Abstract
The abundance and composition of heterochromatin changes rapidly between species and contributes to hybrid incompatibility and reproductive isolation. Heterochromatin differences may also destabilize chromosome segregation and cause meiotic drive, the non-Mendelian segregation of homologous chromosomes. Here we use a range of genetic and cytological assays to examine the meiotic properties of a Drosophila simulans chromosome 4 (sim-IV) introgressed into D. melanogaster. These two species differ by ∼12–13% at synonymous sites and several genes essential for chromosome segregation have experienced recurrent adaptive evolution since their divergence. Furthermore, their chromosome 4s are visibly different due to heterochromatin divergence, including in the AATAT pericentromeric satellite DNA. We find a visible imbalance in the positioning of the two chromosome 4s in sim-IV/mel-IV heterozygote and also replicate this finding with a D. melanogaster 4 containing a heterochromatic deletion. These results demonstrate that heterochromatin abundance can have a visible effect on chromosome positioning during meiosis. Despite this effect, however, we find that sim-IV segregates normally in both diplo and triplo 4 D. melanogaster females and does not experience elevated nondisjunction. We conclude that segregation abnormalities and a high level of meiotic drive are not inevitable byproducts of extensive heterochromatin divergence. Animal chromosomes typically contain large amounts of noncoding repetitive DNA that nevertheless varies widely between species. This variation may potentially induce non-Mendelian transmission of chromosomes. We have examined the meiotic properties and transmission of a highly diverged chromosome 4 from a foreign species within the fruitfly Drosophila melanogaster. This chromosome has substantially less of a simple sequence repeat than does D. melanogaster 4, and we find that this difference results in altered positioning when chromosomes align during meiosis. Yet this foreign chromosome segregates at normal frequencies, demonstrating that chromosome segregation can be robust to major differences in repetitive DNA abundance.
Keywords: heterochromatic threads, meiosis, meiotic drive
HETEROCHROMATIC repeats at and near telomeres and centromeres turn over rapidly at short evolutionary time scales (Charlesworth et al. 1994). A subset of genes involved in meiosis, chromosome and chromatin function, and transposable element defense also show high rates of divergence between sibling species, often with accompanying signatures of adaptive evolution (Malik and Henikoff 2001; Begun et al. 2007; Larracuente et al. 2008; Anderson et al. 2009; Obbard et al. 2009; Raffa et al. 2011; Langley et al. 2012). These patterns suggest that organisms need to mount a continual adaptive response to suppress deleterious consequences caused by heterochromatic repetitive DNAs. Satellite DNAs and transposable elements, the major components of heterochromatin, can increase their copy numbers by unequal crossing over and transposition. These expansions can reduce fitness by increasing genome size and rates of ectopic recombination.
Repetitive DNA evolution can be particularly rapid if it selfishly biases its transmission through meiosis (true meiotic drive) or gametogenesis (gametic drive; we refer to both phenomena collectively as segregation distortion). Meiotic drive is an especially strong driver of chromosomal evolution that takes advantage of asymmetric meioses (that is, females in Drosophila and mammals) where only one meiotic product becomes the egg pronucleus (Pardo-Manuel De Villena and Sapienza 2001; Fabritius et al. 2011). The selfish elements that cause meiotic drive likely result from variation in heterochromatic repeat sequences (Buckler et al. 1999; Fishman and Saunders 2008). Adaptive divergence of centromeric and telomeric proteins may reflect a host response to suppress meiotic drive, as meiotic drivers can have pleiotropic deleterious consequences on host fitness (Zwick et al. 1999; Henikoff et al. 2001).
There are hints that segregation distorters may be prevalent in natural populations (Jaenike 2001; Reed et al. 2005; Bastide et al. 2013), but few specific loci have been identified. Hybrid backgrounds may reveal these loci, if suppressors fail to function or are separated from their targets by segregation (Mercot et al. 1995). Here we take advantage of a rare opportunity to examine meiotic transmission of an entire foreign chromosome, which is D. simulans chromosome 4 (sim-IV) in a heterospecific D. melanogaster background. D. melanogaster and D. simulans are sibling species that can be intercrossed but contain substantial divergence. Alignable synonymous nucleotide sites are ∼12–13% diverged (Begun et al. 2007), and the species are strikingly different in repetitive DNA content and heterochromatin, with D. simulans having substantially fewer transposable elements and less satellite DNA (Lohe and Roberts 1988; Bosco et al. 2007; Lerat et al. 2011). They also have experienced adaptive evolution in genes that are essential for chromosome segregation (Malik and Henikoff 2001; Anderson et al. 2009).
Chromosome 4 has a number of advantages for this study. (1) sim-IV is viable when introgressed into D. melanogaster due to its small size, the only incompatible phenotype being homozygous male sterility (Muller and Pontecorvo 1942). (2) Chromosome 4 is triplo-viable, which allows for novel chromosome segregation assays (Sturtevant 1934). (3) Chromosome 4 contains an interesting mix of heterochromatic and euchromatic properties (Riddle et al. 2009). It has a high proportion of repetitive DNA but a normal abundance of protein coding genes. It is therefore not a gene-poor B or Y chromosome. (4) Chromosome 4 is achiasmatic and segregates in the absence of crossing over. Therefore all divergence on 4 remains linked to the centromere and can potentially impact meiotic segregation. (5) Chromosome 4 segregation nevertheless typically utilizes homology to achieve pairing during meiosis, while also being able to segregate under an alternative homology-independent pathway when homology is absent (Hawley et al. 1992). In short, we propose that we are testing for faithful segregation among the most diverged chromosomes possible in an animal model.
One recent advance in understanding the segregation of nonexchange chromosomes, such as the small 4 chromosomes of Drosophila, is the identification of tethers connecting spatially separated chromosomes during prometaphase of meiosis I in females. These tethers appear to be built from pericentromeric heterochromatin and are proposed to establish tension between chromosomes not held together by chiasmata, thus allowing homologous coorientation to be established (Hughes et al. 2009, 2011). Similar tethers have been inferred by micromanipulation experiments in grasshopper spermatocytes (LaFountain et al. 2002) and by PICH localization to DNA threads connecting mitotic sister kinetochores in mammalian cultured cells (Baumann et al. 2007). While the exact mechanisms of establishing and resolving these tethers are unknown, they are a strong candidate for establishing nonexchange chromosome segregation, as heterochromatic homology is sufficient for coorientation (Hawley et al. 1992). Heterochromatin divergence between species can cause mitotic segregation failure in interspecific hybrids (Ferree and Barbash 2009). Here we address whether a foreign-species chromosome with extensive divergence affects the formation of heterochromatic threads and can segregate properly during female meiosis.
Materials and Methods
Drosophila stocks and nomenclature
We refer to generic fourth chromosomes as 4, and specific fourth chromosomes as IV. Therefore, the unmarked introgressed D. simulans 4th chromosome used in this study is referred to as sim-IV. An exception is the D. melanogaster chromosome 4 containing the visible eye marker svspa-pol, which we refer to simply as pol. The 4 wild-type lines used in triplo-4 segregation assay were obtained from Stuart MacDonald and are described elsewhere (King et al. 2012). We created a D. melanogaster y w sim-IV/ciD stock derived from the sim-IV introgression obtained from J. P. Masly (Masly et al. 2006). All other stocks were from the Hawley lab or obtained from the Bloomington Drosophila Stock Center. We used a w+-marked chromosome 4 (y1 w1118; PBac{w+mC = 5HPw+}CG33978A437), abbreviated as w+-IV as a control chromosome in crosses in Table 1 to measure sim-IV segregation and production of nullo maternal gametes. A y+-marked chromosome 4 (y1 w1118; PBac{y+-attP-9A}VK00024), abbreviated as y+-IV, was used as the opposing chromosome to follow segregation of the sim-IV or control chromosome.
