Abstract
Subsets of CD16-positive monocytes produce proinflammatory cytokines and expand during chronic infection with the human immunodeficiency virus type 1 (HIV). HIV-infected macrophage in tissues may be long lived and contribute to the establishment and maintenance of the HIV reservoir. We found that the (intermediate) CD14++CD16+ and (nonclassical) CD14+CD16++ monocyte subsets are significantly expanded during infection of Rhesus macaques with pathogenic SIVmac251 but not during infection of sooty mangabeys with the nonpathogenic isolate SIVSM. In vitro glucocorticoid (GC) treatment of peripheral blood mononuclear cells (PBMCs) from uninfected or SIVmac251-infected Rhesus macaques and HIV-infected patients treated or not with antiretroviral therapy (ART) resulted in a significant decrease in the frequency of both CD16-positive monocyte subsets. Short-term in vivo treatment with high doses of GC of chronically SIVmac251-infected macaques resulted in a significant decrease in the CD14+CD16++ population and, to a lesser extent, in the CD14++CD16+ monocytes, as well as a significant decrease in the number of macrophages in tissues. Surprisingly, treatment of SIVmac251-infected macaques with ART significantly increased the CD14++CD16+ population and the addition of GC resulted in a significant decrease in only the CD14+CD16++ subset. No difference in SIV DNA levels in blood, lymph nodes, gut, and spleen was found between the groups treated with ART or ART plus GC. Thus, it appears that high doses of GC treatment in the absence of ART could affect both CD16-positive populations in vivo. Whether the efficacy of this treatment at higher doses to decrease virus levels outweighs its risks remains to be determined.
Introduction
Monocytes/macrophage expressing the CD16 surface marker may be a target of HIV infection in vitro.1,2 In contrast to CD4+ T cells, HIV-infected mononuclear phagocytes are highly resistant to the cytopathic effect of HIV3 and might persist for longer periods of time as a cellular reservoir for the virus in vivo. In humans, monocytes can be divided into three main subsets based on the differential expression of CD14 and CD16.1,4 The majority of monocytes are CD14++CD16− (designated as classical) while the remaining two subsets are CD14++CD16+ (intermediate) and CD14+CD16++ (nonclassical) monocytes. There is a developmental relationship between these subsets, with classical being the least and nonclassical being the most differentiated.4–6 Monocytes leave bone marrow as CD14++CD16− cells and, once in the peripheral blood, a fraction of them become CD14++CD16+ cells, which may in turn become CD14+CD16++ cells.5,7 The developmental steps from CD14++CD16− to CD14++CD16+ and to CD14+CD16++ are thought to ultimately generate cells that home to the tissue and give rise to different types of terminally differentiated macrophage.4–10 Indeed, the pan-macrophage marker CD68 is mostly present on CD14+CD16++ subsets, whereas MAC387, the marker that is expressed on monocytes and downregulated on CD14++CD16− macrophage, is not expressed by CD14+CD16++ monocytes.4,11
CD16+ monocyte subsets are expanded in inflammatory and infectious conditions such as in neurological diseases, atherosclerosis, coronary heart disorders, cancer, and aging.4,12–19 Both the CD14++CD16+ and CD14+CD16++ monocyte subsets are expanded in the peripheral blood of HIV-infected subjects, suggesting that infection by HIV of this cellular compartment may contribute to the virus reservoir.20–22 Remarkably, HIV-infected macrophage populations derived from CD16-positive monocytes, in contrast to those derived from CD16-negative monocytes, are better able to transfer the virus to resting CD4+ T cells in vitro.23 Monocyte and macrophage populations infected with HIV have been reported in patients on suppressive antiretroviral therapy24–26 and in some of these patients, the HIV DNA copy number in CD16-positive monocytes was comparable to that of resting CD4+ T cells.20,24,25 Thus, it is possible that CD16-positive monocytes contribute to the maintenance of the HIV reservoir in vivo and that, reciprocally, therapeutic strategies aimed at decreasing the number of CD16-positive monocytes may further decrease this reservoir.
SIVmac251 infection of nonhuman primates provides a valuable model for the analysis of HIV pathogenesis as well as for preclinical testing of potential therapeutic approaches for HIV infection. Since corticosteroid treatment has been found to decrease the number of CD16-positive monocytes in patients with multiple sclerosis or asthma27,28 in a caspase-dependent manner,29 we hypothesized that glucocorticoid (GC) treatment might similarly affect the frequency of CD14++CD16+ and CD14+CD16++ monocytes circulating within the SIVmac251-infected Rhesus macaque. Our results demonstrate that, as in the case in HIV infection of humans, the CD16-positive subsets are expanded in macaques infected with pathogenic SIVmac251. Interestingly, however, no significant change in these subsets was observed in sooty mangabeys infected with the nonpathogenic isolate SIVSM. In vitro GC treatment of peripheral blood mononuclear cells (PBMCs) from SIVmac251-infected macaques resulted in significant depletion of both the CD14++CD16+ and CD14+CD16++ monocyte subsets. Likewise, in vivo treatment of SIVmac251-infected macaques with high doses of GC resulted in a significant decrease in both the CD16-positive monocyte subsets in blood as well as a decrease in the number of tissue macrophage. Prolonged in vivo treatment of SIVmac251-infected macaques with antiretroviral therapy (ART) and lower doses of GC resulted in a significant reduction of the CD14+CD16++ population and GC treatment at this dose did not affect the levels of SIV DNA in blood and tissue.
