Abstract
Agriculture today places great strains on biodiversity, soils, water and the atmosphere, and these strains will be exacerbated if current trends in population growth, meat and energy consumption, and food waste continue. Thus, farming systems that are both highly productive and minimize environmental harms are critically needed. How organic agriculture may contribute to world food production has been subject to vigorous debate over the past decade. Here, we revisit this topic comparing organic and conventional yields with a new meta-dataset three times larger than previously used (115 studies containing more than 1000 observations) and a new hierarchical analytical framework that can better account for the heterogeneity and structure in the data. We find organic yields are only 19.2% (±3.7%) lower than conventional yields, a smaller yield gap than previous estimates. More importantly, we find entirely different effects of crop types and management practices on the yield gap compared with previous studies. For example, we found no significant differences in yields for leguminous versus non-leguminous crops, perennials versus annuals or developed versus developing countries. Instead, we found the novel result that two agricultural diversification practices, multi-cropping and crop rotations, substantially reduce the yield gap (to 9 ± 4% and 8 ± 5%, respectively) when the methods were applied in only organic systems. These promising results, based on robust analysis of a larger meta-dataset, suggest that appropriate investment in agroecological research to improve organic management systems could greatly reduce or eliminate the yield gap for some crops or regions.
Keywords: organic yield, conventional yield, meta-analysis
1. Introduction
While tremendously productive, our current agricultural food system causes many environmental problems, often trading off long-term maintenance of ecosystem services for short-term agricultural production [1,2]. Resultant problems include biodiversity loss, massive soil erosion and degradation, eutrophication and oceanic dead zones, pesticide effects on humans and wildlife, greenhouse gas emissions, and regime shifts in hydrological cycling [3–12]. Furthermore, although agriculture produces a food surplus at the global scale, over 1 billion people are chronically hungry. These problems of hunger, food insecurity and environmental harms will only be exacerbated if current trends in population growth, food and energy consumption, and food waste continue [13–15]. To maintain the Earth's capacity to produce food, it is imperative that we adopt sustainable and resilient agricultural practices as soon as possible [16,17]. Yet it is also broadly perceived that such practices will produce lower yields [17–19], leading to a conundrum—how do we maintain or increase food production without sacrificing sustainability and resilience? Previous analyses have concluded that improving the distribution of food while also reducing waste and meat consumption will greatly contribute to sustainably meeting future global demands [9,15], although how these goals are to be achieved is not yet clear.
Advocates of ‘sustainable intensification’ have focused on increasing production efficiency while minimizing economic and environmental costs. An emphasis on efficiency, however, may not necessarily lead to development of sustainable, resilient production systems that can buffer unexpected changes resulting from the complex socio-ecological interactions that influence agriculture [17,19,20]. To achieve environmental sustainability, we must grow food in a manner that protects, uses and regenerates ecosystem services (e.g. favours natural pest control over the use of synthetic pesticides), rather than replacing them [19,21,22]. Replacing ecosystem services often has unintended, negative consequences (e.g. lethal or sub-lethal effects of pesticides on humans, beneficial insects and wildlife [7,10,12,23]). Broad adoption of sustainable agricultural methods is unlikely, however, unless such methods are similarly productive and/or cost-effective, such that they improve livelihoods. Hence, there is much incentive to determine whether a yield gap exists between ‘conventional’ agriculture (i.e. chemically intensive and biologically simplified) and alternative, more sustainable forms of agriculture, and if so, how it can be reduced or eliminated.
Such systems (e.g. agroecological, ecologically intensive, biologically diversified or regenerative farming systems) use cultivation techniques that, through plot- to landscape-scale diversification, specifically encourage ecological interactions that generate soil fertility, nutrient cycling and retention, water storage, pest/disease control, pollination, and other essential agricultural inputs/ecosystem services [22]. The most widely practised and studied alternative to conventional agriculture is organic, which now takes place on 0.9% of agricultural lands [24]. Organic agriculture is defined as having no synthetic inputs, but organic farms may or may not practise the full suite of cultivation techniques characterizing sustainable agriculture [21,25]. Although the terms ‘organic’ and ‘sustainable’ agriculture are not equivalent, studies of organic agriculture have revealed better performance than conventional systems on some (but not all) sustainability metrics, including species richness and abundance, soil fertility, nitrogen uptake by crops, water infiltration and holding capacity, and energy use and efficiency [26–32]. Here, we provide the most comprehensive calculation of the yield gap between organic and conventional agriculture, building on the work of others [33–36].
