Abstract
Central to the concept of ecological speciation is the evolution of ecotypes, i.e. groups of individuals occupying different ecological niches. However, the mechanisms behind the first step of separation, the switch of individuals into new niches, are unclear. One long-standing hypothesis, which was proposed for insects but never tested, is that early learning causes new ecological preferences, leading to a switch into a new niche within one generation. Here, we show that a host switch occurred within a parasitoid wasp, which is associated with the ability for early learning and the splitting into separate lineages during speciation. Lariophagus distinguendus consists of two genetically distinct lineages, most likely representing different species. One attacks drugstore beetle larvae (Stegobium paniceum (L.)), which were probably the ancestral host of both lineages. The drugstore beetle lineage has an innate host preference that cannot be altered by experience. In contrast, the second lineage is found on Sitophilus weevils as hosts and changes its preference by early learning. We conclude that a host switch has occurred in the ancestor of the second lineage, which must have been enabled by early learning. Because early learning is widespread in insects, it might have facilitated ecological divergence and associated speciation in this hyperdiverse group.
Keywords: host preference, early learning, early adult experience, host switch, ecological speciation, Lariophagus distinguendus
1. Introduction
Insects represent the world's largest group of organisms, comprising over 900 000 described species, which is about 70% of all animal species [1]. Their species richness and biological diversity make insects key players in almost every terrestrial ecosystem. Many explanations for this hyperdiversity focus on the ecological niche and on ecological speciation [2]. Central to the concept of ecological speciation is that populations of the same species become genetically isolated because they specialize in different ecological niches [3–5]. This is assumed to result in the evolution of new species owing to restricted gene flow between populations, in allopatry as well as in sympatry [6]. One problem with the concept of ecological speciation is that it must be initiated by the switch of particular individuals of a population to a new ecological niche (e.g. a new host in herbivorous or parasitoid insects) to which they are not yet adapted. It is unclear why the offspring of these pioneers should remain in this niche and how they become genetically isolated from the rest of the population, despite sympatry. A solution could be that in the larval or pupal stage, some insects memorize the host that they consume during development [7]. As adult insects, they afterwards accept or even prefer this host for oviposition. The subsequent genetic isolation of the pioneers could result from mating on or near the host [8]. This mechanism of early learning was first proposed by Thorpe & Jones in 1937 [9] and does not require mutations of genetically fixed preferences. Therefore, early learning has been repeatedly cited as a potential initial mechanism for the separation of subpopulations and the emergence of new ecological species [7,10,11]. But up to now, there are no studies of speciation in insects combining phylogenetic data with results from behavioural studies on innate and learned host preferences and performance, i.e. the fecundity on different hosts. The role of early learning in speciation thus remains unclear.
One group of insects highly suitable to study mechanisms of speciation are hymenopteran parasitoids [12], which are among the most speciose groups in the animal kingdom [13]. For oviposition, females of these insects choose specific hosts, which are usually immature stages of other insects. The offspring develop in or on the host, thereby killing it [14]. There are a number of well-studied species, mostly parasitoids of agricultural pests. Within some parasitoid species, there are different populations that differ considerably in their reaction to cues from specific hosts, suggesting that they represent host races or even different (cryptic) species [15–18]. As early as 1968, Askew [12] considered chalcidoid parasitoid wasps to be especially susceptible to ecological speciation because of their prevalence of inbreeding, caused by sib-mating in close proximity to the host in combination with monandry. These characteristics help to overcome one of the main obstacles for ecological speciation, that is, a close association between ecological niche and mate choice. In addition, many parasitoid wasp species modify their behaviour through early learning, i.e. learning during development or during and directly after emergence from the host. In some cases, this causes parasitoid females to prefer the host on which they developed for oviposition [19–22]. Early learning could thus lead to the ecological and spatial separation of individuals from different hosts already within one generation.
The cosmopolitan parasitoid wasp Lariophagus distinguendus (Förster) (Hymenoptera: Chalcidoidea: Pteromalidae) is reported to attack the larvae and pupae of 15 different beetle species from seven families [23]. However, parasitization of several of these hosts has been demonstrated only in the laboratory [24–27] or is highly doubtful owing to the non-endophytic biology of reported hosts (e.g. Tribolium sp.), whereas L. distinguendus females clearly prefer endophytic hosts for oviposition. Of 23 samples of L. distinguendus collected worldwide, 10 were collected on larvae of the drugstore beetle Stegobium paniceum (L.) (Ptinidae), 12 were collected on larvae of the weevil genus Sitophilus (Dryophthoridae: Curculionoidea) and one was collected at a location where both hosts occurred (see the electronic supplementary material, table S1). Obviously, drugstore beetles and Sitophilus weevils are the main hosts. However, the latter are not suitable as hosts for all L. distinguendus. A previous study revealed that certain strains of L. distinguendus that were collected from drugstore beetles had a very low fecundity on granary weevils (Sitophilus granarius (L.)) compared with strains collected from this host [28]. This indicates that some host specialization has occurred. Host finding in L. distinguendus was intensively studied [29–32] and is strongly influenced by adult learning [33]. Mating occurs mostly on the natal host patch, and the protandrous males can perceive the presence of conspecific pupae within the grain kernels and wait for emerging females [34,35]. These features make L. distinguendus a suitable candidate species for studying the potential role of early learning in ecological speciation.
Here, we compare different strains of L. distinguendus collected from drugstore beetles and granary weevils as hosts. We examine their innate and learned host preferences, their fecundity on the two main hosts, and their genetic divergence, to study the hypothesis that early learning has been involved in the ecological divergence of subpopulations, as a pre-condition for the emergence of new species.
2. Material and methods
(a). Insects
In total, nine strains of L. distinguendus were studied (electronic supplementary material, table S2). These were collected by us or sent to us by colleagues. Four strains were collected on drugstore beetles (db) as hosts in pantries of private homes: RAVdb (collected 2008 in Ravensburg, Germany), STUdb (2007, Stuttgart, Germany), BIRdb (2011, Stuttgart-Birkach, Germany) and WAGdb (2011, Wageningen, The Netherlands). Five strains were collected on granary weevils (gw) as hosts in grain stores: PFOgw (2005, Pforzheim, Germany), SLOgw (1996, Slough, Great Britain), SATgw (2010, Satrup, Germany), SACgw (2010, Sachsen, Germany) and BYGgw (2005, Bygholm, Denmark). Host preference experiments could not be performed with strains WAGdb, SACgw and BYGgw, and fecundity data are not yet available for BIRdb, because these strains were only recently obtained.
For standard rearing, all strains were reared on their original host, and all insect cultures were kept under constant conditions of 26°C and 45% r.h. and 16 L : 8 D photoperiod, unless stated otherwise. For the experiments, all strains were reared on both hosts. In addition, the granary weevil strains SLOgw and SATgw were reared on bean weevils Acanthoscelides obtectus (Say; Bruchinae) and on cowpeas Vigna unguiculata unguiculata (L.) Walpers, to exclude potential learning of host cues from drugstore beetles or granary weevils during development, thus elucidating their innate host preference for one of the two hosts.
