ABSTRACT
Little is known about the relative, additive, and interactive effects of different population-based treatments for smoking cessation. The goal of this study was to evaluate the main and interactive effects of five different smoking interventions. Using the multiphase optimization strategy (MOST), 1,034 smokers who entered a Web site for smokers (smokefree.gov) were randomly assigned to the “on” and “off” conditions of five smoking cessation interventions: the National Cancer Institute’s (NCI) Web site (www.smokefree.gov vs a “lite” Web site), telephone quitline counseling (vs none), a smoking cessation brochure (vs a lite brochure), motivational e-mail messages (vs none), and mini-lozenge nicotine replacement therapy (NRT vs none). Analyses showed that the NCI Web site and NRT both increased abstinence; however, the former increased abstinence significantly only when it was not used with the e-mail messaging intervention (messaging decreased Web site use). The other interventions showed little evidence of effectiveness. There was evidence that mailed nicotine mini-lozenges and the NCI Web site (www.smokefree.gov) provide benefit as population-based smoking interventions.
Electronic supplementary material
The online version of this article (doi:10.1007/s13142-014-0278-8) contains supplementary material, which is available to authorized users.
KEYWORDS: Smoking cessation, Web site evaluation, MOST research, Telephone quitline, Nicotine replacement
INTRODUCTION
Smoking remains the leading preventable cause of premature death and disability [1] despite the availability of multiple evidence-based interventions (e.g., [2, 3]). This may be because barriers related to access and cost reduce the use of such interventions [4–6]. About 16–20 million smokers in the USA make a quit attempt each year [7, 8]. Thus, it is important to identify smoking interventions that are both effective and that have potentially broad reach.
Progress has been made in identifying smoking interventions that are both effective and disseminable. For instance, research shows that telephone cessation quitlines are effective, cost-effective, and fairly heavily used [2, 9–11]. Similarly, about 40 % of smokers in the USA have used a smoking cessation medication [12], in part because of over-the-counter (OTC) availability. However, while considerable research attests to the efficacy of cessation medications [2, 13], questions have been raised as to their effectiveness in real world use (e.g., via OTC access [14, 15]). Likewise, other interventions have great potential for broad dissemination, but their effectiveness is not well established. For instance, while Web-based resources have great potential reach [16], evidence is mixed as to their effectiveness [17]. Similarly, self-help brochures and brief text messaging interventions have broad potential reach but there is either little evidence regarding their effectiveness (messaging [18, 19]) or the extant evidence is fairly negative (self-help brochures [2]).
The current research explores the effectiveness of five smoking cessation interventions that have potential for broad dissemination, but for which important information is lacking: viz. (1) their benefits in a real-world effectiveness research context (cessation medication, messaging, Web-based interventions), (2) their relative effectiveness (e.g., there are few comparative data on quitline counseling vs Web-based intervention; see [20], and (3) their effects in combination with one another.
The current experiment addressed the above information needs through the use of a screening experiment as suggested by the multiphase optimization strategy (MOST; [21, 22]). MOST is a methodologically principled approach to evaluating interventions (the term “intervention” in this context is used to denote a relatively specific treatment element or component [21]) so that, ultimately, the most efficacious ones can be incorporated into an “optimized” multicomponent treatment or treatment package [21]. The approach involves initial screening of multiple interventions in factorial experiments so that their relative, additive, and interactive effects can be determined. This information is then combined with other relevant evidence (e.g., perhaps from additional parametric studies that explore intervention intensity) in order to identify interventions that should work well together and so be combined in an optimized treatment package. MOST then calls for such an optimized treatment package to be further improved by ongoing programmatic research. The current research is an initial screening experiment of five “active” smoking interventions, some of which could ultimately be combined into a population-based smoking treatment package.
Each of the intervention factors had an “on” level, indicating the delivery of the active intervention, and an “off” level, indicating the delivery of a control intervention or no intervention (Table 1). The following interventions were evaluated: (1) a Web site intervention, smokefree.gov, sponsored by the National Cancer Institute (NCI) which had about 1,555,500 estimated visitors in 2012 (via Webtrends data), a year during which the study was ongoing; (2) quitline counseling, a five-call service provided by the Cancer Information Service (CIS); (3) a brochure, the NCI’s 36-page clearing the air brochure [23]; (4) messaging, the delivery of brief e-mail messages that participants could receive on either computer or mobile platforms and that provided motivational content, basic information about quitting, and quitting tips; and (5) cessation medication, a mailed, free 2-week “starter kit” of nicotine mini-lozenges.
Table 1.
Intervention levels of the five population-based smoking cessation interventions, yielding 32 distinct experimental conditions (see Electronic supplementary material)
| Intervention status | Web site | CIS quitline counseling | Messaging | Brochures | NRT |
|---|---|---|---|---|---|
| On | Smokefree | Yes | Yes | Full | Yes |
| Off | Smokefree lite | No | No | Brief | No |
The active interventions all comprised evidence-based contents as defined in relevant research [2, 3, 13] and therefore overlapped in nature (e.g., involving motivation and skill training components) but differed meaningfully in mode of delivery and intensity.
