Abstract
We have optimized the magnetic alignment of phospholipid bilayered micelles (bicelles) for EPR spectroscopy, by varying lipid composition and temperature. Bicelles have been extensively used in NMR spectroscopy for several decades, in order to obtain aligned samples in a near-native membrane environment and take advantage of the intrinsic sensitivity of magnetic resonance to molecular orientation. Recently, bicelles have also seen increasing use in EPR, which offers superior sensitivity and orientational resolution. However, the low magnetic field strength (less than 1 T) of most conventional EPR spectrometers results in homogeneously oriented bicelles only at a temperature well above physiological. To optimize bicelle composition for magnetic alignment at reduced temperature, we prepared bicelles containing varying ratios of saturated (DMPC) and unsaturated (POPC) phospholipids, using EPR spectra of a spin-labeled fatty acid to assess alignment as a function of lipid composition and temperature. Spectral analysis showed that bicelles containing an equimolar mixture of DMPC and POPC homogeneously align at 298 K, 20 K lower than conventional DMPC-only bicelles. It is now be possible to perform EPR studies of membrane protein structure and dynamics in well-aligned bicelles at physiological temperatures and below.
Keywords: bicelle, EPR, lipid composition, spectral simulation, temperature
INTRODUCTION
For several decades, bicelles have been used in magnetic resonance studies of membrane protein structure and dynamics [1]. A bicelle contains a flat patch of lipid bilayer comprised of long-chain lipids such as 1,2-dimyristoyl-sn-glycero-3-phosphocholine (14:0 PC or DMPC), surrounded by a rim of short-chain lipids such as 1,2-dihexanoyl-sn-glycero-3-phosphocholine (6:0 PC or DHexPC) protecting the hydrophobic core of the bilayer. Due to their negative magnetic susceptibility, bicelles placed in a sufficiently strong magnetic field will align with the membrane symmetry axis (membrane normal) perpendicular to the applied magnetic field [2]. This magnetic susceptibility can be altered by addition of paramagnetic lanthanides, which bind to lipid headgroups within bicelles, allowing for both parallel and perpendicular alignment [3]. In a well-prepared sample containing many bicelles, the alignment homogeneity is excellent, providing a more physiological alternative to other anisotropic membrane systems such as partially dehydrated lipid bilayers supported on glass plates [4] or lipid-containing crystals [5]. Since transmembrane proteins can be uniformly oriented in an aligned bicelle sample, techniques with sensitivity to orientation, such as nuclear magnetic resonance (NMR) and electron paramagnetic resonance (EPR), can be used to measure probe orientation with high accuracy relative to the applied magnetic field [6-8], thus providing crucial information about protein structure and dynamics relative to the membrane.
Magnetically aligned bicelles have been most widely used in NMR, but bicelles applications in EPR using nitroxide spin labels have grown in recent years [9]. Due to the large magnetic moment of the electron spin, EPR offers much greater sensitivity than NMR, making possible much briefer experiments on much smaller samples, at more physiological lipid/protein ratios [10]. However, the substantially lower magnetic field strength (typically ~ 0.35 T of an X-band EPR spectrometer vs 16 T for 700 MHz NMR spectrometer) requires that bicelle composition must be modified to produce adequate alignment homogeneity. In particular, the addition of paramagnetic lanthanides is essential in achieving a suitable bicelle magnetic susceptibility for alignment in EPR experiments [11]. Cholesterol has also been shown to stabilize bicelle rigidity and reduce alignment temperature both for NMR and EPR experiments, reducing extraneous spin label dynamics and improving orientation precision [12, 13].