Table 1. Test of segregation, chromosome loss, and NDJ.
| Regular progeny | Exceptional progeny | |||||
|---|---|---|---|---|---|---|
| Chr. 4 tested | F1 sex | No. inheriting P[y+] | No. inheriting tested chromosome | Segregation ratioa | No. 4 NDJ | 4 NDJ %b |
| w+-IV | Female | 1249 | 1194 | 0.489 | 1 | |
| Male | 1022 | 1095 | 0.517 | 1 | ||
| Both | 2271 | 2289 | 0.502 N.S. | 2 | 0.044 | |
| sim-IV | Female | 1276 | 1147 | 0.473 | 0 | |
| Male | 1031 | 963 | 0.483 | 1 | ||
| Both | 2307 | 2110 | 0.478** | 1 | 0.023 | |
y w; w+-IV females were crossed to w/Y; sim-IV/ciD males. y w/Y; w+-IV/sim-IV sons were then crossed to y w; y+-IV females. y w; y+-IV/w+-IV and y w y+-IV/sim-IV daughters were collected and separately crossed to y1 pn1/Y; C(4)RM, ci1 eyR/O males at 27°.
Defined as the ratio of those inheriting the tested chromosome/total progeny. As each class has a 50% chance of survival due to sperm genotype (Figure 5), significance was tested by comparison to simulation of equal segregation followed by 50% survival with 1,000,000 replicates. N.S., not significant (P > 0.5); **P < 0.002.
Calculated as the number of observed exceptional progeny/total progeny (excluding minutes; see Figure 5 and Materials and Methods). The NDJ rates for the two genotypes were not significantly different (P = 1, Fisher’s exact test).
Drosophila crosses
In the C(4)RM, ci1 eyR stock used in Table 1, the penetrance of the ey phenotype was variable. Among the thousands of progeny, a small number of various developmental defects were observed. Therefore flies were scored as being ci ey only if both wings displayed the ci1 phenotype and at least one eye displayed a small or misshapen eye characteristic of the eyR phenotype. In the experimental cross ci ey females will be y w+, and ci ey males will be y w. Regular progeny with these phenotypes are thus potentially overlapping with C(4)/O if the regular progeny have morphological defects affecting the wings and eyes. Between 2 and 11 flies with morphological defects were found for each sex and genotype in the Table 1 crosses and were predominantly cases where one eye was missing and wings were wild type or where both eyes were wild type and one wing had a defective longitudinal vein 4 or 5. In the control cross ci ey females will be y w+, and ci ey males will be y w. No regular y w males will be produced but regular y w+ daughters are again potentially overlapping with C(4)/O. We also found the minute phenotype associated with haplo-4 challenging to score but classified between 2 and 17 flies of each sex and genotype as minute in Table 1.
To measure nondisjunction (NDJ) in the y w; sim-IV/sim-IV, y w; sim-IV/pol and y w; sim-IV/ciD genotypes, single virgin females were mated to multiple C(1;Y), v f B/O; C(4)RM, ci eyR/O males in vials, allowed to lay eggs for 5 days, and adults removed. X chromosome NDJ could be seen by following y (normal progeny were y+ females and y– males, while progeny of diplo-X or nullo-X eggs were y– females and y+ males, respectively). Progeny of nullo-4 eggs could be identified as being both ci and ey (normal progeny in the sim-IV/ciD cross could be ci alone), but because the sim-IV chromosome is wild type for all chromosome 4 markers, diplo-4 progeny of mothers carrying sim-IV could not be distinguished from normal progeny.
To produce y w; sim-IV/pol females, we crossed y w; sim-IV homozygous females from the introgression stock to males from a y w/y+Y; pol laboratory stock. Then y w/y+Y; pol/sim-IV heterozgous males were collected and backcrossed to y w; pol virgin females to produce y w; pol/sim-IV females.
To produce FM7, y w B/y w; pol/sim-IV and FM7, y w B/y w; sim-IV/sim-IV females, y w/y+Y; sim-IV/pol males from above were crossed to FM7, y w B; pol females, and FM7, y w B/y+Y; sim-IV/pol males and FM7, y w B/y w; sim-IV/pol virgin females were collected. These were sib-mated, which produced FM7, y w B/y w females that were phenotypically pol+. These females could be either pol/sim-IV or sim-IV/sim-IV, which were expected in a 2:1 ratio. These females were mated singly in vials to C(1;Y)/O; C(4)/O tester males to test X and 4 NDJ as above. The maternal 4 genotype was inferred to be sim-IV/pol if any pol minute progeny were produced in a vial. Vials that did not produce any pol minute progeny were also testcrossed by mating multiple F2 females to y w/y+Y; pol males and looking for any pol progeny; all tested vials were confirmed to lack pol, meaning the experimental female in that vial must have been sim-IV/sim-IV. Count data for each vial were then combined by maternal 4 genotype.
To produce y w/y w noda; pol and y w/y w noda; sim-IV/pol progeny, y w noda/y+Y; pol males (from a stock with the X balanced over C(1)DX females) were crossed to FM7, y w B/y w; pol/sim-IV virgin females from above, and virgin females of both genotypes were collected and mated singly in vials to C(1;Y)/O; C(4)/O tester males as above.
To produce triplo-4 females, we used a mutation in nod to increase the rate of nondisjunction. The w+-IV chromosome was crossed into a FM7a, nod background to generate the stock C(1)DX, y1 w1 f1/FM7a, nod4/ /Dp(1;Y)y+; PBac{w+mC = 5HPw+}CG33978A437. We abbreviate the males from this stock as FM7a, nod4/Y; w+-IV. To generate triplo-4 females, we first crossed y w; y+-IV females to FM7a, nod4/Y; w+-IV males. F1 virgin daughters of genotype y w/FM7a, nod4/Y; y+-IV/w+-IV were then mated to males of genotype y w/Y containing different chromosome 4 genotypes. Males containing wild-type chromosome 4s were generated by crossing y w; sim-IV/ciD females to wild-type males and selecting y w/Y; +/ciD sons. Rare y w/y w daughters inheriting both maternal chromosome 4s and a paternal chromosome 4 were identified by their y+ w+ phenotype; where appropriate non-ciD females were selected in order to obtain the desired paternally inherited wild-type chromosome 4. Triplo-4 females were then mated singly to 2 y w/Y males at 25°.
Probability analyses were done in R (cran.r-project.org). To test significance for random segregation with 50% survival in Table 1, a binomial number Nj was generated with a mean of 0.5 and an N of twice the experimental result. The surviving segregation proportion was then simulated as pj = binomial (0.5, Nj)/Nj. This was repeated 1,000,000 times to generate a distribution, with significance determined as the two-tailed likelihood of obtaining the observed result due to chance.
4-4 distance preps
Bottles were cleared of adults and virgin females of the desired genotypes were collected 6 hr later. Females were aged in yeasted vials with sibling males for 42 hr after collection, and so were 42–48 hr posteclosion at the point of dissection. To standardize prep conditions, a timer was started as the vial was anesthetized with CO2, followed by hand dissection of ovaries as quickly as possible in room temperature 1× Robb’s media + 1% BSA (Matthies et al. 2000), transferring ovaries to a second well of media after extraction. After 10 females were dissected, the ovaries were left to incubate in Robb’s until the timer reached 7 min, when buffer plus ovaries were pipetted into a 1.5-ml Eppendorf tube and allowed to settle. At 8 min, the Robb’s was aspirated, and 1.3 ml of room temperature fixative [a 1:1 mix of 16% EM grade paraformaldehyde (Ted Pella) with William’s Hypotonic Oocyte Preservation and Stabilization Solution (Gillies et al. 2013), combined just before use] was applied. After fixation at room temperature for 5 min, oocytes were washed briefly in PBST (PBS + 0.1% Triton X-100), ovarioles were separated by rapid pipetting with a p1000 pipette, washed three times in PBST for 15 min each, stained in PBST plus 1× DAPI for 6 min, washed in PBST (three times quickly followed by two times for 15 min) then mounted on slides in SlowFade Gold (Invitrogen).