The control conditions differed across the different factors. Three of the control conditions, the off conditions, involved no comparison or control intervention (i.e., for the quitline, the messaging intervention, and the nicotine mini-lozenge). However, the Web site and brochure control conditions were brief versions of each intervention (e.g., the “brief” brochure and the “lite” Web site) that were designed to have nearly identical design features and basic information as their respective on or active interventions but provided no putative active intervention components (i.e., no skill training to identify, avoid, and cope with smoking triggers [2]). The use of lite conditions ensured that every individual in the experiment received at least a minimal intervention. Also, for the targeted smokefree.gov Web site evaluation, we wanted to assess not just whether Web site access per se produced benefit, but whether the identified active ingredients of the smokefree.gov Web site produced added benefit. Thus, comparisons with lite components were somewhat conservative in that they tested for effects over and above those of a reduced intervention.
Our primary aim in this research was to assess the effects of two NCI resources, the smokefree.gov Web site, and the CIS quitline, on abstinence outcomes when evaluated in conditions approximating real-world use. The inclusion of other interventions (e.g., the messaging condition and NRT) was intended to allow us to determine how effective the primary interventions were relative to other interventions, and whether any of these other interventions would produce interactive effects with regard to the Web site or quitline. Thus, this research allowed us to determine the following: (1) which of the active interventions significantly increased abstinence rates and the relative size of these effects and (2) whether any of the interventions interacted with one another, e.g., whether participants receiving both active quitline counseling and smokefree.gov attained abstinence rates that exceeded levels that could be explained by their individual (i.e., “main”) effects. The information yielded by this research would therefore suggest which interventions should and should not be used together, whether in the context of an optimized treatment package or more generally.
METHODS
Recruitment
Potential participants were individuals who spontaneously accessed the smokefree.gov Web site portal. Inclusion criteria were the following: age >17 years, daily smoking ≥5 cpd, interest in quitting smoking within the next 30 days but not actively engaged in quitting, having a phone and home Internet access (including an e-mail address), no prior use of the smokefree.gov Web site, suitability for NRT (e.g., no allergies to NRT, not pregnant [2]), willingness to perform study procedures and have use of the assigned smokefree.gov Web site tracked.
Recruitment in the study involved a five-step process: (1) the person clicked either a static button (or pop-up, see below) on the smokefree.gov home page inviting those interested in quitting to learn more about a research study; (2) completion of a series of demographic and eligibility screening questions; (3) if screened successfully, individuals reviewed consent information and then clicked a button to indicate their understanding of the requirements and their willingness to participate; (4) completion of a baseline questionnaire; and (5) these potential participants were then asked to make a “confirmation” call to an automated answering system and confirm their willingness to perform study procedures. The confirmation call was intended to increase the likelihood that individuals would take treatment and follow-up calls and to reduce the likelihood that individuals would sign up for the study multiple times to obtain research payments. Randomization occurred immediately after the confirmation call, and participants completing this step were sent an automated e-mail welcoming them to the study and outlining services they would receive (based on their randomization). The study invitation was displayed through either a button on the computer screen or via a pop-up (see Electronic supplementary material on recruitment).
Interventions
The five factors each comprised two intervention conditions or levels (see Table 1), and participants were randomly assigned to one of these two levels. Thus, half of the participants were randomly assigned to one level of each factor (e.g., active mini-lozenge), and the other half were assigned to the other level of each factor (e.g., no mini-lozenge, see Table 1). This randomization scheme across the 2 levels of 5 factors results in 32 different combinations of intervention components, with 1/32 of the participants being assigned to receive all of the on or active levels and 1/32 of the participants being assigned to receive all of the control (lite or off levels), with the majority of the participants receiving a mix of active and control conditions or levels (Table 1; also, see Supplementary Table 1).
Web site
Each type of Web site (the active or lite Web sites) was provided in both a standard version and one that was tailored for women. Users had their choice of accessing either the standard or women’s version of each type of Web site (the former via smokefree.gov, the latter through women.smokefree.gov). The active versions represented the existing Web site content at the time of the study. “Cookies” were placed on participants’ Web browsers upon study entry, allowing us to automatically connect participants to the smokefree Web site to which they had been randomly assigned (active or lite) when they subsequently tried to enter the Web site.
Smokefree.gov
Participants in the on condition for this component received the standard smokefree.gov Web site content that included resources to motivate quitting and a step-by-step quitting guide that provided a skill-based intervention for preparing to quit, quitting, and maintaining abstinence. In addition, the active Web site offered encouragement and support, motivational information, and interactive features and referral links. However, the active Web site did not include direct interaction with users (e.g., live help) or interactive audio or video content. User engagement features included task charts for behavior change, self-monitoring tools (e.g., for cravings and self-assessment), creation of a personal calendar, links to a quitline or to a counselor via text message for live help, and social support through social media [24]. No tailoring, feedback, or outbound reminders were in use (note: since the completion of this research, additional services were added, e.g., a downloadable app and a text messaging service).