Materials and Methods
Animals and treatment
In the first study, we used 12 sooty mangabeys, six uninfected and six infected with SIVSM (Table 1), and 23 Indian Rhesus macaques (Macaca mulatta). Of these, eight were uninfected and 15 had been infected with SIVmac251 (Table 1). Of the 15 infected animals six were cultured with GC for 24 h in vitro and five were treated intravenously with 3.5 mg/kg of methylprednisolone for 4 days; on days 5 and 6 the daily dose of methylprednisolone was further increased to 21 mg/kg. Blood was collected during treatment and tissues were collected at time of euthanasia at day 7. The plasma virus level and CD4+ T cell levels in all these animals are summarized in Table 1.
Table 1.
Plasma Virus Levels and CD4+ T Cell Levels of Animals
| Animal number | CD4+ T cells/m3 | Plasma viral (RNA) copies/ml | Glucocorticoid treatment (high dose) |
|---|---|---|---|
| Sooty mangabeys: infected | |||
| FAv | 811 | 45,300 | − |
| FFv | 1,032 | 8,330 | − |
| FYs | 430 | 158,000 | − |
| FKN | 832 | 22,800 | − |
| FLI | 1,078 | 372,000 | − |
| FSG | 437 | 79,500 | − |
| Sooty mangabeys: uninfected | |||
| FYY | 1,314 | − | − |
| FHQ | 770 | − | − |
| FCR | 503 | − | − |
| FGA | 1,276 | − | − |
| FMZ | ND | − | − |
| FFZ | ND | − | − |
| Rhesus macaques: uninfected | |||
| 733 | 1,493 | − | − |
| 734 | 1,472 | − | − |
| 735 | 1,051 | − | − |
| 736 | 951 | − | − |
| 737 | 1,681 | − | − |
| 738 | 1,447 | − | − |
| 739 | 1,671 | − | − |
| 740 | 800 | − | − |
| Rhesus macaques: infected | |||
| 439 | 2,155 | <50 | + |
| 442 | 2,096 | <50 | + |
| P126 | 315 | 19,643 | −a |
| P127 | 133 | 193,240 | −a |
| P132 | 383 | 91,813 | −a |
| P135 | 106 | 7,085,377 | −a |
| P146 | 227 | 21,509,892 | −a |
| P797 | 87 | 11,213,357 | −a |
| 421 | 1,530 | 15,850 | + |
| 444 | 1,509 | 530 | + |
| 507 | 1,661 | 2,213 | + |
| P064 | 290 | <50 | −b |
| P149 | 167 | 5,548,303 | −b |
| P161 | 1,203 | 8,798 | −b |
| M903 | 586 | <50 | −b |
In vitro glucocorticoid treatment.
Antiretroviral therapy: didanosine+stavudine+tenofovir.
In a second study (shown in Fig. 4A), 15 SIVmac251-infected Indian Rhesus macaques were treated with ART and, of those, eight were also treated with glucocorticoid at a low dose (2 mg/kg) daily for 5 weeks. Details about the prior treatment, the plasma virus levels, and the CD4+ T cell levels of these monkeys are summarized in Table 2. These 15 macaques were exposed intravaginally to SIVmac251 and treated with ART 6 months after infection. Viral levels in these animals ranged from 59,004 to 9,583,762 viral RNA copies per/ml (Table 2). All 15 macaques were treated for 11 weeks with tenofovir (20 mg/kg/day) and emtricitabine (50 mg/kg/day) subcutaneously, alongside stavudine (1.2 mg/kg/bid) and raltegravir (100 mg/bid) orally. Eight macaques also received solumedrol (2 mg/kg/day) intravenously for 5 weeks in addition to ART. Blood and tissues were collected at week 2 and the animals were sacrificed at week 11. Rhesus macaques were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International, and the study was reviewed and approved by the animal care and use committee at Advanced Bioscience Laboratories (Rockville, MD). Sooty mangabeys were housed at the Yerkes National Primate Research Center of Emory University and maintained in accordance with U.S. National Institutes of Health guidelines. Anesthesia was used for all blood and tissue collections.
FIG. 4.