Fluorescent in-situ hybridization preps
Females were aged for 2 or 3 days posteclosion in yeasted vials with males. A timer was started as females were anesthetized with CO2, transferred to a CO2 plate for 1 min, then the gas was turned off, flies were covered with a Petri dish lid, and allowed to rest on the plate. At 6 min, the CO2 was turned back on, and ovaries were dissected as quickly as possible in Robb’s (above). Once all ovaries were dissected, they were left to incubate in Robb’s until 15 min from the start of the procedure, when they were transferred to an Eppendorf tube. Oocytes were allowed to settle for 1 min, the Robb’s was aspirated, and 1.3 ml of prewarmed 39° fixative (above) was applied. Oocytes were fixed for 4 min at 39°, washed briefly in 2× SSCT (saline sodium citrate + 0.1% Tween 20), and ovarioles separated by pipetting. Oocytes were washed in 2× SSCT three times for 10 min, washed 10 min each in 2× SSCT containing 20, 40, and 50% formamide, then incubated in 2× SSCT + 50% formamide for 2 hr at 37°. As much buffer as possible was aspirated, and 40 µl of hybridization solution (36 µl of 1.1× hybridization solution (1.0 g dextran sulfate, 1.5 ml 20× SSC, 5.0 ml formamide, dilute to 9.0 ml with ddH2O) plus 4 µl of probe mix) was added. All probes were synthesized with fluorophores by www.idtdna.com and diluted to 200 ng/µl in ddH2O. Probe mixes were prepared by combining 2 µl of each probe to be used, then diluting to a total volume of 96 µl in ddH2O, then storing at −20°. For each prep, 4 µl of probe mix was used, resulting in 16.7 ng of each probe in each prep. Probes used were 2L-3L (AATAACATAG)3 and 4 (AATAT)6 (Dernburg 2000) and X (TTT-TCC-AAA-TTT-CGG-TCA-TCA-AAT-AAT-CAT) (Ferree and Barbash 2009).
After the hybridization solution was added, DNA was denatured at 92° followed by overnight hybridization at 32°. Oocytes were washed twice for 15 min in 2× SSCT + 50% formamide at 32°, for 10 min each in 2× SSCT containing 40, 20, and 0% formamide, then stained in 2× SSCT + 1× DAPI for 10 min. Oocytes were washed in 2× SSCT (two times briefly and two times for 10 min), then mounted in SlowFade Gold.
Immunofluorescent preps
Two-day mated females were dissected as per fluorescent in-situ hybridization (FISH) preps (1 min CO2, 5 min rest, quickly dissected then incubated for up to 10 min in Robb’s), followed by fixation at room temperature in 1.3 ml fixative. Oocytes were then washed briefly in PBST, ovarioles separated by pipetting, and washed three times for 10 min in PBST. Oocytes were dechorionated by rolling between frosted glass slides, washed three times briefly in PBST, transferred to an 0.5 ml Eppendorf tube, and blocked for 1 hr in PBST-NGS (Matthies et al. 2000). Fresh PBST-NGS with primary antibodies (Serotec MCA786 rat antitubulin at 1:250 and Millipore rabbit antiphosphorylated histone H3 at serine 10 at 1:500) was added and hybridized overnight, followed by washing in PBST (three times briefly and once for 15 min), 1 hr blocking in PBST-NGS, and then either a 4-hr incubation at room temperature or overnight at 4°, in PBST-NGS plus secondary antibodies (goat antirat IgG with Alexa Fluor 647 conjugate and goat antirabbit IgG with Alexa Fluor 568 conjugate, Invitrogen, both at 1:250). A total of 2.5 µl of 200× DAPI was added and incubated for 6 min, followed by PBST washes (three times briefly and twice for 15 min) and mounting in SlowFade Gold.
Imaging and quantification
To ensure oocytes were not missed or double counted, microscope slides were photographed on a dissection microscope and a print of the photo was used as a map to mark oocytes. Oocytes were viewed at low magnification and marked using the LAS AF software (www.leica.com) “mark and find” panel. All confocal images were collected with the ×63 objective on a Leica TCS SPE II confocal microscope using LAS AF, and presented images were deconvolved using Huygens Essential (www.svi.nl).
Estimation of 4-4 distances was done by combining XY distances (determined by the LAS AF line tool in projected stacks) with Z distances (determined by multiplying the number of confocal sections between the centers of the 4 light cones by the section thickness in orthogonal projections) using the Pythagorean theorem (distance = sqrt(xy2 + z2)) in Excel. Measurement was restricted to oocytes that had at least one 4 out on the spindle. This was determined by whether there was at least a 50% dip in background-subtracted fluorescent intensity, measured on the 4 and the space between the 4 and the adjacent chromosome using the line ROI tool. Oocytes with both 4s on the same side of the spindle, with additional nonexchange chromosomes, or with chromosomes in the “slippage” configuration (Hughes et al. 2011) were counted as having chromosomes out on the spindle, but their 4-4 distances were not included in the analysis. Plots and t-tests were then done in R.
To calculate chromosome 4 brightness ratios, figures where both 4 chromosomes were fully separated from other chromosomes were selected, identically sized regions of interest (ROI) were placed over each 4 and on nearby empty space, and the summed pixel intensity for each ROI was recorded. The brightness ratio (lower intensity − background)/(higher intensity − background) was calculated for 10 oocytes for each genotype.
Results
Reduced heterochromatin of sim-IV
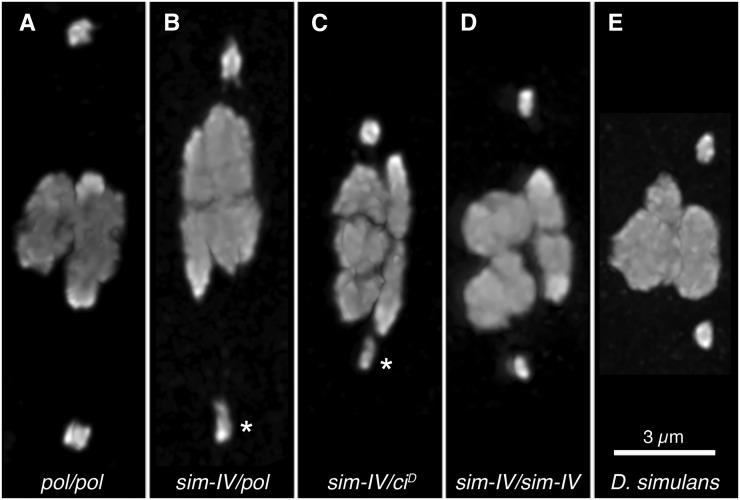
In examining sim-IV, in comparison with pure-strain D. melanogaster and D. simulans oocytes, we found that sim-IV is dimmer than its D. melanogaster homolog in DAPI fluorescence. This was readily apparent even in the ocular, and caused an asymmetry between the 4s in heterozygous females (Figure 1, A–C). This dimness, without asymmetry, was also observed in introgressed sim-IV homozygotes (Figure 1D) as well as D. simulans females (Figure 1E). This result is not unexpected; the AATAT heterochromatin repeat, which primarily labels the 4 in females (Dernburg 2000), is considerably less abundant in the D. simulans genome, comprising only 1.9% of the genome vs. 3.1% in D. melanogaster (Lohe and Brutlag 1987).
Figure 1.