Early reviews comparing organic to conventional agriculture found yield gaps of 8–9% in developed countries [33,34], but yield gains of as much as 180% in developing countries. Two recent meta-analyses, however, found organic yields to be 20–25% lower than conventional yields [35,36]. That these studies differed so much in their conclusions can probably be attributed to two factors. First, each study used different criteria for selecting the data to be included in its review or meta-analysis. For example, for developing countries, Badgley et al. [34] focused primarily on comparing sites using techniques of sustainable agriculture with ‘resource-poor’ sites, rather than strict organic versus conventional comparisons, accounting for the yield gains they found for ‘organic management’ in developing countries [18,21,37]. Second, each of the above studies used different analytical methods to combine the data across the different sub-studies. For example, the reviews of de Ponti et al. [35], Stanhill [33] and Badgley et al. [34] did not account for the sampling variance within studies, which is the recommended practice to deal with heteroscedasticity in the sample of studies [38]. Seufert et al.'s [36] meta-analysis, while accounting for sampling variance, combined nested data (e.g. several experiments reported within the same study) without accounting for the hierarchy (electronic supplementary material, §S1). This introduced pseudo-replication that effectively understated the Type I error rate of their analysis by an order of magnitude (electronic supplementary material, figure S1), and biased estimates of the yield gap and its statistical uncertainty (electronic supplementary material, figure S2). Given these methodological and data-related critiques, a new study is needed to produce a more robust estimate of the gap between organic and conventional yields.
Here, we develop a hierarchical meta-analytic framework that overcomes the methodological pitfalls of previous studies by accounting for both the multi-level nature of the data and the yield variation within studies. Furthermore, via a literature search we compiled a more extensive and up-to-date meta-dataset, comprising 1071 organic versus conventional yield comparisons from 115 studies—over three times the number of observations of any of the previous analyses. Our meta-dataset includes studies from 38 countries and 52 crop species over a span of 35 years.
2. Material and methods
(a). Search details
In our search, we used similar terms to those employed by Seufert et al. [36] and de Ponti et al. [35]. The search term used was a complex Boolean search containing (i) the term ‘organic’ or ‘ecological’ and (ii) the term ‘agriculture’, ‘farming’, ‘production’ or ‘cropping’ in combination with (iii) terms equal or similar to the terms ‘yield’ and ‘compare’. We used the search engines Academic Compete Search, Google Scholar and Web of Science. The last search was conducted in January 2013. The complete list of studies and yield data are provided in the electronic supplementary material, table S5.
(b). Inclusion criteria
We adopted Seufert et al.'s [36] rigorous inclusion criteria, except we excluded (i) comparisons of subsistence yields (unimproved agriculture) against improved agricultural methods (e.g. [34,39]) and (ii) comparisons of yields taken from different years. Additionally, in cases where the means of organic and conventional yields were reported but the variance of those means were not (a necessary component for inclusion in meta-analysis), we obtained an estimate of the variance directly from the original authors, whenever possible. Of the 99 studies lacking variance estimates, we obtained variance estimates or original data from the authors of 28 of them. In cases where the authors did not reply and there were multiple years of data reported, we took the mean and variance across years (59 studies, 232 organic to conventional comparisons), as did Seufert et al. [36]. Because the variance across years is not a perfect estimate of the within-year variation, however, we also conducted analyses excluding these studies (electronic supplementary material, §S2.5 and figure S2). Together, the search and data request yielded 115 studies from which we extracted 1071 organic versus conventional comparisons.
(c). Meta-analytic model
We built a hierarchical meta-analytic model to generate an estimate of the yield gap (see the electronic supplementary material, §S2, for details). Following standard practice, we compared the natural log of the ratios between organic and conventional yields (the ‘response ratio’) across studies [36,40]. The response ratio is more normally distributed than the raw ratio and independent of the units of measurement used within a study, and thus is comparable across studies [40].
To analyse the yield data, which contained several levels of hierarchy, we employed two methods. First, for studies that compare multiple treatments (usually organic) with one control treatment (usually conventional), and are thus not independent [41,42], we calculated a combined response ratio and corresponding standard error for the entire study using the method presented in eqn 3 and 8 in [41], and then used these combined response ratios in the nested analysis.
Next, we constructed a hierarchical regression model in a Bayesian framework to account for the dependencies in the yield data. We expanded on the traditional random effects model [43] by considering three additional sources of random variation (i.e. random effects): (1) between studies, (2) within a study between years and (3) within a year between response ratios (e.g. across replicated trials of a crop planted at different times in the season). We also considered whether the variances of the random effect distributions for (2) and (3) were shared across studies, or study-specific.
