Abstract
HIV latency is the foremost barrier to clearing HIV infection from patients. Reactivation of latent HIV-1 represents a promising strategy to deplete these viral reservoirs. Here, we report a novel approach to reactivate latent HIV-1 provirus using artificially designed transcription activator-like effector (TALE) fusion proteins containing a DNA-binding domain specifically targeting the HIV-1 promoter and the herpes simplex virus-based transcriptional activator VP64 domain. We engineered four TALE genes (TALE1–4) encoding TALE proteins, each specifically targeting different 20-bp DNA sequences within the HIV-1 promoter, and we constructed four TALE-VP64 expression vectors corresponding to TALE1–4. We found that TALE1-VP64 effectively reactivated HIV-1 gene expression in latently infected C11 and A10.6 cells. We further confirmed that TALE1-VP64 reactivated latent HIV-1 via specific binding to the HIV-LTR promoter. Moreover, we also found that TALE1-VP64 did not affect cell proliferation or cell cycle distribution. Taken together, our data demonstrated that TALE1-VP64 can specifically and effectively reactivate latent HIV-1 transcription, suggesting that this strategy may provide a novel approach for anti-HIV-1 latency therapy in the future.
Introduction
Despite the fact that highly active antiretroviral treatment (HAART) can substantially suppress the replication of HIV-1, latent reservoirs remain the primary obstacle to the eradication of infection.1–5 Therefore, the search for effective strategies to eliminate these reservoirs has gained a great deal of attention. One strategy, referred to as “shock and kill,” is currently the most widely discussed.6–8 In this strategy, the “shock” phase is administered to reactivate latent proviruses, and the “kill” phase is used to block the new infection of cells via HAART and to eliminate the HIV-1-producing cells via immune responses and viral cytopathogenicity. In devising this strategy, much interest has recently centered on finding ways to reactivate latent HIV-1. To this end, several types of small molecule activators have been studied in vitro or in early clinical trials.1,8,9 However, although these compounds have been successful, they are toxic, ineffective, and even have the potential for significant off-target effects.10–12 These phenomena may partly result from the fact that most existing drugs cannot target HIV-1-infected cells or the proviral genome. Thus, better and more specific latency activators are urgently needed.
Transcription activator-like effectors (TALEs), originating from Xanthomonas plant pathogens, are a class of transcriptional activators that specifically binds and manipulates plant genes during pathogenesis.13–16 The recognition specificity of TALEs is determined by their DNA-binding domain, which is composed of tandemly arranged 33–35 amino acid repeats, with each repeat specifying one target base via two adjacent amino acids (12 and 13) referred to as the “repeat variable diresidues” (RVDs).13,17–19 This characteristic of the “one repeat to one base” code enables the assembly of TALE repeat arrays that bind to any user-defined target sequence. Several recent studies have demonstrated that artificially designed TALE fusion proteins containing transactivation domains can be generated to effectively and specifically induce the expression of the desired target genes.13,20–25 These studies have increased our interest in the potential use of TALEs as site-specific activators to reactivate latent HIV-1.
Here we report the generation of a chimeric transcriptional activator, referred to as TALE1-VP64, that targets the highly conserved HIV-1 long terminal repeat (LTR) promoter sequence. Our data reveal that TALE1-VP64 selectively induced the expression of a reporter gene under the control of the HIV-1 LTR promoter and increased the transcription of HIV-1 in latently infected cells. Thus, we demonstrate that the designed TALE1-VP64 protein may be used as a specific compound to perform anti-HIV latency therapy in the future.
Materials and Methods
Design and construction of TALE-VP64 plasmids
The TAL effector target sequences and their corresponding RVD sequences were identified using the online tool TAL Effector Targeter (http://boglabx.plp.iastate.edu/TALENT/). All of the TALE expression plasmids were assembled using the Golden Gate TALEN and TAL effector kit (Addgene, catalog number 1000000016) constructed by Cermak et al.25 and subsequently subcloned into the mammalian expression vector pcDNA3.1(-) using XhoI and AflII. The sequences between NheI and XbaI were then replaced with a triple-FLAG tag sequence synthesized by Sangon Biotech (Shanghai, China). The transcription activation domain VP64 sequence, described previously,26 was assembled via polymerase chain reaction (PCR) (using the forward primer 5′-AAGATATCGGACGGGCTGACGCATTGGACG-3′ and the reverse primer 5′-GCGCTTAAGGTTAATCAGCATG TCCAGGTCG-3′) and fused to the C-terminus of the synthesized TALE expression plasmids using EcoRV and AflII. These new constructs were referred to as TALE-VP64. The DNA and protein sequences of each construct are presented in Supplementary Fig. S1 (Supplementary Data are available online at www.liebertpub.com/aid).
Cell culture
C11 cells, an HIV latently infected cell line used for HIV latency studies,27–30 were constructed in our laboratory. A10.6 cells were kindly provided by the NIH AIDS Research and Reference Reagent Program.31,32 C11 cells and A10.6 cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco, Grand Island, NY), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Shanghai, China) at 37°C in 5% CO2. Human embryonic kidney 293 (HEK-293) cells were cultured in Dulbecco′s modified Eagle′s medium (DMEM) (Gibco) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2.
Construction of reporter vectors and transient transfection assays
To produce LTR-Luc, a full-length LTR fragment was PCR amplified from pNL4-3-EGFP using the forward primer F-LTR: 5′-CGGGGTACCTGGAAGGGCTAATTCACTCC CAAAG-3′ and the reverse primer R-LTR: 5′-CCGCTC GAGCAGGTCCCTGTTCGGGCGCC-3′. This PCR product was gel purified, digested using the restriction enzymes KpnI and XhoI, and then ligated into the KpnI and XhoI restriction sites of the pGL3-basic plasmid (Promega, Madison, WI). The HIV-1 LTR-Luc reporter vector lacking the TALE1-VP64 target site [LTR(ΔTALE1)-Luc] was constructed via overlapping PCR using the following primers: forward 5′-CTGGGGAGTGGCGAGGCTGCATATAAG CAG-3′ and reverse 5′- CTGCTTATATGCAGCCTCGCC ACTCCCCAG-3′.
