Abstract
The vast majority of research into the mechanisms of camouflage has focused on forms that confound visual perception. However, many organisms primarily interact with their surroundings using chemosensory systems and may have evolved mechanisms to ‘blend in’ with chemical components of their habitat. One potential mechanism is ‘chemical crypsis' via the sequestration of dietary elements, causing a consumer's odour to chemically match that of its prey. Here, we test the potential for chemical crypsis in the coral-feeding filefish, Oxymonacanthus longirostris, by examining olfactory discrimination in obligate coral-dwelling crabs and a predatory cod. The crabs, which inhabit the corals consumed by O. longirostris, were used as a bioassay to determine the effect of coral diet on fish odour. Crabs preferred the odour of filefish fed their preferred coral over the odour of filefish fed a non-preferred coral, suggesting coral-specific dietary elements that influence odour are sequestered. Crabs also exhibited a similar preference for the odour of filefish fed their preferred coral and odour directly from that coral, suggesting a close chemical match. In behavioural trials, predatory cod were less attracted to filefish odour when presented alongside the coral it had been fed on, suggesting diet can reduce detectability. This is, we believe, the first evidence of diet-induced chemical crypsis in a vertebrate.
Keywords: camouflage, predator–prey interactions, olfaction, coral reefs, Oxymonacanthus longirostris, Acropora
1. Introduction
Many organisms have evolved mechanisms to match elements of their environment, to avoid detection or recognition by either predators or prey [1]. Research has focused on forms of visual camouflage, such as crypsis, likely owing to our own reliance on vision [2]. However, organisms often rely on non-visual sensory systems to interact with their environments [3], and recent empirical evidence suggests a variety of taxa have also evolved mechanisms to ‘blend in’ with various non-visual habitat components (reviewed in [2]). For instance, many species have highly developed olfactory capabilities, relying on chemical, not visual, cues to locate and identify predators or prey [4,5]. To counteract this, an organism could employ mechanisms that render it chemically insignificant, either by limiting the chemical cues it produces [6,7], reducing the ability of a receiver to exploit chemical cues [8,9] or by altering these cues to match chemical signatures present within its habitat [10].
One potential pathway by which an organism's chemical signature can be altered is via its diet and sequestering chemical compounds from prey species [11,12]. If habitat-specific, diet-derived compounds are sequestered, this may infer a cryptic benefit if the organism's chemical signature is altered such that it is either not detected, or misidentified, by a potential receiver. Compelling evidence for this mechanism has come from the herbivorous Biston robustum caterpillar [13]. Ingested plant-specific compounds are incorporated in the caterpillar's exterior cuticle, reducing its detectability to predatory ants. While this ‘chemical crypsis' has also been reported in other herbivorous invertebrates, where dietary elements are incorporated into the exoskeleton during development [14,15], evidence from other taxa is scarce. If similar processes occur in species without external hard structures, or with non-plant based diets, this would indicate that chemical crypsis may be more widespread in the animal kingdom than currently recognized.
On coral reefs, the high number of potential predator–prey interactions may make camouflage a particularly important process. Recent analysis has revealed that many of the apparently gaudy colour patterns seen in reef fishes have an underlying cryptic function [16] with camouflage implicated in the colour patterns of both predators [17] and prey [18]. Reef fishes also rely on non-visual senses, such as olfaction [5,19], for detecting predators and prey. As corals form the primary shelter for many species, chemically resembling coral habitat may prove advantageous during predator–prey interactions.
Coral-feeding fishes represent a good model for testing the potential importance of chemical crypsis on coral reefs as some use corals for both food [20] and shelter in a manner analogous to herbivorous insect–plant systems. One particular example, the harlequin filefish, Oxymonacanthus longirostris (Monacanthidae), feeds almost exclusively on corals from one genus, Acropora, and is also selective between species [21]. Furthermore, it associates closely with these corals, particularly during crepuscular and nocturnal periods when it shelters among them—visually resembling a coral branch [22]. However, whether or not it sequesters chemical compounds from its diet is unknown.
The objective of this study was to investigate whether O. longirostris sequesters dietary elements that affect odour, and if this infers a cryptic benefit. Specifically, we determined (i) whether filefish odour resembled the prey coral odour, but differed from that of a non-prey coral, suggesting sequestration of coral-specific dietary elements that affect odour. If this was the case, we determined (ii) how similar a filefish's odour and the odour of its coral prey were. Finally, we determined (iii) whether chemically resembling a coral imparts a cryptic benefit to the filefish by reducing its detectability to reef predators.
2. Methods
(a). Study location and species
Experiments were conducted at Lizard Island Research Station, Great Barrier Reef, Australia (14°40′ S; 145°27′ E), between August 2013 and February 2014. O. longirostris is relatively common at Lizard Island, feeding mostly on Acropora corals (R. M. Brooker 2013, personal observation). Fish were collected using hand nets and clove oil, and maintained in aquaria.