The traditional random effects meta-analytic model includes a random effect of study (electronic supplementary material, equation S1), but individual response ratios must be nested within study so that studies, rather than individual organic to conventional yield comparisons, are treated as replicates. This avoids the pseudo-replication and resulting Type I error inflation of previous studies (electronic supplementary material, figure S1). We then added the additional random effects sequentially and determined whether the posterior distribution of the added parameter was clearly differentiated from zero (electronic supplementary material, Section S2). If it was, we concluded that the data supported adding that layer of hierarchy. We confirmed our model selection using the deviance information criterion, which can be problematic but agreed in this case [44,45]. The full possible model, prior to model selection, with all sources of random variation is
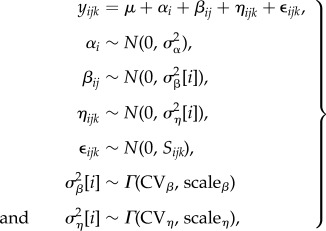 |
2.1 |
where yijk is the observed magnitude of the kth response ratio from the jth year of the ith study, μ is the mean response ratio across studies, αi is the effect of ith study, βij is the effect of jth year of the ith study, ηijk is the effect of the kth response ratio of the jth year of ith study and εijk is the residual. 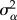 is the between-study variance,
is the between-study variance, 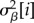 is the between-year variance of study i,
is the between-year variance of study i, 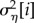 is the within-year, between-response ratio variance of study i, and Sijk is the variance of response ratio yijk as reported by its study. CVβ and CVη and scaleβ and scaleη are the coefficient of variation and scale parameters of the gamma distributions of the study-specific between- and within-year variances. When response ratios that shared a common control were combined, yijk corresponds to the aggregate within-study response ratio (eqn 3 in [41]) and Sijk is its pooled variance (eqn 8 in [41]).
is the within-year, between-response ratio variance of study i, and Sijk is the variance of response ratio yijk as reported by its study. CVβ and CVη and scaleβ and scaleη are the coefficient of variation and scale parameters of the gamma distributions of the study-specific between- and within-year variances. When response ratios that shared a common control were combined, yijk corresponds to the aggregate within-study response ratio (eqn 3 in [41]) and Sijk is its pooled variance (eqn 8 in [41]).
We also extended this model in order to accommodate analyses of study characteristics such as crop type and management practices. We analyse these additional explanatory variables one at a time because not all studies reported all explanatory variables. In these analyses, for cases where multiple organic treatments represented different categories for a specific explanatory variable, they could not be combined using Lajeunesse's method [41]. The potential bias resulting from non-independence of the response ratios in these cases, however, would be minimized by the fact that they are not pooled together in the analysis [41].
Letting h index the categories for a particular explanatory variable (e.g. crop species), we then have
| 2.2 |
where γh is the effect of the hth category, and the rest of the model parallels that given in equation (2.1).
In order to facilitate comparison between our results and those of previous analyses, we used the same categories as those defined by Seufert et al. [36]. We also examined the sensitivity of our results to explanatory variables related to study quality, again using the study quality categories defined by Seufert et al. [36]. The coefficients of explanatory variables were considered to be different from each other if the posterior of the difference between the 95% credibility intervals around the group means did not overlap zero.
We used JAGS through the R package rjags interface [46,47] to implement Markov chain Monte Carlo (MCMC) sampling. Inference was made from three chains each with 103 samples of the posterior distribution after a burn-in of 104 and with a thin rate of 103. We used Gaussians with large variances to define priors, except for variance terms, where we used a uniform (0, 100) prior on the standard deviation. Initial values were chosen randomly. Convergence was assessed by visual assessment of MCMC chains and using the Gelman–Rubin statistic (‘Rhat’ in R package R2JAGS, with values less than 1.1 indicating convergence [48]). Credible intervals around parameter estimates were calculated as the 2.5% and 97.5% quantiles of the posterior. We also checked for bias in our meta-dataset using a funnel plot and QQ-plot (electronic supplementary material, §S3 [49]).
3. Results
We found the data supported including random variation between studies and study-specific variation with a year, but not random variation between years of a study (electronic supplementary material, table S1 and figure S6). Using the selected hierarchical model, we found a smaller yield gap between conventional and organic cropping systems than that reported in recent meta-analyses [35,36]. We found that organic yields were 19.2% lower than conventional yields, with a 95% credible interval ranging from 15.5% to 22.9% (figure 1). Conventional yields were significantly higher than organic for all crop types and the yield ratios of most crop types did not vary significantly from one another (figure 1). At the finer scale of crop species, however, yield ratios differed significantly between some pairs of species (electronic supplementary material, figure S3).
Figure 1.
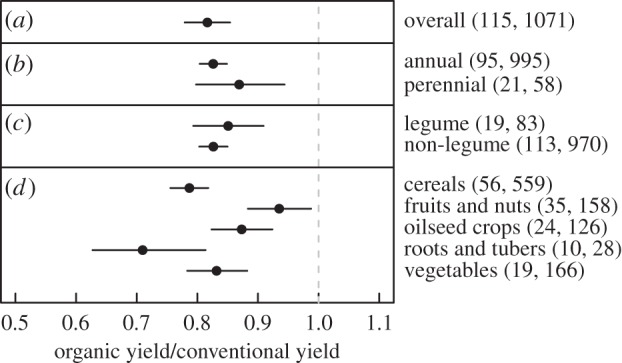
The organic-to-conventional yield ratio of (a) all crops, (b,c) plant types and (d) different crop types. Values are mean effect sizes with 95% credible intervals (i.e. 95% of the posterior distribution). The number of studies and observations in each category are shown in parentheses. Only categories with at least 10 yield comparisons from greater than five studies are shown. Organic and conventional yields were deemed significantly different from each other if the 95% credible interval of the yield ratio did not overlap one. Different levels of explanatory variables were considered to be significantly different if the posterior of the difference between the group means did not overlap zero.