Smokefree.gov lite
Participants in this condition received the lite version of the Web site (developed by the investigators for this research) that included information about smoking and health such as benefits of quitting and information about withdrawal, medications, and stress but contained no interactive features or content that would support skill training. The look and graphics directly mirrored the full smokefree.gov Web site, but the number of Web pages was reduced from over 50 to 16, and external links to resources were virtually eliminated.
Quitline telephone counseling
Participants in the on condition received telephone cessation quitline counseling. These proactive calls initiated by CIS included an initial call (30 min), targeted to occur within 3 days of enrollment, followed by four additional counseling calls (up to 15 min each) that were scheduled to occur on the quit day or the day after, and then weekly for the next 3 weeks. The content of the counseling calls was the same as used in the CIS quitline service, focusing initially on motivating quitting and then setting a quit date, providing support, building self-efficacy, and skill training [9]. Counselors were the same thoroughly trained counselors who delivered the CIS quitline counseling in nonexperimental applications. Counseling was conducted at a CIS call center based at the Fred Hutchinson Cancer Research Center, Seattle, WA, and counselors were blind as to the participants’ other treatment assignments. Participants in the off condition received no quitline counseling.
Messaging
Participants in the on condition received brief e-mail messages designed expressly for this study and that could be accessed by any computer or mobile device that allowed e-mail receipt. Messages were intended to provide the following: (1) motivation/encouragement; (2) quitting tips and information; (3) adherence/education prompts (to use available resources as recommended and facts about quitting, e.g., “Did you know that feeling stressed or upset is the number one reason people turn back to smoking when trying to quit?” And “Most ex-smokers say that the first couple of weeks are the toughest.”); and (4) relapse prevention content (e.g., [2, 25–27]). These e-mailed prompts were sent twice/day for 2 weeks, once/day for an additional month, and then one every 3rd day for an additional 6 weeks (constituting a 3-month-long intervention). Participants in the off condition did not receive e-mail messages.
Brochures
Longer brochure
Participants in the on condition received the NCI’s 36-page clearing the air brochure [23], containing a detailed guide for preparing to quit, quitting, and preventing relapse as well as suggested resources.
Brief brochure
Those in the off condition received a smaller 12-page booklet developed by the investigators. The content of this booklet was the same as contained in the larger booklet except that information directly relevant to coping skill training (identification of smoking triggers, making coping plans) was removed.
Nicotine replacement therapy (NRT)
Participants in the on condition for this component received a 2-week starter package of nicotine mini-lozenges, with dose based on time since first cigarette of the day as per package instructions. Each package contained two mini-lozenge dispensers (162 lozenges total) and instructions on proper use (e.g., to use 6–10 lozenges/day). Participants were encouraged to start lozenge use the morning of their quit day. The 2-week duration of NRT mimics the manner in which it is often used in population-based interventions, i.e., as 2-week “starter packs” [28, 29]. Participants in the off condition received no pharmacotherapy.
Assessment plan and measures
Following the baseline data collection, all participants were asked to complete six additional assessment surveys; three brief weekly e-mail assessments at weeks 1–3, followed by more lengthy e-mailed assessments at 1, 3, and 7 months. The timing of all surveys was based on the date of enrollment. Participants were compensated for the baseline assessment/enrollment and the three later assessments (a maximum total of $95). Payment by check was distributed as follows: $10 for enrollment, $20 each for the 1- and 3-month surveys, and $45 for the 7-month survey.
The baseline questionnaire assessed demographic factors, tobacco dependence (via the Fagerstrom Test of Nicotine (or Cigarette) Dependence [30] and selected items from the Wisconsin Inventory of Smoking Dependence Motives-Brief version (i.e., the WISDM: [31]), Internet experience and resources, smoking history information, social support, alcohol use and problems, relapse proneness (via the WI-PREPARE: [32]), and withdrawal symptoms (via the Wisconsin Smoking Withdrawal Scale [33]). The brief weekly e-mail assessments gathered information on smoking status, ratings of access to quitting resources (support, information, skills, and motivation), and withdrawal symptoms. Follow-up assessments elicited information on smoking status (7-day point prevalence abstinence via a smoking calendar), tobacco dependence (via selected WISDM items), and symptom and treatment process measures such as perceived social support, withdrawal symptoms (via selected WSWS items), use of smoking treatments, treatment satisfaction, and affect (via selected PANAS items [34]). Follow-up interviewers were blind as to treatment assignment.
Other key assessment data were used to track intervention use. For instance, cookies were placed on participants’ Web browsers at the point of initial randomization (with participants’ consent) in order to automatically link them to their assigned Web site and track their Web use from tagged browsers (note: the initial study registration process included e-mailed instructions about registering each device the participant used). In addition, data on successful and unsuccessful call completion were collected automatically by CIS quitline service software.