(b). Diet treatments
Oxymonacanthus longirostris were fed an exclusive diet, Acropora spathulata or Pocillopora damicornis, over four weeks. Four fish were allocated to each of four 500 l tanks, two tanks per diet, ensuring equal size distributions. As corallivores often forage continuously diurnally, tanks were supplied with sufficient coral to permit foraging to satiation. Diet treatments began 3 days prior to trials, allowing accumulation of coral-specific, odour-influencing compounds. As waste products may affect odour, fish from alternate tanks were used each night. Prior to trials, coral was removed from the tank, ensuing no feeding for greater than or equal to 12 h. The absence of faeces in the gut after this time was confirmed from inspection of five euthanized individuals. As condition may affect odour, the length : weight ratio of fishes fed each coral was compared using a Mann–Whitney U-test. Median length : weight ratio was not significantly different between filefish fed each coral (U = 125, z = −1.171, p = 0.252), suggesting no diet effect on condition.
(c). Experimental evaluation of the effect of coral diet on filefish odour: a bioassay
The olfactory preferences of coral-dwelling crabs were used to determine the effect of coral diet on filefish odour. These small crabs live exclusively between the branches of their preferred coral, where they largely feed on coral mucus, tissue and eggs [23]. Two specialized species were used: the Pocillopora-obligate Trapezia cymodoce and the Acropora-obligate Tetralia glaberrima [24]. Olfactory responses to filefish fed different diets, and corals themselves, were tested in pair-wise choice experiments (electronic supplementary material, figure S1). Diet effects on odour were examined by testing (i) whether crabs preferred the odour of their preferred coral over that of non-preferred coral, and if so (ii) whether crabs preferred the odour of filefish fed their preferred coral over filefish fed non-preferred coral. Similarity between coral and filefish odour was examined by testing (iii) whether crabs preferred the odour of preferred coral over the odour of filefish fed that coral. This was examined further by testing (iv) whether crabs preferred the odour of filefish fed their preferred coral over non-preferred coral odour.
Trials were conducted overnight in aquaria (L 60 cm × W 25 cm × H 40 cm), with a sand bottom (10 cm) creating a flat, textured surface on which crabs could move. A section of perforated pipe was located at each end, into which a size-matched coral fragment or filefish was placed prior to trials, with a third non-perforated outflow pipe located centrally. Perforations released olfactory cues while restricting visual cues. Inflow was split, so equal water entered each perforated pipe, creating a flow gradient towards the centre, whereas an air stone in each pipe aided mixing. Beside each pipe, a skeletal coral fragment provided shelter, whereas a 2 cm section of 15 ml pipe, placed between fragments, allowed covered movement (electronic supplementary material, figure S2). Following sunset, individual crabs were collected from coral and placed by the centre tube, with their subsequent location recorded at first light. If a crab was located on a fragment or within 2 cm of a tube, this was regarded as a choice.
Data were analysed using chi-squared goodness-of-fit tests (χ2), with H0 being that crabs would settle next to each choice (choice A, choice B, centre) equally, suggesting no preference. Where a significant χ2 was found, standardized residuals (sr) determined what choices were driving the deviation from H0. An sr > 2.00 meant a choice was selected significantly more than expected, whereas an sr < −2.00 meant a choice was selected less [25].
(d). Coral diet and ability of a predator to detect Oxymonacanthus longirostris
An experiment using predatory cod (Cephalopholis spp.) examined whether a coral diet affects detectability. Cod were run through six randomly ordered 30 min trials, each presenting combinations of two filefish odours and corals (electronic supplementary material, figure S1). Two questions were examined, (i) did treatment affect activity level and (ii) when presented with filefish odour that matched the live coral versus filefish odour that did not match the live coral, did predators spend equal time near each?
Cod, collected on hand lines, were acclimated to captivity for 3 days. Prior to experimentation (24 h), individuals were placed into circular tanks (1.2 m diameter), supplied with seawater. Tanks contained a shelter, extending into the tank from the wall opposite the inflow pipe. For each trial, two sections of perforated tube were placed in the tank opposite each other, equal distance from the shelter. Air stones in each tube created outwards flow, confirmed in dye tests. One filefish was placed in each tube and, when appropriate, a small coral colony was placed alongside (electronic supplementary material, figure S2). Trials were filmed (GoPro, Woodman Labs) for subsequent analysis, with the cod's location recorded each 30 s. Similarity between estimated and actual activity levels in a random subset of videos showed this technique provided accurate behavioural measurements.
To test the relationship between activity level and treatment, a linear mixed effects analysis was conducted using the nlme package in R [26]. Treatment was included in the model as a fixed effect, with fish included as a random effect. To permit parametric analysis, data were logit-transformed following [27], using log[y + e/(1 − y + e)], with e the smallest non-zero proportion in the dataset. To test the relative proportion of time associated with filefish whose diet matched, or differed from, the associated coral, treatments 3 and 4 data (electronic supplementary material, figure S1) were analysed using a Kruskal–Wallis H-test with post hoc comparisons [28]. These treatments consisted of either two A. spathulata corals or two P. damicornis corals, along with one filefish whose diet matched, and one filefish whose diet differed from, their associated coral.