Homogeneous bicelle alignment is significantly influenced by lipid phase transitions, which are especially important in the case of the comparatively weak EPR magnetic field. At lower temperatures (~270 K), bicelles exist in an isotropic phase that is not readily aligned magnetically [14]. At higher temperatures (~290 K), bicelles transition into a nematic crystal phase, where they may be partially aligned, but are characterized by flexible lipid interactions that result in overall disorder [14]. At still higher temperatures (ca 320 K), bicelles transition into a smectic crystal phase, with rigid lipid interactions [15]. Bicelles in this smectic crystal phase can be identified by a substantial increase in sample viscosity and opacity, compared with the nematic and isotropic phases [16]. The typical procedure for magnetic alignment of bicelles for EPR involves increasing temperature to drive the bicelle mixture from the isotropic phase to the smectic phase in the presence of a strong magnetic field (~ 1 T, the maximum field available on X-band EPR spectrometers). It has been shown that this alignment remains stable for hours after decreasing the field to 0.35 T for X-band EPR measurements [15]. The need for high temperatures to achieve the smectic phase presents a problem for studies of many proteins, as increased dynamics and thermal degradation complicate orientation measurements [17]. A possible solution is suggested by NMR studies in which the unsaturated phospholipid 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (16:0-18:1 PC or POPC) has been shown to improve bicelle alignment at temperatures down to 300 K in the absence of cholesterol, and down to 290 K in the presence of cholesterol [4, 13].
METHODS
In the present study, we optimize bicelle lipid composition with DMPC and POPC, in order to determine the minimum temperature for homogeneous alignment in X-band EPR experiments. Bicelles were prepared based on methods previously described [18]. Briefly, 5-DOXYL-stearic acid (5-DSA, Sigma Aldrich, St. Louis, MO) was combined with lipids (DMPC, POPC, DHexPC, Avanti Polar Lipids, Alabaster, AL), and cholesterol (Sigma Aldrich), all dissolved in chloroform. This produced bicelles with 700 long-chain lipids per 5-DSA [10], 13% cholesterol (mol cholesterol / mol long-chain lipid) [12, 18], and q (molar ratio of long- to short-chain lipids [4]) = 4. Organic solvent was evaporated under N2 gas and then completely removed by storage in a vacuum desiccator overnight. 100 mM HEPES buffer (pH 7.0) was added up to 25% (lipid mass / buffer volume), then the sample was vortexed for 2 min and placed on ice for 5 min. After repeating this process twice, samples were sonicated in an ice bath for 30 min [18]. Prepared lanthanide stock (TmCl3*6H2O (Sigma Aldrich, St. Louis, MO) in 100 mM HEPES) was added to the sample up to 20% (mol lanthanide / mol long-chain lipid), then vortexed for 2 min [18]. Samples were then flash-frozen in liquid N2 three times to further homogenize, yielding a clear, viscous fluid. 16 μL of the sample was pipetted into a 0.6/0.84 mm ID/OD glass capillary (Vitrocom, Mountain Lakes, NJ), in order to span the active portion of the EPR resonator. The capillary was then plugged with Critoseal (Krackeler Scientific, Albany, NY). Randomly oriented samples (vesicles) were prepared identically to bicelles, with the omission of the short-chain lipid DHexPC and lanthanide.
EPR experiments were performed on a Bruker EleXsys E500 spectrometer equipped with an ER 4122 SHQ spherical resonator. Sample temperature was maintained at ± 0.2 K using a quartz dewar attached to a nitrogen gas temperature controller. Samples were first placed in the resonator and equilibrated at 273 K for 5 min. To align bicelles, the field strength was raised to 1.1 T [15], then the temperature was raised 2 K per minute up to the final (alignment) temperature, at which point the sample was incubated for 20 min at 1.1 T field strength. The EPR spectrum was then immediately acquired at X-band field strength (~3400 G) at the desired alignment temperature. For randomly oriented vesicle samples, spectra were acquired immediately after the initial 5 minute equilibration at the desired temperature. Spectra were acquired with the following parameters: 2 mW microwave power, 1 G peak-to-peak modulation amplitude, 120 G sweep width, and 20.48 ms time constant and conversion time.