Asymmetry in sim-IV heterozygotes. pol and ciD are visible markers on different D. melanogaster chromosome 4s. Representative oocytes from 42- to 48-hr-old mated females from the DAPI-only preps used for 4-4 distance measurement, scaled to the same size. The differences in the brightness of the 4s are not as clear in these projected images as in the ocular, so the background-subtracted intensity of each 4 was determined, and the brightness ratio (dimmer 4/brighter 4) calculated, for 10 oocytes per genotype, with the mean (and range) reported. (A) Homozygous control pol/pol oocyte. Mean brightness ratio: 0.87 (0.77–0.98). (B) Heterozygous sim-IV/pol oocyte made from outcrossing the introgression stock. The dimmer sim-IV chromosome is indicated (asterisk). Mean brightness ratio: 0.63 (0.40–0.76). (C) Heterozygous sim-IV/ciD oocyte from the introgression stock. The dimmer sim-IV chromosome is indicated (asterisk). Mean brightness ratio: 0.66 (0.57–0.89). (D) Homozygous sim-IV/sim-IV oocyte from the introgression stock. The 4s are dimmer but not asymmetric. Mean brightness ratio: 0.88 (0.73–0.96). (E) Pure-strain D. simulans oocyte. The 4s are also dimmer but not asymmetric. Mean brightness ratio: 0.94 (0.78–0.99).
Positioning of sim-IV during female meiosis
Because recent work has identified heterochromatin tethers that can incorporate the AATAT repeat (Hughes et al. 2009), we asked whether these tethers were also present in D. simulans. We were able to detect them by both a phospho-specific histone antibody that can highlight threads (Hughes et al. 2011) and by FISH of an AATAT probe (Figure 2). However, during this experiment, we noticed that it was much more difficult to find oocytes that had their chromosome 4s positioned far enough out on the spindle to have detectable threads, in both D. simulans and introgressed sim-IV females. Instead, while roughly similar numbers of oocytes appeared to have chromosomes out on the spindle (and therefore also roughly equal durations of time spent in prometaphase), those chromosomes were positioned much closer to the main mass of chiasmate chromosomes. To quantify this, we did preps under tightly controlled aging and dissection conditions and measured the 4-4 distances for oocytes from pure-strain D. melanogaster, introgressed sim-IV hetero- and homozygotes, and pure-strain D. simulans (Figure 3). To limit consideration to 4-4 distances under comparable conditions of prometaphase and congression, we excluded those oocytes where other chromosomes besides the 4 were spontaneously nonexchange, as well as oocytes that were fixed while chromosomes were in transient configurations such as having both homologs on the same side of the spindle (Hughes et al. 2009) or in the slippage configuration where the chiasmate autosomes are positioned end to end (Hughes et al. 2011).
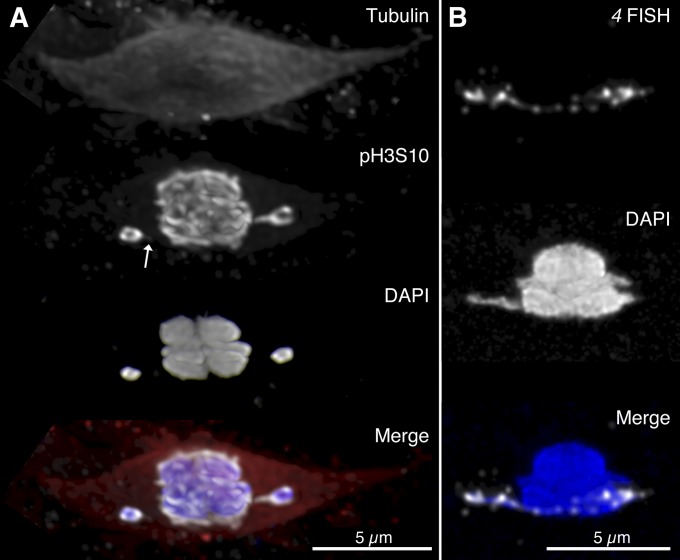
Figure 2.
Heterochromatin threads in D. simulans. (A) Fixed oocyte from a 2-day-old mated D. simulans female, visualized by immunofluorescence with anti-tubulin (red), anti-pH3S10 (white), and DAPI (blue) staining. Threads are detectable by anti-pH3S10 the right chromosome has a clear and complete thread while a very dim spur can be seen on the left chromosome (arrow). (B) Fixed oocyte from a 3-day-old mated D. simulans female, visualized by heterochromatin FISH (white) against the AATAT repeat primarily found on chromosome 4. A complete thread can be detected running between the 4 chromosomes.
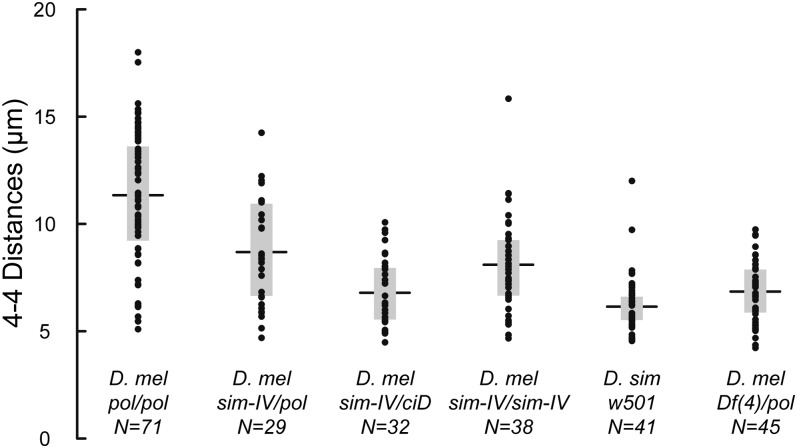
Figure 3.
4-4 distance measurements. pol and ciD are visible markers on different D. melanogaster chromosome 4s. (A) The mean distances for each genotype (horizontal lines) and the inner quartile ranges (boxes) are indicated, along with the number of measurements. The first four sets are for D. melanogaster, including the pol/pol control, the outcrossed sim-IV/pol heterozygote, the introgressed sim-IV/ciD heterozygote, and the introgressed sim-IV/sim-IV homozygote, while the fifth set is for pure-strain D. simulans females. The sixth set is D. melanogaster females heterozygous for the deletion Df(4)m101-62f/pol (see Figure 4).
Consistent with our initial qualitative observations, we found that the mean 4-4 distances in pure-strain (pol/pol) D. melanogaster females (11.3 µm) were nearly twice as large as in D. simulans (6.1 µm). Interestingly, the introgressed sim-IV chromosome was more intermediate when homozygous in D. melanogaster (sim-IV/sim-IV: 8.1 µm), suggesting that genetic background affects chromosome positioning. This may also contribute to the difference between the two heterozygous genotypes (sim-IV/pol: 8.7 µm, sim-IV/ciD: 6.79 µm). Note that because the 4 chromosomes are normally positioned near the centromeres of the other chromosomes, the minimum 4 separation is the normal karyosome width, ∼4.5 µm. Therefore the proportional separation of 4 chromosomes from the main mass is considerably larger in pure-strain D. melanogaster. Many of these comparisons, including all comparisons involving pure-strain D. melanogaster, were highly statistically significant as determined by pairwise t-tests (supporting information, Figure S1).
This novel observation that the 4th chromosomes from these two closely related species have notably different behavior provides strong evidence that the amount of heterochromatin on a chromosome has a functional consequence. A speculative further interpretation is that if the repeats on a chromosome are forming threads that connect nonexchange homologs, then having a greater amount of those repeats may increase thread length and enable those homologs to move farther apart from each other before the tether pulls tight enough to prevent further movement.