3. Results
(a). Experimental evaluation of the effect of coral diet on filefish odour
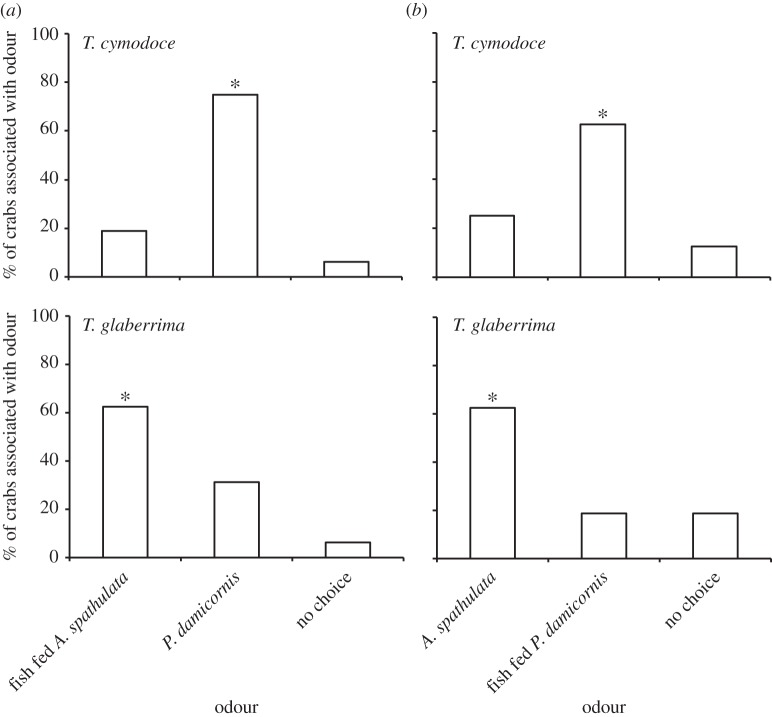
Both crab species exhibited a strong olfactory preference for filefish fed their preferred coral species over those fed on a non-preferred coral (table 1 and figure 1). As expected, both crab species preferred the odour of a preferred coral habitat over the odour of a non-preferred coral habitat, with T. cymodoce preferring the odour of P. damicornis and T. glaberrima preferring the odour of A. spathulata (figure 1). There was no side preference in controls.
Table 1.
Summarized χ2 goodness-of-fit test results for olfactory choice trials using two species of coral-dwelling crab; the Pocillopora-obligate Trapezia cymodoce, and the Acropora-obligate Tetralia glaberrima. Trials are as indicated in figure 4 and electronic supplementary material, figure S1, using the odours of Pocillopora damicornis (magenta), Acropora spathulata (green), filefish fed P. damicornis (orange), and filefish fed A. spathulata (blue). Where significant χ2-values were identified, standardized residuals (sr) identified variables selected more or less than expected by chance, with key variables indicated in the ‘driven by’ column. If crabs were associated with the central habitat patch more or less than expected under the null hypothesis, this is indicated as ‘no choice’. Crabs per trial, n = 16.
| trial | χ2 (d.f.) | p-value | driven by | sr | |
|---|---|---|---|---|---|
| T. cymodoce |  |
16.63 (2) | <0.01 | coral P | 3.22 |
 |
9.88 (2) | <0.01 | fish P | 2.46 | |
 |
1.63 (2) | >0.05 | — | — | |
 |
1.63 (2) | >0.05 | — | — | |
 |
4.63 (2) | >0.05 | — | — | |
 |
6.5 (2) | 0.04 | fish P | 2.02 | |
 |
12.88 (2) | <0.01 | coral P | 2.89 | |
| T. glaberrima |  |
21.73 (2) | <0.01 | coral A | 3.76 |
 |
9.13 (2) | 0.01 | fish A | 2.46 | |
 |
3.5 (2) | >0.05 | — | ||
 |
0.13 (2) | >0.05 | — | ||
 |
9.5 (2) | <0.01 | coral A | 2.02 | |
| no choice | −2.31 | ||||
 |
6.13 (2) | 0.04 | coral A | 2.02 | |
 |
7.63 (92) | 0.02 | fish A | 2.02 |
Figure 1.
Percentage of coral-obligate crabs associated with each odour in olfactory choice trials comparing two different coral odours (white), or odours of filefish fed each coral exclusively (black). (a) Results for the Pocillopora-obligate Trapezia cymodoce. (b) Results for the Acropora-obligate Tetralia glaberrima. Coral/diet odours are Pocillopora damicornis and Acropora spathulata. Asterisks indicate where significant standardized residuals were identified. Crabs per trial, n = 16.
