasmid containing a σA-dependent promoter element, sinP3, for expression of mCherry. All three plasmids were transformed in E. coli Bl21(DE3) cells grown at 37°C up to an OD (at 595 nm) of 0.4 and induced with 0.5 mM IPTG under different growth conditions. (B) Results of an in vitro transcription assay to assess the interactions between the M. tuberculosis RNAP core and E. coli σ70 and between the E. coli RNAP core with M. tuberculosis σA. sinP3 was used as the DNA template. Runoff transcripts of 30 nucleotides (nt) were produced. Lane 1, M. tuberculosis (Mtb) RNAP core; lane 2, M. tuberculosis RNAP core plus M. tuberculosis σA; lane 3, M. tuberculosis RNAP core plus E. coli (Ec) σ70; lane 4, E. coli RNAP plus E. coli σ70; lane 5, E. coli RNAP plus M. tuberculosis σA. (C) Results for the recombinant M. tuberculosis reporter assays. The bars represent mCherry fluorescence of E. coli cells containing the pFPVmCherry-sinP3 plasmid in the presence of the following: control, with no M. tuberculosis RNAP; M. tuberculosis RNAP core (pAcYc Duet rpoA-rpoZ plus pCOLA Duet rpoB-rpoC); M. tuberculosis RNAP holo (as in panel A); σA (pAcYc Duet-sigA). The data represent means of three replicates, and error bars represent standard deviations. (Left) Cells were induced with 0.5 mM IPTG at 37°C for 6 h. (Right) Cells were induced with 0.5 mM IPTG at 16°C for 16 h. (D) FACS data. Aliquots of cells from the above-described assay were scanned for the mCherry (610 nm) and Cy7 (760 nm) fluorescence channels. (Left) Control, pFPVmCherry-sinP3 plasmid; (middle) all three plasmids as described above, with protein induction at 37°C; (right) all three plasmids, with protein induction at 16°C. The number of fluorescent cells in the right panel is approximately twice than that in the middle panel.
As a control, the assays were performed with E. coli BL21(DE3) harboring the dual plasmid (pAcYc Duet rpoA-rpoZ plus pCOLA Duet rpoB-rpoC) for RNAP core expression or plasmid pAcYc Duet-sigA for σA expression, along with pFPVmCherry-sinP3 (or pFPVmCherry-rrnA) or pFPVmCherry-sinP3 alone. pAcYc Duet rpoA-sigA plus pCOLA Duet rpoB-rpoC along with pFPVmCherry-sinP3 were used for M. tuberculosis holo expression. Identical assays were conducted with pFPVmCherry-rrnA plasmid.
To optimize the bacterial growth duration at which the fluorescence signal versus background ratio was highest, we monitored the OD and fluorescence intensities of the bacterial cultures at regular intervals. Aliquots of 100 μl of the cultures were taken, and the OD (at 595 nm) and fluorescence intensities (excitation wavelength, 592 nm; emission wavelength, 610 nM) were measured at each interval. The normalized fluorescence intensity (fluorescence intensity/OD ratio) data were plotted against the nearest single-decimal OD value (for example, if X was the fluorescence intensity of a bacterial culture at an OD of 0.37, the normalized fluorescence intensity, which was calculated as [X × (0.4/0.37)], was plotted against an OD of 0.4). The assays were repeated twice, and the mCherry expression kinetic curves were plotted (see Fig. S1 in the supplemental material). The duration of bacterial growth at which the optimum fluorescence signal-to-background ratio was obtained was observed either at 37°C for 6 h or at 16°C for 16 h.
Assay with M. tuberculosis σE.
The assays with σE and a σE-specific promoter were conducted essentially as described above, except that σA was replaced with σE and the sinP3 promoter was replaced with sigBpr. The three-plasmid expression system for σE is shown in Fig. 2A, below.
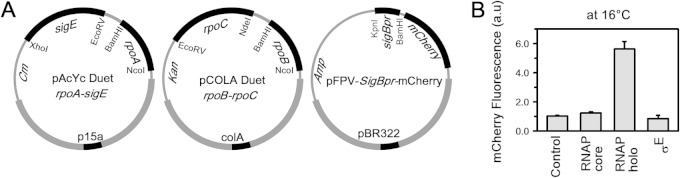
FIG 2.