Analytic methods
Logistic regression and analysis of variance were used to test for differences in demographic characteristics across the different treatment conditions. Analysis plans called for the evaluation of 7-day point prevalence abstinence outcomes both longitudinally and at individual time points. Abstinence analyses included the full sample of randomized participants (i.e., intention to treat or ITT analyses); participants with missing abstinence data were assumed to be smoking. Generalized linear mixed model (GLMM) analysis was used to analyze abstinence at particular follow-up time points in longitudinal analyses; abstinence at individual time points was analyzed via logistic regression. Treatment effects in these models were coded using effect coding; all models tested treatment main effects and all possible two-way treatment interactions. Longitudinal analyses of treatment effects on abstinence rates were computed using SuperMix (Scientific Software, Inc.), using data from the 1-, 3-, and 7-month follow-up time points. SuperMix models use maximum likelihood estimates of the model parameters via numerical quadrature; a logistic (logit) link function was used, and the dependency (correlation) of the repeated abstinence outcomes was explicitly modeled as a random effect [35]. SPSS [35, 36] was used for all other analyses. Follow-up analyses were conducted with and without covariate adjustment (covariates were gender, race, age, smoking rate at baseline, and FTND score) to determine robustness with regard to covariate-related error control. Very similar effects were obtained with the two types of models; for reasons of parsimony, results reflect unadjusted models.
Power
A priori power analyses were conducted to determine sample sizes needed to detect a 7 % difference in point prevalence abstinence with alpha at 0.05, with a two-tailed test, and power = 0.80. This effect size was deemed appropriate for evaluating population-based interventions. Because power values depend on the abstinence rate attained in the control condition, we calculated the power to detect an increment in abstinence rate of 7 % across multiple control values (i.e., 5, 10, and 15 %). Our N (1,034) comfortably exceeded the largest needed sample size generated by those analyses (i.e., 964), permitting detection of a 7 % difference in prevalence rates across reasonable control condition abstinence rates.
RESULTS
Recruitment outcomes
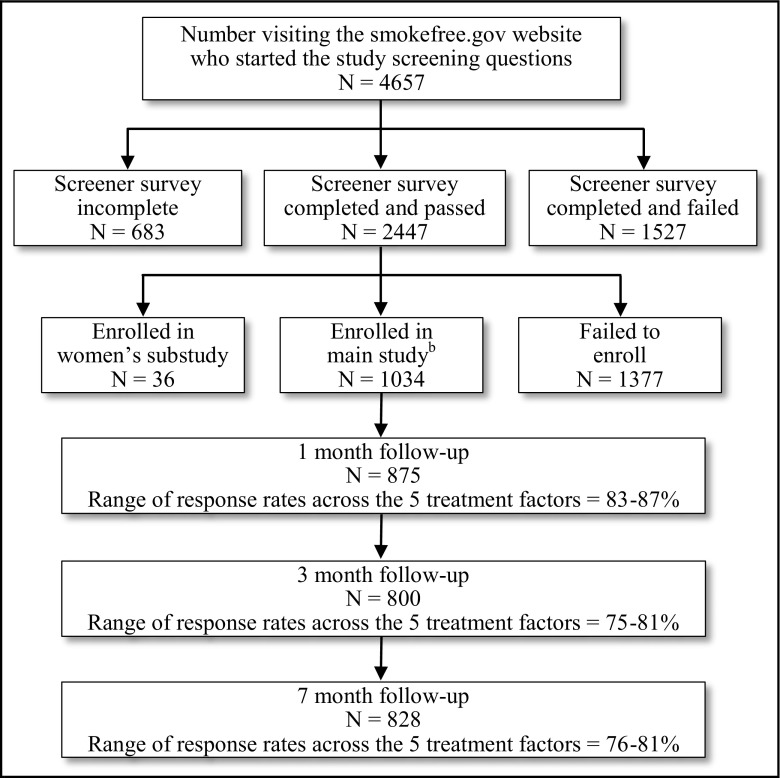
Figure 1 shows the CONSORT diagram for the study. Of those failing screening (N = 1,527), the most common reasons were already being engaged in a smoking cessation treatment, having no regular access to a phone and/or computer, and use of too many Web-linked devices for the study to be able to monitor Web usage (four or more). Among those who failed to enroll after completing the baseline survey and screening (N = 1,373), by far, the most common reason was failure to make the confirmation phone call. The Ns assigned to the various treatment conditions are depicted in Table 2.
Fig. 1.
Recruitment flow charta
Table 2.