EPR spectra were background-corrected and normalized to the double integral [19]. Least-squares minimization fitting was performed in MultiComponent, an EPR simulation program that is optimized for fitting spectra to models involving multiple populations, using a model of microscopic order, macroscopic disorder (MOMD) [20]. Starting values for the DOXYL g-factor and hyperfine tensors were obtained from a previous study by Gaffney and McConnell [21]. Specifically, g = [2.0088, 2.0061, 2.0027] and A = [8.46, 7.83, 45.06]. These values were further refined with the spectrum of a 5-DSA vesicle sample acquired at 200 K (see Supplemental Information for fitting details). The electron g tensor was assumed not to vary significantly between different samples, while the hyperfine tensor was allowed to vary to account for changes in environment polarity [22]. The model for vesicle samples assumed a single, randomly oriented component with isotropic rotational diffusion and Lorentzian linewidth tensors, as well as an orienting potential determined by c20. Oriented bicelle spectra were fit to a two-component model: randomly oriented and aligned. The randomly oriented component was assumed to be the same as that of the corresponding vesicle sample. For the aligned component, all parameters were fixed to those of the random component, with the exceptions that NORT (the number of orientations calculated) was reduced from 20 to 1, and the orienting potential coefficients c20, c22, and c40 were allowed to vary. Rigorous fitting revealed that constraining the dynamics (τR) and magnetic parameters (g, A) for the aligned component to those from the randomly oriented component requires additional orienting coefficients, presumably to account for subtle orientation disorder in the aligned component. Alignment homogeneity was characterized by the mole fraction of the aligned component relative to the randomly oriented component (“fraction aligned” below). Aligned bicelle spectra were also fit to a single aligned component, allowing disorder in the orienting potential, but these fits gave much higher χ2 values than the two-component model described above. Standard error of the mean (SEM) for fit parameters was determined by least-squares minimization fitting in NLSL (Freed et al) [23, 24]. All spectra are centered at 3356 G with a baseline of 120 G, and are to scale, as normalized by the double integral.
RESULTS
We began by verifying the bicelle alignment methodology established previously [18], preparing conventional DMPC bicelles containing 5-DSA, and using thulium to align bicelles parallel to the magnetic field up to 318 K and 298 K. Figure 1 shows EPR spectra of 5-DSA in randomly oriented vesicles (top) and parallel aligned bicelles (bottom). There is a substantial change in lineshape upon alignment of bicelles at 318 K, reflecting a random orientation distribution of the 5-DSA spin label. These spectra are consistent with previous studies of 5-DSA in DMPC bicelles [25]. When this experiment was repeated using an alignment temperature of 298 K (Figure 1, right), there was no significant spectral change between the vesicle and bicelle samples, indicating that DMPC bicelles are not uniformly aligned at 298 K.
Figure 1.
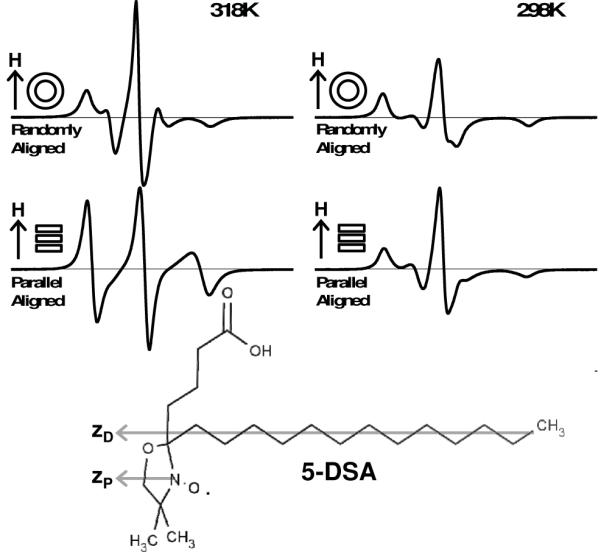
EPR spectra of 5-DSA in conventional DMPC vesicles (top) and bicelles with membrane normal oriented parallel to the instrument magnetic field (bottom) aligned at 318 K (left) and 298 K (right). All samples contain 13% cholesterol (mol cholesterol / mol long-chain lipid). Structural formula for 5-DSA (bottom) indicating that probe principal magnetic axis (zP) is parallel with probe principal diffusion axis (zD).