(b). Experimental evaluation of the similarity between filefish and coral odours
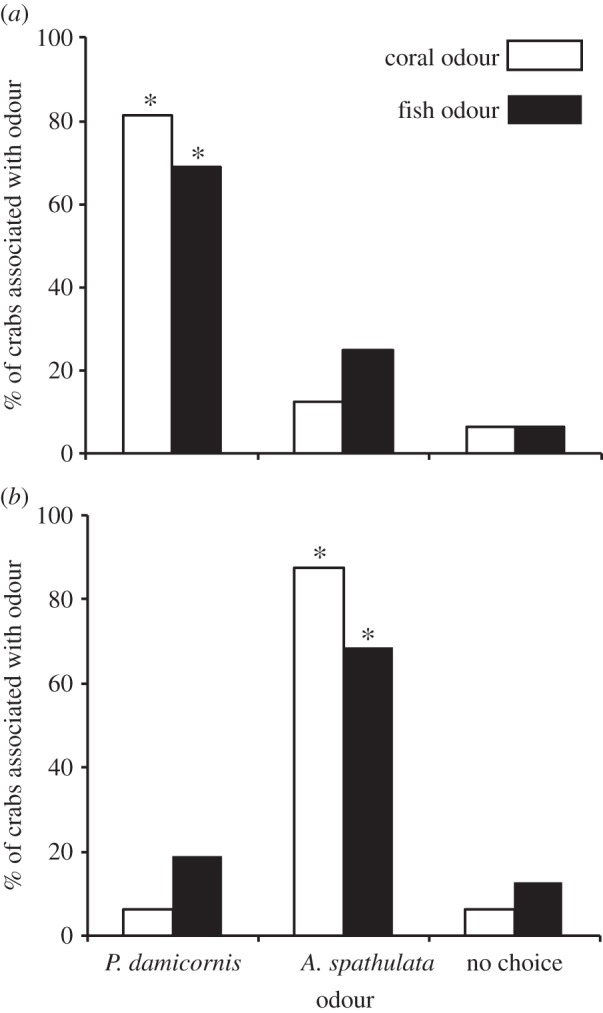
Offered a choice between the odour of their preferred coral and of a filefish fed that preferred coral, a high proportion of both species selected the filefish odour (table 1 and figure 2). No significant preference between odours was identified in T. cymodoce, though a slightly higher proportion selected the coral odour. In T. glaberrima, significantly more crabs selected the coral odour than expected under H0. However, a high percentage (37.5%) still selected the filefish odour, with the significant χ2 driven primarily by individuals that made no choice. Finally, while both species preferred the odour of their preferred coral over the odour of filefish fed a non-preferred coral, they preferred the odour of a filefish fed their preferred coral over the odour of a non-preferred coral (table 1 and figure 3).
Figure 2.
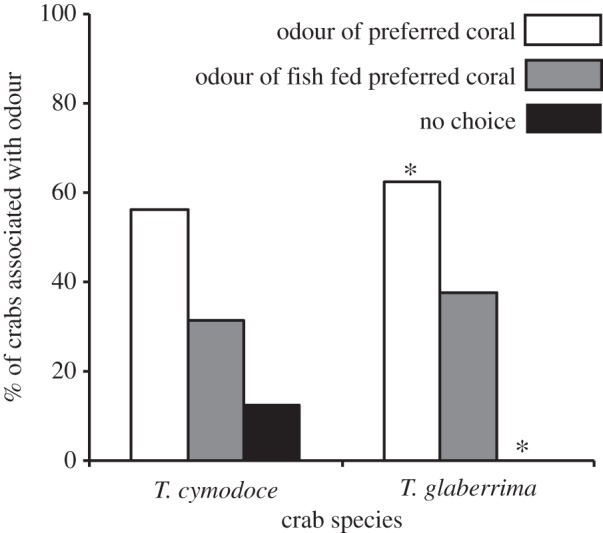
Percentage of coral-obligate crabs associated with preferred coral odour versus the odour of filefish fed that coral exclusively. Crab species are Trapezia cymodoce, which prefers Pocillopora damicornis coral, and Tetralia glaberrima, which prefers Acropora spathulata coral. Asterisks indicate where significant standardized residuals were identified. Crabs per trial, n = 16.
Figure 3.
Percentage of coral-obligate crabs associated with non-preferred coral odour versus the odour of a filefish fed preferred coral, and vice versa. Odours are Pocillopora damicornis, filefish fed P. damicornis, Acropora spathulata, and filefish fed A. spathulata. (a) Trials using the Pocillopora-obligate Trapezia cymodoce. (b) Trials using the Acropora-obligate Tetralia glaberrima. Asterisks indicate where significant standardized residuals were identified. Crabs per trial, n = 16.
(c). Coral diet and detectability to predators
Odour treatment had a significant effect on the relative activity level of predatory cod (F5,130 = 15.24, p < 0.001; figure 4). Cod were least active in treatments where all corals and filefish diets matched (p < 0.001), with no significant difference in activity level identified between the all P. damicornis, or all A. spathulata treatments (p = 0.843). While the relative activity level of cod was significantly higher in all treatments where one or more of the fish's diets did not match the associated coral, or no coral was present, no significant difference was identified between these treatments (p > 0.05; figure 4). Where cod had a choice between the odour of a filefish whose diet matched, or differed from, the associated coral, cod spent more time near the filefish whose diet differed (P. damicornis, 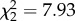 , p < 0.5; A. spathulata,
, p < 0.5; A. spathulata, 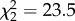 , p < 0.05; figure 5). For the P. damicornis treatment, more time was spent near A. spathulata-fed filefish (Mdn = 60%) than P. damicornis-fed filefish (Mdn = 10.27%) (p < 0.05). For the A. spathulata treatment, more time was spent near P. damicornis-fed filefish (Mdn = 66.96%) than A. spathulata-fed filefish (Mdn = 6.67%; p < 0.05), or not associated with either odour source (Mdn = 0%; p < 0.05; figure 5).
, p < 0.05; figure 5). For the P. damicornis treatment, more time was spent near A. spathulata-fed filefish (Mdn = 60%) than P. damicornis-fed filefish (Mdn = 10.27%) (p < 0.05). For the A. spathulata treatment, more time was spent near P. damicornis-fed filefish (Mdn = 66.96%) than A. spathulata-fed filefish (Mdn = 6.67%; p < 0.05), or not associated with either odour source (Mdn = 0%; p < 0.05; figure 5).