Intent-to-treat 7-day point prevalence abstinence and treatment main effects and N’s
| Intent-to-treat 7-day point prevalence abstinence, % | Quitline counseling | NRT | Messaging | Web site | Brochures | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Off (n = 581) | On (n = 453) | Off (n = 516) | On (n = 518) | Off (n = 508) | On (n = 526) | Lite (n = 525) | Full (n = 509) | Brief (n = 517) | Full (n = 517) | |
| Month 1 | 22.9 % | 25.6 % | 20.5 %a | 27.6 %a | 24.8 % | 23.4 % | 22.6 % | 25.5 % | 24.4 % | 23.8 % |
| Month 3 | 26.3 % | 27.8 % | 23.4%a | 30.5 %a | 27.8 % | 26.2 % | 24.4 %b | 29.5 %b | 27.7 % | 26.3 % |
| Month 7 | 28.4 % | 27.6 % | 26.9 % | 29.2 % | 31.5 %a | 24.7 %a | 26.5 % | 29.5 % | 27.7 % | 28.4 % |
Note: Point prevalence abstinence rates without statistical adjustment for covariates. The active(“on”) and comparison or control (“off”) conditions differ statistically at p < 0.05 (a) or at p < 0.07 (b). Also, note that the relatively small number of participants associated with the active CIS quitline condition was due to a problem in the electronic transfer of data from the research coordinating site to CIS, i.e., CIS was not notified that some subjects had been randomized to the CIS intervention and, thus, did not deliver the intervention to them. This lack of transfer of information occurred due to a mechanical failure and was not related to subject characteristics. Because the individuals in question received all the other intervention components consistent with their randomization, and because the information transfer failure occurred at random, these individuals were retained in the study and were coded as being in the quitline counseling “off” condition
Subject characteristics
The sample (N = 1,034) had the following demographic features: 68 % were female (range across all treatment conditions = 65.1–70.2 %), 84.8 % were white (range = 83.2–86.1 %), 6.6 % were African-American (range = 5.1–8.4 %), 49 % were employed (range = 47.3–50.8 %), 33.7 % were married (range = 32.2–34.8 %), 48.6 % had a partner or spouse who smoked (range = 46.2–52.7 %); 47 % had an income <$30,000 (range = 43.8–50.3 %), they smoked an average of 19.3 cpd (SD = 8.9), their FTND scores averaged 5.3 (SD = 2.1), and they had a mean age of 39.3 (SD = 12.3). Their highest level of education completed was the following: 3.9 % below high school education (range = 2.8–5.3 %), 20.4 % high school only (range = 17.8–23.1), 56.1 % had a high school degree and some college (range = 53.7–58.4 %), and 19.7 % were college graduates (range = 17.8–21.5 %).
Smoking outcomes
Follow-up contact
Figure 1 shows the follow-up contact rates for the three major follow-up time points. There were no significant differences in these rates across any of the intervention components.
Longitudinal analyses of abstinence data
The GLMM model with no covariates showed a significant main effect due to NRT (estimate = −0.3895; SE = 0.1413; p = 0.0058) and a significant effect of time (estimate = −0.2107; SE = 0.0729; p = .0039). In addition, two effects approached significance: (1) an interaction between NRT and time (estimate = 0.1379; SE = 0.0724; p = 0.0569) and an interaction between the Web site and messaging factors (estimate = 0.1181; SE = 0.067; p = 0.0768). These reflected, respectively, the following across the three follow-up time points: (1) higher abstinence rates in the active NRT condition versus the control (off) NRT condition; (2) increased abstinence rates over time for all conditions, especially from the 1- to the 3-month mark; (3) a trend toward different patterns of abstinence across time in the active versus the control NRT conditions, with relatively high abstinence rates across all follow-up points in the former, and low initial levels in the control condition that rose across time (e.g., see Table 2); and finally (4) a trend for the active Web site condition to produce higher abstinence rates than the control condition when neither was paired with the active messaging condition. To illustrate this last trend, the average abstinence rates across the three follow-up time points were 23.9 and 32 %, respectively, in the conditions where the full Web site was paired, and then unpaired, with messaging. Table 3 suggests that among the individuals in the no-messaging condition, the Web site effect was most pronounced at months 1 and 3. When the longitudinal analysis is restricted to these follow-up time points, there is a significant interaction between the Web site and messaging condition (estimate = 0.2747; SE = 0.1265; p = 0.0299).
Table 3.
Abstinence rates for the follow-up time points in the four conditions formed by the Web site and messaging factors
| Follow-up time point | Full Web site + no messaging | Lite Web site + no messaging | Full Web site + messaging | Lite Web site + messaging |
|---|---|---|---|---|
| 1 month | 28.4 | 21.4 | 23.0 | 23.8 |
| 3 months | 33.5 | 21.8 | 25.7 | 26.8 |
| 7 months | 34.2 | 28.6 | 24.9 | 24.5 |
Abstinence rates at individual follow-up time points
1-, 3-, and 7-month follow-up. Table 2 shows that there were significant effects due to NRT at both the 1-month (Wald = 6.67, p = 0.01) and the 3-month marks (Wald = 4.82, p < 0.05). Two additional significant effects occurred at the 3- and 7-month follow-up time points (see Table 2): (1) e-mail messaging decreased abstinence at month 7 (Wald = 4.51, p = 0.034). In addition, there was a significant two-way interaction between the Web site and messaging factors at month 3 (Wald = 4.96, p = 0.026). This interaction occurs because the full Web site produced stronger effects relative to the lite Web site when individuals received no messaging (33.5 vs 21.8 %, respectively, p = 0.003), versus when they receive messaging (25.7 vs 26.8 %, respectively, p = 0.762) (Table 3).