For the transient transfection assays, HEK-293 cells were seeded on 24-well plates at a density of 1×105 cells per well. After 24 h, the cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The transfection mixture included 100 ng of the reporter plasmid, either LTR-Luc or LTR(ΔTALE1)-Luc, 600 ng of a TALE-VP64 expression plasmid, and 10 ng of pLR-SV40 (Promega) with or without 100 ng of the Tat expression plasmid pCMV-Tat. The amount of DNA was kept constant by adding pUC57 up to 1.0 μg of total DNA. The cells were lysed in 1×PLB lysis buffer (Promega) 48 h after transfection. Firefly and Renilla luciferase activities were measured using a luminometer with the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions.
Green fluorescent protein (GFP) expression visualization and flow cytometry analysis
A total of 2×106 C11 cells or A10.6 cells were transfected with 2.0 μg of pcDNA3.1(-) or the TALE1-VP64 expression plasmid via nucleofection using the Amaxa Cell Line Nucleofector Kit V (Lonza, Gaithersburg, MD). The expression of GFP as a marker for the reactivation of the HIV-1 promoter was detected using a Nikon fluorescence microscope 24–72 h posttransfection. The images were captured using a Nikon E2 digital camera. At the indicated time points after transfection, the percentage of GFP-positive cells was measured via flow cytometry to determine the extent of reactivation. The cell suspension was centrifuged at 1000 rpm for 5 min, and after removal of the supernatant, the cell pellet was resuspended in 0.4 ml of phosphate-buffered saline (PBS). GFP expression was measured using a FACScan flow cytometer, and the FACS data were analyzed using CellQuest software (Macintosh, Sunnyvale, CA). A total of 10,000 gated events were collected, and the data represent the percentage of GFP-expressing cells out of the total gated events.
Isolation of human peripheral blood mononuclear cells (PBMCs)
Whole peripheral blood from healthy donors was purchased from the Blood Center of Shanghai (Shanghai, China). The PBMCs isolation was made by the difference of gradient density Ficoll-Hypaque (density=1.077 g/ml, Haoyang Biological Manufacture, Tianjin, China). After centrifugation (400×g; 30 min at room temperature), the PBMCs were found at the plasma/Ficoll-Hypaque interphase and collected carefully with a Pasteur pipette. The cells were then washed in PBS twice (240×g for 10 min) and resuspended in RPMI 1640 medium containing 4.5 g/liter glucose supplemented with 2 mM l-glutamine, 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Cell proliferation assay and cell cycle distribution analysis
Cell proliferation was evaluated via Cell Counting Kit-8 (CCK-8) (Dojindo Molecular technologies, Gaithersburg, MD) detection assays. Briefly, PBMCs or HEK-293 cells were transfected with pcDNA3.1(-) or the TALE1-VP64 expression plasmid. Then, 48 h posttransfection, the cells were treated with 10 μl of CCK-8 solution and incubated at 37°C for 4 h. The absorbance was directly quantified using an enzyme-linked immunosorbent assay reader at 450 nm. All experiments were performed independently at least three times.
For cell cycle analysis, the cells were detached via trypsinization, and the centrifuged cells were immediately fixed with 500 μl of 70% ethanol and incubated at 4°C overnight. The cells were then washed three times with cold PBS to remove ethanol and were stained using propidium iodide (50 μg/ml propidium iodide and 100 μg/ml RNase A in PBS) and analyzed via flow cytometry using a FACSCalibur. All experiments were performed independently at least three times.
Chromatin immunoprecipitation (ChIP) assay
The ChIP experiments were performed according to the instructions provided in the EZ-ChIP Chromatin Immunoprecipitation Kit (Millipore, Billerica, MA) and a previously described procedure.27,30 Briefly, 2×106 C11 cells were transfected with 2 μg of pcDNA3.1(-) or TALE1-VP64 via nucleofection for 48 h preceding the ChIP assay. The cells were cross-linked using formaldehyde at a final concentration of 1% for 20 min at 37°C and quenched using 0.125 M glycine for 5 min. The cells were then washed twice with ice-cold PBS, resuspended in sodium dodecyl sulfate (SDS) lysis buffer, and incubated for 10 min on ice. After lysis, the chromatin was sheared using a sonicator for a total of 8 min (8 s on, 8 s off) on ice to obtain DNA fragments 200–1,000 bp in length. Then 10% of the total sheared chromatin DNA was used as the input DNA. Other samples of the sheared chromatin were incubated in antibodies against the triple-FLAG tag (Abcam) or normal mouse IgG (Millipore) as a negative control. Protein G agarose beads were added to collect the immunocomplexes. The samples were then reverse cross-linked by incubating overnight at 65°C.
The immunoprecipitated DNA was isolated and analyzed via a quantitative real-time PCR assay (ABI Prism 7900 Real-Time PCR System) at 30 cycles with Taq Mastermix (Invitrogen) using primers specific to the 5′-LTR of HIV-1 (forward: 5′-AGACTGCTGACATCGAGCTTTCT-3′; reverse: 5′-GTGGGTTCCCTAGTTAGCCAGAG-3′). The amount of PCR product obtained from each immunoprecipitated chromatin sample was normalized to the amount of PCR product obtained from the input DNA. The fold-change in occupancy of anti-FLAG at the LTR relative to the control vector was calculated.