Reducing AATAT content also affects positioning of D. melanogaster 4
This simple model suggests that deleting some of the 4 heterochromatin should reduce the 4-4 distance during prometaphase. Few deletions on the D. melanogaster 4 chromosome are available, but Df(4)M101-62f deletes proximal gene-containing sequence and extends into the centromeric heterochromatin for an unknown distance (J. Locke, personal communication). We crossed this deletion to the same pol stock used above to produce Df(4)m101-62f/pol females. We found that the deficiency chromosome was noticeably smaller than pol and hybridized less strongly to the AATAT FISH probe (Figure 4), consistent with the deletion of some of the 4 heterochromatin. Then, we measured the 4-4 distances in oocytes from Df(4)m101-62f/pol females and found a highly significant reduction in the mean 4-4 distance (6.8 µm, Figure 3 and Figure S1). These results strongly support our conclusion that 4-4 distances are proportional to the amount of 4 heterochromatin.
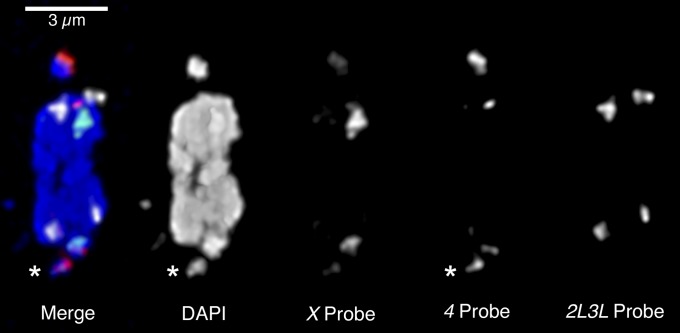
Figure 4.
Asymmetry in Df(4)m101-62f heterozygotes. A fixed oocyte from a mated 2-day-old heterozygous Df(4)m101-62f/pol female is shown, with FISH staining of the 359-bp satellite (X probe, green), the AATAT repeat (4 probe, red), and the AATAACATAG repeat (2L3L probe, white) along with DAPI (blue). The Df(4) chromosome (asterisk) stains less brightly with both DAPI and the 4 probe, consistent with the deletion of some AATAT heterochromatin from this chromosome.
Segregation of sim-IV in D. melanogaster females
To test whether sim-IV segregates properly in a foreign species, we assayed sim-IV by making it heterozygous over a y+-marked D. melanogaster reference chromosome in D. melanogaster females. We also performed in parallel a control cross using a w+-marked D. melanogaster chromosome 4 that was heterozygous over the same reference chromosome (Figure 5). Over 4400 progeny were scored in each experiment (Table 1). In the control cross the two progeny classes were not significantly different from the expected 1:1 ratio. In the experimental cross, sim-IV progeny were recovered at slightly below Mendelian expectations (47.8%). This deficit, however, is significantly below 50% (P < 0.002, binomial simulation). The experiment and control are also significantly different when compared directly in a contingency table (P < 0.05, chi square).
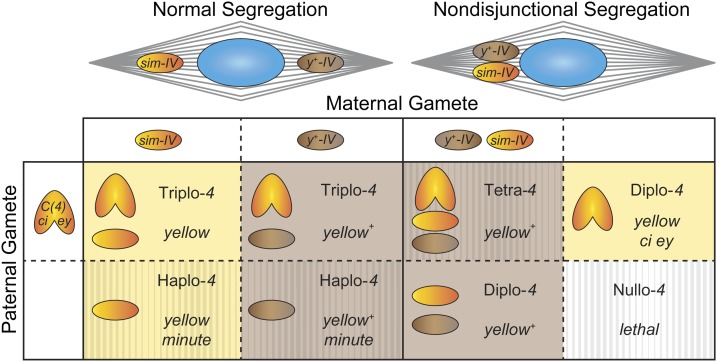
Figure 5.
Expected progeny from the cross in Table 1 to measure the sim-IV segregation ratio. At top are two spindle diagrams, showing normal segregation (left) and meiosis I nondisjunctional segregation (right). As either spindle pole can form the egg pronucleus, those poles drop down to four types of female gametes in the table. Chromosome loss is also possible but not diagrammed; in that case, nullo-4 gametes equivalent to the last column will be produced. Females are mated to compound-4 bearing males of genotype C(4), ci ey, who produce either diplo-4 or nullo-4 gametes. Progeny will be y+ if the maternal y+-IV is transmitted, and are otherwise y mutant, indicated by the background color. The hatching pattern indicates progeny that are semiviable or lethal. Haplo-4 leads to minute phenotypes with poor viability, while nullo-4 is always lethal. Tetra-4 flies from nondisjunctional oocytes are usually lethal, but can survive under some circumstances (Grell 1972). Note that the normal yellow+ triplo-4 progeny are indistinguishable from the nondisjunctional diplo-4 progeny (as well as any tetra-4 progeny that survive). Therefore only the yellow ci ey class of progeny from NDJ can be observed. A similar situation arises in most of the crosses in Table 2, where sim-IV/pol progeny arising from nondisjunction are phenotypically wild type and cannot be distinguished from triplo-4 regular progeny. In both Table 1 and Table 2, progeny inheriting no maternal 4 are products of either maternal nondisjunction or chromosome loss and are detected by their ey ci phenotype. Although only half of the exceptional progeny are therefore detectable, we have calculated 4 NDJ without doubling the number of nullo-4 progeny observed, as spontaneous 4 NDJ events in wild-type and nod– heterozygous backgrounds yielded 11 nullo events and only 1 diplo event across multiple experimental controls (Zhang and Hawley 1990; Rasooly et al. 1991; Gillies et al. 2013), suggesting these arise primarily from loss events rather than nondisjunction. Products of meiosis II nondisjunction are not shown, but again only those inheriting no maternal 4 are phenotypically distinguishable.
Normal disjunction of sim-IV in D. melanogaster
These differences might reflect a true segregation disadvantage of sim-IV, but also could result from small viability differences between D. melanogaster flies heterozygous for sim-IV vs. mel-IV that cannot be easily detected. We therefore performed a range of additional assays. First we measured NDJ within the above cross, since it can result from chromosome loss, the most plausible cause of reduced transmission. The absolute rate in sim-IV/y+ females was 2.3 × 10−4, lower than in the corresponding control and consistent with wild-type rates for pure-strain D. melanogaster from other published studies (see Figure 5).
We further tested the meiotic behavior of sim-IV by crossing to males from a standard NDJ tester stock that allows estimation of both X and 4 NDJ. We observed no X or 4 NDJ within the sim-IV introgression stock, either as sim-IV/ciD heterozygotes or sim-IV/sim-IV homozygotes (Table 2). We also outcrossed the stock to a standard laboratory stock with the 4th chromosome marked with pol, to create sim-IV/pol females, and again saw no X or 4 NDJ in this genotype. Because of these negative results, we considered the possibility that any defect in sim-IV may be weak. We reasoned that if this were the case, we might see NDJ if we sensitized the genetic background to increase NDJ, as has been done for assaying natural variation (Zwick et al. 1999). We performed two sensitizations, one by testing sim-IV in a background carrying a single dose of the meiotic mutant nod, and the other by testing sim-IV in females heterozygous for the X chromosome balancer FM7. Even in these sensitized backgrounds, we saw no increase in NDJ (Table 2). Furthermore, the transmission rates appear roughly equal for both 4th chromosomes, by comparing the pol− minute and pol+ minute progeny of heterozygous sim-IV/pol females. Therefore, the genetic evidence from a range of genetic backgrounds strongly suggests that the introgressed sim-IV chromosome is fully competent for normal segregation in female meiosis.