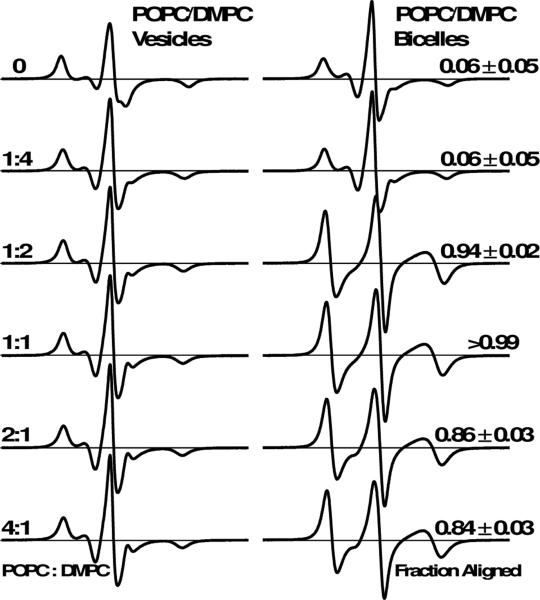
Next we optimized the POPC:DMPC molar ratio. We aligned bicelle samples at 298 K, consistent with previous NMR studies showing that POPC improves the alignment of DOPC bicelles [4, 13, 26]. Figure 2 shows EPR spectra of 5-DSA in vesicles and bicelles at 298 K as a function of the POPC:DMPC molar ratio. Both the control (without POPC) and 1:4 POPC:DMPC bicelle samples were poorly aligned. These samples possess similar spectra for vesicles and bicelles, which also show low alignment fractions. Upon increasing the POPC:DMPC molar ratio to 1:2, there was a substantial change in the EPR lineshape, indicating improved alignment homogeneity. The aligned component for this sample accounted for most of the spectrum (fraction aligned = 0.94). At 1:1 POPC:DMPC, the bicelle sample was homogeneously aligned, with a fraction aligned greater than 0.99. This spectrum is well-fit with only the aligned component. For higher POPC:DMPC molar ratios, the fraction aligned was slightly degraded. Thus Figure 2 indicates that a 1:1 molar ratio of POPC:DMPC is the optimum value for aligned bicelle EPR.
Figure 2.
EPR spectra of 5-DSA in POPC/DMPC vesicles (left) and bicelles (right) as a function of POPC:DMPC molar ratio (left text). All samples were aligned at 298 K, and all spectra were acquired at 298 K. All samples contain 13% cholesterol (mol cholesterol / mol long-chain lipid). The relative amount of 5-DSA in uniformly oriented bicelles is indicated as fraction aligned (right text).
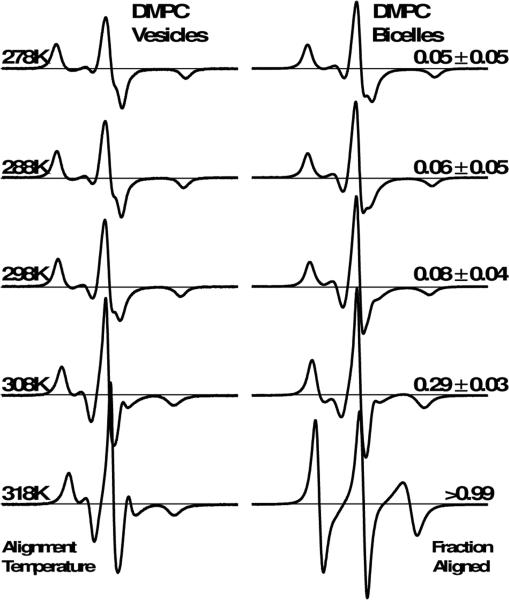
Next we characterized the alignment homogeneity of conventional DMPC bicelles as a function of temperature. Figure 3 shows the EPR spectra of 5-DSA in DMPC-only vesicles and conventional bicelles, using alignment temperatures between 278 K and 318 K. Aside from small changes in splitting and linewidth due to thermally excited rotational dynamics, the bicelle spectra from 278 K to 298 K are nearly identical, with aligned fractions below 0.1. At 308 K, the bicelle spectrum changes slightly, particularly in the region immediately flanking the central resonance, and the fraction aligned increases to 0.29. In contrast, the 318 K spectrum possesses a strikingly different lineshape, and is well-fit by only the aligned component. Therefore, a temperature of 318 K is required for homogeneous alignment of conventional DMPC bicelles. This result is consistent with previous EPR experiments using this conventional bicelle method, achieving complete alignment only at 318 K or higher [11, 12, 15].
Figure 3.