Figure 4.
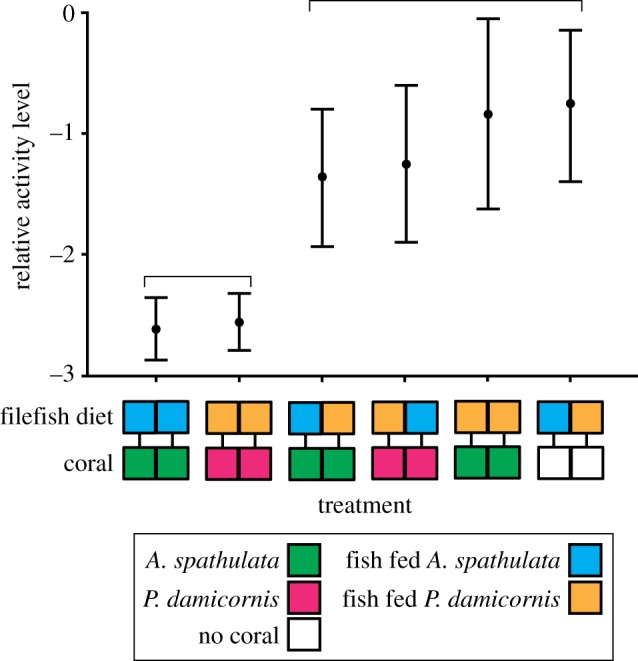
Relative cod activity level during each of the six coral/fish odour treatments. The y-axis represents logit-transformed proportional activity (mean ± s.e.). Treatments are as indicated using the odours of Pocillopora damicornis (magenta), Acropora spathulata (green), filefish fed P. damicornis (orange) and fish fed A. spathulata (blue). Horizontal braces indicate treatments where activity level did not significantly differ in linear mixed effects analysis. Sample size per treatment, n = 27.
Figure 5.
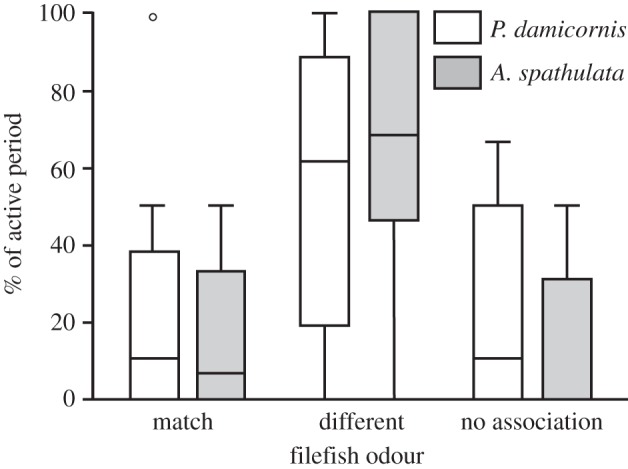
Box-and-whisker plot showing relative % of cod activity spent near the odour of a filefish whose diet matched, versus one whose diet differed from, the associated coral. White bars represent P. damicornis treatment, whereas grey bars represent A. spathulata treatment. Boxes equal median and inter-quartile range, while whiskers extend to the non-outlier upper and lower data values. Points indicate outliers. In each treatment, one filefish was fed P. damicornis, and one A. spathulata. NA represents the % of activity not associated with either odour/coral combination. Trials per treatment, n = 18.
4. Discussion
Our results provide compelling evidence of a diet-induced chemical crypsis mechanism in O. longirostris. Biological assays indicate that filefish odour closely resembles that of its coral prey, suggesting sequestration of coral-specific dietary elements that affect odour. The Acropora-dwelling crab clearly preferred the smell of filefish fed Acropora over those fed Pocillopora and vice versa. The ‘coral’ olfactory signal coming from filefish was so strong that some crabs could not distinguish it from the coral itself. Behavioural experiments clearly show that predators have difficulty using olfactory cues to distinguish filefish from the corals they eat. This is the first result of this kind, but is potentially of general significance across the range of corallivorous fishes.
The biochemical pathway from ingestion to sequestration to odour is not yet known, although the lack of gut contents suggests coral-specific cues were originating from the fish itself as opposed to waste products such as faeces. In uninjured fish, odourants can originate from a number of sources, from specific chemicals released to mediate social behaviours, i.e. pheromones, to others that are released passively as a by-product of non-related physiological processes [29]. Small variations in the composition of these odourants could affect how distinguishable a fish is from its background [30]. As an example, both fish and scleractinian corals produce external mucus that is constantly sloughed off into the water column [31,32]. The mucus contains a diverse suite of amino acids [33,34], some of which are used by fish to detect and orientate towards specific prey [35]. There is evidence that the amino acid composition of fish mucus can closely match that found in the diet [36], suggesting crypsis-relevant links between diet and a fish's biochemical signature could exist. Decapod crustaceans and teleost fishes have evolved comparable chemosensory systems, using similar metabolites to gather information about their environment [30]. This suggests that the chemical signals used by crabs to distinguish between odours would also be those received by predatory fish.