Recombinant in vivo M. tuberculosis reporter assay using M. tuberculosis σE and its specific promoter, sigBpr. (A) The strategy for this experiment was the same as that shown in Fig. 1A, except sigA was replaced by sigE in the dual-plasmid system and sinP3 was preplaced by sigBpr in pFPVmCherry. (B) Results for recombinant M. tuberculosis reporter assays: The bars represent mCherry fluorescence of E. coli cells containing pFPVmCherry-sigBpr plasmid in the presence of the following: no M. tuberculosis RNAP (control); M. tuberculosis RNAP core (pAcYc Duet rpoA plus pCOLA Duet rpoB-rpoC); M. tuberculosis RNAP-sigE holo (as in panel A); σE (pAcYc Duet-sigE). The data represent means of three replicates, and error bars represent standard deviations.
Assay with M. tuberculosis CRP.
The assay with CRP and a CRP-specific promoter were conducted according to the above-described method and using the plasmids shown in Fig. 3A and B, below. As control, the assays were performed with E. coli BL21(DE3) harboring the following plasmid(s): (i) pFPVmCherry-WhiB1, to test whether E. coli RNAP induces mCherry expression at the WhiB1 promoter; (ii) pFPVmCherry-WhiB1-CRP, to test whether E. coli RNAP in the presence of M. tuberculosis CRP induces mCherry expression at the WhiB1 promoter; (iii) pAcYc Duet rpoA-sigA plus pCOLA Duet rpoB-rpoC plus pFPVmCherry-WhiB1, to test whether M. tuberculosis RNAP induces mCherry expression at the WhiB1 promoter; (iv) pAcYc Duet rpoA-sigA plus pCOLA Duet rpoB-rpoC plus pFPVmCherry-WhiB1-CRP, to test whether M. tuberculosis RNAP in the presence of M. tuberculosis CRP induces mCherry expression at the WhiB1 promoter (see Fig. 3).
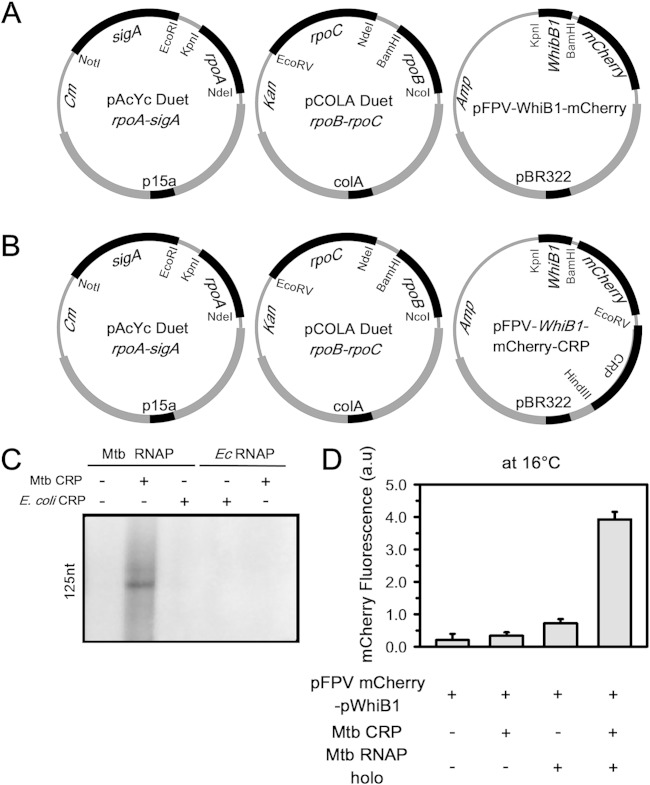
FIG 3.
Recombinant in vivo M. tuberculosis reporter assay using M. tuberculosis CRP and its specific promoter, WhiB1. (A) Strategy for three-plasmid expression system, with dual plasmids (first two plasmids) for expression of M. tuberculosis RNAP holo (as in Fig. 1A) and the third plasmid containing M. tuberculosis CRP-dependent promoter element WhiB1 for expression of mCherry. (B) In pFPV-WhiB1-mCherry, M. tuberculosis CRP was inserted under the control of the T7 promoter (pFPV-WhiB1-mCherry-CRP). (C) In vitro transcriptional activity assay to study the interactions of M. tuberculosis (Mtb) and E. coli (Ec) RNAP and CRP on WhiB1 promoter DNA fragments. Runoff transcripts of 125 nucleotides (nt) were produced. Lane 1, M. tuberculosis RNAP holo; lane 2, M. tuberculosis RNAP holo plus 400 nM M. tuberculosis CRP plus 400 μM cAMP; lane 3, M. tuberculosis RNAP holo plus 400 nM E. coli CRP plus 400 μM cAMP; lane 4, E. coli RNAP holo plus 400 nM E. coli CRP plus 400 μM cAMP; lane 5, E. coli RNAP holo plus 400 nM M. tuberculosis CRP plus 400 μM cAMP. (D) Results for recombinant M. tuberculosis reporter assays. The bars represent mCherry fluorescence of E. coli cells containing the pFPVmCherry-WhiB1 plasmid in the presence of the following; no M. tuberculosis RNAP holo or M. tuberculosis CRP; M. tuberculosis RNAP CRP (pFPV-WhiB1-mCherry-CRP); M. tuberculosis RNAP holo (all three plasmids in panel A); M. tuberculosis RNAP holo and M. tuberculosis CRP (all three plasmids in panel B). The data represent the means of three replicates, and error bars represent standard deviations.