To rear drugstore beetles, about 1 g of newly emerged unsexed adult drugstore beetles (about 700 beetles) was placed in a Petri dish on 80 g food pellets for koi fish (Hikari Friend, Kamihata Fish Industry Group, Kyorin Corporation, Japan) or on 40 g wheat grain (cultivar: Batis; Saaten-Union GmbH, Hannover, Germany) moistened with 1 ml H2O for oviposition and left there until they died. After six weeks, L. distinguendus were placed on the infested koi pellets or the infested wheat grain, depending on the experiment. To rear granary weevils, 2.7 g of unsexed adult granary weevils (about 600 beetles) were placed in jars (diameter 12 cm, 16 cm height) with a ventilated lid on 40 g of moistened wheat grain (40 ml H2O on 1 kg grain) for oviposition. After one week, adult beetles were removed. After three weeks, L. distinguendus were placed on the infested grain. To rear A. obtectus, 6.7 g of unsexed adults (about 1600 beetles) were placed in jars (1 l capacity, 15.5 cm height) on 500 g of cowpeas for oviposition and left there until they died. After three weeks, L. distinguendus were placed on the infested cowpeas. Cultures were kept at 27 ± 1°C, 65 ± 5% r.h. and natural daylight conditions.
(b). Host preference experiments
To study host preferences of wasps with different experience from strains RAVdb, STUdb, BIRdb, PFOgw, SLOgw and SATgw, experiments were performed in a small Petri dish (5.5 cm diameter). Wasps were released in the centre of the dish. One grain kernel infested by a drugstore beetle larva, and one grain kernel infested by a granary weevil larva were placed on opposite sides at a distance of 2 cm from the centre. The number of times a wasp inserted her ovipositor into the infested grains (frequency of drilling) [29] was observed for 600 s using a stereo-microscope, and registered using the software ‘The Observer v. 5.0’ (Noldus, Wageningen, The Netherlands). Frequency of drilling was used to quantify the preference of the wasps, as it is stimulated by external chemical cues [29].
(i). Innate host preference and host preference after pre-imaginal experience
To study innate host preference, and/or host preference after pre-imaginal experience, wasps were reared on drugstore beetles and on granary weevils. Developing wasps were dissected out of the grain in the late pupal stage, when the wasp pupae had turned black [35], and cleaned with wet paint brushes. Then, they were kept singly in Eppendorf tubes under standard rearing conditions until emergence from the pupal case. Wasps were then transferred to small Petri dishes (5.5 cm diameter) and kept under the same conditions for another two days until the preference test (see §2b) was performed. Because these wasps only had pre-imaginal host contact and moulted in the absence of any host-associated chemical cues, their preference must have been innate or owing to pre-imaginal learning. To exclude pre-imaginal experience, and to identify the innate preference for either drugstore beetles or granary weevils in wasps from strains SLOgw and SATgw, they were additionally reared on bean weevils and treated as described above.
(ii). Host preference after early imaginal experience
To study host preference after early imaginal experience, wasps were reared on their original host, i.e. strains RAVdb, STUdb, BIRdb were reared on drugstore beetles, and strains PFOgw, SLOgw and SATgw were reared on granary weevils. To enable early imaginal experience with chemical cues from their original host, wasps were allowed to emerge normally. Directly after emergence from the grains, females were collected from the cultures and kept in a Petri dish for 24 h on grain material infested by their original host from which host beetles had already emerged. The wasps thus received experience with chemical host cues but were not able to associatively learn these cues in direct host encounters by oviposition or host feeding [33,36]. To enable early imaginal experience with chemical cues from the alternative host, wasps were dissected out of the grains in the pupal stage and treated as described above until emergence from the pupal case. Then, they were placed for 24 h on grain material infested by the alternative host from which the beetles had already emerged. The preference test (see §2b) was performed after 24 h. Thus, wasps obtained experience with chemical host cues as early adults in the first 24 h after emergence from the grain. Any difference in host preference between these wasps and those that had pre-imaginal experience on their original hosts must be owing to early imaginal learning.
(c). Fecundity on different hosts
The fecundity of the strains RAVdb, STUdb, PFOgw, SLOgw and SATgw was studied on both hosts. One female and two males were placed in a Petri dish with 15 g koi pellets infested by drugstore beetles (1085 ± 197 beetles per 15 g, n = 6) or 12 g wheat grain infested by granary weevils (213 ± 8 beetles per 12 g, n = 14) and kept under standard culture conditions. From exploratory work, it was known that this amount of hosts is sufficient to study the weekly fecundity of L. distinguendus females. The survival of wasps was checked daily. Wasps were placed on new hosts after one week, and for a maximum of 18 days. Afterwards, the emerging offspring were counted.
(d). Molecular data and phylogenetic analysis
(i). DNA extraction, PCR amplification and sequencing
DNA from two specimens of each L. distinguendus strain and one specimen of Nasonia vitripennis (Walker, 1836) as an outgroup was extracted following the manufacturer's instructions either using DNeasy Blood & Tissue Kit (Qiagen) for analysis of the partial mitochondrial cytochrome oxidase I (COI) and partial nuclear carbamoylphosphate synthetase (CAD) or using the QiaAmp mini kit (Qiagen) for the three nuclear LOC markers (anonymous markers developed based on genome comparisons in Hymenoptera [37]) LOC100123206 (LOC1), LOC100123909 (LOC2) and LOC100117339 (LOC3) and for the nuclear internal transcribed spacer 2 (ITS2). The LOC markers, which span introns [37], and ITS2 were chosen because of their high proportion of variable regions. For amplification, oligonucleotide primers in electronic supplementary material, table S3 were used. In contrast to Hartig et al. [37], the LOC oligonucleotide primers were used without sequencing tails for the sake of simplicity. For studying COI, PCR amplification was performed as follows: denaturation temperature 95°C for 2 min, followed by 40 cycles at 94°C for 1 min, 58°C for 1 min and 72°C for 1.5 min. Final extension was 10 min at 72°C. CAD was amplified by a hot start PCR with denaturation at 95°C for 5 min, followed by 40 cycles at 94°C for 1 min, 55°C for 1 min and 72°C for 1.5 min. Final extension was 10 min at 72°C. Positive PCR products were bidirectionally sequenced by Seqlab (Göttingen, Germany). For studying ITS2 and the LOC markers, PuReTaq PCR beads (GE healthcare) were used according to the manufacturer's protocol, with 1.5 μl of 10 mM primer added to a final volume of 20 μl. A typical PCR cycle started with denaturation 94°C for 4 min followed by 35–38 cycles of 94°C for 1 min, 1 min at the primers' annealing temperature, and elongation at 72°C for 1.5 min, ending with a 5 min elongation period. Touchdown PCRs were chosen when applying some of the more degenerative primers (i.e. for LOC100123206 and LOC100123909), and started with two cycles at annealing temperature + 4°C, two cycles at +2°C and 33–36 cycles at the primers' annealing temperature. PCR products were sequenced on an ABI 377 automated sequencer using Big Dye Terminator technology (Applied Biosystems) at the Swedish Natural History Museum in Stockholm. Chromatograms were checked for ambiguous positions, edited after comparing the forward and reverse sequences of each individual, assembled using the program GENtle v. 1.9.4 (by Magnus Manske, University of Cologne, released under GPL 2003, v. 1.9.4) and aligned in MAFFT v. 7 [38] with the L-INS-i algorithm [39]. Heterozygous positions were included using IUPAC ambiguity codes. Protein-coding regions of all genes were translated into amino acid sequences using the program ‘Virtual Ribosome’ [40] based on the translation tables of standard genetic code or the code for invertebrate mitochondria in case of COI, and compared with orthologous sequences from N. vitripennis. No unexpected stop codons or gaps were observed. Newly obtained sequences were submitted to GenBank (electronic supplementary material, table S4). For the outgroup N. vitripennis, we also used the nucleotide sequence EU746610.1 (COI) [41] as well as XM_001607631.2 (LOC100123909) and KC213163 (CAD) [42] from NCBI.