Statistical analysis
Data are representative of three independent experiments, and error bars represent standard deviation (SD). Paired samples t tests were performed using SPSS version13.0 (SPSS Inc., Chicago), and statistical significance was indicated at *p<0.05, **p<0.01, or ***p<0.001.
Results
Design and construction of TALE-VP64 targeting the HIV-1 LTR promoter
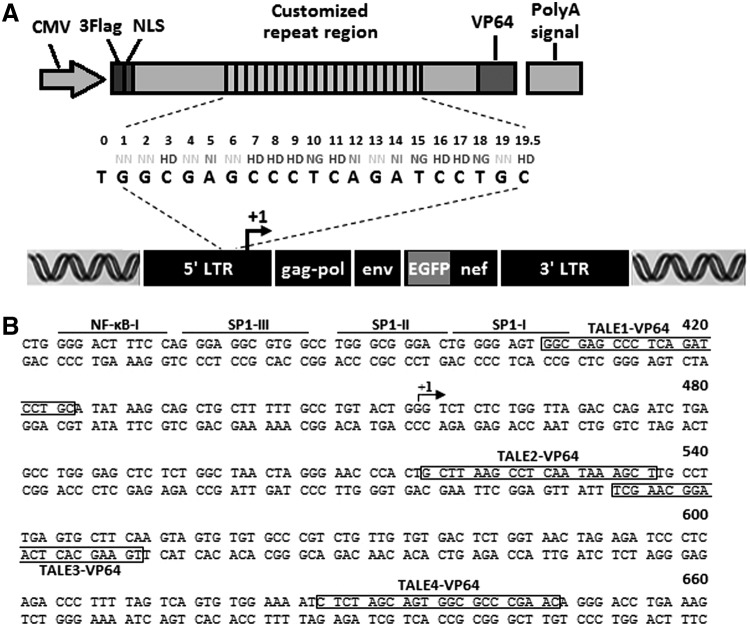
The HIV-1 LTR is highly conserved across all HIV-1 clades and represents an attractive and ideal anti-HIV-1 target. To design TALEs specifically targeting the HIV-1 LTR, DNA sequences in the HIV-1 LTR promoter were selected and included based on the following criteria: (1) potential binding sites in the HIV-1 promoter estimated to both be accessible based on the chromatin structure and display a highly conserved sequence structure among the various HIV-1 subtypes,33 (2) recognition sites preceded by a T,19,34 (3) and about 20 bp long. Potential target sites were then examined for the presence of identical or similar sites in the human genome based on a BLAST search of the Homo sapiens DNA sequences using the GenBank database (data not shown). Finally, four candidate DNA sequences in HIV-1 LTR were selected that were located in either the template DNA strand or the nontemplate DNA strand (Fig. 1).
FIG. 1.
Basic structure and target sites of transcription activator-like effector (TALE)-VP64. (A) Schematic representation of TALE-VP64-mediated activation of HIV-1 expression. The diagram illustrates the composition of the tandem repeat of a TALE-VP64 and its corresponding 20-bp DNA binding target site on the 5′-LTR of the HIV-1 genome. NLS, nuclear localization signal; VP64, synthetic transcription activation domain. (B) The DNA sequence of the HIV-1 (HXB2 strain) 5′-LTR. Several features of the HIV-1 promoter [binding sites for NF-κB, Sp1, and the transcription initiation site (arrow)] are indicated. The sites targeted by TALE-VP64 are overlaid on the promoter sequence.
The corresponding TALE expression plasmids (TALE1–4) coding for TALE proteins, each specifically targeting one of the selected 20-bp DNA sequences, were constructed by the “Golden Gate cloning” method as described previously.25 TALE-VP64 fusion constructs were generated by inserting the VP64 transcription activation domain,26 a tetrameric repeat of the VP16's minimal activation domain, directly to the C-terminal end of the TALE expression plasmids and cloning into the pcDNA3.1(-) mammalian expression vector (Invitrogen). For the ChIP analysis experiments, the expression vectors included an additional triple-FLAG tag cloned upstream of the nuclear localization signal (NLS). Ultimately, we generated four TALE-VP64 constructs, referred to as TALE1-VP64, TALE2-VP64, TALE3-VP64, and TALE4-VP64. Each coding protein contained one TALE DNA-binding module, a triple-FLAG tag, and an NLS at its N-terminus and the VP64 domain at its C-terminus (Fig. 1 and Supplementary Fig. S1).
TALE1-VP64 induced the expression of a reporter gene under the control of the HIV-1 LTR
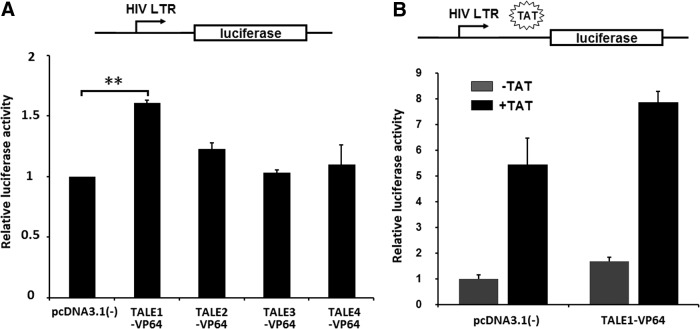
The activity of each TALE-VP64 construct was first analyzed via transient transfection and luciferase assays. HEK-293 cells were cotransfected with a designed TALE-VP64 construct and LTR-Luc, a luciferase reporter driven by the HIV-1 LTR, as described previously.35 The luciferase levels were measured 48 h posttransfection. A 1.7-fold increase in reporter expression was detected when TALE1-VP64 was present, while a slight activation was detected for TALE2-VP64 (Fig. 2A). Based on these data, we selected TALE1-VP64 for further study.