The most dramatic difference between our findings and earlier work is an almost complete lack of significant differences between groups for all of the explanatory variables investigated. Unlike Seufert et al. [36], we did not find significant differences in yields for leguminous and non-leguminous crops nor for perennials and annuals (figure 1). Nor did we find a difference between the yield gaps for studies conducted in developed versus developing countries (electronic supplementary material, table S2; see also [34]). Our results were robust to including a between-year random effect (though including this parameter was not supported by the data; electronic supplementary material, table S1 and figure S6).
A likely explanation for the completely different outcomes for the majority of the explanatory variables between our study and that of Seufert et al. [36] (see electronic supplementary material, tables S2–S4, for a summary of the differences) is that Seufert et al. [36] did not account for all sources of shared variation in their analyses, which resulted in an inflated Type 1 error (electronic supplementary material, figure S1). This would increase the probability of accepting non-significant relationships as significant.
Our analysis also differs from that of Seufert et al. [36] in that the latter concluded that a number of management practices might minimize the differences between organic and conventional yields. For example, Seufert et al. [36] found significant differences in yield gaps related to irrigation practices, time since conversion from conventional to organic, and whether best management practices were used in the organic system. We found, however, no such differences between treatments within any of these categories (electronic supplementary material, table S3). We also included a new explanatory variable, phosphorus input, but again found no significant differences in yield when phosphorus input in the organic treatment was higher or lower than in the conventional treatment (electronic supplementary material, figure S4).
Seufert et al. [36] also found significantly larger yield gaps when levels of nitrogen input were similar in the organic and conventional treatments or greater in the conventional treatment, compared with cases where nitrogen input was higher in the organic treatment (electronic supplementary material, table S3). Our findings differed: we found a significantly smaller yield gap when N inputs were similar between treatments (9 ± 4%), compared with when N inputs were greater in conventional treatments (30 ± 4%). When N inputs were higher in organic treatments, the yield gap was intermediate and more variable (17 ± 6%), and marginally significantly different from the yield ratio with similar N input (figure 2). Similarly, low-input conventional systems have a smaller yield gap than high-input (electronic supplementary material, figure S4), a result also found by Seufert et al. [36].
Figure 2.
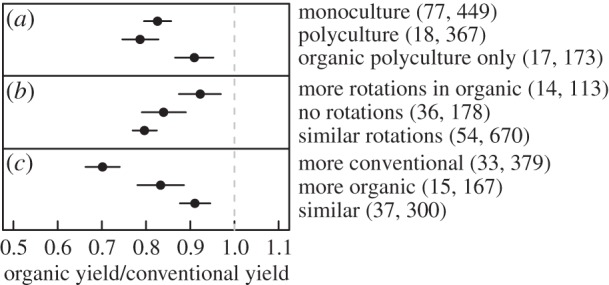
The influence of (a) cropping system, (b) rotation and (c) nitrogen input on the organic-to-conventional yield ratio. Values are mean effect sizes with 95% credible intervals. The number of studies and observations in each category are shown in parentheses.
We found that two management practices that diversify crop fields in space or over time, multi-cropping and crop rotations, can improve yields in organic systems. The yield gap between organic polycultures and conventional monocultures (9 ± 4%) was significantly smaller than when both treatments were monocultures (17 ± 3%) or both polycultures (21 ± 6%). We found a similar result with crop rotations. The yield gap was smaller when the organic system had more rotations (8 ± 5%) compared with when both treatments had a similar number of rotations (20 ± 2%) or did not have crop rotations at all (16 ± 5%). These results also suggest that polyculture and crop rotations increase yields in both organic and conventional cropping systems (figure 2). Seufert et al. [36] found no such differences between cropping or rotation systems. There is some overlap between studies that reported the yields of organic polycultures with more rotations, so these practices could work synergistically to close the yield gap, or one of the practices could be producing the majority of the effect.
We found evidence of bias in the meta-dataset towards studies reporting higher conventional yields relative to organic (electronic supplementary material, Section S3). We also detected a trend towards larger yield gaps in more recent studies, though it is difficult to determine the causal mechanism for this trend (electronic supplementary material, Section S3). Our results should therefore be interpreted as presenting an estimate of the yield gap from the available literature that is likely favouring studies reporting higher conventional yields than organic.