(ii). Data analysis
COI was partitioned in first/second and third codon positions (electronic supplementary material, table S5). DNA sequences of nuclear-encoded genes (nDNA) were manually concatenated in MEGA6 [43] into a single multiple nucleotide sequence alignment owing to the small number of variable sites in each single marker, which does not permit the separate estimation of substitution model parameters for each of them. The nDNA dataset was partitioned into first/second codon positions (p1) and third codon positions (p2) when referring to exonic nucleotides, the intronic nucleotides of CAD and the LOC markers (p3), and ITS2 (p4; electronic supplementary material, table S5).
The models for nucleotide substitutions used in the analyses were selected for each partition by applying the Bayesian information criterion [44] using the program PartitionFinder v. 1.1.1 [45] (electronic supplementary material, table S5). Gaps were treated as missing data.
Phylogenetic relationships were examined using maximum-likelihood (ML) analysis in Garli v. 2.01 [46] with 100 independent search replicates using the default configuration settings. Bootstrap replicates (1000 ML) were calculated under the same setting but with only two independent search replicates per bootstrap dataset. Bootstrap values were visualized by PAUP* 4.0b10 [47]. Uncorrected p-distances were calculated using MEGA6 [43] (electronic supplementary material, figure S6).
(e). Statistical analysis
We used the software package Statistica v. 6.0 (StatSoft Inc. Tulsa, OK) for statistical comparisons. Frequency of drilling on the two grain kernels was compared using the Wilcoxon matched pairs test for paired samples. Outliers were eliminated by using the three-sigma-interval. Statistical differences in fecundity between strains and hosts were calculated using the Mann–Whitney U-test followed by sequential Bonferroni correction.
3. Results
(a). Host preference experiments
(i). Innate host preference and host preference after pre-imaginal experience
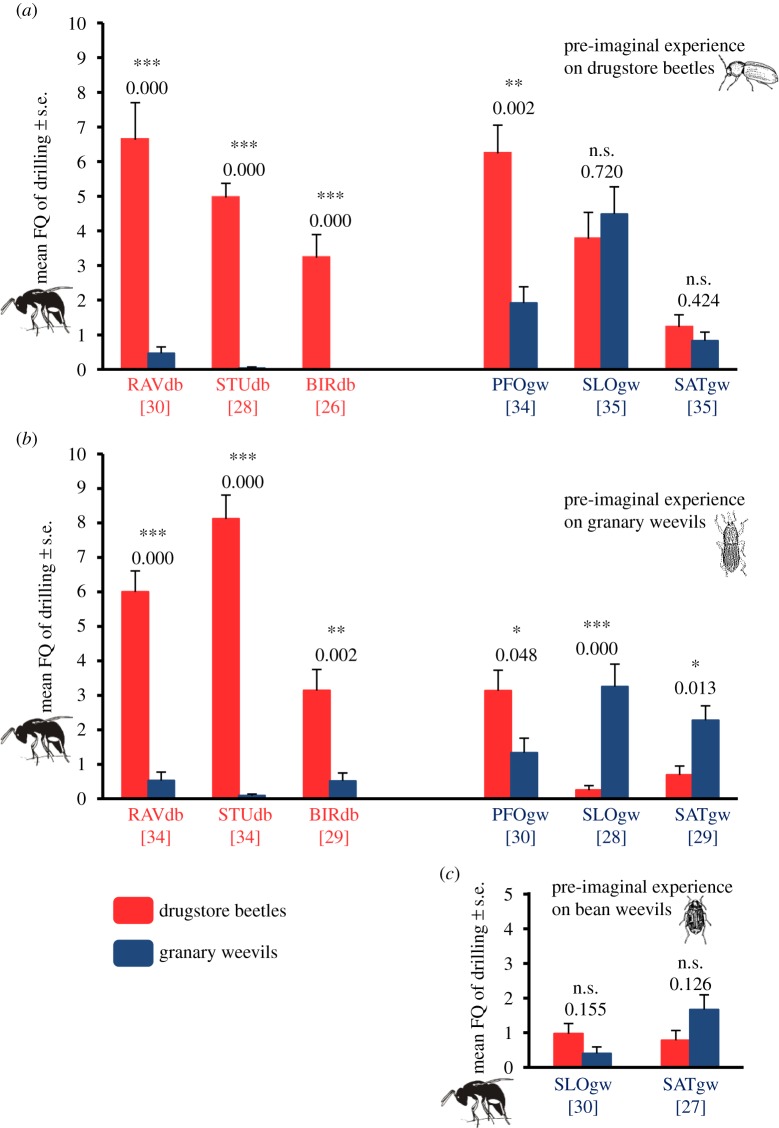
Regardless of whether females from RAVdb, STUdb, BIRdb and PFOgw developed on drugstore beetles or on granary weevils up to the black pupal stage, and therefore had pre-imaginal experience with these hosts, they all showed a significant preference for grains infested by drugstore beetles over grains infested by granary weevils (figure 1a,b). Obviously, the host on which the wasps developed did not influence the preference in these strains. This demonstrates that they had an innate preference for drugstore beetles that was not influenced by pre-imaginal learning experience. Remarkably, this was not only true for strains collected on drugstore beetles, but also for PFOgw, which was collected on granary weevils and also, according to the molecular analysis, clearly belongs to the granary weevil group (see below).
Figure 1.
Mean frequency (FQ) of drilling behaviour in 10 min observation time of female wasps from different strains of L. distinguendus with different pre-imaginal experience: (a) development on drugstore beetles, (b) development on granary weevils and (c) development on bean weevils. Light grey bars (red online): grains infested by drugstore beetles. Dark grey bars (blue online): grains infested by granary weevils. RAVdb, STUdb, BIRdb, PFOgw, SLOgw, SATgw: different strains of L. distinguendus. Numbers in brackets refer to the number of replicates. Means were compared using the Wilcoxon matched pairs test (n.s., not significant; *p < 0.05; **p < 0.01; ***p < 0.001). (Online version in colour.)
In contrast, host experience during development influenced the preference of females from the strains SLOgw and SATgw that were collected on granary weevils. They did not show any preference after development on drugstore beetles (figure 1a), but significantly preferred granary weevils when they had developed on this host (figure 1b). This demonstrates that pre-imaginal learning takes place in these strains. To exclude the effect of pre-imaginal experience and identify their innate host preference, both these strains were reared on bean weevils. These wasps had no significant preference for drugstore beetles or granary weevils, indicating that they had no innate preference at all (figure 1c).