Intervention use
Data on Web site use showed that participants made 1,392 visits to the full Web site (smokefree.gov) and 1,198 visits to the lite Web site (mean number of visits in the two conditions were 2.7 and 2.35, respectively). In terms of quitline counseling, only 30 % of the participants assigned to the on counseling condition actually accepted any calls. Among those who did, the average number of calls taken was 2.3. About 70 % of the participants assigned to the lozenge on condition reported using the lozenge within 1 month of study entry. Participants reported little use of nonstudy treatment resources (see “Use of Non-study Resources” Electronic supplementary material).
DISCUSSION
This research was designed to evaluate which smoking cessation intervention components most strongly warrant support in population-based application. The primary purpose of this research was to explore the benefits of the NCI smokefree.gov Web site and the CIS quitline counseling service, both in terms of their main effects and interactions.
The smokefree.gov Web site did not produce a significant main effect in the longitudinal analysis. However, there was evidence of significant effectiveness as revealed by interaction effects. A significant Web site X messaging interaction was found when the analyses were restricted to either the 3-month, or the 1- and 3-month, marks. These interaction effects revealed that the full Web site produced considerably greater benefit than the lite Web site when the Web site was not paired with e-mail messaging (vs when paired). The increase in abstinence of the full Web site over the lite Web site was about 6–11 percentage points across the three follow-up time points in the absence of messaging (Table 3). This suggests that the specific active treatment elements in the smokefree.gov Web site (e.g., skill training elements) did indeed enhance cessation outcomes, when they were not paired with messaging.
Why might the full Web site intervention have been hampered by the active messaging condition? There was evidence that the iterative e-mail messages in the on messaging condition discouraged Web site use. When actual tracked use of the full Web site was dichotomized, individuals in the on messaging condition were significantly less likely to use the active Web site three or more times than those in the off messaging condition (31 vs 39 % B = 176 [SE = 0.067], p = 0.008). Self-report data also suggested that the messaging condition discouraged Web site use (p < 0.05). The causes of this association are not known, but messaging may have decreased Web site use because it increased perceived treatment burden, or because the iterative proactive messages may have led participants to believe that messaging was the primary, or a sufficient, treatment resource.
This research showed no statistically significant evidence of quitline counseling benefit across any of the follow-up time points (see Table 2). This lack of effectiveness of quitline counseling is surprising given the extensive evidence that shows its effectiveness [2, 3, 9, 11]. The low success rate observed may reflect the recruitment method; recruiting through a Web site may have inadvertently selected individuals who were unwilling to engage in person-to-person interaction (via the phone) as a quit strategy (see [20], which reported a similar phenomenon). This account is consistent with the unusually low rate of quitline utilization in this study, with only 30 % of those assigned to the on condition taking any calls (cf. [37]). Thus, the outcomes observed in this experiment may reflect the recruitment channel used, intervention use rates, and other factors such as the motivational status of the sample.
The intervention component producing the most consistent benefit was the 2-week starter pack of the nicotine mini-lozenge. NRT mini lozenges produced significant benefit at the 1- and 3-month time points and also exerted a significant main effect in the longitudinal analysis. The provision of a 2-week supply of the mini-lozenge increased abstinence rates by about 7 percentage points at the 1- and 3-month marks. The benefits of the lozenge provide additional evidence of the benefit of NRT in conditions closely reflecting real-world use (cf. [15, 38]).
The other two tested intervention components did not significantly boost abstinence rates. The lack of efficacy of the active brochure (vs a brief, ostensibly inert brochure) is consistent with a great deal of other research showing no or very little benefit of brochures (e.g., [2]). The current work adds to this literature, showing that the active brochure tested did not enhance cessation outcomes through interactive effects. The lack of effectiveness of the messaging intervention component may be due to features such as its periodicity and duration, its content, a lack of interactivity and tailoring [39], or the fact that it was not targeted at mobile devices [39, 40]. Prior positive findings obtained with interactive intervention resources targeted at mobile devices [19, 41] encourage further research into such intervention strategies.
Limitation of this research include the following: (1) No placebo medication was used for the NRT condition nor were time/attention controls used for the quitline counseling condition (features that are consistent with a pragmatic trial such as this one [42, 43]); (2) biochemical verification was not used to confirm follow-up self-reports of abstinence (although evidence supports the validity of self-reports in low-contact research contexts [44, 45]; (3) the on messaging condition was developed for this trial per se and did not comprise features that may be essential to the optimal effects of such interventions; (4) extra-experimental intervention resources may have been used differentially across conditions (but self-report data argue against this); (5) study requirements (e.g., the confirmation call) may have biased outcomes by affecting the nature of the population that participated (e.g., by removing those highly concerned with privacy); and (6) greater statistical power might have revealed additional significant effects.