Table 2. Tests for sim-IV nondisjunction in multiple genetic backgrounds.
| Genotype | Normal progeny | 4-only NDJ | X-only NDJ | X and 4 double NDJ | pol+ minutea | pol– minutea | X NDJ %b | 4 NDJ %b |
|---|---|---|---|---|---|---|---|---|
| y w; sim-IV/sim-IV | 181 | 0 | 0 | 0 | 0 | — | 0 | 0 |
| y w; sim-IV/ciD | 230 | 0 | 0 | 0 | 2 | — | 0 | 0 |
| y w; sim-IV/pol | 1641 | 0 | 0 | 0 | 119 | 56 | 0 | 0 |
| y w/y w noda; pol | 509 | 2 | 0 | 0 | — | 235 | 0 | 0.39 |
| y w/y w noda; sim-IV/pol | 866 | 4 | 1 | 0 | 133 | 135 | 0.23 | 0.46 |
| FM7/y w; sim-IV/pol | 1405 | 1 | 5 | 1 | 189 | 134 | 0.85 | 0.21 |
| FM7/y w; sim-IV/sim-IV | 1127 | 3 | 7 | 0 | 314 | — | 1.22 | 0.26 |
Females of the indicated genotypes were crossed to C(1;Y), v f B/O; C(4)RM, ci eyR/O males.
The missing class of minutes cannot be produced by these crosses.
The number of X NDJ progeny was doubled for calculation of X NDJ, to account for inviable classes (Zeng et al. 2010). Number of X and 4 double NDJ progeny was therefore also doubled for calculation of both X NDJ and 4 NDJ. In calculating percentage of X NDJ and 4 NDJ, the number of NDJ progeny was divided by the sum of the total progeny, not including minutes.
Normal sim-IV segregation in triplo-4 D. melanogaster females
Females carrying three chromosome 4s are viable and fertile. Such females are expected to produce three types of meiotic segregation at equal frequencies (Figure 6). Sturtevant (1934, 1936) discovered, however, that in many crosses with triplo 4s, the segregation ratios differ substantially from equal frequencies. He further determined that different chromosome 4s from wild-type and marker strains display a characteristic “preference” for whether they tend to segregate with one of the other chromosome 4s being tested (classes I and III in Figure 6), or instead, segregate away from the other two chromosome 4s (class II). The genetic basis of this curious preference property remains unexplained. In our scheme, we arranged in a triplo-4 female the unmarked chromosome to be tested against chromosome 4s dominantly marked with either y+ or w+ (Figure 6). We reasoned that if sim-IV is perceived by D. melanogaster as being a foreign chromosome, then the two marked D. melanogaster 4s would segregate away from each other and sim-IV would segregate analogous to a free duplication. This would result in a deficit of type II segregation below the random expectation of 1/3, which would manifest as a deficit of y+ w+ and y w phenotypes.
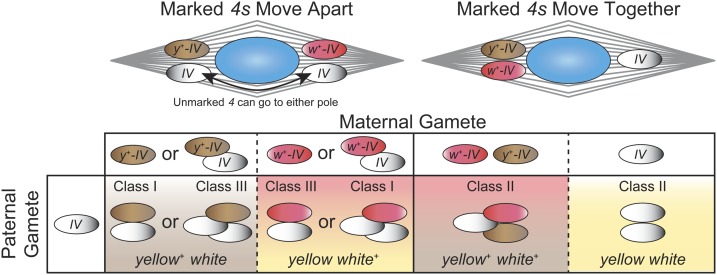
Figure 6.
Expected segregation types and phenotypic classes of progeny from triplo-4 females. The unmarked 4 being tested is indicated as “IV.” Triploid females of chromosome 4 genotype y+-IV/w+-IV/IV were mated to y w/Y males with unmarked 4s. Female chromosome 4s can segregate in three possible classes to generate six different gametes. However, not all gametes can be distinguished because the tested 4 is unmarked, leading to the same phenotype from different genotypes, as indicated by background colors. When the two marked 4s segregate to opposite poles, the unmarked chromosome will segregate to either pole. This leads to class I segregations (y+-IV <=> w+-IV/IV) and class III segregations (w+-IV <=> y+-IV/IV), which both produce progeny carrying only one of the two 4-linked markers. Conversely, in class II segregations, the two marked 4 chromosomes move to the same pole, leading to progeny that are either wild type or mutant for both markers together. If segregation is equal, then all six classes of progeny are equally likely, leading to an expected 2: 2: 1: 1 ratio of the phenotypes y+ w: y w+: y+ w+: y w.
Contrary to this expectation we found that class II segregations were significantly overrepresented with sim-IV, but also in four of the five control crosses with D. melanogaster chromosome 4s derived from different marker and wild-type stocks (Table 3). The one outlier with a significant deficit of class II segregations involved chromosome 4 from the wild-type stock BS 1. The wide range of values is consistent with results from Sturtevant (1936). This variation is not due to aberrant production or recovery of the two reciprocal classes within the three segregation types, because in most crosses the number of y+ progeny was similar to w+ progeny produced by class I and III segregations, and likewise for y+ w+ and y w progeny produced by class II segregation. Instead we conclude that sim-IV segregation falls within the normal range of variation observed for D. melanogaster chromosome 4s.
Table 3. Triplo-4 segregation tests.
| Source of Chr. 4 tested | No. y+ | No. w+a | No. y+ w+ | No. y wb | % class II freq.c |
|---|---|---|---|---|---|
| BS 1 | 725 | 714 | 285 | 307 | 29.1*** |
| BOG 1 | 165 | 216** | 151 | 133 | 42.7*** |
| sim-IV | 356 | 383 | 295 | 333 | 45.9*** |
| VAG 1 | 151 | 167 | 136 | 171* | 49.1*** |
| Wild 5B | 141 | 131 | 140 | 131 | 49.9*** |
| y w | 670 | 760* | 741 | 901*** | 53.5*** |
y w; y+-IV / w+-IV / 4 females, where 4 represents the unmarked chromosome 4 being tested, were crossed to y w/Y males. *P < 0.05; **P < 0.01; ***P < 0.001 in chi-squared tests.
y+ and w+ classes were tested for deviation of a 1:1 ratio.
y+ w+ and y w classes were tested for deviation of a 1:1 ratio.
y+: w+: y+ w+: y w classes were tested for deviation from a 2:2:1:1 ratio.
Discussion
The function of heterochromatic threads in meiosis
The heterochromatic threads connecting homologous chromosomes in female meiosis are the leading candidate mechanism for how nonexchange chromosomes achieve proper coorientation (Hughes et al. 2009), as they can explain a variety of experimental observations, such as heterochromatic homology being sufficient to achieve segregation (Hawley et al. 1992). We found that sim-IV has shortened 4-4 distances. and is positioned more closely to the other chromosomes compared to mel-IV. We suggest that this correlation reflects a role of threads in chromosome positioning, but acknowledge that differential positioning might have other causes such as variation in microtubule capture or centromere strength. Regardless, we have also found that both properties correlate with differences in heterochromatin abundance, both between mel-IV and sim-IV, and between wild-type mel-IV and a heterochromatic deletion. Our results therefore provide evidence that the amount of heterochromatin on the 4 changes its positioning.
In addition to unresolved questions of the proximal mechanism (such as how threads are established, how they regulate coorientation, and how they are finally resolved), there is also the evolutionary question of why these chromosomes move out on the spindle at all. We suggest that because chromosome 4 is fully achiasmatic, it may be acting as an “organizing center” for threads emanating from other chromosomes. This idea is conceptually similar to a proposal by Carpenter (1991), with chromatin threads fulfilling the role previously proposed for interchromosomal microtubules. There is some circumstantial evidence for this organizational role; for example, the microtubule mass along the spindle arc between prometaphase 4 chromosomes is substantially denser than elsewhere in the spindle (Hawley and Theurkauf 1993) and in some figures, threads that appear to originate from other chromosomes can also lead toward the 4s (Hughes et al. 2009). We further suggest that increased amounts of heterochromatin on 4 cause longer threads. These longer threads may more efficiently capture or associate with heterochromatic threads from facultatively achiasmate chromosomes and increase their probability of correct segregation.