In vivo reporter assay in the presence of the ω-subunit.
E. coli BL21(DE3) was transformed with two plasmids (pAcYc Duet rpoA-sigA plus pCOLA Duet rpoB-rpoC or pAcYc Duet rpoA-sigE plus pCOLA Duet rpoB-rpoC) harboring genes for the M. tuberculosis RNAP holo (σA or σE) without the ω-subunit (see Fig. 1A and 2A). These cotransformed cells were grown in 200 ml LB broth at 37°C with appropriate antibiotics (chloramphenicol at 35 μg/ml, kanamycin at 50 μg/ml) to an OD (at 595 nm) of 0.4. Cells were harvested at 5,000 × g for 10 min at 4°C. Cell pellets were resuspended in 20 ml of prechilled 100 mM MgCl2 and incubated on ice for 30 min, followed by centrifugation at 5,000 × g for 10 min at 4°C. Cell pellets were resuspended in 50 ml of prechilled 100 mM CaCl2 and incubated for 4 h on ice. Finally, cells were harvested at 5,000× g for 10 min at 4°C, resuspended in 3 ml of mixture containing prechilled 85 mM CaCl2 and 15% absolute glycerol, and incubated for 12 h at −80°C to gain competency. These competent cells containing the above-described one of the two-plasmid combinations were further transformed either with the respective plasmid alone (pFPVmCherry-sinP3, pFPVmCherry-rrnA, pFPVmCherry-sigBpr, or pFPVmCherry-WhiB1-CRP) or with the respective plasmid along with pCDF 1b-rpoZ. These cotransformed cells, containing either three or four plasmids, were grown in 50 ml LB broth at 37°C with appropriate antibiotics (chloramphenicol at 35 μg/ml, ampicillin at 100 μg/ml, kanamycin at 50 μg/ml, and streptomycin at 50 μg/ml) up to an OD (at 595 nm) of 0.4, induced by the addition of 0.5 mM IPTG, and further grown at 16°C for 16 h (until the OD595 reached 1.5). mCherry fluorescence was determined by using a fluorescence scanner (Typhoon Trio+; GE Healthcare) and quantified using ImageQuant TL software. The changes in mCherry fluorescence were determined based on the ratio of signal in the presence versus absence of M. tuberculosis RNAP.
To monitor the expression of M. tuberculosis RNAP with the ω-subunit in the above-described in vivo reporter assays, RNAP was purified from 50-ml E. coli cultures via Ni-nitrilotriacetic acid (NTA) chromatography following the protocol described by Banerjee et al. (26). The subunit composition of M. tuberculosis RNAP was analyzed by 10% SDS-PAGE followed by Coomassie staining. As a control, the identical method was followed for an assay in which M. tuberculosis RNAP was expressed without the ω-subunit.
FACS analysis of the cells from the reporter assay.
Aliquots (2 ml) of cell cultures from the above-described reporter assays were harvested at 5,000 × g for 5 min at 4°C. The pellets were dissolved in 1 ml phosphate-buffered saline (PBS; pH 7.5) and transferred to fluorescence-activated cell sorting (FACS) tubes (BD Biosciences). A total of 20,000 cells were measured for each sample, and every sample had three replicates. Data were acquired on a FACS-ARIA apparatus (BD BioSciences) and analyzed using the FACS-Diva software.
RESULTS
Reporter assay with σA-RNAP holo.
Previously, we developed a method to assemble M. tuberculosis RNAP holo enzymes in vivo in E. coli by employing a dual-plasmid expression system (26). These methods yield large amounts of transcriptionally active enzymes, free of E. coli RNAP contamination. In order to develop a reporter assay for M. tuberculosis promoters in E. coli cells that express M. tuberculosis RNAP, we introduced a third plasmid that harbors an mCherry cassette under an M. tuberculosis σA-dependent promoter element (Fig. 1A). We expected that the recombinant M. tuberculosis RNAP in association with σA would induce mCherry expression from the M. tuberculosis σA-dependent promoter element, resulting in fluorescent E. coli cells. On the other hand, the endogenous E. coli RNAP holo enzyme, the E. coli RNAP core enzyme in association with M. tuberculosis σA, or the M. tuberculosis RNAP core in association with E. coli σ70 would not induce mCherry expression from the σA-dependent promoter. To test the above-described possibilities, we first performed in vitro transcription assays with M. tuberculosis promoters and all of the above-described three RNAPs or RNAP combinations (Fig. 1B). The results showed that only the M. tuberculosis RNAP holo was able to initiate transcription from M. tuberculosis σA-specific promoter DNA fragments, indicating that E. coli RNAP and/or σ70 did not interfere with the reporter assay.