FIG. 2.
Luciferase assays of TALE-VP64-mediated HIV-1 5′-LTR activity. (A) Transient reporter assays comparing the activation potential of various designer TALE-VP64 transcription factors. HEK-293 cells were transfected with an HIV LTR-luciferase reporter plasmid and a plasmid expressing the chimeric protein indicated; the empty vector pcDNA3.1(-) was used as a control. Then, 48 h posttransfection, the relative luciferase activity was measured. The data represent the mean±standard deviation of three independent experiments. *p<0.05, **p<0.01, ***p<0.001; paired t test. (B) Synergistic activation of HIV-1 by TALE1-VP64 and Tat. HEK-293 cells were transfected with the TALE1-VP64 expression plasmid in the presence or absence of Tat; the empty vector pcDNA3.1(-) was used as a control. Then, 48 h posttransfection, the relative luciferase activity was measured. The data represent the mean±standard deviation of three independent experiments.
The viral regulatory protein Tat has been demonstrated to be a powerful transactivator of HIV gene expression.36–38 It acts by binding to the transactivation response element (TAR), located in the R region of the 5′-LTR, and activating transcription initiation and elongation from the LTR promoter.39,40 To determine whether Tat and TALE1-VP64 can function synergistically, the HIV-1 LTR-Luc reporter vector was cotransfected with TALE1-VP64 in the presence or absence of a Tat expression vector. The results revealed that Tat alone could induce an approximately 5.4-fold increase in luciferase reporter expression; when cotransfected with TALE1-VP64, the induction level rose to 7.9-fold (Fig. 2B). These data indicate that TALE-VP64 and Tat function synergistically to promote HIV-1 LTR transcription.
TALE1-VP64 efficiently mediated HIV-1 provirus reactivation in latently infected cells
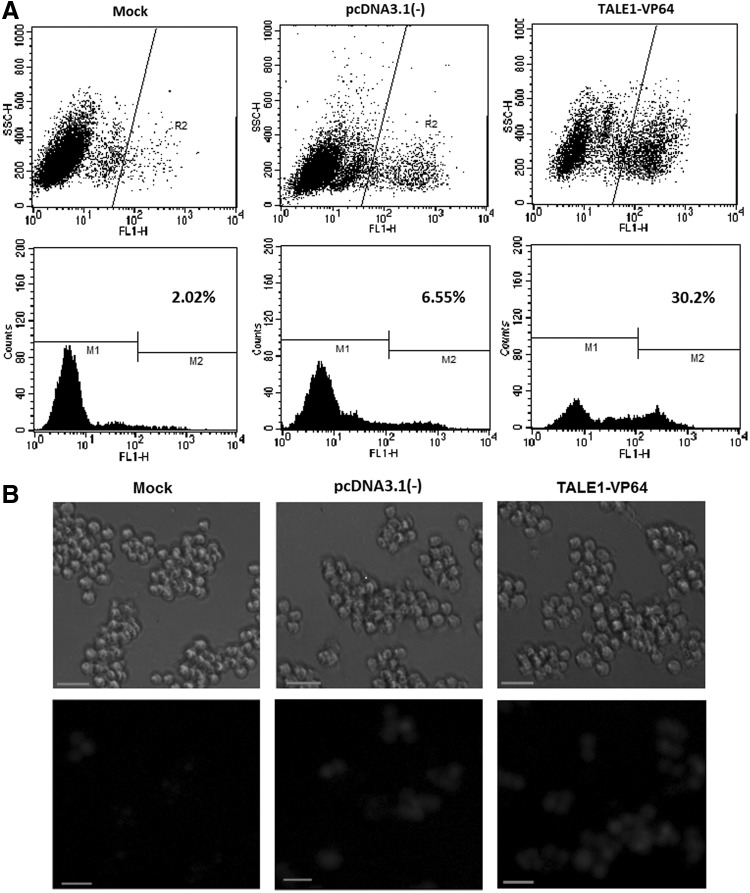
Next, we explored whether TALE1-VP64 could induce the expression of HIV-1 in latently infected cells. C11 cells, which are latently infected Jurkat cells integrated with a full-length HIV-1 genome containing the GFP open reading frame under the control of the HIV-1 LTR,27–30 were transfected with TALE1-VP64 or pcDNA3.1(-) via nucleofection. The percentage of GFP-positive cells, representing the transcriptional activity of the HIV-1 promoter, was then measured via flow cytometry. As expected, we detected a dramatic increase in GFP expression after transfection with TALE1-VP64 compared to the controls. At 48 h posttransfection, the percentage of GFP-positive cells was found to be as high as 30.2% in the TALE1-VP64 group, which was approximately 15-fold greater than that of the nontransfected cells (Fig. 3A).
FIG. 3.
Reactivation of latent HIV-1 in latently infected C11 cells by TALE1-VP64. (A) C11 cells were untransfected (mock) or transfected with pcDNA3.1(-) (2 μg) or TALE1-VP64 (2 μg). Then, 48 h posttreatment, the percentage of cells expressing green fluorescent protein (GFP) was measured via flow cytometry to determine the extent of reactivation. The results are presented as fluorescence histograms. (B) Fluorescence microscopic images of TALE1-VP64-induced GFP expression in C11 cells 48 h posttransfection. Scale bar, 100 μm.
These results were also confirmed visually via fluorescence microscopy (Fig. 3B). Furthermore, the results revealed a time dependence of TALE1-VP64 on HIV-1 reactivation in C11 cells (Fig. 4A). Similar results were found using an alternate cell line latently infected with HIV-1 (J-Lat clone A10.6 cells) (Fig. 4B and Supplementary Fig. S2). Collectively, these data indicate that TALE1-VP64 could potently activate latent HIV-1 replication.