EPR spectra for 5-DSA in DMPC-only vesicles (left) and bicelles (right) as a function of alignment temperature (left text). All samples contain 13% cholesterol (mol cholesterol / mol long-chain lipid). The relative amount of 5-DSA in uniformly oriented bicelles is indicated as fraction aligned (right text).
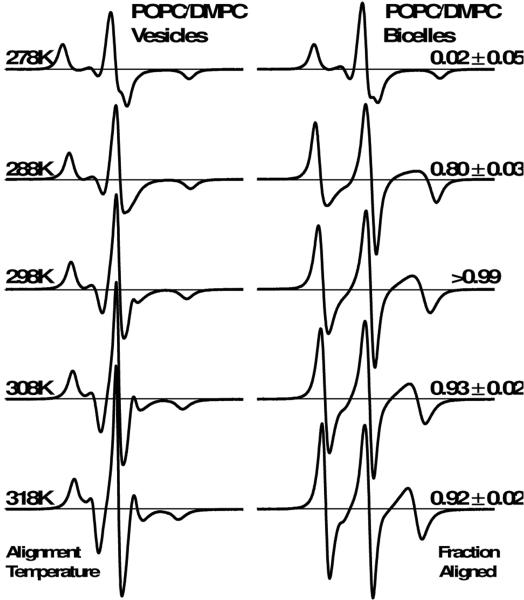
Figure 4 shows the EPR spectra of 5-DSA in vesicles and bicelles with 1:1 POPC:DMPC, using alignment temperatures between 278 K and 318 K. Although the 278 K spectra indicate poor alignment, substantial alignment is observed beginning at 288 K (fraction aligned = 0.80). At 298 K, the fraction aligned is greater that 0.99, indicative of homogeneous alignment. When the temperature was increased further, to 308 K and 318 K, the fraction aligned decreases slightly. Importantly, the alignment homogeneity of these POPC/DMPC bicelles at 298 K is comparable to that of conventional DMPC-only bicelles at 318 K, as both spectra are well-fit by a single oriented spectral component (fraction aligned ~ 1).
Figure 4.
EPR spectra for 5-DSA in 1:1 POPC:DMPC vesicles (left) and bicelles (right) as a function of alignment temperature (left text). All samples contain 13% cholesterol (mol cholesterol / mol long-chain lipid). The relative amount of 5-DSA in uniformly oriented bicelles is indicated as fraction aligned (right text).
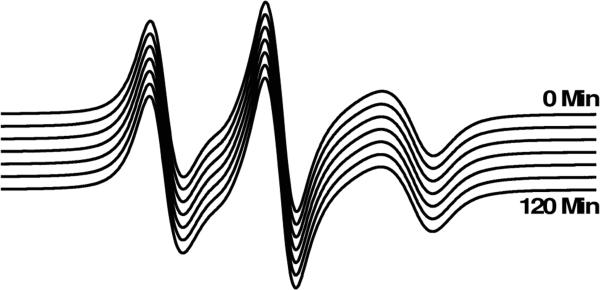
Figure 5 shows the EPR spectra of 5-DSA in bicelles with 1:1 POPC:DMPC, aligned at 298 K, as a function of time after the alignment procedure is complete and the magnetic field has been reduced to X-band strength for spectral acquisition. The EPR spectrum indicates homogenous bicelle alignment, and is stable over a period of at least 2 hours at 298 K and X-band magnetic field strength. Therefore, high magnetic field strength is not required to maintain homogeneous bicelle alignment after it has been produced. Rather, sufficiently high temperature for bicelle smectic phase is required to maintain this alignment.
Figure 5.
EPR spectra of 5-DSA in 1:1 POPC/DMPC bicelles with 13% cholesterol (mol cholesterol / mol long-chain lipid) as a function of time (20 min intervals between adjacent spectra) after alignment and reduction of magnetic field strength from 11000 G to 3356 G for spectral acquisition. All spectra were acquired at 298 K.