4. Discussion
Our extensive dataset including over three times more yield comparisons than previous studies [35,36] and our hierarchical analytical framework, provides the most up-to-date estimate of the yield gap between organic and conventional agriculture, and how this yield gap is influenced, or not, by management practices and crop types. The lower bound of our credible interval around the yield ratio overlaps the upper bounds of the two previous meta-analyses [35,36], but because these analyses did not account for the hierarchy of their data and/or the sampling variance within studies, these prior estimates are subject to high levels of Type 1 error (underestimated uncertainty), which likely results in inaccuracy in estimating the yield gap and its statistical uncertainty. Further, we found entirely different effects of crop types and management practices on the yield gap then previous studies [36].
The results of our analysis are limited by modelling considerations and the studies available for inclusion. We modelled as many layers of non-independence in our meta-dataset as the data supported, but others may exist. In addition, we found a bias towards reporting of higher conventional to organic yield ratios in the literature; therefore, even though our estimate of the yield gap is more robust and smaller than previous analyses, it may still be an overestimate.
The estimate of the organic-to-conventional yield ratio is an average over many disparate systems and crop types. The over-representation of specific practices or crops in the dataset may therefore excessively influence the estimate of the yield gap. For example, cereal crops, which exhibit the greatest difference in yield of the crop types between organic and conventional systems, were greatly over-represented (53% of comparisons). The finding that cereal productivity (including wheat, barley, rice and maize) is lower in organic systems is of interest because of its central importance in the human diet and predominance in cultivated land area. This larger difference, however, is not surprising, given the extensive efforts since the Green Revolution to increase cereal yields by breeding high-yielding cereal varieties adapted to work well with conventional inputs [50,51].
Given that there is such a diversity of management practices used in both organic and conventional farming, a broad-scale comparison of organic and conventional production may not provide the most useful insights for improving management of organic systems. Instead, it might be more productive to investigate explicitly and systematically how specific management practices (e.g. intercrop combinations, crop rotation sequences, composting, biological control, etc.) could be altered in different cropping systems to mitigate yield gaps between organic and conventional production. Historically, research and development of organic cropping systems has been extensively underfunded relative to conventional systems [16,52,53]; thus, research priorities would need to shift to provide for this needed work. Our meta-analyses found relatively small, and potentially overestimated, differences in yield between organic and conventional agriculture (i.e. between 15.5 and 22.9%), despite historically low rates of investment in organic cropping systems. These yield differences dropped to 9 ± 4% and 8 ± 5% when diversification techniques (multi-cropping and crop rotations, respectively) were used. We therefore suggest that further investment in agroecological research has the potential to improve productivity of sustainable agricultural methods to equal or better conventional yields in various cropping systems, as has indeed been demonstrated through long-term studies (e.g. [54,55]).
Further, many comparisons between organic and conventional agriculture use modern crop varieties selected for their ability to produce under high-input (conventional) systems. Such varieties are known to lack important traits needed for productivity in low-input systems, potentially biasing towards finding lower yields in organic versus conventional comparisons. By contrast, few modern varieties have yet been developed to produce high yields under organic conditions [50,56]; generating such breeds would be an important first step towards reducing yield gaps when they occur. Finally, reducing the yield gap between organic and conventional agriculture (or, more accurately, between biologically diversified versus chemically intensive farming systems) has the potential benefit of reducing the loss of biodiversity and ecosystem services often associated with conventional agricultural methods [1,2], and thus promoting a high-yielding agriculture that is relatively environmentally beneficial and wildlife-friendly compared with conventional systems [21,28,57,58]. There is some evidence that biodiversity decreases with increased yields on organic farms [59], but this might not apply to yield increases on biologically diversified farming systems.
As others have pointed out, agricultural yields, in and of themselves, are not sufficient to address the twin crises of hunger and obesity, both associated with poverty, that are seen in the world today. Current global caloric production greatly exceeds that needed to supply the world's population, yet social, political and economic factors prevent many people from accessing sufficient food for a healthy life [15,16,60,61]. A focus solely on increased yields will not solve the problem of world hunger. Increased production is, however, critical for meeting the economic needs of poor farmers who make up the largest portion of the world's chronically hungry people [21,39,60], and agroecological methods provide low-cost methods for doing so (e.g. [54]). Further, environmentally sustainable, resilient production systems will become an increasingly urgent necessity in a world where many planetary boundaries have already been reached or exceeded [19,62,63]. We believe it is time to invest in analytically rigorous, agroecological and socio-economic research oriented at eliminating yield gaps between sustainable and conventional agriculture (when they occur), identifying barriers to adoption of sustainable techniques and improving livelihoods of the rural poor.