FIG. 4.
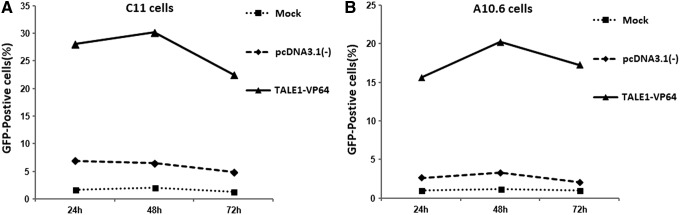
Time dependence of the effect of TALE1-VP64 on C11 and A10.6 cells. The cells were untransfected (mock) or transfected with pcDNA3.1(-) (2 μg) or TALE1-VP64 (2 μg) for the indicated periods. The level of GFP expression in (A) C11 cells or (B) A10.6 cells was measured at 24, 48, and 72 h using standard flow cytometric techniques. The data are presented as the means±standard deviations of three independent experiments.
Reactivation of latent HIV-1 in latently infected C11 cells by TALE2-VP64
TALE2-VP64 displays a slight activation potential based on the luciferase reporter assay. To determine whether TALE2-VP64 could also effectively reactivate HIV-1 expression in latently infected cells, C11 cells were transfected with TALE2-VP64 or pcDNA3.1(-) via nucleofection. At the indicted time point, the percentage of GFP-positive cells was measured via flow cytometry. Similar to the results with TALE1-VP64, we found that virus activation occurred as soon as 24 h posttransfection and the optimal activation efficiency appeared 48 h posttransfection. The results also revealed a time dependence of TALE2-VP64 on HIV-1 reactivation (Supplementary Fig. S3).
TALE1-VP64 reactivates HIV-1 by specifically binding to the HIV-LTR promoter
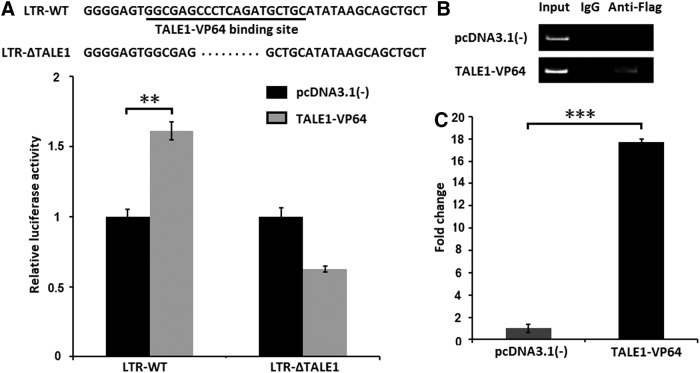
Nonspecific transcriptional activation might result in cellular toxicity and limit the application of this approach. To validate whether TALE1-VP64 reactivates HIV-1 via direct interaction with the 5′-LTR, HEK-293 cells were cotransfected with the TALE1-VP64 expression plasmid and either LTR-Luc or a mutant HIV-1 LTR-Luc reporter vector lacking the TALE1-VP64 target site [LTR(ΔTALE1)-Luc]. We found that TALE1-VP64 induced a 1.6-fold increase in the stimulation of the wild-type LTR-Luc reporter relative to the control vector pcDNA3.1(-), but failed to induce the expression of the HIV-1 LTR(ΔTALE1)-Luc reporter (Fig. 5A), indicating that TALE1-VP64-mediated activation occurred via targeting the HIV-1 LTR promoter.
FIG. 5.
TALE1-VP64 activates latent HIV-1 replication by binding to the HIV-1 LTR promoter. (A) HEK-293 cells were transfected with the HIV-1 LTR-Luc or HIV-1 LTR(ΔTALE1)-Luc reporter plasmid and the TALE1-VP64 expression plasmid; the empty vector pcDNA3.1(-) was used as a control. Then, 48 h posttransfection, the relative luciferase activity was measured. The data represent the mean±standard deviation of three independent experiments. *p<0.05, **p<0.01, ***p<0.001; paired t test. (B) C11 cells were treated with pcDNA3.1(-) (2 μg) or TALE1-VP64 (2 μg). Then, 48 h posttreatment, ChIP assays were performed using an anti-FLAG antibody or normal mouse IgG. PCR primers corresponding to the LTR promoter were used to amplify the DNA isolated from the immunoprecipitated chromatin as described in the Materials and Methods section. (C) ChIP analysis data are presented as fold change (mean value±standard error). Each ChIP experiment was repeated three times to confirm the reproducibility of the results. *p<0.05, **p<0.01, ***p<0.001; paired t test.
To further confirm the direct association between TALE1-VP64 and the HIV-1 LTR, a ChIP assay was performed. C11 cells were nucleofected with TALE1-VP64 or the control vector pcDNA3.1(-). The cells were then cross-linked with formaldehyde, and the chromatin fragments were immunoprecipitated with an antibody against the FLAG tag at the N-terminus of TALE1-VP64 or with normal mouse IgG. The amount of LTR DNA in the immunoprecipitates was assessed via PCR using primers specific for the HIV-1 LTR. Ethidium bromide visualization of the PCR products revealed that the anti-FLAG antibody efficiently immunoprecipitated the HIV-1 LTR promoter region from the TALE1-VP64-transfected cells but not the pcDNA3.1(-)-transfected cells, and no immunoprecipitated DNA fragments were detected via PCR in the normal mouse IgG controls (Fig. 5B).