There appears to be a clear selective advantage to sequestering coral chemical signals. In behavioural trials, predatory cod were least active when all filefish odours were presented alongside the coral they had fed on, regardless of coral species, and were more attracted to the odour of filefish that differed from the associated coral. The cognitive process underlying this behaviour was not determined; however, it is highly suggestive of crypsis as the filefish's odour was presented, as would be the case in nature, against an extensive background of coral odour rather than in isolation [37]. In addition, cod were more attracted to filefish odours that did not match the associated coral, suggesting cod perceived a filefish's odour, when presented against a mismatched background, as potential prey. These results indicate that diet-induced chemical crypsis can have a fitness benefit by reducing detectability.
For O. longirostris, chemically resembling a coral would most likely be advantageous during crepuscular and nocturnal periods when individuals maintain a stationary resting position among branching Acropora [22]. Diel patterns of predation on reefs are non-uniform, with crepuscular and nocturnal periods hypothesized to be when predation risk is highest [38]. Many reef piscivores are nocturnally active (i.e. the squirrelfish, snapper and grunts) and these species often have adaptations to heighten sensory acuity under low-light conditions, including an increased reliance on olfactory cues to locate prey [39]. When stationary in among Acropora, O. longirostris is also visually cryptic [22]. This finely tuned combination of visual and chemical camouflage may be an efficient anti-predator strategy if the effectiveness of each mode of camouflage varies depending on the distance and location of a potential predator relative to the fish, or owing to environmental factors present.
Coral reefs are home to a diverse assemblage of coral species, all of which presumably have unique chemical signatures. How closely the diet of O. longirostris needs to match the associated coral habitat to benefit the fish is not known. In this study, a mismatch between coral diet and habitat at the genus level appears to limit any benefit. In the wild, O. longirostris is known to consume a range of Acropora species. It is possible that consuming a mix of Acropora corals would mask a fish's odour sufficiently for a general, if not optimized, crypsis to occur against a range of Acropora backgrounds [40]. Whether fish consciously select prey to alter their chemical signature, or if this is simply a fortunate by-product of their specialized diet, is not clear. If fish do play an active role, this may explain why a large proportion of the diet is often composed of branching species of limited nutritional value [21]. Likewise, the results suggest Pocillopora is a nutritionally valuable prey for O. longirostris, but these corals are rarely consumed or used as habitat in the wild [21,41]. Some nutritionally rich, non-branching species of Acropora appear essential for reproduction [21]. Adult O. longirostris are presumably less vulnerable to predation than juveniles, and may need to trade-off maximizing any diet-induced anti-predator defence with the high energetic requirements of reproduction.
These results suggest that similar mechanisms of sequestration could have evolved wherever there is a close coupling between an organism's food and habitat. On coral reefs, a diverse range of fishes feed on scleractinian corals [20]. While many species are highly mobile as adults, they are often very site-attached as juveniles. For example, following settlement to the reef, corallivorous butterflyfish juveniles (Chaetodontidae) often use a single coral colony exclusively for shelter and food [42]. Scleractinian corals are also home to a diverse assemblage of small corallivorous invertebrates [24], which may incorporate cnidarian-specific elements into their tissue via digestion [43–45]. There is therefore potential for a wide variety of corallivorous organisms to be, either actively or passively, incorporating this mechanism into their anti-predator defences. In addition, these findings show diet-induced crypsis can occur in vertebrates, and without the need for the signal-receiver to come into direct contact with the cryptic individual for a benefit to occur, increasing the likelihood of similar mechanisms occurring in a range of aquatic, and potentially terrestrial, systems.
In conclusion, this study adds a new component to the already complex array of predator avoidance mechanisms observed on coral reefs. Given that visual camouflage is often a central component of predator–prey interactions, it seems logical that organisms existing in multi-sensory environments would also employ non-visual mechanisms to conceal themselves. This study showed that, via digestion, a corallivorous fish was able to chemically resemble its coral habitat, which reduced its detectability to predators. This is, we believe, the first evidence that diet-induced chemical crypsis as a mechanism can meaningfully be applied to vertebrates, and provides further evidence that non-visual camouflage plays an important role in predator–prey interactions in a variety of ecosystems.
Supplementary Material
Supplementary Material
Acknowledgements
Thanks to Lizard Island Research Station, M. Cowlin and J. Stella for logistical support and assistance.
Ethics statement
This work corresponds to Australian laws and regulations under GBRMPA permit G13.36166.1 and animal ethics permit A1920.
Data accessibility
Data are available from the Dryad digital repository (doi:10.5061/dryad.911p1).
Funding statement
Financial support was provided by the ARC Centre of Excellence for Coral Reef Studies and the Ecological Society of Australia.