(ii). Host preference after early imaginal experience
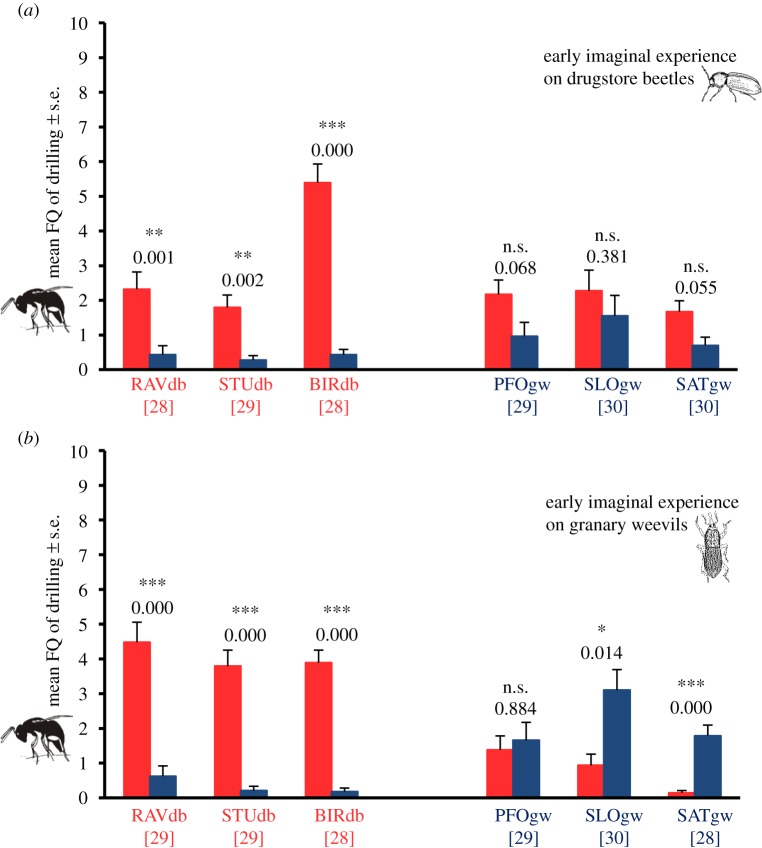
When wasps had 24 h experience with chemical cues from grain material infested by drugstore beetles or granary weevils, females from RAVdb, STUdb and BIRdb again showed a significant preference for drugstore beetles, regardless of the host experienced (figure 2a,b). Thus, early imaginal experience did not influence preference in these wasps either, as we already demonstrated for pre-imaginal experience. In contrast, females from PFOgw had a strong, albeit not significant tendency towards drugstore beetles after experience with cues from this host, but no preference for either host after experience with granary weevil cues (figure 2a,b). Early imaginal learning with chemical cues from granary weevils obviously changed the innate preference for drugstore beetles in this strain. Conversely, early imaginal experience with drugstore beetle cues in SLOgw and SATgw changed the preference for granary weevils, which was induced in these strains after pre-imaginal learning. This led to an equal acceptance of both hosts in SLOgw and even a slight, non-significant preference for drugstore beetles in SATgw. Thus, early imaginal learning influenced host preference in the strains that were collected on granary weevils, but not in the strains from drugstore beetles.
Figure 2.
Mean frequency (FQ) of drilling behaviour in 10 min observation time of female wasps from different strains of L. distinguendus with different early imaginal experience: (a) 24 h experience on grain material formerly infested by drugstore beetles, (b) 24 h experience on grain material formerly infested by granary weevils. Light grey bars (red online): grains infested by drugstore beetles. Dark grey bars (blue online): grains infested by granary weevils. RAVdb, STUdb, BIRdb, PFOgw, SLOgw, SATgw: different strains of L. distinguendus. Numbers in brackets refer to the number of replicates. Means were compared using the Wilcoxon matched pairs test (n.s., not significant; *p < 0.05; **p < 0.01; ***p < 0.001). (Online version in colour.)
(b). Fecundity
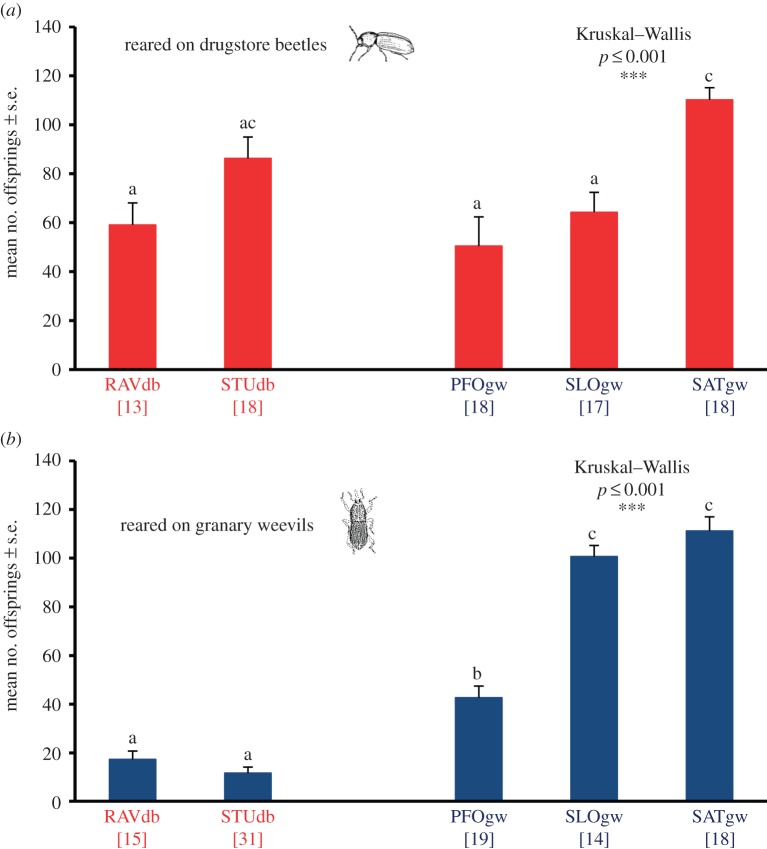
Analysis of fecundity data from experiments with drugstore beetles as host using Kruskal–Wallis ANOVA revealed significant differences between the strains, caused by strain SATgw having superior fecundity relative to all strains, with the exception of STUdb (figure 3a). Apart from that, the number of offspring was similar in all strains. In contrast, the fecundity on granary weevils was very low in RAVdb and STUdb, intermediate in PFOgw and high in SLOgw and SATgw (figure 3b). When comparing the fecundity between strains after providing them with different hosts, RAVdb and STUdb had a higher fecundity on drugstore beetles when compared with granary weevils (RAVdb: U = 19.5, p < 0.001; STUdb: U = 21.5, p < 0.001). PFOgw and SATgw showed no differences between the two hosts (PFOgw: U = 169.5, p < 0.963; SATgw: U = 159.5, p < 0.937), and SLOgw had a higher fecundity on granary weevils when compared with drugstore beetles (U = 41.0, p < 0.002). Thus, the two strains collected on drugstore beetles performed well only on drugstore beetles, whereas strains from granary weevils were indifferent or even showed a higher fecundity on granary weevils.
Figure 3.
Mean fecundity of female wasps from different strains of L. distinguendus on different hosts: (a) drugstore beetles as hosts, (b) granary weevils as hosts. RAVdb, STUdb, BIRdb, PFOgw, SLOgw, SATgw: different strains of L. distinguendus. Numbers in brackets refer to the number of replicates. Different letters indicate a significant difference at the 0.05 level using pairwise Mann–Whitney U-tests followed by sequential Bonferroni correction for multiple comparisons. (Online version in colour.)