The percentage of input for each immunoprecipitation was calculated, and the fold-change in occupancy of anti-FLAG at the LTR relative to the control vector was calculated. We found that immunoprecipitation using the anti-FLAG antibody enriched the TALE1-VP64 binding region of the HIV-1 promoter by 17.7-fold in chromatin relative to the nonspecific IgG control (Fig. 5C). These results further confirmed that the detected reactivation of HIV-1 occurred via a direct interaction between TALE1-VP64 and the 5′- LTR.
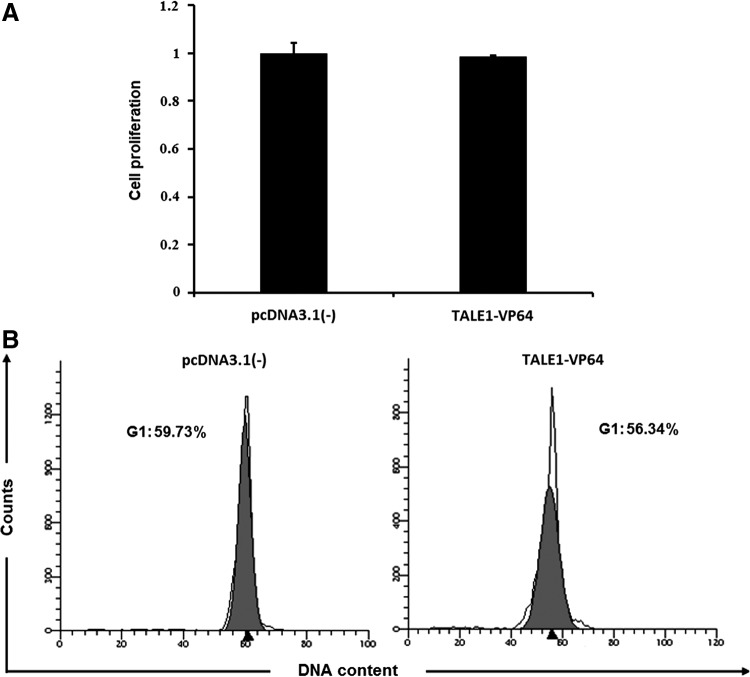
TALE1-VP64 does not affect cell proliferation or cell cycle distribution
To determine the safety of TALE1-VP64, we investigated the effects of TALE1-VP64 on cell proliferation and cell cycle distribution in PBMCs or HEK-293 cells. The cells were transfected with pcDNA3.1(-) or TALE1-VP64. After 48 h, the amount of cell proliferation was determined using a CCK-8 kit. The results revealed that equivalent to the pcDNA3.1(-)-transfected group, the TALE1-VP64-transfected group displayed no significant difference in cell proliferation (Fig. 6A and Supplementary Fig. S4A). Analysis of the cell cycle was performed by staining these cells with propidium iodide and analyzing the DNA content using a FACScan flow cytometer (Becton Dickinson). The data indicated that TALE1-VP64 did not have any substantive effect on the cell cycle distribution (Fig. 6B and Supplementary Fig. S4B).
FIG. 6.
Effects of TALE1-VP64 on cell proliferation and cell cycle distribution in human peripheral blood mononuclear cells (PBMCs). (A) PBMCs were transfected with pcDNA3.1(-) or TALE1-VP64. Then, 48 h posttransfection, the cell viability was measured using a CCK-8 kit (Dojindo). The cell viability of the transfected groups was normalized to that of the untransfected group. The data are presented as the means±standard deviations of three independent experiments. (B) PBMCs were transfected with pcDNA3.1(-) or TALE1-VP64. Then, 48 h posttransfection, the distribution of the cells in each phase of the cell cycle was determined based on their DNA content via propidium iodide staining and FACS analysis.
Discussion
HAART has been demonstrated to very successfully control HIV infection and significantly prolong the life span of infected individuals. However, due to the persistence of HIV-1 in a latent state in different cellular and anatomical reservoirs,3,41 HAART cannot completely eradicate the virus. Currently, extensive efforts are being directed toward the development of effective strategies to eliminate latent HIV-1 reservoirs. Most of these recent studies have focused on the “shock and kill” strategy, as mentioned above, and many small-molecule drugs targeting different mechanisms of HIV-1 latency have been explored. Aside from these drugs, other strategies for eradication include gene therapy approaches that directly remove integrated HIV genomes from infected cells28,42,43 and HIV-dependent suicide genes that selectively kill HIV-infected cells.44,45
Recently, our team described a novel gene therapy approach to eliminate the provirus DNA that is based on the “shock and kill” strategy.46 We demonstrated that ZF-VP64 with HIV-1 LTR promoter-specific affinity could reactivate HIV-1 expression from latently infected cells. Here, based on the same principle of reactivation of latent HIV-1, we indicated that a chimeric protein, termed TALE1-VP64, designed to target the highly conserved HIV-1 LTR promoter sequence, can also specifically and effectively reactivate latent HIV-1 transcription in vitro. To our knowledge, this was the first evidence that a fusion TALE-VP64 protein that specifically binds to the HIV-1 LTR promoter can reactivate HIV-1 expression in latently infected cells.
The primary task in generating a successful TALE activator is selecting the DNA site to be targeted. The ideal target site should be located in a chromatin region that is accessible in the chromatin structure, highly conserved in sequence structure among the various HIV-1 subtypes, and close to known transcriptional regulators of the gene of interest.47 Four candidate DNA sequences in the HIV-1 LTR were selected as described in the Materials and Methods section. All of these target sites are accessible to these DNA-binding proteins and are located in the vicinity of the transcription initiation site. Furthermore, these target sites were also confirmed to be well conserved across various HIV-1 subtypes (data not shown). One target site, that of TALE1-VP64, was immediately adjacent to NF-κB and Sp1 cis-regulatory sites (Fig. 1B). The Sp1 sites have been shown to be essential for the function of HIV-1 proviral DNA-mediated gene expression, and activators that are positioned close together in this region often act synergistically to boost transcription47; this may partially explain why TALE1-VP64 exhibited the highest activation potential.