As expected, when the pFPVmCherry-sinP3 plasmid was introduced into E. coli cells, we observed a small amount of mCherry expression. These data served as a control, set the background level, and indicated a lack of interaction of E. coli RNAP with the sinP3 promoter in vivo. When the M. tuberculosis RNAP core was expressed in E. coli containing the pFPVmCherry-sinP3 plasmid, we observed a 2-fold increase in the fluorescence signal over the background control, indicating nonspecific induction of mCherry expression by the M. tuberculosis RNAP core. However, when M. tuberculosis RNAP holo was expressed in the above-described assay system, we observed a 10-fold increase in the fluorescence signal (Fig. 1C, left panel). In all the above-described events, mCherry fluorescence was monitored by growing the cells at 37°C for 6 h. When the growth temperature of the above-described system was lowered from 37°C to 16°C (for 16 h), we observed a 16-fold increase in the fluorescence signal (Fig. 1C, right panel). This is in line with our previous report that the yield of RNAP holo assembly increases at the lower temperature, resulting in higher induction of mCherry expression (26). To exclude the possibility of association of M. tuberculosis σA with E. coli RNAP core to induce mCherry expression in vivo, we performed the above-described assay by introducing a plasmid containing the gene for M. tuberculosis σA into our mCherry expression system. In this assay, the M. tuberculosis σA along with E. coli RNAP core induced mCherry expression to some extent, comparable to mCherry expression by M. tuberculosis RNAP core and close to the background level. The growth conditions for the assays mentioned above (37°C for 6 h or 16°C for 16 h after induction) were the conditions that provided the best signal-to-background ratio (see Fig. S1 in the supplemental material). FACS analyses of the cells from the above-described assays also revealed that 40 to 50% of the cells were fluorescent at the mCherry channel (610 nm) when grown at 37°C, whereas 80 to 90% of the cells were fluorescent when grown at 16°C (Fig. 1D).
When the reporter assay was conducted with another M. tuberculosis σA-specific promoter rrnA (21), the results followed the same trend as those with sinP3 (see Fig. S2B in the supplemental material).
All of the above-described results indicated that the E. coli RNAP in association with either E. coli σ70 or with M. tuberculosis σA was not functional at M. tuberculosis promoters. Thus, the reporter assay would allow us to monitor the interactions with M. tuberculosis RNAP with its promoters in vivo in E. coli.
Reporter assay with σE-RNAP holo.
In order to test whether the reporter assay could be used to monitor the interaction of alternative σ factors with their cognate promoters, we conducted the assay with a representative M. tuberculosis alternative σ factor, σE (Fig. 2) on its promoter, sigBpr (30). Using an in vitro transcription assay, we confirmed that RNAP-sigE holo was able to initiate transcription from the sigBpr promoter DNA fragment (see Fig. S3 in the supplemental material). When the pFPVmCherry-sigBpr plasmid was introduced into either E. coli cells or E. coli cells expressing M. tuberculosis RNAP core, we observed mCherry expression at a background level, as observed for the assay with σA. However, when M. tuberculosis RNAP-sigE holo was expressed in E. coli containing the pFPVmCherry-sigBpr plasmid at 16°C for 12 h, we observed a 6-fold increase in the fluorescence signal (Fig. 2B). These results indicated that the assay could be used with the alternative σ factors of M. tuberculosis.
Reporter assay with the M. tuberculosis transcriptional regulator CRP.