If so, this role suggests parallels between the evolution of heterochromatin and other aspects of meiosis. While D. melanogaster has many common polymorphic chromosome inversions, D. simulans is monomorphic with no common inversions (Lemeunier and Aulard 1992). As inversions block crossing over, increasing the abundance of inversions will make meioses with nonexchange chromosomes more common. In D. melanogaster, nonexchange chromosomes move out on the spindle during prometaphase I. While the significance of this movement is not known, we speculate that it may be involved in how the oocyte achieves proper nonexchange chromosome coorientation and metaphase-arrested karyosome structure. Because nonexchange chromosomes in D. melanogaster are positioned between the 4s near the spindle poles and the exchange chromosomes at the metaphase plate, having the 4s further out would provide more space for additional nonexchange chromosomes to also move fully out onto the spindle. If this additional space is beneficial (such as reducing the time needed to complete prometaphase, or avoiding deleterious entanglements between multiple nonexchange chromosomes), then the greater amount of space on the spindle provided by the longer 4-4 tethers in D. melanogaster may help this species to tolerate common inversions. Note that the causal relationship in this model is unknown; it could be that longer 4-4 tethers evolved first, which allowed inversions to accumulate in the population, or alternatively, accumulating inversions favored the evolution of longer tethers to accommodate their segregation. Either way, this model predicts that Drosophila species with common inversions should have greater 4-4 distances than species that lack them. This would be particularly interesting to examine in species such as D. virilis, which has a large genome with a high satellite DNA content (Bosco et al. 2007), yet appears to lack inversions in natural populations (Evgen’ev et al. 2000). This hypothesis also may explain why dot chromosomes persist in many Drosophila species (Ashburner et al. 2005).
Heterochromatin divergence and meiotic drive
There is a resurgence of interest in heterochromatin variation, due to evidence that it affects gene expression (Lemos et al. 2010) and to new methods to detect and quantitate such variation (Aldrich and Maggert 2014). Strong meiotic drive is typically associated with cytologically detectable differences in heterochromatin between chromosomes (Fishman and Saunders 2008; Dawe 2009). Our results here show that a large difference in abundance of the AATAT satellite between D. simulans and D. melanogaster chromosome 4s does not result in similarly dramatic levels of meiotic drive. We suggest that location as well as abundance influences whether satellite DNA blocks affect centromere behavior or take on neocentromere function, analogous to heterochromatin position effects that are proposed to influence whether or not circularized sex chromosomes cause mitotic defects (Ferree et al. 2014). Our results further suggest that strong meiotic drive is not an inevitable consequence of even extensive chromosome divergence. It remains an open question whether meiotic drivers are truly rare in nature, or instead whether higher frequency variants exist that cause lower level drive that is beyond the limit of detection in small-scale experiments. A major hurdle in resolving this question is the difficulty of reliably detecting weak meiotic drive effects, one example being the maize chromosomal knob K10L2 (Kanizay et al. 2013).
Faithful segregation of sim-IV
Our diplo-segregation assay did reveal a small (∼2%) but statistically significant deficit in sim-IV-containing progeny. However this deficit is well within the range of potential viability effects. Distinguishing subtle viability effects vs. a meiotic segregation difference would require precise tracking and quantification of egg to adult viability for many thousands of animals. We instead pursued two additional approaches to examine sim-IV segregation. First we quantitated nondisjunction in a manner that includes the detection of chromosome loss events. We found no excess in NDJ for sim-IV, most strikingly even when sensitizing the genetic background using either a nod mutation or an achiasmate X chromosome balancer.
Segregation of sim-IV in triplo-4 females
Our second approach took advantage of the very high levels of nonrandom disjunction that are often seen in triplo-4 females. We constructed D. melanogaster females containing sim-IV as the tester chromosome and two marked D. melanogaster 4s, as well as five control lines with different tester D. melanogaster 4s. We expected that if sim-IV is “perceived” as being foreign or distinct from D. melanogaster 4s, then the two D. melanogaster 4s would preferentially segregate away from each other, resulting in an excess of class I and III segregations and a deficit of class II (Table 3). Instead we saw the opposite pattern, with 45.9% class II segregations compared to the random expectation of 33.3%.
It is instructive to compare this result to cases where chromosome 4 derivatives or aberrations have been introduced into diplo-4 backgrounds, even if the use of different reference 4s between studies precludes precise quantitative comparisons. Table 3 in Hawley et al. (1992) examined the effects of a series of Dp(1;4) chromosomes containing varying amounts of chromosome 4 heterochromatin on segregation of two marked chromosome 4s. NDJ of these two 4s is analogous to class II segregation in Figure 6. NDJ ranged from ∼12 to 33% and showed a positive correlation with abundance of chromosome 4 heterochromatin. Interestingly, a deletion derivative, Dp(1;4)M5D, that appears to remove some chromosome 4 heterochromatin induced very low NDJ. Similarly, Bauerly et al. (2014) recently discovered D. melanogaster strains containing B chromosomes that are predominantly composed of AATAT satellite and may be derived from chromosome 4s. These B chromosomes induced 27.1% chromosome 4 NDJ. These results make all the more striking the fact that sim-IV induces a very high frequency of class II segregations despite having reduced AATAT content.
Acknowledgments
We thank J. P. Masly, Stuart MacDonald, and the Bloomington Drosophila Stock Center (supported by National Institutes of Health, NIH P40OD018537) for stocks and Giovanni Bosco, Keith Maggert, Sarah Zanders, and Kevin Wei for helpful comments. This work was supported by NIH GM074737 to D.A.B. and NIH GM099054 to W.D.G.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.172072/-/DC1.