References
- 1.Stevens M, Merilaita S. 2009. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B 364, 423–427. ( 10.1098/rstb.2008.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruxton GD. 2009. Non-visual crypsis: a review of the empirical evidence for camouflage to senses other than vision. Phil. Trans. R. Soc. B 364, 549–557. ( 10.1098/rstb.2008.0228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shichida Y, Yamashita T, Imai H, Kishida T. 2013. Evolution and senses. Berlin, Germany: Springer. [Google Scholar]
- 4.Conover MR. 2007. Predator-prey dynamics, the role of olfaction. New York, NY: CRC Press. [Google Scholar]
- 5.Ferrari MCO, Wisenden BD, Chivers DP. 2010. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. 88, 698–724. ( 10.1139/Z10-029) [DOI] [Google Scholar]
- 6.Lambardi D, Dani F, Turillazzi S, Boomsma J. 2007. Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav. Ecol. Sociobiol. 61, 843–851. ( 10.1007/s00265-006-0313-y) [DOI] [Google Scholar]
- 7.Resetarits WJ, Binckley CA. 2013. Is the pirate really a ghost? Evidence for generalized chemical camouflage in an aquatic predator, pirate perch Aphredoderus sayanus. Am. Nat. 181, 690–699. ( 10.1086/670016) [DOI] [PubMed] [Google Scholar]
- 8.Barimo JF, Walsh PJ. 2006. Use of urea as a chemosensory cloaking molecule by a bony fish. J. Exp. Biol. 209, 4254–4261. ( 10.1242/jeb.02533) [DOI] [PubMed] [Google Scholar]
- 9.Raffa K, Hobson K, LaFontaine S, Aukema B. 2007. Can chemical communication be cryptic? Adaptations by herbivores to natural enemies exploiting prey semiochemistry. Oecologia 153, 1009–1019. ( 10.1007/s00442-007-0786-z) [DOI] [PubMed] [Google Scholar]
- 10.Piskorski R, Trematerra P, Dorn S. 2010. Cuticular hydrocarbon profiles of codling moth larvae, Cydia pomonella (Lepidoptera: Tortricidae), reflect those of their host plant species. Biol. J. Linn. Soc. 101, 376–384. ( 10.1111/j.1095-8312.2010.01511.x) [DOI] [Google Scholar]
- 11.Venzon M, Janssen A, Pallini A, Sabelis MW. 2000. Diet of a polyphagous arthropod predator affects refuge seeking of its thrips prey. Anim. Behav. 60, 369–375. ( 10.1006/anbe.2000.1483) [DOI] [PubMed] [Google Scholar]
- 12.Rosell F, Holtan LB, Thorsen JG, Heggenes J. 2013. Predator-naïve brown trout (Salmo trutta) show antipredator behaviours to scent from an introduced piscivorous mammalian predator fed conspecifics. Ethology 119, 303–308. ( 10.1111/eth.12065) [DOI] [Google Scholar]
- 13.Akino T, Nakamura KI, Wakamura S. 2004. Diet-induced chemical phytomimesis by twig-like caterpillars of Biston robustum Butler (Lepidoptera: Geometridae). Chemoecology 14, 165–174. ( 10.1007/s00049-004-0274-4) [DOI] [Google Scholar]
- 14.Fishlyn DA, Phillips DW. 1980. Chemical camouflaging and behavioral defenses against a predatory seastar by three species of gastropods from the surfgrass Phyllospadix community. Biol. Bull. 158, 34–48. ( 10.2307/1540756) [DOI] [Google Scholar]
- 15.Portugal AHA, Trigo JR. 2005. Similarity of cuticular lipids between a caterpillar and its host plant: a way to make prey undetectable for predatory ants? J. Chem. Ecol. 31, 2551–2561. ( 10.1007/s10886-005-7613-y) [DOI] [PubMed] [Google Scholar]
- 16.Marshall J, Johnsen S. 2011. Camouflage in marine fish. In Animal camouflage, mechanisms and function. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Munday PL, Eyre PJ, Jones GP. 2003. Ecological mechanisms for coexistence of colour polymorphism in a coral-reef fish: an experimental evaluation. Oecologia 137, 519–526. ( 10.1007/s00442-003-1356-7) [DOI] [PubMed] [Google Scholar]
- 18.Marshall NJ. 2000. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. Lond. B 355, 1243–1248. ( 10.1098/rstb.2000.0676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixson DL, Pratchett MS, Munday PL. 2012. Reef fishes innately distinguish predators based on olfactory cues associated with recent prey items rather than individual species. Anim. Behav. 84, 45–51. ( 10.1016/j.anbehav.2012.04.001) [DOI] [Google Scholar]
- 20.Cole AJ, Pratchett MS, Jones GP. 2008. Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fisheries 9, 286–307. ( 10.1111/j.1467-2979.2008.00290.x) [DOI] [Google Scholar]
- 21.Brooker RM, Jones GP, Munday PL. 2013. Prey selectivity affects reproductive success of a corallivorous reef fish. Oecologia 172, 409–416. ( 10.1007/s00442-012-2521-7) [DOI] [PubMed] [Google Scholar]
- 22.Brooker RM, Munday PL, Jones GP. 2011. Coral obligate filefish masquerades as branching coral. Coral Reefs 30, 803 ( 10.1007/s00338-011-0779-6) [DOI] [Google Scholar]