Despite the limitations of this research, the results support the use of mailed nicotine mini-lozenges and smoking Web sites such as smokefree.gov, as population-based interventions for smoking cessation. As per the MOST research strategy guiding this research design, these intervention components would constitute good candidates for incorporation into an optimized population-based smoking treatment package. The results also provide a warning that adding intervention components into multicomponent packages incurs risk; the negative interaction between the smokefree.gov Web site and messaging clearly suggests that more is not necessarily better. Finally, this research supports the feasibility and usefulness of the MOST approach to the experimental analysis of intervention components [21, 22]. At relatively modest cost in terms of time, sample size, and money, this approach permitted a well powered experimental evaluation of the main and interactive effects of five different types of intervention components.
Electronic supplementary material
(DOCX 14 kb)
(DOCX 19 kb)
Acknowledgments
This was an investigator-designed study undertaken in response to a contract to the University of Wisconsin from Matthews Media Group, underwritten by ARRA funding to the National Cancer Institute. Additional funding for Timothy Baker was provided by the National Cancer Institute (5K05CA139871). The funders worked under the direction of the investigators to implement the study-required design and procedures in the Smokefree.gov Web site, providing required data reports on enrollment and Web site usage. The funders played no role in the design, conduct or analysis of the study, nor in the interpretation and reporting of the study findings. The researchers were independent from the funders. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The project was funded through a contract to our university from Matthews Media Group, underwritten by ARRA funding to the National Cancer Institute. Additional funding was provided by the National Cancer Institute (5K05CA139871).
Conflict of interest
David Fraser, Kate Kobinsky, Stevens Smith, Jason Kramer, Wendy Theobald, and Timothy Baker declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Footnotes
Implications
Practice: Clinicians should encourage the use of even brief courses of nicotine replacement therapy and the use of the Smokefree.gov Web site as population-based aids to quitting smoking.
Policy: Those designing or funding population-based preventive interventions should seek information on the additive and interactive effects of different types of interventions since some interventions can add benefit, while other reduce benefit, when they are used with other interventions.
Research: Even in the effectiveness research context, researchers should consider using factorial designs because they can yield evidence of relative effectiveness and interaction effects with regard to multiple interventions.
References
- 1.World Health Organization . WHO global report: mortality attributable to tobacco. Geneva, Switzerland: WHO Press; 2012. [Google Scholar]
- 2.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 3.Fiore MC, Baker TB. Treating smokers in the health care setting. N Engl J Med. 2011;365(13):1222–1231. doi: 10.1056/NEJMcp1101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aveyard P, Begh R, Parsons A, et al. Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction. 2012;107(6):1066–1073. doi: 10.1111/j.1360-0443.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 5.Gollust SE, Schroeder SA, Warner KE. Helping smokers quit: understanding the barriers to utilization of smoking cessation services. Milbank Q. 2008;86(4):601–627. doi: 10.1111/j.1468-0009.2008.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raupach T, West R, Brown J. The most “successful” method for failing to quit smoking is unassisted cessation. Nicotine Tob Res. 2013;15(3):748–749. doi: 10.1093/ntr/nts164. [DOI] [PubMed] [Google Scholar]
- 7.Borland R, Partos TR, Yong HH, et al. How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction. 2012;107(3):673–682. doi: 10.1111/j.1360-0443.2011.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Quitting smoking among adults - United States 2001-2010. MMWR. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 9.Lichtenstein E, Zhu SH, Tedeschi GJ. Smoking cessation quitlines: an underrecognized intervention success story. Am Psychol. 2010;65(4):252–261. doi: 10.1037/a0018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAfee TA. Quitlines a tool for research and dissemination of evidence-based cessation practices. Am J Prev Med. 2007;33(6 Suppl):S357–S367. doi: 10.1016/j.amepre.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 11.North American Quitline Consortium. All quitline facts: an overview of the NAQC 2009 Annual Survey of Quitlines 2010. Available from: http://www.naquitline.org/resource/resmgr/Survey_2009/2009-survey-all-quitline-fac.pdf. Accessibility verified April 28, 2014.
- 12.Hyland A, editor Perceptions of NRT safety and efficacy: results from the International Tobacco Control (ITC) Four Country Collaboration. Society for Research on Nicotine and Tobacco (SRNT) Preconference Symposium; 2007; Austin, TX.