Prolonged GC treatment at low doses in ART-treated SIVmac251-infected Rhesus macaques. Study design (A) (n=7). All animals were treated with ART and eight also received GC. Changes of the absolute number of CD14++CD16−, CD14++CD16+, and CD14−CD16++ monocytes during 11 weeks of ART or ART plus GC significantly (p=0.0023) decreased the CD144++CD16− subset whereas an intermediate subset (CD14++CD16+) significantly increased (p=0.0105) (B). ART plus GC treatment significantly decreased the CD14++CD16+ subset (p=0.0002) (C). The Mann–Whitney t-test was used to compare each group at given time points.
Table 2.
Prior Treatment, Plasma Virus Levels, and CD4+ T Cell Levels of Animals
| Animal numbera | CD4+T cells/m3 | Plasma viral (RNA) copies/ml at start of ART treatment | ART/GC treatment |
|---|---|---|---|
| P455 | 808 | 51,529 | ± |
| P458 | 338 | 204,289 | ± |
| P459 | 767 | 9,583,762 | ± |
| P463 | 57 | 52,232 | ± |
| P469 | 507 | 33,508 | ± |
| P768 | 91 | 520,989 | ± |
| P792 | 66 | 1,259,330 | ± |
| P446 | 900 | 345,907 | +/+ |
| P453 | 900 | 587,252 | +/+ |
| P462 | 291 | 172,895 | +/+ |
| P465 | 269 | 82,226 | +/+ |
| P466 | 1,176 | 65,497 | +/+ |
| P775 | 335 | 701,216 | +/+ |
| P778 | 85 | 4,263,399 | +/+ |
| P789 | 336 | 59,004 | +/+ |
All the animals were part of a prior study (Shari Gordon et al., 2013, submitted) and became viremic following vaginal challenge and SIVmac251.
ART, antiretroviral therapy; GC, glucocorticoid.
Human samples
PBMC samples were obtained from healthy volunteers and HIV-infected individuals treated or not with ART. Samples were obtained after informed consent and the2 protocol was approved by the institutional ethical committee in Bialystok. Details on the CD4+ T cell counts, plasma virus levels, and drug regimens in each HIV-infected individual are summarized in Table 3.
Table 3.
CD4+ T Cell Counts, Plasma Virus Levels, and Drug Regimens of HIV-Infected Individuals
| Humans | ||||
|---|---|---|---|---|
| Patient | Sex | Absolute CD4+ counts | Viral loads (RNA copies/ml plasma) | ART |
| 1 | M | 635 | 1,830 | — |
| 2 | F | 500 | 8,650 | — |
| 3 | F | 345 | 7,110 | — |
| 4 | F | 514 | 1,670 | — |
| 5 | M | 17 | 58,700 | — |
| 6 | F | 370 | <17 | Emtricitabine/tenofovir/efavirenz |
| 7 | M | 427 | 134 | Lopinavir/ritonavir+lamivudine/zidovudine |
| 8 | M | 313 | 716 | Tenofovir/abacavir/atazanavir |
| 9 | F | 1,129 | <50 | Lamivudine/zidovudine/maraviroc |
| 10 | M | 514 | <20 | Lopinavir/ritonavir/atazanavir |
| 11 | F | 662 | <47 | Tenofovir/emtricitabine saquinavir/ritonavir |
Cell culture and flow cytometry
PBMCs from animals or humans were isolated from EDTA-anticoagulated whole blood samples by density gradient sedimentation as described previously.30 Then 1×106 PBMCs were cultured with either medium plus methylprednisolone at a 10–5 M concentration or medium alone as a negative control. The cultures were incubated at 37°C in a humidified 5% CO2 atmosphere for 24 h. After incubation, cells were washed in Dulbecco's phosphate-buffered saline (D-PBS, Invitrogen) with 2% fetal bovine serum, harvested, and processed immediately for surface staining. PBMCs were washed twice with D-PBS, and the concentration was adjusted to 2×105 cells/ml. Cells were stained with 5 μl of fluorochrome-conjugated monoclonal antibodies: anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD16, and anti-CD195 (CCR5) (BD Biosciences). Cells were incubated for 30 min at room temperature, washed twice with D-PBS, and fixed with CellFix (BD Biosciences). For the analysis of tumor necrosis factor (TNF)-α release, cells were incubated for 24 h with lipopolysaccharide (LPS) at 1 μg/ml (Sigma) in the presence of GolgiPlug (1 μl/ml) (BD Biosciences) with methylprednisolone at a 10–5 M concentration or medium alone as a negative control. Cell aliquots prepared for intracellular cytokine analysis were permeabilized with Perm/Wash (BD Biosciences) and incubated for 30 min at room temperature with 20 μl of anti-Ki-67 and anti-FoxP3 or 15 μl of TNF-α monoclonal antibodies and appropriate isotype-matched controls (all from BD Biosciences). Events were acquired on a FACSCalibur (BD Biosciences) for the first part of the study and the LSR11 (BD Bioscience) for the second part of the study. Data analysis was done with CellQuest Pro or FlowJo 9.4 (TreeStar) software, using isotype controls and fluorescence-minus-one control as negative controls for each population.