Data accessibility
The full meta-dataset is available at Dryad data repository, doi:10.5061/dryad.hf305.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the authors who conducted the studies included in our meta-dataset, especially those supplying original data. All references are included as an appendix. We appreciate the correspondence between our group and Verena Seufert, Jonathan Foley and Navin Ramankutty. Laura Driscoll, Kelly Garbach, Lisa Kelley, Andrew Rominger and Nathan Van Schmidt assisted with data management and discussion. We also appreciate our correspondence with John Vandermeer regarding publication bias. We would like to thank Jonathan Foley and two anonymous reviewers for their insightful comments and revisions; one reviewer, in particular, provided statistical feedback that proved extraordinarily helpful.
Funding statement
Funding for L.C.P. was provided by an NSF Graduate Research Fellowship and for L.K.M. by an NSERC Postdoctoral Fellowship.
References
- 1.Raudsepp-Hearne C, Peterson GD, Tengö M, Bennett EM, Holland T, Benessaiah K, MacDonald GK, Pfeifer L. 2010. Untangling the environmentalist's paradox: why is human well-being increasing as ecosystem services degrade? BioScience 60, 576–589. ( 10.1525/bio.2010.60.8.4) [DOI] [Google Scholar]
- 2.Foley JA, et al. 2005. Global consequences of land use. Science 309, 570–574. ( 10.1126/science.1111772) [DOI] [PubMed] [Google Scholar]
- 3.Lal R. 2004. Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623–1627. ( 10.1126/science.1097396) [DOI] [PubMed] [Google Scholar]
- 4.Gordon LJ, Peterson GD, Bennett EM. 2008. Agricultural modifications of hydrological flows create ecological surprises. Trends Ecol. Evol. 23, 211–219. ( 10.1016/j.tree.2007.11.011) [DOI] [PubMed] [Google Scholar]
- 5.Gibbs HK, Ruesch AS, Achard F, Clayton MK, Holmgren P, Ramankutty N, Foley JA. 2010. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl Acad. Sci. USA 107, 16 732–16 737. ( 10.1073/pnas.0910275107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcove DS, Koh LP. 2010. Addressing the threats to biodiversity from oil-palm agriculture. Biodivers. Conserv. 19, 999–1007. ( 10.1007/s10531-009-9760-x) [DOI] [Google Scholar]
- 7.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. 2007. Organophosphate pesticide exposure and neurodevelopment in young Mexican–American children. Environ. Health Perspect. 115, 792–798. ( 10.1289/ehp.9828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz RJ, Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929. ( 10.1126/science.1156401) [DOI] [PubMed] [Google Scholar]
- 9.Foley JA, et al. 2011. Solutions for a cultivated planet. Nature 478, 337–342. ( 10.1038/nature10452) [DOI] [PubMed] [Google Scholar]
- 10.Hayes TB, et al. 2010. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc. Natl Acad. Sci. USA 107, 4612–4617. ( 10.1073/pnas.0909519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosso SJD, Cavigelli MA. 2012. Climate stabilization wedges revisited: can agricultural production and greenhouse-gas reduction goals be accomplished? Front. Ecol. Environ. 10, 571–578. ( 10.1890/120058) [DOI] [Google Scholar]
- 12.Gill RJ, Ramos-Rodriguez O, Raine NE. 2012. Combined pesticide exposure severely affects individual-and colony-level traits in bees. Nature 491, 105–108. ( 10.1038/nature11585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA 108, 20 260–20 264. ( 10.1073/pnas.1116437108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FAO. 2009. How to feed the world in 2050. Rome, Italy: Food and Agriculture Organization. [Google Scholar]
- 15.Tomlinson I. 2013. Doubling food production to feed the 9 billion: a critical perspective on a key discourse of food security in the UK. J. Rural Stud. 29, 81–90. [Google Scholar]
- 16.McIntyre BD. 2009. IAASTD international assessment of agricultural knowledge, science and technology for development: global report. Washington, DC: Island Press. [Google Scholar]
- 17.Garnett T, et al. 2013. Sustainable intensification in agriculture: premises and policies. Science 341, 33–34. ( 10.1126/science.1234485) [DOI] [PubMed] [Google Scholar]
- 18.Connor D. 2008. Organic agriculture cannot feed the world. Field Crops Res. 106, 187–190. ( 10.1016/j.fcr.2007.11.010) [DOI] [Google Scholar]
- 19.Bennett E, et al. 2014. Toward a more resilient agriculture. Solutions 5, 65–75. [Google Scholar]
- 20.Loos J, Abson DJ, Chappell MJ, Hanspach J, Mikulcak F, Tichit M, Fischer J. 2014. Putting meaning back into ‘sustainable intensification’. Front. Ecol. Environ. 12, 356–361. ( 10.1890/130157) [DOI] [Google Scholar]
- 21.Kremen C, Miles A. 2012. Ecosystem services in biologically diversified versus conventional farming systems: benefits, externalities, and trade-offs. Ecol. Soc. 17, 40. [Google Scholar]