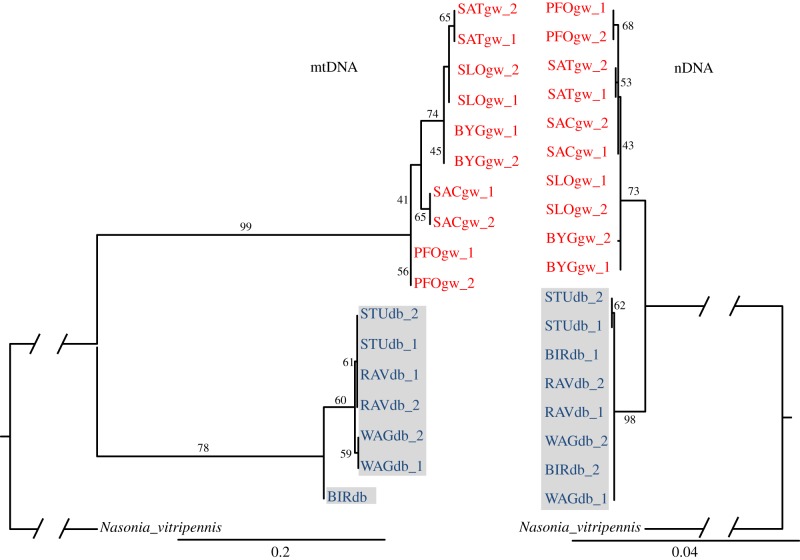
(c). Molecular analysis
Phylogenetic analysis of mitochondrial and nuclear DNA revealed a clear separation of the strains into two clades (figure 4), supported by high bootstrap values and corresponding to hosts and habitats in which the strains were collected. RAVdb, STUdb, BIRdb and WAGdb belonged to a drugstore beetle group collected in private homes and PFOgw, SLOgw, SATgw, SACgw and BYGgw belonged to a granary weevil group from grain stores. The molecular divergence between these two groups was remarkably high for COI (median of uncorrected p-distances: 0.138; minimum: 0.125; maximum: 0.141; n = 17) in contrast to the low genetic differences within both groups (median within drugstore beetle group: 0.006; minimum: 0.000; maximum: 0.028; n = 7); median within granary weevil group: 0.022; minimum: 0.000; maximum: 0.028; n = 10; electronic supplementary material, figure S6).
Figure 4.
Maximum-likelihood phylogenetic relationships of strains of Lariophagus distinguendus inferred from analyses of the partial mitochondrial gene COI (mtDNA) and combined sequences from the nuclear markers CAD, LOC100123206, LOC100123909, LOC100117339 and ITS2 (nDNA). COI was partitioned in first/second and third codon positions (electronic supplementary material, table S5). The nDNA dataset was partitioned into first/second codon positions (p1) and third codon positions (p2) when referring to exonic nucleotides, the intronic nucleotides of CAD and the LOC markers (p3), and ITS2 (p4; electronic supplementary material, table S5). Branch support values are given in percentages for maximum-likelihood bootstrapping (1000 bootstrap replicates). Light grey (red online): strains collected from drugstore beetles in private homes. Dark grey (blue online) with grey shading: strains collected from granary weevils in grain stores. (Online version in colour.)
4. Discussion
(a). The role of early learning for host differentiation in Lariophagus distinguendus
To evaluate the hypothesis that early learning enables the switch between hosts during ecological speciation in insects, we studied the host preference, the fecundity and the relatedness of different strains of L. distinguendus from two different hosts and habitats. Molecular analysis revealed that our strains can be divided into two distinct lineages that differ in their ecology, one parasitizing drugstore beetles in households, the other one granary weevils in grain stores. Strains from drugstore beetles have a fixed innate preference for this host that cannot be altered by experience. In addition, these strains only perform well on drugstore beetles, but have a very low number of offspring on granary weevils. In contrast, the strains collected from granary weevils change their preference according to pre-imaginal and/or early imaginal experience, by learning to accept or even to prefer one or the other host. Their fecundity on drugstore beetles is comparable to that of the other strains, but they are able to develop on granary weevils as well as or even better than on drugstore beetles. Thus, the ability for early learning in the studied strains of L. distinguendus is associated with host and habitat differentiation.
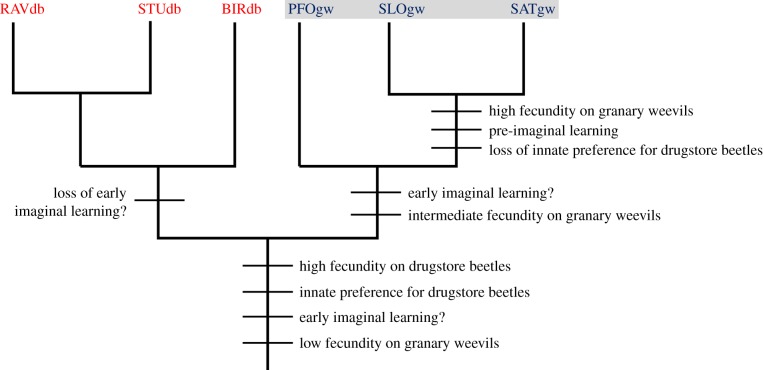
Because all strains have a high fecundity on drugstore beetles and one strain collected from granary weevils has an innate preference for drugstore beetles, the latter were most likely the hosts of the common ancestor of both lineages (figure 5). Therefore, a host switch must have occurred in the ancestor of the strains collected on granary weevils. While such a switch is difficult to conceive in wasps with a fixed, innate preference for drugstore beetles, it can be easily explained by the ability for pre-imaginal and/or early imaginal learning, as demonstrated in the strains collected on granary weevils. It remains unclear whether the ability for early imaginal learning was present in the ancestor of the two lineages and was lost in the drugstore beetle lineage, or if it did emerge only in the ancestor of the granary weevil group. In any case, the ability for early learning would have resulted in an increased exposure to granary weevils, leading to an improved adaptation and increased fecundity on the new host, and to a loss of the innate preference for drugstore beetles.
Figure 5.
Hypothetical scenario of character evolution in L. distinguendus. Light grey (red online): strains collected from drugstore beetles in private homes. Dark grey (blue online) with grey shading: strains collected from granary weevils in grain stores. (Online version in colour.)
Although our molecular data clearly show that the PFOgw strain belongs to the granary weevil group, it seems to have a transitional position. It has retained the probably plesiomorphic innate preference for drugstore beetles, but its host preference is influenced by early imaginal learning and it has an improved ability to use granary weevils as host, the species on which it was collected in the field.
Our molecular analysis revealed a very high uncorrected pairwise distance between the two lineages in COI of 0.138. For comparison, the thresholds for the delimitation of putative species in DNA barcoding of insects, which uses the same gene region, are usually set at 0.02 or 0.03 uncorrected p-distance [48,49]. Furthermore, we also found consistent differences between the two lineages in all the nuclear markers. Although mating experiments are required to demonstrate reproductive isolation, this suggests that the two lineages are in fact two biological species, with no or only very little genetic exchange.