In order to assess whether the reporter assay could be adapted to monitor the interactions of M. tuberculosis transcriptional regulators with their cognate promoters, we performed the assay with a representative M. tuberculosis transcriptional activator, CRP (Fig. 3) (28, 31). We chose M. tuberculosis CRP for the assay, as our in vitro transcription assay results indicated that E. coli CRP, although possessing structural and functional similarities with M. tuberculosis CRP, could not function with the M. tuberculosis transcriptional machinery (Fig. 3C). On the other hand, M. tuberculosis CRP was not functional in E. coli due to its inability to interact with the E. coli transcriptional machinery (28). Therefore, we expected that the endogenous CRP from E. coli would not interfere with the assay. As a control, we used the plasmid pFPV-WhiB1-mCherry, in which mCherry expression is under the control of an M. tuberculosis CRP-dependent promoter, WhiB1 (31, 32) (Fig. 3A). The M. tuberculosis CRP gene was subsequently cloned under an inducible promoter and inserted into the above-described plasmid to generate pFPV-WhiB1-mCherry-CRP (Fig. 3B). When the assay was performed using either pFPV-WhiB1-mCherry or pFPV-WhiB1-mCherry-CRP, we observed a low level of fluorescent signal. The results indicated that E. coli RNAP holo alone or in combination with E. coli CRP or M. tuberculosis CRP could not induce mCherry expression on the M. tuberculosis CRP-dependent promoter (WhiB1). Similarly, in the absence of M. tuberculosis CRP, M. tuberculosis RNAP holo could not induce mCherry expression from the above-described promoter. When BL21(DE3) cells containing pFPV-WhiB1-mCherry-CRP expressed M. tuberculosis RNAP holo, we observed a 5-fold increase in the fluorescent signal over the signal obtained with RNAP holo (Fig. 3D). The results showed that M. tuberculosis CRP is able to regulate its cognate promoter only in the presence of M. tuberculosis RNAP.
Reporter assay in the presence of the RNAP ω-subunit.
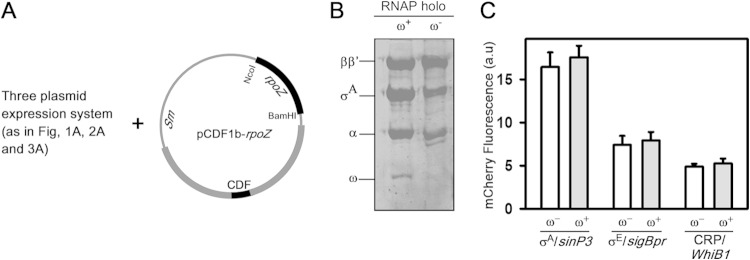
All three of the above-described assays were performed with M. tuberculosis RNAP without the ω-subunit. Previous reports suggested that the ω-subunit of M. tuberculosis is essential for optimal growth of the bacterium (33) and that removal of the ω-subunit from the genome leads to diverse phenotypes (33, 34). Thus, the ω-subunit could play an important role in M. tuberculosis RNAP expression in vivo or could be essential for the function of some of the transcriptional regulators of M. tuberculosis, as in the case of E. coli, where the ω-subunit is essential for the function of ppGpp during the stringent response (35, 36). Therefore, we performed the above-described reporter assays by incorporating the ω-subunit in each of the above-described three cases. A plasmid containing the gene that encodes the ω-subunit, pCDF-rpoZ (26), was inserted into E. coli BL21(DE3) cells within the existing three-plasmid expression system (Fig. 4A). To make sure that ω was incorporated into M. tuberculosis RNAP, we purified Ni-NTA chromatography on RNAP from the E. coli cells harboring the above-described four-plasmid expression system, in which the σA of M. tuberculosis RNAP was His tagged. The subunit composition of the affinity-purified RNAP by using SDS-PAGE and Coomassie staining confirmed the presence of ω in RNAP (Fig. 4B). The assay results suggested that E. coli cells can accommodate up to four compatible plasmids simultaneously. Comparing the data of the reporter assays in the presence and absence of ω in each of the above-described three cases (for σA, σE, and CRP), we observed no significant changes in the promoter activities (in the presence of ω, mCherry fluorescence increased by 5 to 10%, which was within error limit) (Fig. 4C). This result was consistent with our previous findings and also with the results of in vitro transcription assays (see Fig. S3 in the supplemental material) indicating that the ω-subunit of M. tuberculosis may not play any role in RNAP assembly or the transcriptional activity of the enzyme.
FIG 4.
Reporter assay in the presence of the M. tuberculosis ω-subunit. (A) Strategy for the four-plasmid expression systems. Plasmid pCDF1b-rpoZ harboring genes for the M. tuberculosis ω-subunit was incorporated into E. coli harboring the three-plasmid expression system shown in Fig. 1A, 2A, and 3A. (B) SDS-PAGE analysis of expression of M. tuberculosis RNAP with and without the ω-subunit in an in vivo reporter assay. (C) Results for recombinant M. tuberculosis reporter assays in the presence (+) or absence (−) of the ω-subunit. Fold changes in mCherry fluorescence of E. coli BL21(DE3) cells with respect to cells without M. tuberculosis RNAP expression are shown. (Left) M. tuberculosis RNAP-σA holo; the plasmid used was pFPVmCherry-sinP3. (Middle) M. tuberculosis RNAP-σE holo; the plasmid used was pFPVmCherry-sigBpr. (Right) M. tuberculosis CRP and M. tuberculosis RNAP-σA holo. The plasmid used was pFPVmCherry-WhiB1-CRP. The data represent the means of three replicates, and error bars represent standard deviations.