Next, the corresponding TALE-VP64 plasmids were constructed, and their potential ability to activate HIV-1 expression was assessed in luciferase reporter-transfected cells and in latently infected cells. Among the four TALE-VP64 expression plasmids that we examined, TALE1-VP64 potently activated the expression of the HIV LTR-Luc reporter, indicating that the activation potential of TALE-VP64 may be due to the location of its binding site, similar to previous reports.23,24 The effectiveness of TALE1-VP64 as a transcriptional activator was also confirmed in latently infected cells. The activation efficiency was as high as 30.2% and 20.3% in latently infected C11 cells and A10.6 cells, respectively (Fig. 4), a proportion greater than approximately 15-fold that of the untreated cells. Interestingly, the C11 cells transfected with TALE2-VP64 via nucleofection also displayed a remarkable increase in GFP expression.
Encouraged by our achievements, the safety and specificity of TALE1-VP64 were further investigated. We performed cytotoxicity assays and found that TALE1-VP64 did not have any substantive effect on cell proliferation or cell cycle distribution in PBMCs and HEK-293 cells. Then, to determine the activation specificity of TALE1-VP64, luciferase assays were performed as described above. We found that if the target site of TALE1-VP64 was deleted from Luc-LTR, the activation of the LTR promoter disappeared completely. Furthermore, the direct association between TALE1-VP64 and the HIV-1 LTR promoter was demonstrated via ChIP assays. All of these results provide strong evidence that TALE1-VP64 specifically targets the LTR, representing the primary advantage over currently existing drugs using this strategy.
In summary, this study demonstrated that the chimeric transcriptional activator TALE1-VP64 can specifically and effectively reactivate HIV-1 with no significant toxicity. However, it is too early to suggest that this method can be used in anti-HIV-1 latency therapy since numerous hurdles are still present, such as the delivery systems and possible off-target effects, which are also widely present in other gene therapies and are constantly under development. The strategy that specifically reactivates latent HIV-1 such as the one described here will be a useful basis for the development of HIV gene therapy in the future.
Supplementary Material
Acknowledgments
We thank the NIH AIDS Research and Reference Reagent Program for providing the J-Lat clone A10.6 cells. We also thank Dr. Jianqing Xu (Shanghai Medical College of Fudan University) for providing the pNL4-3-EGFP plasmid. This study was supported by the National Grand Program on Key Infectious Disease (2014ZX10001003) and the National Natural Science Foundation of China (31271418 to H.Z.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Katlama C, Deeks SG, Autran B, et al. : Barriers to a cure for HIV: New ways to target and eradicate HIV-1 reservoirs. Lancet 2013;381(9883):2109–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyagi M. and Bukrinsky M: Human immunodeficiency virus (HIV) latency: The major hurdle in HIV eradication. Mol Med 2012;18(1):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iglesias-Ussel MD. and Romerio F: HIV reservoirs: The new frontier. AIDS Rev 2011;13(1):13–29 [PubMed] [Google Scholar]
- 4.Finzi D, Hermankova M, Pierson T, et al. : Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997;278(5341):1295–1300 [DOI] [PubMed] [Google Scholar]
- 5.Siliciano JD, Kajdas J, Finzi D, et al. : Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003;9(6):727–728 [DOI] [PubMed] [Google Scholar]
- 6.Shen L. and Siliciano RF: Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immun 2008;122(1):22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsden MD. and Zack JA: Eradication of HIV: Current challenges and new directions. J Antimicrob Chemother 2009;63(1):7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richman DD, Margolis DM, Delaney M, et al. : The challenge of finding a cure for HIV infection. Science 2009;323(5919):1304–1307 [DOI] [PubMed] [Google Scholar]
- 9.Barton KM, Burch BD, Soriano-Sarabia N, et al. : Prospects for treatment of latent HIV. Clin Pharmacol Ther 2012;93(1):46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr JS, Galloway S, Lagrutta A, et al. : Nonclinical safety assessment of the histone deacetylase inhibitor vorinostat. Int J Toxicol 2010;29(1):3–19 [DOI] [PubMed] [Google Scholar]
- 11.Matalon S, Rasmussen TA, and Dinarello CA: Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir. Mol Med 2011;17(5–6):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pion M, Jordan A, Biancotto A, et al. : Transcriptional suppression of in vitro-integrated human immunodeficiency virus type 1 does not correlate with proviral DNA methylation. J Virol 2003;77(7):4025–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JC, Tan S, Qiao G, et al. : A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 2010;29(2):143–148 [DOI] [PubMed] [Google Scholar]
- 14.Römer P, Hahn S, Jordan T, et al. : Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science 2007;318(5850):645–648 [DOI] [PubMed] [Google Scholar]
- 15.Kay S, Hahn S, Marois E, et al. : A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 2007;318(5850):648–651 [DOI] [PubMed] [Google Scholar]
- 16.Bogdanove AJ, Schornack S, and Lahaye T: TAL effectors: Finding plant genes for disease and defense. Curr Opin Plant Biol 2010;13(4):394–401 [DOI] [PubMed] [Google Scholar]
- 17.Moscou MJ. and Bogdanove AJ: A simple cipher governs DNA recognition by TAL effectors. Science 2009;326(5959):1501. [DOI] [PubMed] [Google Scholar]
- 18.Scholze H. and Boch J: TAL effector-DNA specificity. Virulence 2010;1(5):428–432 [DOI] [PubMed] [Google Scholar]