CONCLUSIONS
Through optimization of lipid composition and alignment temperature, we found that bicelles containing a 1:1 mixture of POPC and DMPC homogeneously align at 298 K, which is 20 K less than the alignment temperature required for conventional DMPC-only bicelles. This decrease in alignment temperature is probably due to POPC reducing the lipid phase transition temperature (TM), since TM for pure POPC is 26 K lower than that for pure DMPC. The EPR spectra of randomly oriented vesicles are quite similar, indicating similar dynamics and magnetic parameters, indicating that the lipid environments are also similar. The POPC/DMPC bicelles described in this work should be excellent reconstitution systems for orientation studies of proteins with structural dynamics sensitive to temperature, such as the regulatory proteins sarcolipin and phospholamban, as well as the sarcoplasmic reticulum Ca-ATPase SERCA. However, variations in lipid and bicelle phase transitions may occur due to specific protein-lipid interactions, so additional methodology refinement in each case may be necessary.
Supplementary Material
HIGHLIGHTS.
Conventional DMPC-only lipid bicelles align at 318 K for EPR spectroscopy.
Addition of 1:1 POPC:DMPC to bicelle reduces alignment temperature to 298 K.
POPC:DMPC bicelles align homogeneously just as DMPC-only bicelles do.
Spectral simulation was used to quantify aligned and unaligned populations.
Alignment was measured using 5-DOXYL stearic acid lipid spin label.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants GM27906 and AR057220 to DDT. JEM was supported by NIH Training Grant AR007612, and ZMJ was supported by NIH Training Grants AR007612 and GM008700. EPR experiments were performed at the Biophysical Spectroscopy Center, and computational resources were provided by the Minnesota Supercomputing Institute. We thank Edmund Howard and Yuri Nesmelov for EPR simulation assistance, Kaustubh Mote for helpful bicelle preparation discussions, and Octavian Cornea for assistance in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPORTING INFORMATION
Additional EPR spectra and fitting parameters can be found in the SI. This material is available free of charge via the Internet at <INSERT_ADDRESS>.
REFERENCES
- 1.Ram P, Prestegard JH. Magnetic field induced ordering of bile salt/phospholipid micelles: new media for NMR structural investigations. Biochimica et biophysica acta. 1988;940(2):289–94. doi: 10.1016/0005-2736(88)90203-9. [DOI] [PubMed] [Google Scholar]
- 2.Cho G, Fung BM, Reddy VB. Phospholipid bicelles with positive anisotropy of the magnetic susceptibility. Journal of the American Chemical Society. 2001;123(7):1537–8. doi: 10.1021/ja005605+. [DOI] [PubMed] [Google Scholar]
- 3.Prosser RS, Volkov VB, Shiyanovskaya IV. Solid-state NMR studies of magnetically aligned phospholipid membranes: taming lanthanides for membrane protein studies. Biochimie et biologie cellulaire. 1998;76(2-3):443–51. doi: 10.1139/bcb-76-2-3-443. [DOI] [PubMed] [Google Scholar]
- 4.De Angelis AA, Opella SJ. Bicelle samples for solid-state NMR of membrane proteins. Nature Protocols. 2007;2(10):2332–8. doi: 10.1038/nprot.2007.329. [DOI] [PubMed] [Google Scholar]
- 5.Landau EM, Rosenbusch JP. Lipidic cubic phases: a novel concept for the crystallization of membrane proteins. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14532–5. doi: 10.1073/pnas.93.25.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi L, et al. Tilt and azimuthal angles of a transmembrane peptide: a comparison between molecular dynamics calculations and solid-state NMR data of sarcolipin in lipid membranes. Biophysical journal. 2009;96(9):3648–62. doi: 10.1016/j.bpj.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Angelis AA, et al. Structure determination of a membrane protein with two trans-membrane helices in aligned phospholipid bicelles by solid-state NMR spectroscopy. Journal of the American Chemical Society. 2006;128(37):12256–67. doi: 10.1021/ja063640w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inbaraj JJ, et al. Determining the topology of integral membrane peptides using EPR spectroscopy. Journal of the American Chemical Society. 2006;128(29):9549–54. doi: 10.1021/ja0622204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber SM, Lorigan GA, Howard KP. Magnetically oriented phospholipid bilayers for spin label EPR studies. Journal of the American Chemical Society. 1999;121(13):3240–3241. doi: 10.1021/ja005574i. [DOI] [PubMed] [Google Scholar]