DISCUSSION
The reporter assay remains an important tool in biological research for monitoring the functions of transcriptional factors in a biological network (37–40). However, the development of a reporter assay in M. tuberculosis has its limitation, since the preparation of knockout or knockdown variants of the species is difficult because it requires handling of pathogenic bacteria that also have an intrinsically slow growth rate (11–14). Here, we have described a novel recombinant reporter assay that could provide a rapid and efficient avenue for monitoring the interactions among M. tuberculosis RNAP, σ factors, transcription factors, and their cognate promoters in vivo without the hazard of handling the pathogenic slow-growing bacterium.
The reporter assay has been validated for the principal σ factor, a representative alternative σ factor, a transcriptional regulator from M. tuberculosis, σE, and CRP. Therefore, we propose that this novel recombinant reporter assay could be used to assess the ability of RNAP to interact with other M. tuberculosis σ factors and/or transcriptional regulators for transcriptional regulation at M. tuberculosis promoters in vivo. Using the assay with M. tuberculosis CRP, we have been able to show that the hypothesis made by Bai et al. (28) is indeed true. Our assays showed that the functional interaction of M. tuberculosis CRP is restricted to M. tuberculosis RNAP, not with E. coli RNAP. The assay results in the presence of the ω-subunit support our previous finding that the ω-subunit may not be involved in vivo assembly of M. tuberculosis RNAP. However, the development of the assay to include incorporation of ω might be useful for monitoring the function of a transcriptional regulator(s) in cases where the presence of ω in RNAP is essential for the function of the regulator(s). The assay performed in E. coli may not show the same level of promoter activity as when performed in M. tuberculosis, due to different micromilieus within the two bacteria. Nonetheless, any level of promoter activity in this recombinant assay validates the interactions of the promoter with the transcriptional regulator. Also, performance of the recombinant assay in E. coli would be much less time-consuming than in M. tuberculosis. In addition, the in vivo assays involving functional analysis of transcriptional regulators in the knockout host might be often misrepresented due to cross talk among regulators that have overlapping functions. Anticipating that there are no significant functional overlaps between E. coli transcriptional regulators and their counterparts in M. tuberculosis, as observed by us with M. tuberculosis CRP, this recombinant assay would be free from the influence of other interfering M. tuberculosis transcriptional regulators. Although we cannot rule out the possibility of interference of the E. coli RNAP with M. tuberculosis promoters and transcriptional regulators, this interference could easily be detected in a control experiment where reporter assays are performed in the absence of M. tuberculosis RNAP or transcriptional regulators, as shown in the assays here.
We envisage that this assay could be further used to screen/identify promoter elements specific to a particular M. tuberculosis σ factor or transcriptional regulator by inserting 200 to 300 bp of random M. tuberculosis genomic DNA fragments in the promoter region of the mCherry structural gene (in place of the sinP3 promoter element [Fig. 1]). If a DNA fragment contains a promoter element for the σ factor (or transcriptional regulator), mCherry expression will result in a fluorescent bacterial colony. The DNA sequence of the promoter region of the mCherry gene in fluorescent bacteria would identify the promoter element (this work is in progress).
Overall, the recombinant reporter assay would be useful in establishing the interaction of M. tuberculosis RNAP with its cognate promoters in association with any σ factors and/or transcriptional regulators and thus help increase the understanding of the mechanism of gene regulation by these factors in vivo. Although the assay was developed to monitor the function of transcriptional regulators of M. tuberculosis, the approach could be expanded for other slow-growing pathogenic bacterial systems. This novel reporter assay is the first example in which the host transcriptional machinery was not used for expression of the reporter gene. Since the assay employs recombinant transcriptional machinery, the technique could be used to develop synthetic circuits to assess biological pathways of other bacteria.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by research grants from the Department of Biotechnology, India (BT/PR 3178/GBD/27/212/2009 and BT/PR 5270/BRB/10/1066/2012). R.B., P.R., and A.S. are recipients of CSIR India fellowships.
We thank R. Sur (University of Calcutta) for careful reading of the manuscript and suggestions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02445-14.