- 22.Kremen C, Iles A, Bacon C. 2012. Diversified farming systems: an agroecological, systems-based alternative to modern industrial agriculture. Ecol. Soc. 17, 44. [Google Scholar]
- 23.Henry M, Beguin M, Requier F, Rollin O, Odoux J-F, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350. ( 10.1126/science.1215039) [DOI] [PubMed] [Google Scholar]
- 24.Willer H, Kilcher L. 2011. The world of organic agriculture, statistics and emerging trends. Bonn, Germany: International Federation of Organic Agriculture Movements (IFOAM). [Google Scholar]
- 25.Guthman J. 2000. Raising organic: An agro-ecological assessment of grower practices in California. Agric. Hum. Values 17, 257–266. ( 10.1023/A:1007688216321) [DOI] [Google Scholar]
- 26.Bengtsson J, Ahnström J, Weibull A-C. 2005. The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J. Appl. Ecol. 42, 261–269. ( 10.1111/j.1365-2664.2005.01005.x) [DOI] [Google Scholar]
- 27.Mäder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U. 2002. Soil fertility and biodiversity in organic farming. Science 296, 1694–1697. ( 10.1126/science.1071148) [DOI] [PubMed] [Google Scholar]
- 28.Hole D, Perkins A, Wilson J, Alexander I, Grice P, Evans A. 2005. Does organic farming benefit biodiversity? Biol. Conserv. 122, 113–130. ( 10.1016/j.biocon.2004.07.018) [DOI] [Google Scholar]
- 29.Lotter D, Seidel R, Liebhardt W. 2003. The performance of organic and conventional cropping systems in an extreme climate year. Am. J. Altern. Agric. 18, 146–154. ( 10.1079/AJAA200345) [DOI] [Google Scholar]
- 30.Lynch DH, MacRae R, Martin RC. 2011. The carbon and global warming potential impacts of organic farming: does it have a significant role in an energy constrained world? Sustainability 3, 322–362. ( 10.3390/su3020322) [DOI] [Google Scholar]
- 31.Nissen TM, Wander MM. 2003. Management and soil-quality effects on fertilizer-use efficiency and leaching. Soil Sci. Soc. Am. J. 67, 1524–1532. ( 10.2136/sssaj2003.1524) [DOI] [Google Scholar]
- 32.Tuck SL, Winqvist C, Mota F, Ahnström J, Turnbull LA, Bengtsson J. 2014. Land-use intensity and the effects of organic farming on biodiversity: a hierarchical meta-analysis. J. Appl. Ecol. 51, 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanhill G. 1990. The comparative productivity of organic agriculture. Agric. Ecosyst. Environ. 30, 1–26. ( 10.1016/0167-8809(90)90179-H) [DOI] [Google Scholar]
- 34.Badgley C, Moghtader J, Quintero E, Zakem E, Chappell MJ, Aviles-Vazquez K, Samulon A, Perfecto I. 2007. Organic agriculture and the global food supply. Renew. Agric. Food Syst. 22, 86–108. ( 10.1017/S1742170507001640) [DOI] [Google Scholar]
- 35.de Ponti T, Rijk B, van Ittersum M. 2012. The crop yield gap between organic and conventional agriculture. Agric. Syst. 108, 1–9. ( 10.1016/j.agsy.2011.12.004) [DOI] [Google Scholar]
- 36.Seufert V, Ramankutty N, Foley JA. 2012. Comparing the yields of organic and conventional agriculture. Nature 485, 229–232. ( 10.1038/nature11069) [DOI] [PubMed] [Google Scholar]
- 37.Cassman K. 2007. Editorial response by Kenneth Cassman: can organic agriculture feed the world-science to the rescue? Renew. Agric. Food Syst. 22, 83–84. [Google Scholar]
- 38.Gurevitch J, Hedges LV. 1999. Statistical issues in ecological meta-analyses. Ecology 80, 1142–1149. ( 10.1890/0012-9658(1999)080[1142:SIIEMA]2.0.CO;2) [DOI] [Google Scholar]
- 39.Pretty JN, Hine R. 2001. Reducing food poverty with sustainable agriculture: a summary of new evidence. Colchester, UK: University of Essex. [Google Scholar]
- 40.Hedges L, Gurevitch J, Curtis P. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. ( 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2) [DOI] [Google Scholar]
- 41.Lajeunesse MJ. 2011. On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology 92, 2049–2055. ( 10.1890/11-0423.1) [DOI] [PubMed] [Google Scholar]
- 42.Gleser LJ, Olkin I. 1994. Stochastically dependent effect sizes. In The handbook of research synthesis (eds Cooper H, Hedges LV.), pp. 339–355. New York, NY: Russell Sage Foundation. [Google Scholar]
- 43.Hedges LV, Olkin I. 1985. Statistical methods for meta-analysis. New York, NY: Academic Press. [Google Scholar]
- 44.Gelman A, Hill J. 2006. Data analysis using regression and multilevel/hierarchical models. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Kéry M, Schaub M. 2012. Bayesian population analysis using WinBUGS: a hierarchical perspective. New York, NY: Academic Press. [Google Scholar]
- 46.Plummer M. 2013. rjags: Bayesian graphical models using MCMC. R package v. 3–10. See http://CRAN.R-project.org/package=rjags. [Google Scholar]
- 47.Plummer M. 2003. Jags: a program for analysis of Bayesian graphical models using Gibbs sampling. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing, 20–22 March, Vienna, Austria. See www.r-project.org/conferences/DSC-2003/Proceedings/Plummer.pdf.