- 23.Knudsen JW. 1967. Trapezia and Tetralia (Decapoda, Brachyura, Xanthidae) as obligate ectoparasites of pocilloporid and acroporid corals. Pac. Sci. 21, 51–57. [Google Scholar]
- 24.Stella JS, Pratchett MS, Hutchings PA, Jones GP. 2011. Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr. Mar. Biol. 49, 43–104. [Google Scholar]
- 25.Agresti A. 2013. Categorical data analysis. New York, NY: Wiley. [Google Scholar]
- 26.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team. 2014. nlme: linear and nonlinear mixed effects models. R package version 3.1-115. (http://CRAN.R-project.org/package=nlme)
- 27.Warton DI, Hui FKC. 2010. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92, 3–10. ( 10.1890/10-0340.1) [DOI] [PubMed] [Google Scholar]
- 28.Dunn OJ. 1964. Multiple comparisons using rank sums. Technometrics 6, 241–252. ( 10.1080/00401706.1964.10490181) [DOI] [Google Scholar]
- 29.Sorensen PW, Stacey NE. 2004. Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes. N. Z. J. Mar. Freshw. Res. 38, 399–417. ( 10.1080/00288330.2004.9517248) [DOI] [Google Scholar]
- 30.Derby C, Sorensen P. 2008. Neural processing, perception, and behavioral responses to natural chemical stimuli by fish and crustaceans. J. Chem. Ecol. 34, 898–914. ( 10.1007/s10886-008-9489-0) [DOI] [PubMed] [Google Scholar]
- 31.Shephard K. 1994. Functions for fish mucus. Rev. Fish. Biol. Fish. 4, 401–429. ( 10.1007/BF00042888) [DOI] [Google Scholar]
- 32.Brown BE, Bythell JC. 2005. Perspectives on mucus secretion in reef corals. Mar. Ecol. Prog. Ser. 296, 291–309. ( 10.3354/meps296291) [DOI] [Google Scholar]
- 33.Ducklow H, Mitchell R. 1979. Composition of mucus released by coral reef coelenterates. Limnol. Oceanogr. 24, 706–714. ( 10.4319/lo.1979.24.4.0706) [DOI] [Google Scholar]
- 34.Chong K, Sock Ying T, Foo J, Toong Jin L, Chong A. 2005. Characterisation of proteins in epidermal mucus of discus fish (Symphysodon spp.) during parental phase. Aquaculture 249, 469–476. ( 10.1016/j.aquaculture.2005.02.045) [DOI] [Google Scholar]
- 35.Mitchel W. 2006. Chemoreception. In The physiology of fishes (eds Evans D, Claiborne J.), pp. 471–498. New York, NY: CRC Press. [Google Scholar]
- 36.Saglio P, Fauconneau B. 1985. Free amino acid content in the skin mucus of goldfish, Carassius auratus L.: influence of feeding. Comp. Biochem. Physiol. A 82, 67–70. ( 10.1016/0300-9629(85)90705-4) [DOI] [PubMed] [Google Scholar]
- 37.Skelhorn J, Rowland HM, Speed MP, Ruxton GD. 2010. Masquerade: camouflage without crypsis. Science 327, 51 ( 10.1126/science.1181931) [DOI] [PubMed] [Google Scholar]
- 38.Danilowicz BS, Sale PF. 1999. Relative intensity of predation on the French grunt, Haemulon flavolineatum, during diurnal, dusk, and nocturnal periods on a coral reef. Mar. Biol. 133, 337–343. ( 10.1007/s002270050472) [DOI] [Google Scholar]
- 39.Myrberg AA, Jr, Fuiman LA. 2002. The sensory world of coral reef fishes. In Coral reef fishes (ed. Sale PF.), pp. 123–148. New York, NY: Academic Press. [Google Scholar]
- 40.Merilaita S, Tuomi J, Jormalainen V. 1999. Optimization of cryptic coloration in heterogeneous habitats. Biol. J. Linn. Soc. 167, 151–161. ( 10.1111/j.1095-8312.1999.tb01858.x) [DOI] [Google Scholar]
- 41.Hobbs JPA. 2013. Obligate corallivorous filefish (Oxymonacanthus longirostris) switches diet from Acropora to Pocillopora corals following habitat loss. Mar. Biodivers. 43, 175–176. ( 10.1007/s12526-013-0155-6) [DOI] [Google Scholar]
- 42.Pratchett MS, Berumen ML, Marnane MJ, Eagle JV, Pratchett DJ. 2008. Habitat associations of juvenile versus adult butterflyfishes. Coral Reefs 27, 541–551. ( 10.1007/s00338-008-0357-8) [DOI] [Google Scholar]
- 43.Cronin G, Hay ME, Fenical W, Lindquist N. 1995. Distribution, density, and sequestration of host chemical defenses by the specialist nudibranch Tritonia hamnerorum found at high densities on the sea fan Gorgonia ventalina. Mar. Ecol. Prog. Ser. 119, 177–189. ( 10.3354/meps119177) [DOI] [Google Scholar]
- 44.Burghardt I, Stemmera K, Wägele H. 2008. Symbiosis between Symbiodinium (Dinophyceae) and various taxa of Nudibranchia (Mollusca: Gastropoda), with analyses of long-term retention. Org. Divers. Evol. 8, 66–76. ( 10.1016/j.ode.2007.01.001) [DOI] [Google Scholar]
- 45.Greenwood PG. 2009. Acquisition and use of nematocysts by cnidarian predators. Toxicon 54, 1065–1070. ( 10.1016/j.toxicon.2009.02.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad digital repository (doi:10.5061/dryad.911p1).