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis report. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en. [Google Scholar]
- 2.Dye C, Espinal MA, Watt CJ, Mbiaga C, Williams BG. 2002. Worldwide incidence of multidrug-resistant tuberculosis. J Infect Dis 185:1197–1202. doi: 10.1086/339818. [DOI] [PubMed] [Google Scholar]
- 3.Raviglione MC, Gupta R, Dye CM, Espinal MA. 2001. The burden of drug-resistant tuberculosis and mechanisms for its control. Ann N Y Acad Sci 953:88–97. doi: 10.1111/j.1749-6632.2001.tb11364.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigue S, Provvedi R, Jacques PE, Gaudreau L, Manganelli R. 2006. The sigma factors of Mycobacterium tuberculosis. FEMS Microbiol Rev 30:926–941. doi: 10.1111/j.1574-6976.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 5.Manganelli R, Dubnau E, Tyagi S, Kramer FR, Smith I. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol Microbiol 31:715–724. doi: 10.1046/j.1365-2958.1999.01212.x. [DOI] [PubMed] [Google Scholar]
- 6.Manganelli R, Provvedi R, Rodrigue S, Beaucher J, Gaudreau L, Smith I. 2004. Sigma factors and global gene regulation in Mycobacterium tuberculosis. J Bacteriol 186:895–902. doi: 10.1128/JB.186.4.895-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontan PA, Aris V, Alvarez ME, Ghanny S, Cheng J, Soteropoulos P, Trevani A, Pine R, Smith I. 2008. Mycobacterium tuberculosis sigma factor E regulon modulates the host inflammatory response. J Infect Dis 198:877–885. doi: 10.1086/591098. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Geiman DE, Bishai WR. 2008. Role of stress response sigma factor SigG in Mycobacterium tuberculosis. J Bacteriol 190:1128–1133. doi: 10.1128/JB.00511-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Karakousis PC, Bishai WR. 2008. Roles of SigB and SigF in the Mycobacterium tuberculosis sigma factor network. J Bacteriol 190:699–707. doi: 10.1128/JB.01273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigue S, Brodeur J, Jacques PE, Gervais AL, Brzezinski R, Gaudreau L. 2007. Identification of mycobacterial sigma factor binding sites by chromatin immunoprecipitation assays. J Bacteriol 189:1505–1513. doi: 10.1128/JB.01371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl JL, Kraus CN, Boshoff HI, Doan B, Foley K, Avarbock D, Kaplan G, Mizrahi V, Rubin H, Barry CE III. 2003. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc Natl Acad Sci U S A 100:10026–10031. doi: 10.1073/pnas.1631248100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrt S, Schnappinger D. 2006. Controlling gene expression in mycobacteria. Future Microbiol 1:177–184. doi: 10.2217/17460913.1.2.177. [DOI] [PubMed] [Google Scholar]
- 13.Parish T, Smith DA, Roberts G, Betts J, Stoker NG. 2003. The senX3-regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology 149:1423–1435. doi: 10.1099/mic.0.26245-0. [DOI] [PubMed] [Google Scholar]
- 14.Rickman L, Scott C, Hunt DM, Hutchinson T, Menendez MC, Whalan R, Hinds J, Colston MJ, Green J, Buxton RS. 2005. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol Microbiol 56:1274–1286. doi: 10.1111/j.1365-2958.2005.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo XV, Monteleone M, Klotzsche M, Kamionka A, Hillen W, Braunstein M, Ehrt S, Schnappinger D. 2007. Silencing Mycobacterium smegmatis by using tetracycline repressors. J Bacteriol 189:4614–4623. doi: 10.1128/JB.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamionka A, Bertram R, Hillen W. 2005. Tetracycline-dependent conditional gene knockout in Bacillus subtilis. Appl Environ Microbiol 71:728–733. doi: 10.1128/AEM.71.2.728-733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl DA, Urbance JW. 1990. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. J Bacteriol 172:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curcic R, Dhandayuthapani S, Deretic V. 1994. Gene expression in mycobacteria: transcriptional fusions based on xylE and analysis of the promoter region of the response regulator mtrA from Mycobacterium tuberculosis. Mol Microbiol 13:1057–1064. doi: 10.1111/j.1365-2958.1994.