In agreement with our hypothesis, these data strongly suggest that a host switch from drugstore beetles to granary weevils occurred within L. distinguendus, which was enabled by the ability for early learning. Given that L. distinguendus is monandrous and females generally mate on the host substrate directly after emergence, often with their own siblings [50], we are tempted to assume that the ecological divergence in two populations with different hosts resulted in increased separation, reproductive isolation and finally speciation within L. distinguendus.
We are currently testing alternative mechanisms of reproductive isolation, for example hybrid breakdown based on genetic differences [51], cytoplasmic incompatibility caused by Wolbachia infection [52] and sexual isolation owing to different pheromones [53]. Remarkably, a recent study revealed that cuticular hydrocarbons, which are used as mate recognition pheromones in L. distinguendus, change within one generation after a host switch [54]. Together with early learning and on-host mating, this could further promote reproductive isolation of wasps from different hosts. In addition, we are studying which of the two species agrees with the type specimen of L. distinguendus and which has to be assigned a new name or one of the former synonyms [55].
(b). Hopkins' host selection principle and neo-Hopkins host selection principle
Our study sheds new light on the processes of early learning in insects. Based on the observation by Hopkins [56], that adult insects choose for oviposition the host on which they had developed, the ‘Hopkins' host selection principle’ was established. It states that a memory for chemical cues learned during development can be transferred through the pupal stage, affecting the host choice behaviour of the adult insect [22]. Alternative to this pre-imaginal learning, the phenomenon described by Hopkins can also be explained by early imaginal learning of chemical cues that are present at the emergence site of the adult insect [57]. This process was termed ‘neo-Hopkins host selection principle’ by Jaenike [58]. In addition, Corbet suggested the ‘chemical legacy hypothesis', proposing that traces of chemical cues that have been transferred from the larva could be learned by the early adult [59]. Convincing demonstrations of Hopkins' host selection principle were first presented with odour-shock conditioning in Drosophila [60], and by the transfer of neural cells from trained to untrained house fly larvae, which stimulated odour preferences in recipient adult flies that reflected the training of the donor larvae [61]. For parasitoid wasps, the Hopkins' host selection principle was first demonstrated by Gandolfi et al. [62]. Our study revealed that pre-imaginal learning according to the Hopkins' host selection principle is present in the strains SLOgw and SATgw of L. distinguendus, whereas early imaginal learning according to the neo-Hopkins host selection principle occurs in all three strains from the granary weevil lineage (PFOgw, SLOgw, SATgw). Obviously, both forms of learning represent separate processes that evolve independently, but can also be present in the same species.
(c). Implications of early learning for classical biological control
The finding that a host switch in insects is associated with the ability for early learning is important from an applied point of view. In classical biological control, exotic pests are controlled by introducing their co-evolved natural antagonists from the same place of origin [63]. It is crucial to introduce only those natural enemies that are not expected to switch to non-target organisms [64]. Our results indicate that studies on early learning ability should be integrated in risk assessments of exotic natural enemies used in classical biological control, as it could facilitate the switch to non-target, native host species.
5. Conclusion
To the best of our knowledge, our study is the first experimental support in insects for the long-standing hypothesis that early learning can be a first step for ecological divergence as pre-condition for ecological speciation. Using mathematical models, it has been demonstrated that speciation through learning of habitat features is possible in theory [65]. However, empirical data supporting this hypothesis exist only for parasitic African indigobirds. In these finches, nestlings are reared by different host species and acquire the songs of their respective host by imprinting. As a consequence, male indigobirds mimic host songs and females use these songs to choose their mates and the nests they parasitize. This provides a mechanism for reproductive isolation after colonization of a new host [66–68]. Interestingly, the induction of host and habitat preferences seems to be widespread in herbivorous and parasitoid insects [7,69], which represent the most species-rich insect groups. Therefore, the induction of new ecological preferences by early learning, leading to a switch to a new niche within one generation, might well be a general mechanism in speciation, helping to explain the huge diversity of insects. To further address this hypothesis, behavioural and molecular studies with different host races and/or closely related species of herbivorous and parasitoid insects with different ecology are required.
Supplementary Material
Acknowledgements
We thank Lise Hansen (Department of IPM, Aarhus University, Danmark), Steffi Niedermayer (Department of Animal Ecology, University of Hohenheim, Germany), Sabine Prozell and Matthias Schöller (BiP GmbH, Berlin, Germany) and Hans Smid (Laboratory of Entomology, Wageningen University, The Netherlands) for providing us with the L. distinguendus strains BYGdb, PFOgw, SATgw, WAGdb, and Natalie Dale-Skey Papilloud (Natural History Museum London, UK) for the loan of material. We greatly appreciate the help of Matthias Tisler (Department of Zoology, University of Hohenheim, Germany) in preparing the figures, and of the whole animal ecology working group of the University of Hohenheim for good advice and proofreading. Joachim Ruther (Department of Chemical Ecology, University Regensburg, Germany) kindly improved an earlier version of the manuscript and Elsa Obrecht (Natural History Museum Bern, Switzerland) revised the English of the manuscript. We thank three unknown reviewers for their careful reading as well as their helpful suggestions and comments.
Data accessibility
DNA sequences: Genbank accessions KJ867375–KJ867409 and KJ923849–KJ923923. For details, see the electronic supplementary material, table S4. Original data of experiments on preference and fecundity: see electronic supplementary material, tables S7 and S8.
Funding statement
Kerstin König was financially supported by the Landesgraduiertenförderung Baden-Württemberg.