- 10.James ZM, et al. Protein-protein interactions in calcium transport regulation probed by saturation transfer electron paramagnetic resonance. Biophys J. 2012;103(6):1370–8. doi: 10.1016/j.bpj.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caporini MA, et al. Investigating magnetically aligned phospholipid bilayers with various lanthanide ions for X-band spin-label EPR studies. Biochimica et biophysica acta. 2003;1612(1):52–8. doi: 10.1016/s0005-2736(03)00085-3. [DOI] [PubMed] [Google Scholar]
- 12.Lu JX, Caporini MA, Lorigan GA. The effects of cholesterol on magnetically aligned phospholipid bilayers: a solid-state NMR and EPR spectroscopy study. Journal of magnetic resonance. 2004;168(1):18–30. doi: 10.1016/j.jmr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Cho HS, Dominick JL, Spence MM. Lipid domains in bicelles containing unsaturated lipids and cholesterol. The journal of physical chemistry. B. 2010;114(28):9238–45. doi: 10.1021/jp100276u. [DOI] [PubMed] [Google Scholar]
- 14.Harroun TA, et al. Comprehensive examination of mesophases formed by DMPC and DHPC mixtures. Langmuir : the ACS journal of surfaces and colloids. 2005;21(12):5356–61. doi: 10.1021/la050018t. [DOI] [PubMed] [Google Scholar]
- 15.Cardon TB, Tiburu EK, Lorigan GA. Magnetically aligned phospholipid bilayers in weak magnetic fields: optimization, mechanism, and advantages for X-band EPR studies. Journal of magnetic resonance. 2003;161(1):77–90. doi: 10.1016/s1090-7807(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 16.Nieh MP, et al. Structural phase behavior of high-concentration, alignable biomimetic bicelle mixtures. Macromolecular Symposia. 2004;219:135–145. [Google Scholar]
- 17.Bigelow DJ, Squier TC, Thomas DD. Temperature dependence of rotational dynamics of protein and lipid in sarcoplasmic reticulum membranes. Biochemistry. 1986;25(1):194–202. doi: 10.1021/bi00349a028. [DOI] [PubMed] [Google Scholar]
- 18.Ghimire H, et al. Probing the helical tilt and dynamic properties of membrane-bound phospholamban in magnetically aligned bicelles using electron paramagnetic resonance spectroscopy. Biochim Biophys Acta. 2012;1818(3):645–50. doi: 10.1016/j.bbamem.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin AY, et al. Large-scale opening of utrophin's tandem CH domains upon actin binding, by an induced-fit mechanism. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1106453108. p. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durer ZA, et al. Structural states and dynamics of the D-loop in actin. Biophysical journal. 2012;103(5):930–9. doi: 10.1016/j.bpj.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaffney BJM, H. M. The Paramagnetic Resonance Spectra of Spin Labels in Phospholipid Membranes. Journal of Magnetic Resonance. 1974;16(1):1–28. [Google Scholar]
- 22.Snipes W, et al. Electron spin resonance analysis of the nitroxide spin label 2,2,6,6-tetramethylpipidone-N-oxyl (Tempone) in single crystals of the reduced Tempone matrix. Biophysical journal. 1974;14(1):20–32. doi: 10.1016/s0006-3495(74)85900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider DJF. J. H., Calculating Slow Motional Magnetic Resonance Spectra. In: Berliner, J. LJR, editor. Biological Magnetic Resonance. Vol. 8. Springer US; New York: 1989. [Google Scholar]
- 24.Budil DE, et al. Nonlinear-least-squares analysis of slow-motion EPR spectra in one and two dimensions using a modified Levenberg-Marquardt algorithm. Journal of Magnetic Resonance Series A. 1996;120(2):155–189. [Google Scholar]
- 25.Cardon TB, et al. Magnetically aligned phospholipid bilayers at the parallel and perpendicular orientations for X-band spin-label EPR studies. J Am Chem Soc. 2001;123(12):2913–4. doi: 10.1021/ja005574i. [DOI] [PubMed] [Google Scholar]
- 26.Lau TL, et al. Structure of the integrin beta3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry. 2008;47(13):4008–16. doi: 10.1021/bi800107a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.