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 19.Das Gupta SK, Bashyam MD, Tyagi AK. 1993. Cloning and assessment of mycobacterial promoters by using a plasmid shuttle vector. J Bacteriol 175:5186–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timm J, Lim EM, Gicquel B. 1994. Escherichia coli-mycobacteria shuttle vectors for operon and gene fusions to lacZ: the pJEM series. J Bacteriol 176:6749–6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma A, Sampla AK, Tyagi JS. 1999. Mycobacterium tuberculosis rrn promoters: differential usage and growth rate-dependent control. J Bacteriol 181:4326–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bashyam MD, Kaushal D, Dasgupta SK, Tyagi AK. 1996. A study of mycobacterial transcriptional apparatus: identification of novel features in promoter elements. J Bacteriol 178:4847–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahl JL, Arora K, Boshoff HI, Whiteford DC, Pacheco SA, Walsh OJ, Lau-Bonilla D, Davis WB, Garza AG. 2005. The relA homolog of Mycobacterium smegmatis affects cell appearance, viability, and gene expression. J Bacteriol 187:2439–2447. doi: 10.1128/JB.187.7.2439-2447.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalo-Asensio J, Soto CY, Arbues A, Sancho J, del Carmen Menendez M, Garcia MJ, Gicquel B, Martin C. 2008. The Mycobacterium tuberculosis phoPR operon is positively autoregulated in the virulent strain H37Rv. J Bacteriol 190:7068–7078. doi: 10.1128/JB.00712-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stallings CL, Stephanou NC, Chu L, Hochschild A, Nickels BE, Glickman MS. 2009. CarD is an essential regulator of rRNA transcription required for Mycobacterium tuberculosis persistence. Cell 138:146–159. doi: 10.1016/j.cell.2009.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee R, Rudra P, Prajapati RK, Sengupta S, Mukhopadhyay J. 2014. Optimization of recombinant Mycobacterium tuberculosis RNA polymerase expression and purification. Tuberculosis (Edinb) 94:397–404. doi: 10.1016/j.tube.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay J, Mekler V, Kortkhonjia E, Kapanidis AN, Ebright YW, Ebright RH. 2003. Fluorescence resonance energy transfer (FRET) in analysis of transcription-complex structure and function. Methods Enzymol 371:144–159. doi: 10.1016/S0076-6879(03)71010-6. [DOI] [PubMed] [Google Scholar]
- 28.Bai G, McCue LA, McDonough KA. 2005. Characterization of Mycobacterium tuberculosis Rv3676 (CRPMt), a cyclic AMP receptor protein-like DNA binding protein. J Bacteriol 187:7795–7804. doi: 10.1128/JB.187.22.7795-7804.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 30.Song T, Song SE, Raman S, Anaya M, Husson RN. 2008. Critical role of a single position in the −35 element for promoter recognition by Mycobacterium tuberculosis SigE and SigH. J Bacteriol 190:2227–2230. doi: 10.1128/JB.01642-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stapleton M, Haq I, Hunt DM, Arnvig KB, Artymiuk PJ, Buxton RS, Green J. 2010. Mycobacterium tuberculosis cAMP receptor protein (Rv3676) differs from the Escherichia coli paradigm in its cAMP binding and DNA binding properties and transcription activation properties. J Biol Chem 285:7016–7027. doi: 10.1074/jbc.M109.047720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith LJ, Stapleton MR, Fullstone GJ, Crack JC, Thomson AJ, Le Brun NE, Hunt DM, Harvey E, Adinolfi S, Buxton RS, Green J. 2010. Mycobacterium tuberculosis WhiB1 is an essential DNA-binding protein with a nitric oxide-sensitive iron-sulfur cluster. Biochem J 432:417–427. doi: 10.1042/BJ20101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 34.Mathew R, Chatterji D. 2006. The evolving story of the omega subunit of bacterial RNA polymerase. Trends Microbiol 14:450–455. doi: 10.1016/j.tim.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Ross W, Vrentas CE, Sanchez-Vazquez P, Gaal T, Gourse RL. 2013. The magic spot: a ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation. Mol Cell 50:420–429. doi: 10.1016/j.molcel.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vrentas CE, Gaal T, Ross W, Ebright RH, Gourse RL. 2005. Response of RNA polymerase to ppGpp: requirement for the omega subunit and relief of this requirement by DksA. Genes Dev 19:2378–2387. doi: 10.1101/gad.1340305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang T, Xing B, Rao J. 2008. Recent developments of biological reporter technology for detecting gene expression. Biotechnol Genet Eng Rev 25:41–75. doi: 10.5661/bger-25-41. [DOI] [PubMed] [Google Scholar]
- 38.Carroll P, James J. 2009. Assaying promoter activity using LacZ and GFP as reporters. Methods Mol Biol 465:265–277. doi: 10.1007/978-1-59745-207-6_18. [DOI] [PubMed] [Google Scholar]
- 39.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. 1994. Green fluorescent protein as a marker for gene expression. Science 263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 40.Barker LP, Brooks DM, Small PL. 1998. The identification of Mycobacterium marinum genes differentially expressed in macrophage phagosomes using promoter fusions to green fluorescent protein. Mol Microbiol 29:1167–1177. doi: 10.1046/j.1365-2958.1998.00996.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.