Communicating editor: J. A. Sekelsky
Literature Cited
- Aldrich J. C., Maggert K. A., 2014. Simple quantitative PCR approach to reveal naturally occurring and mutation-induced repetitive sequence variation on the Drosophila y chromosome. PLoS ONE 9: e109906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. A., Gilliland W. D., Langley C. H., 2009. Molecular population genetics and evolution of Drosophila meiosis genes. Genetics 181: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Golic K. G., Hawley R. S., 2005. Drosophila: A Laboratory Handbook, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Bastide H., Gérard P. R., Ogereau D., Cazemajor M., Montchamp-Moreau C., 2013. Local dynamics of a fast-evolving sex-ratio system in Drosophila simulans. Mol. Ecol. 22: 5352–5367. [DOI] [PubMed] [Google Scholar]
- Bauerly E., Hughes S. E., Vietti D. R., Miller D. E., McDowell W., et al. , 2014. Discovery of supernumerary B chromosomes in Drosophila melanogaster. Genetics 196: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C., Körner R., Hofmann K., Nigg E. A., 2007. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell 128: 101–114. [DOI] [PubMed] [Google Scholar]
- Begun D. J., Holloway A. K., Stevens K., Hillier L. W., Poh Y.-P., et al. , 2007. Population genomics: whole-genome analysis of polymorphism and divergence in Drosophila simulans. PLoS Biol. 5: e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G., Campbell P., Leiva-Neto J. T., Markow T. A., 2007. Analysis of Drosophila species genome size and satellite DNA content reveals significant differences among strains as well as between species. Genetics 177: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler E. S., Phelps-Durr T. L., Buckler C. S., Dawe R. K., Doebley J. F., et al. , 1999. Meiotic drive of chromosomal knobs reshaped the maize genome. Genetics 153: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T., 1991. Distributive segregation: Motors in the polar wind? Cell 64: 885–890. [DOI] [PubMed] [Google Scholar]
- Charlesworth B., Sniegowski P., Stephan W., 1994. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371: 215–220. [DOI] [PubMed] [Google Scholar]
- Dawe, R. K., 2009 Maize centromeres and knobs (neocentromeres), pp. 239–250 in Handbook of Maize. Springer-Verlag, New York. [Google Scholar]
- Dernburg, A. F., 2000 In situ hybridization to somatic chromosomes, pp. 22–55 in Drosophila Protocols. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [DOI] [PubMed] [Google Scholar]
- Evgen’ev M. B., Zelentsova H., Poluectova H., Lyozin G. T., Veleikodvorskaja V., et al. , 2000. Mobile elements and chromosomal evolution in the virilis group of Drosophila. Proc. Natl. Acad. Sci. USA 97: 11337–11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabritius A. S., Ellefson M. L., McNally F. J., 2011. Nuclear and spindle positioning during oocyte meiosis. Curr. Opin. Cell Biol. 23: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree P. M., Barbash D. A., 2009. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol. 7: e1000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree P. M., Gomez K., Rominger P., Howard D., Kornfeld H., et al. , 2014. Heterochromatin position effects on circularized sex chromosomes cause filicidal embryonic lethality in Drosophila melanogaster. Genetics 196: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman L., Saunders A., 2008. Centromere-associated female meiotic drive entails male fitness costs in monkeyflowers. Science 322: 1559–1562. [DOI] [PubMed] [Google Scholar]
- Gillies S. C., Lane F. M., Paik W., Pyrtel K., Wallace N. T., et al. , 2013. Nondisjunctional segregations in Drosophila female meiosis I are preceded by homolog malorientation at metaphase arrest. Genetics 193: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell R. F., 1972. Viability of tetra-4 flies. Drosoph. Inf. Serv. 48: 69. [Google Scholar]
- Hawley R. S., Theurkauf W. E., 1993. Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet. 9: 310–317. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Irick H., Zitron A. E., Haddox D. A., Lohe A., et al. , 1992. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13: 440–467. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Ahmad K., Malik H. S., 2001. The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293: 1098–1102. [DOI] [PubMed] [Google Scholar]
- Hughes S. E., Beeler J. S., Seat A., Slaughter B. D., Unruh J. R., et al. , 2011. Gamma-tubulin is required for bipolar spindle assembly and for proper kinetochore microtubule attachments during prometaphase I in Drosophila oocytes. PLoS Genet. 7: e1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. E., Gilliland W. D., Cotitta J. L., Takeo S., Collins K. A., et al. , 2009. Heterochromatic threads connect oscillating chromosomes during prometaphase I in Drosophila oocytes. PLoS Genet. 5: e1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J., 2001. Sex chromosome meiotic drive. Annu. Rev. Ecol. Syst. 32: 25–49. [Google Scholar]
- Kanizay L. B., Albert P. S., Birchler J. A., Dawe R. K., 2013. Intragenomic conflict between the two major knob repeats of maize. Genetics 194: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. G., Merkes C. M., McNeil C. L., Hoofer S. R., Sen S., et al. , 2012. Genetic dissection of a model complex trait using the Drosophila Synthetic Population Resource. Genome Res. 22: 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFountain J. R., Cole R. W., Rieder C. L., 2002. Partner telomeres during anaphase in crane-fly spermatocytes are connected by an elastic tether that exerts a backward force and resists poleward motion. J. Cell Sci. 115: 1541–1549. [DOI] [PubMed] [Google Scholar]
- Langley C. H., Stevens K., Cardeno C., Lee Y. C. G., Schrider D. R., et al. , 2012. Genomic variation in natural populations of Drosophila melanogaster. Genetics 192: 533–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larracuente A. M., Sackton T. B., Greenberg A. J., Wong A., Singh N. D., et al. , 2008. Evolution of protein-coding genes in Drosophila. Trends Genet. 24: 114–123. [DOI] [PubMed] [Google Scholar]
- Lemeunier, F., and S. Aulard, 1992 Inversion polymorphism in Drosophila melanogaster, pp. 339–405 in Drosophila Inversion Polymorphism. CRC, Boca Raton, FL. [Google Scholar]
- Lemos B., Branco A. T., Hartl D. L., 2010. Epigenetic effects of polymorphic Y chromosomes modulate chromatin components, immune response, and sexual conflict. Proc. Natl. Acad. Sci. USA 107: 15826–15831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerat E., Burlet N., Biémont C., Vieira C., 2011. Comparative analysis of transposable elements in the melanogaster subgroup sequenced genomes. Gene 473: 100–109. [DOI] [PubMed] [Google Scholar]
- Lohe A., Roberts P., 1988. Evolution of satellite DNA sequences in Drosophila, pp. 148–186 in Heterochromatin, Molecular and Structural Aspects, edited by Verma R. S., Cambridge University Press, Cambridge, UK. [Google Scholar]
- Lohe A. R., Brutlag D. L., 1987. Identical satellite DNA sequences in sibling species of Drosophila. J. Mol. Biol. 194: 161–170. [DOI] [PubMed] [Google Scholar]
- Malik H. S., Henikoff S., 2001. Adaptive evolution of Cid, a centromere-specific histone in Drosophila. Genetics 157: 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masly J. P., Jones C. D., Noor M. A. F., Locke J., Orr H. A., 2006. Gene transposition as a cause of hybrid sterility in Drosophila. Science 313: 1448–1450. [DOI] [PubMed] [Google Scholar]
- Matthies, H. J., M. J. Clarkson, R. B. Saint, R. Namba, and R. S. Hawley, 2000 Analysis of meiosis in fixed and live oocytes by light microscopy, Drosophila: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Mercot H., Atlan A., Jacques M., Montchamp-Moreau C., 1995. Sex-ratio distortion in Drosophila simulans: co-occurence of a meiotic drive and a suppressor of drive. J. Evol. Biol. 8: 283–300. [Google Scholar]
- Muller H. J., Pontecorvo G., 1942. Recessive genes causing interspecific sterility and other disharmonies between Drosophila melanogaster and simulans. Genetics 27: 157. [Google Scholar]
- Obbard D. J., Gordon K. H. J., Buck A. H., Jiggins F. M., 2009. The evolution of RNAi as a defence against viruses and transposable elements. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364: 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena F., Sapienza C., 2001. Nonrandom segregation during meiosis: the unfairness of females. Mamm. Genome 12: 331–339. [DOI] [PubMed] [Google Scholar]
- Raffa G. D., Ciapponi L., Cenci G., Gatti M., 2011. Terminin: a protein complex that mediates epigenetic maintenance of Drosophila telomeres. Nucleus 2: 383–391. [DOI] [PubMed] [Google Scholar]
- Rasooly R. S., New C. M., Zhang P., Hawley R. S., Baker B. S., 1991. The lethal(1)TW-6cs mutation of Drosophila melanogaster is a dominant antimorphic allele of nod and is associated with a single base change in the putative ATP-binding domain. Genetics 129: 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed F. A., Reeves R. G., Aquadro C. F., 2005. Evidence of susceptibility and resistance to cryptic X-linked meiotic drive in natural populations of Drosophila melanogaster. Evolution 59: 1280–1291. [PubMed] [Google Scholar]
- Riddle N. C., Shaffer C. D., Elgin S. C. R., 2009. A lot about a little dot: lessons learned from Drosophila melanogaster chromosome 4. Biochem. Cell Biol. 87: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1934. Preferential segregation of the fourth chromosomes in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 20: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. H., 1936. Preferential segregation in triplo-IV females of Drosophila melanogaster. Genetics 21: 444–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Li H., Schweppe N. M., Hawley R. S., Gilliland W. D., 2010. Statistical analysis of nondisjunction assays in Drosophila. Genetics 186: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Hawley R. S., 1990. The genetic analysis of distributive segregation in Drosophila melanogaster. II. Further genetic analysis of the nod locus. Genetics 125: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M. E., Salstrom J. L., Langley C. H., 1999. Genetic variation in rates of nondisjunction: association of two naturally occurring polymorphisms in the chromokinesin nod with increased rates of nondisjunction in Drosophila melanogaster. Genetics 152: 1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]