- 48.Kery M. 2010. Introduction to WinBUGS for ecologists: Bayesian approach to regression, ANOVA, mixed models and related analyses. New York, NY: Academic Press. [Google Scholar]
- 49.Rothstein HR, Sutton AJ, Borenstein M. 2006. Publication bias in meta-analysis: prevention, assessment and adjustments. New York, NY: John Wiley and Sons. [Google Scholar]
- 50.Lammerts van Bueren E, Jones S, Tamm L, Murphy K, Myers J, Leifert C, Messmer M. 2011. The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: a review. NJAS Wageningen J. Life Sci. 58, 193–205. ( 10.1016/j.njas.2010.04.001) [DOI] [Google Scholar]
- 51.Evenson RE, Gollin D. 2003. Assessing the impact of the Green Revolution, 1960 to 2000. Science 300, 758–762. ( 10.1126/science.1078710) [DOI] [PubMed] [Google Scholar]
- 52.Vanloqueren G, Baret PV. 2009. How agricultural research systems shape a technological regime that develops genetic engineering but locks out agroecological innovations. Res. Policy 38, 971–983. ( 10.1016/j.respol.2009.02.008) [DOI] [Google Scholar]
- 53.Carlisle L, Miles A. 2013. Closing the knowledge gap: how the USDA could tap the potential of biologically diversified farming systems. J. Agric. Food Syst. Commun. Dev. 3, 219–225. ( 10.5304/jafscd.2013.034.025) [DOI] [Google Scholar]
- 54.Khan Z, Midega C, Pittchar J, Pickett J, Bruce T. 2011. Push–pull technology: a conservation agriculture approach for integrated management of insect pests, weeds and soil health in Africa: UK Government's foresight food and farming futures project. Int. J. Agric. Sustain. 9, 162–170. ( 10.3763/ijas.2010.0558) [DOI] [Google Scholar]
- 55.Rodale Institute. 2012. Farming systems trial, 30-year report. Technical report, Rodale Institute, Kutztown, PA, USA. [Google Scholar]
- 56.Murphy KM, Campbell KG, Lyon SR, Jones SS. 2007. Evidence of varietal adaptation to organic farming systems. Field Crops Res. 102, 172–177. ( 10.1016/j.fcr.2007.03.011) [DOI] [Google Scholar]
- 57.Batáry P, Báldi A, Kleijn D, Tscharntke T. 2011. Landscape-moderated biodiversity effects of agri-environmental management: a meta-analysis. Proc. R. Soc. B 278, 1894–1902. ( 10.1098/rspb.2010.1923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Philpott SM, et al. 2008. Biodiversity loss in Latin American coffee landscapes: review of the evidence on ants, birds, and trees. Conserv. Biol. 22, 1093–1105. ( 10.1111/j.1523-1739.2008.01029.x) [DOI] [PubMed] [Google Scholar]
- 59.Gabriel D, Sait SM, Kunin WE, Benton TG. 2013. Food production versus biodiversity: comparing organic and conventional agriculture. J. Appl. Ecol. 50, 355–364. ( 10.1111/1365-2664.12035) [DOI] [Google Scholar]
- 60.Holt-Giménez E, Shattuck A, Altieri M, Herren H, Gliessman S. 2012. We already grow enough food for 10 billion people… and still can't end hunger. J. Sustain. Agric. 36, 595–598. ( 10.1080/10440046.2012.695331) [DOI] [Google Scholar]
- 61.Smil V. 2005. Feeding the world: how much more rice do we need? In Rice is life: scientific perspectives for the 21st century (ed. K Toriyama, KL Heong, B Hardy), pp. 21–23. Manila, PH: International Rice Research Institute. [Google Scholar]
- 62.Rockström J, et al. 2009. A safe operating space for humanity. Nature 461, 472–475. ( 10.1038/461472a) [DOI] [PubMed] [Google Scholar]
- 63.Godfray HCJ, et al. 2010. Food security: the challenge of feeding 9 billion people. Science 327, 812–818. ( 10.1126/science.1185383) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full meta-dataset is available at Dryad data repository, doi:10.5061/dryad.hf305.