- 19.Boch J, Scholze H, Schornack S, et al. : Breaking the code of DNA binding specificity of TAL-type III effectors. Science 2009;326(5959):1509–1512 [DOI] [PubMed] [Google Scholar]
- 20.Bultmann S, Morbitzer R, Schmidt CS, et al. : Targeted transcriptional activation of silent oct4 pluripotency gene by combining designer TALEs and inhibition of epigenetic modifiers. Nucleic Acids Res 2012;40(12):5368–5377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremblay JP, Chapdelaine P, Coulombe Z, et al. : Transcription activator-like effector proteins induce the expression of the frataxin gene. Hum Gene Ther 2012;23(8):883–890 [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Cong L, Lodato S, et al. : Programmable sequence-specific transcriptional regulation of mammalian genome using designer TAL effectors. Nat Biotechnol 2011;29(2):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeder ML, Linder SJ, Reyon D, et al. : Robust, synergistic regulation of human gene expression using TALE activators. Nat Methods 2013;10(3):243–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Pinera P, Ousterout DG, Brunger JM, et al. : Synergistic and tunable human gene activation by combinations of synthetic transcription factors. Nat Methods 2013;10(3):239–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cermak T, Doyle EL, Christian M, et al. : Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 2011;39(12):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang F, Cong L, Lodato S, et al. : Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol 2011;29(2):149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding D, Qu X, Li L, et al. : Involvement of histone methyltransferase GLP in HIV-1 latency through catalysis of H3K9 dimethylation. Virology 2013;440(2):182–189 [DOI] [PubMed] [Google Scholar]
- 28.Qu X, Wang P, Ding D, et al. : Zinc-finger-nucleases mediate specific and efficient excision of HIV-1 proviral DNA from infected and latently infected human T cells. Nucleic Acids Res 2013;41(16):7771–7782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying H, Zhang Y, Zhou X, et al. : Selective histone deacetylase inhibitor M344 intervenes in HIV-1 latency through increasing histone acetylation and activation of NF-kappaB. PloS One 2012;7(11):e48832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, Qu X, Wang X, et al. : As2O3 synergistically reactivate latent HIV-1 by induction of NF-κB. Antiviral Res 2013;100(3):688–697 [DOI] [PubMed] [Google Scholar]
- 31.Jordan A, Defechereux P, and Verdin E: The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J 2001;20(7):1726–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan A, Bisgrove D, and Verdin E: HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 2003;22(8):1868–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segal DJ, Gonçalves J, Eberhardy S, et al. : Attenuation of HIV-1 replication in primary human cells with a designed zinc finger transcription factor. J Biol Chem 2004;279(15):14509–14519 [DOI] [PubMed] [Google Scholar]
- 34.Moscou MJ. and Bogdanove AJ: A simple cipher governs DNA recognition by TAL effectors. Science 2009;326(5959):1501. [DOI] [PubMed] [Google Scholar]
- 35.Eberhardy SR, Goncalves J, Coelho S, et al. : Inhibition of human immunodeficiency virus type 1 replication with artificial transcription factors targeting the highly conserved primer-binding site. J Virol 2006;80(6):2873–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaynor R: Regulation of HIV-1 gene expression by the transactivator protein Tat. Curr Top Microbiol 1995;193:51–77 [DOI] [PubMed] [Google Scholar]
- 37.Sakane N, Kwon H-S, Pagans S, et al. : Activation of HIV transcription by the viral Tat protein requires a demethylation step mediated by lysine-specific demethylase 1 (LSD1/KDM1). PLoS Pathog 2011;7(8):e1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romani B, Engelbrecht S, and Glashoff RH: Functions of Tat: the versatile protein of human immunodeficiency virus type 1. J Gen Virol 2010;91(1):1–12 [DOI] [PubMed] [Google Scholar]
- 39.Marcello A, Zoppé M, and Giacca M: Multiple modes of transcriptional regulation by the HIV‐1 Tat transactivator. IUBMB Life 2001;51(3):175–181 [DOI] [PubMed] [Google Scholar]
- 40.Ott M, Geyer M, and Zhou Q: The control of HIV transcription: Keeping RNA polymerase II on track. Cell Host Microbe 2011;10(5):426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saksena NK. and Potter SJ: Reservoirs of HIV-1 in vivo: Implications for antiretroviral therapy. AIDS Rev 2003;5(1):3–18 [PubMed] [Google Scholar]
- 42.Sarkar I, Hauber I, Hauber J, et al. : HIV-1 proviral DNA excision using an evolved recombinase. Science 2007;316(5833):1912–1915 [DOI] [PubMed] [Google Scholar]
- 43.Ebina H, Misawa N, Kanemura Y, et al. : Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep 2013;3:2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macías D, Oya R, Saniger L, et al. : A lentiviral vector that activates latent human immunodeficiency virus-1 proviruses by the overexpression of tat and that kills the infected cells. Hum Gene Ther 2009;20(11):1259–1268 [DOI] [PubMed] [Google Scholar]
- 45.Huelsmann PM, Hofmann AD, Knoepfel SA, et al. : A suicide gene approach using the human pro-apoptotic protein tBid inhibits HIV-1 replication. BMC Biotechnol 2011;11(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang P, Qu X, Wang X, et al. : Specific reactivation of latent HIV-1 with designer zinc-finger transcription factors targeting the HIV-1 5′-LTR promoter. Gene Ther 2014;21:491–495 [DOI] [PubMed] [Google Scholar]
- 47.Gräslund T, Magnenat L, Popkov M, et al. : Exploring strategies for the design of artificial transcription factors targeting sites proximal to known regulatory regions for the induction of γ-globin expression and the treatment of sickle cell disease. J Biol Chem 2005;280:3707–3714 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.