- 13.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Hughes JR, Peters EN, Naud S. Effectiveness of over-the-counter nicotine replacement therapy: a qualitative review of nonrandomized trials. Nicotine Tob Res. 2011;13(7):512–522. doi: 10.1093/ntr/ntr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlam TR, Baker TB. Interventions for tobacco smoking. Invited review. Annu Rev Clin Psychol. 2013;9:675–702. doi: 10.1146/annurev-clinpsy-050212-185602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett GG, Glasgow RE. The delivery of public health interventions via the internet: actualizing their potential. Annu Rev Public Health. 2009;30:273–292. doi: 10.1146/annurev.publhealth.031308.100235. [DOI] [PubMed] [Google Scholar]
- 17.Civljak M, Sheikh A, Stead LF, et al. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev. 2010;9 doi: 10.1002/14651858.CD007078.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Devries KM, Kenward MG, Free CJ. Preventing smoking relapse using text messages: analysis of data from the txt2stop trial. Nicotine Tob Res. 2013;15(1):77–82. doi: 10.1093/ntr/nts086. [DOI] [PubMed] [Google Scholar]
- 19.Whittaker R, McRobbie H, Bullen C, et al. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Graham AL, Cobb NK, Papandonatos GD, et al. A randomized trial of internet and telephone treatment for smoking cessation. Arch Intern Med. 2011;171(1):46–53. doi: 10.1001/archinternmed.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins LM, Baker TB, Mermelstein RJ, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41(2):208–226. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins LM, Murphy SA, Nair VN, et al. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med. 2005;30(1):65–73. doi: 10.1207/s15324796abm3001_8. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Clearing the air: how to quit smoking . . . and quit for keeps. (NIH publication no. 95-1647.). 1993.
- 24.Lehto T, Oinas-Kukkonen H. Persuasive features in web-based alcohol and smoking interventions: a systematic review of the literature. J Med Internet Res. 2011;13(3):e46. doi: 10.2196/jmir.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abroms LC, Padmanabhan N, Thaweethai L, et al. iPhone apps for smoking cessation: a content analysis. Am J Prev Med. 2011;40(3):279–285. doi: 10.1016/j.amepre.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etter JF. A list of the most popular smoking cessation web sites and a comparison of their quality. Nicotine Tob Res. 2006;8(Suppl 1):S27–S34. doi: 10.1080/14622200601039923. [DOI] [PubMed] [Google Scholar]
- 27.Mermelstein R, Hedeker D, Wong SC. Extended telephone counseling for smoking cessation: does content matter? J Consult Clin Psychol. 2003;71(3):565–574. doi: 10.1037/0022-006X.71.3.565. [DOI] [PubMed] [Google Scholar]
- 28.Bush TM, McAfee T, Deprey M, et al. The impact of a free nicotine patch starter kit on quit rates in a state quit line. Nicotine Tob Res. 2008;10(9):1511–1516. doi: 10.1080/14622200802323167. [DOI] [PubMed] [Google Scholar]
- 29.Krupski L, Cummings KM, Hyland A, et al. Nicotine replacement therapy distribution to light daily smokers calling a quitline. Nicotine Tob Res. 2013;15(9):1572–1577. doi: 10.1093/ntr/ntt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, et al. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith SS, Piper ME, Bolt DM, et al. Development of the brief Wisconsin inventory of smoking dependence motives. Nicotine Tob Res. 2010;12(5):489–499. doi: 10.1093/ntr/ntq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolt DM, Piper ME, McCarthy DE, et al. The Wisconsin predicting patients’ relapse questionnaire. Nicotine Tob Res. 2009;11(5):481–492. doi: 10.1093/ntr/ntp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welsch SK, Smith SS, Wetter DW, et al. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. doi: 10.1037/1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 34.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 35.Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corporation IBM. IBM SPSS statistics for windows, version 22.0. Armonk, NY: IBM Corporation; 2013. [Google Scholar]
- 37.Smith SS, Keller PA, Kobinsky KH, et al. Enhancing tobacco quitline effectiveness: identifying a superior pharmacotherapy adjuvant. Nicotine Tob Res. 2013;15(3):718–728. doi: 10.1093/ntr/nts186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes JR, Cummings KM, Foulds J, et al. Effectiveness of nicotine replacement therapy–a rebuttal. Addiction. 2012;107(8):1527–1528. doi: 10.1111/j.1360-0443.2012.03925.x. [DOI] [PubMed] [Google Scholar]
- 39.Jamison J, Sutton S, Gilbert H. Delivering tailored smoking cessation support via mobile phone text messaging: a feasibility and acceptability evaluation of the Quittext program. J Appl Biobehav Res. 2012;17(1):38–58. doi: 10.1111/j.1751-9861.2012.00075.x. [DOI] [Google Scholar]
- 40.Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev. 2005;3 doi: 10.1002/14651858.CD001118.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Riley W, Augustson EM. Mobile phone-based smoking cessation interventions increase long-term quit rates compared with control programmes, but effects of the interventions are heterogeneous. Evid Based Nurs. 2013;16(4):108–109. doi: 10.1136/eb-2012-101204. [DOI] [PubMed] [Google Scholar]
- 42.Glasgow RE, Magid DJ, Beck A, et al. Practical clinical trials for translating research to practice: design and measurement recommendations. Med Care. 2005;43(6):551–557. doi: 10.1097/01.mlr.0000163645.41407.09. [DOI] [PubMed] [Google Scholar]
- 43.March J, Kraemer HC, Trivedi M, et al. What have we learned about trial design from NIMH-funded pragmatic trials? Neuropsychopharmacology. 2010;35(13):2491–2501. doi: 10.1038/npp.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–1093. doi: 10.2105/AJPH.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)
(DOCX 19 kb)