References
- 1.Resh VH, Cardé RT. 2003. Encyclopedia of insects, pp. XXV New York, NY: Academic Press. [Google Scholar]
- 2.Mayhew PJ. 2007. Why are there so many insect species? Perspectives from fossils and phylogenies. Biol. Rev. Camb. Philos. Soc. 82, 425–454. ( 10.1111/j.1469-185X.2007.00018.x) [DOI] [PubMed] [Google Scholar]
- 3.Schluter D. 2009. Evidence for ecological speciation and its alternative. Science 323, 737–739. ( 10.1126/science.1160006) [DOI] [PubMed] [Google Scholar]
- 4.Nosil P. 2012. Ecological speciation. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Funk DJ, Filchak KE, Feder JL. 2002. Herbivorous insects: model systems for the comparative study of speciation ecology. Genetics 116, 251–267. ( 10.1007/978-94-010-0265-3_10) [DOI] [PubMed] [Google Scholar]
- 6.Funk DJ, Nosil P, Etges WJ. 2006. Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc. R. Soc. B 103, 3209–3213. ( 10.1073/pnas.0508653103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis JM, Stamps JA. 2004. The effect of natal experience on habitat preferences. Trends Ecol. Evol. 19, 411–416. ( 10.1016/j.tree.2004.04.006) [DOI] [PubMed] [Google Scholar]
- 8.Feder JL, Opp SB, Wlazlo B, Reynolds K, Go W, Spisak S. 1994. Host fidelity is an effective premating barrier between sympatric races of the apple maggot fly. Proc. Natl Acad. Sci. USA 91, 7990–7994. ( 10.1073/pnas.91.17.7990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorpe W, Jones F. 1937. Olfactory conditioning in a parasitic insect and its relation to the problem of host selection. Proc. R. Soc. Lond. B 124, 56–81. ( 10.1098/rspb.1937.0072) [DOI] [Google Scholar]
- 10.Immelmann K. 1975. Significance of imprinting and early learning. Annu. Rev. Ecol. Evol. Syst. 6, 15–37. ( 10.1146/annurev.es.06.110175.000311) [DOI] [Google Scholar]
- 11.Dukas R. 2008. Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160. ( 10.1146/annurev.ento.53.103106.093343) [DOI] [PubMed] [Google Scholar]
- 12.Askew RR. 1968. Considerations on speciation in Chalcidoidea (Hymenoptera). Evolution 22, 642–645. ( 10.2307/2406886) [DOI] [PubMed] [Google Scholar]
- 13.Godfray HCJ. 1994. Parasitoids: behavioral and evolutionary ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Quicke DLJ. 1997. Parasitic wasps, ch. 10, pp. 302–328. London, UK: Chapman & Hall. [Google Scholar]
- 15.Pak GA, Buis HCEM, Heck ICC, Hermans MLG. 1986. Behavioural variations among strains of Trichogramma spp.: host-age selection. Entomol. Exp. Appl. 40, 247–258. ( 10.1111/j.1570-7458.1986.tb00508.x) [DOI] [Google Scholar]
- 16.Vos M, Vet LEM. 2004. Geographic variation in host acceptance by an insect parasitoid: genotype versus experience. Evol. Ecol. Res. 6, 1021–1035. [Google Scholar]
- 17.Forbes AA, Hood GR, Feder JL. 2010. Geographic and ecological overlap of parasitoid wasps associated with the Rhagoletis pomonella (Diptera: Tephritidae) species complex. Ann. Entomol. Soc. Am. 103/6, 908–915. ( 10.1603/AN10046) [DOI] [Google Scholar]
- 18.Heimpel GE, Antolin MF, Franqui RA, Strand MR. 1997. Reproductive isolation and genetic variation between two ‘strains’ of Bracon hebetor (Hymenoptera: Braconidae). Biol. Control 9, 149–156. ( 10.1006/bcon.1997.0529) [DOI] [Google Scholar]
- 19.Caubet Y, Jaisson P. 1991. A post-eclosion early learning involved in host recognition by Dinarmus basalis Rondani (Hymenoptera: Pteromalidae). Anim. Behav. 42, 977–980. ( 10.1016/S0003-3472(05)80150-2) [DOI] [Google Scholar]
- 20.Hare JD. 1996. Priming Aphytis: behavioral modification of host selection by exposure to a synthetic contact kairomone. Entomol. Exp. Appl. 78, 263–269. ( 10.1111/j.1570-7458.1996.tb00790.x) [DOI] [Google Scholar]
- 21.Villagra CA, Pennacchio F, Niemeyer HM. 2007. The effect of larval and early adult experience on behavioural plasticity of the aphid parasitoid Aphidius ervi (Hymenoptera, Braconidae, Aphidiinae). Naturwissenschaften 94, 903–910. ( 10.1007/s00114-007-0269-4) [DOI] [PubMed] [Google Scholar]
- 22.Barron AB. 2001. The life and death of Hopkins’ host selection principle. J. Ins. Behav. 14, 725–737. ( 10.1023/A:1013033332535) [DOI] [Google Scholar]
- 23.Noyes JS. 2014. Universal chalcidoidea database. See http://www.nhm.ac.uk/chalcidoids.
- 24.Goodrich ES. 1921. Note on the Hymenoptera parasite in beetles infesting grains. Report of the Grain Pest (War) Committee of the Royal Society of London 9, 5–7. Cambridge Scholars Publishing.
- 25.Kaschef AH. 1961. Gibbium psylloides Czemp. (Col., Ptinidae), new host of Lariophagus distinguendus Foerst. (Hym., Pteromalidae). Z Parasitenk. 21, 65–70. ( 10.1007/BF00260177) [DOI] [PubMed] [Google Scholar]
- 26.Bellows TS., Jr 1985. Effects of host age and host availability on developmental period, adult size, sex ratio, longevity and fecundity in Lariophagus distinguendus Förster (Hymenoptera: Pteromalidae). Res. Popul. Ecol. 27, 55–64. ( 10.1007/BF02515479) [DOI] [Google Scholar]
- 27.Niedermayer S, Steidle JLM. 2013. The Hohenheimer Box – a new way to rear and release Lariophagus distinguendus to control stored product pest insects. Biol. Control 64, 263–269. (doi:101016/j.biocontrol.2012.12.005) [Google Scholar]
- 28.Steidle JLM, Schöller M. 2002. Fecundity and ability of the parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae) to parasitize larvae of the granary weevil Sitophilus granarius (Coleoptera: Curculionidae) in bulk grain. J. Stored Prod. Res. 38, 43–53. ( 10.1016/S0022-474X(00)00044-8) [DOI] [Google Scholar]
- 29.Steidle JLM. 2000. Host recognition cues of the granary weevil parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). Entomol. Exp. Appl. 95, 185–192. ( 10.1023/A:1003934224535) [DOI] [Google Scholar]
- 30.Steidle JLM, Steppuhn A, Reinhard J. 2001. Volatile cues from different host complexes used for host location by the generalist parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). Basic Appl. Ecol. 2, 45–51. ( 10.1078/1439-1791-00035) [DOI] [Google Scholar]
- 31.Steidle JLM, Steppuhn A, Ruther J. 2003. Specific foraging kairomones used by a generalist parasitoid. J. Chem. Ecol. 29, 131–143. ( 10.1023/A:1021932731350) [DOI] [PubMed] [Google Scholar]
- 32.Steidle JLM. 1998. Learning pays off: influence of experience on host finding and parasitism in Lariophagus distinguendus (Hymenoptera: Pteromalidae). Ecol. Ent. 23, 451–456. ( 10.1046/j.1365-2311.1998.00144.x) [DOI] [Google Scholar]
- 33.Collatz J, Müller C, Steidle JLM. 2006. Protein-synthesis dependent long-term memory induced by one single associative training trial in the parasitic wasp Lariophagus distinguendus. Learn. Mem. 13, 263–266. ( 10.1101/lm.192506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruther J, Homann M, Steidle JLM. 2000. Female-derived sex pheromone mediates courtship behaviour in the parasitoid Lariophagus distinguendus. Entomol. Exp. Appl. 96, 265–274. ( 10.1046/j.1570-7458.2000.00705.x) [DOI] [Google Scholar]
- 35.Steiner S, Steidle JLM, Ruther J. 2005. Female sex pheromone in immature insect males: a case of pre-emergence chemical mimicry? Behav. Ecol. Sociobiol. 58, 111–120. ( 10.1007/s00265-005-0930-x) [DOI] [Google Scholar]
- 36.Schurmann D, Sommer C, Schinko APB, Greschista M, Smid H, Steidle JLM. 2012. Demonstration of long-term memory in the parasitic wasp Nasonia vitripennis (Hymenoptera: Pteromalidae). Entomol. Exp. Appl. 143, 199–206. ( 10.1111/j.1570-7458.2012.01253.x) [DOI] [Google Scholar]
- 37.Hartig G, Peters RS, Borner J, Etzbauer C, Misof B, Niehuis O. 2012. Oligonucleotide primers for targeted amplification of single-copy nuclear genes in apocritan Hymenoptera. PLoS ONE 7/6, e39826 ( 10.1371/journal.pone.0039826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. ( 10.1093/molbev/mst010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh K, Kuma K, Toh H, Miyata T. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33, 511–518. ( 10.1093/nar/gki198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wernersson R. 2006. Virtual ribosome: a comprehensive DNA translation tool with support for integration of sequence feature annotation. Nucleic Acids Res. 34, W385–W388. ( 10.1093/nar/gkl252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira DCSG, Raychoudhury R, Lavrov DV, Werren JH. 2008. Rapidly evolving mitochondrial genome and directional selection in mitochondrial genes in the parasitic wasp Nasonia (Hymenoptera: Pteromalidae). Mol. Biol. Evol. 25, 2167–2180. ( 10.1093/molbev/msn159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klopfstein S, Vilhelmsen L, Heraty JM, Sharkey M, Ronquist F. 2013. The hymenopteran tree of life: evidence from protein-coding genes and objectively aligned ribosomal data. PLoS ONE 8, e69344 ( 10.1371/journal.pone.0069344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis v. 6.0. Mol. Biol. Evol. 30, 2725–2729. ( 10.1093/molbev/mst197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarz GE. 1978. Estimating the dimension of a model. Ann. Stat. 6, 461–464. ( 10.1214/aos/1176344136) [DOI] [Google Scholar]
- 45.Lanfear R, Calcott B, Ho SYW, Guindon S. 2012. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701. ( 10.1093/molbev/mss020) [DOI] [PubMed] [Google Scholar]
- 46.Zwickl DJ. 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD dissertation, The University of Texas at Austin, TX. [Google Scholar]
- 47.Swofford DL. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods). (version 4.0b10). Sunderland, MA: Sinauer Associates. [Google Scholar]
- 48.Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 270, 313–321. ( 10.1098/rspb.2002.2218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.N'gendo RN, Osiemo ZB, Brandl R. 2012. DNA barcodes for species identification in the hyperdiverse ant genus Pheidole (Formicidae: Myrmicinae). J. Insect Sci. 13, 27 ( 10.1673/031.013.2701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruther J, Steiner S. 2008. Costs of female odour in males of the parasitic wasp Lariophagus distinguendus (Hymenoptera: Pteromalidae). Naturwissenschaften 95, 547–552. ( 10.1007/s00114-008-0357-0) [DOI] [PubMed] [Google Scholar]
- 51.Gibson JD, Niehuis O, Peirson BRE, Cash EI, Gadau J. 2013. Genetic and developmental basis of F2 hybrid breakdown in Nasonia parasitoid wasps. Evolution 67, 2124–2132. ( 10.1111/evo.12080) [DOI] [PubMed] [Google Scholar]
- 52.Werren JH. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42, 587–609. ( 10.1146/annurev.ento.42.1.587) [DOI] [PubMed] [Google Scholar]
- 53.Niehuis O, et al. 2013. Behavioural and genetic analyses of Nasonia shed light on the evolution of sex pheromones. Nature 494, 345–348. ( 10.1038/nature11838) [DOI] [PubMed] [Google Scholar]
- 54.Kuehbandner S, Hacker K, Niedermayer S, Steidle JLM, Ruther R. 2012. Composition of cuticular lipids in the pteromalid wasp Lariophagus distinguendus is host dependent. Bull. Entomol. Res. 102, 610–617. ( 10.1017/S000748531200017X) [DOI] [PubMed] [Google Scholar]
- 55.Graham MWRDV. 1969. The Pteromalidae of north-western Europe (Hymenoptera: Chalcidoidea). Bull. Br. Mus. (Nat. Hist.) Entomol. Suppl. 16, 1–908. [Google Scholar]
- 56.Hopkins AD. 1917. A discussion of CG. Hewitt's paper on ‘Insect behaviour’. J. Econ. Entomol. 10, 92–93. [Google Scholar]
- 57.van Emden HF, Sponagl B, Wagner E, Baker T, Ganguly S, Douloumpaka S. 1996. Hopkins’ ‘host selection principle’, another nail in its coffin. Physiol. Entomol. 21, 325–328. ( 10.1111/j.1365-3032.1996.tb00873.x) [DOI] [Google Scholar]
- 58.Jaenike J. 1983. Induction of host preference in Drosophila melanogaster. Oecologia 58, 320–325. ( 10.1007/BF00385230) [DOI] [PubMed] [Google Scholar]
- 59.Corbet S. 1985. Insect chemosensory responses: a chemical legacy hypothesis. Ecol. Entomol. 10, 143–153. ( 10.1111/j.1365-2311.1985.tb00543.x) [DOI] [Google Scholar]
- 60.Tully T, Cambiazo V, Kruse L. 1994. Memory through metamorphosis in normal and mutant Drososphila. J. Neurosci. 14, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ray S. 1999. Survival of olfactory memory through metamorphosis in the fly Musca domestica. Neurosci. Lett. 259, 37–40. ( 10.1016/S0304-3940(98)00892-1) [DOI] [PubMed] [Google Scholar]
- 62.Gandolfi M, Mattiacci L, Dorn S. 2003. Preimaginal learning determines adult response to chemical stimuli in a parasitic wasp. Proc. R. Soc. Lond. B 270, 2623–2629. ( 10.1098/rspb.2003.2541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eilenberg J, Hajek A, Lomer C. 2001. Suggestions for unifying the terminology in biological control. Biocontrol 46, 387–400. ( 10.1023/A:1014193329979) [DOI] [Google Scholar]
- 64.Louda SM, Pemberton RW, Johnson MT, Follett PA. 2003. Nontarget effects: the Achilleś heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Annu. Rev. Entomol. 48, 365–396. ( 10.1146/annurev.ento.48.060402.102800) [DOI] [PubMed] [Google Scholar]
- 65.Beltman JB, Haccou P. 2005. Speciation through learning of habitat features. Theor. Popul. Biol. 67, 189–202. ( 10.1016/j.tpb.2005.01.001) [DOI] [PubMed] [Google Scholar]
- 66.Payne RB, Payne LL, Woods JL. 1998. Song learning in brood-parasitic indigobirds Vidua chalybeata: song mimicry of the host species. Anim. Behav. 55/6, 1537–1553. ( 10.1006/anbe.1997.0701) [DOI] [PubMed] [Google Scholar]
- 67.Payne RB, Payne LL, Woods JL, Sorenson MD. 2000. Imprinting and the origin of parasite–host species associations in female brood parasitic indigobirds, Vidua chalybeata. Anim. Behav. 59, 69–81. ( 10.1006/anbe.1999.1283) [DOI] [PubMed] [Google Scholar]
- 68.Sorenson MD, Sefc KM, Payne RB. 2003. Speciation by host switch in brood parasitic indigobirds. Nature 424, 928–931. ( 10.1038/nature01863) [DOI] [PubMed] [Google Scholar]
- 69.Schoonhoven LM, van Loon JJA, Dicke M. 2005. Insect plant biology, pp. 209–232, ch. 8 Oxford, UK: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DNA sequences: Genbank accessions KJ867375–KJ867409 and KJ923849–KJ923923. For details, see the electronic supplementary material, table S4. Original data of experiments on preference and fecundity: see electronic supplementary material, tables S7 and S8.