Bronchoalveolar lavage samples were collected on day 0 and day 7 of the glucocorticoid treatment. The samples were centrifuged and the cells stained with CD3 Alexa700 (Clone SP-34-2), CD45 PE-Cy7 (Clone D058-1283), CD16 Pacific Blue (Clone 3G8), CD14 FITC (Clone M5E2) (all BD Biosciences), and CD8b ECD (Clone 2ST8.5H7, Beckman Coulter). Viability was determined using the Yellow Live/Dead Amine Binding Dye (Invitrogen). All samples were acquired on an LSRII flow cytometer with FACS DIVA 6.2 software (BD Biosciences) and analyzed using FlowJo 9.4 software (TreeStar).
Cell counts in the blood
CD4+, CD8+, and CD20+ T cell counts were periodically determined from whole blood and by fluorescence-activated cell sorting analysis according to the FACS/Lyse kit (BD Immunocytometry Systems, San Jose, CA), and analyses were performed using a FACSCalibur Flow Cytometer (BD Biosciences, San Jose, CA). The antibodies used were CD3 (clone SP34) and CD4 (clone L200) from BD Biosciences, CD8 (clone DK25) from DakoCytomation (Carpinteria, CA), and CD20 (clone B9E9) from Beckman Coulter (Fullerton, CA).
Plasma and cell-associated virus measurements
SIV and viral RNA in plasma was measured by NASBA.31 Plasma was clarified by centrifugation at 2,300×g for 3 min. Nucleic acid was isolated as described previously4 and then analyzed by real-time nucleic acid sequence-based amplification, as described before.4 The copy number was expressed per 100 μl of plasma. The real-time nucleic acid sequence-based amplification assay had a lower limit of sensitivity of 50 copies of RNA.
Genomic DNA was extracted from the tissues, spleen, lymph node, and jejunum, with the DNeasy Blood & Tissue kit (Qiagen, Valencia, CA) according to the manufacturer's protocol except for the DNA elution step.32 The quantity and quality of the DNA were assessed by OD260 measurements using an ND-1000 spectrophotometer (NanoDrop, Wilmington, DE). The TaqMan probe and PCR primers for the real-time PCR were designed within the conserved gag gene of SIVmac239,33 and published probe and primer sequences were used for the monkey albumin gene detection.34 Briefly, the reaction conditions were as follows: the 25 μl PCR mixture consisted of 500 ng of DNA extracted from tissues; 200 nM primers; 100 nM probe; 2×TaqMan Universal PCR Mastermix (Applied Biosystems, Foster City, CA) consisting of 10 mM Tris–HCl (pH 8.3); 50 mM KCl; 5 mM MgCl2; 300 μM each of dATP, dCTP, and dGTP; 600 μM dUTP; 0.625 U of AmpliTaq Gold DNA polymerase; and 0.25 U uracil N-glycosylase (UNG). Amplification was performed using 1 cycle at 50°C for 2 min and 1 cycle at 95°C for 10 min followed by a two-step PCR procedure consisting of 50 cycles of 15 s at 95°C and 1 min at 60°C. PCR amplification was performed using the ABI Prism 7500 Sequence Detector System (Applied Biosystems, Foster City, CA).
Immunohistochemistry
FFPE rectal tissues from five macaques were deparaffinized and then digested with protease, Type VIII enzyme (Sigma P5380), after which the endogenous alkaline phosphatase was blocked with cold 20% acetic acid followed by serum block. The primary antibody, mouse antihuman macrophage (HAM56 DAKO), was added and detected on tissue sections using the ABC-alkaline phosphatase detection method (Vector Labs) and Vector Red substrate. The samples were counterstained with hematoxylin and photographed with a Zeiss Axio Imager Z1 microscope. Image analysis was conducted as follows: 10 random high-powered fields (HPF) were collected from each tissue (rectal biopsies and rectal tissue from necropsy). Each group of 10 photos was analyzed with the Axiovision Measurement program supplied by Zeiss. Total numbers of macrophage per HPF were determined by this method.
Statistical analysis
Graph Prism 6 was used for statistical analysis of the data sets. We used either t-tests, a one-way ANOVA tests or Mann–Whitney tests to analyze statistically significant differences among groups. The paired t-test was used to analyze the pretreatment and posttreatment values. In all tests, statistically significant results were identified by a p value of<0.05.
Results
Differential distribution of monocyte subsets in pathogenic and nonpathogenic lentiviral infection of Rhesus macaques, sooty mangabeys, and humans
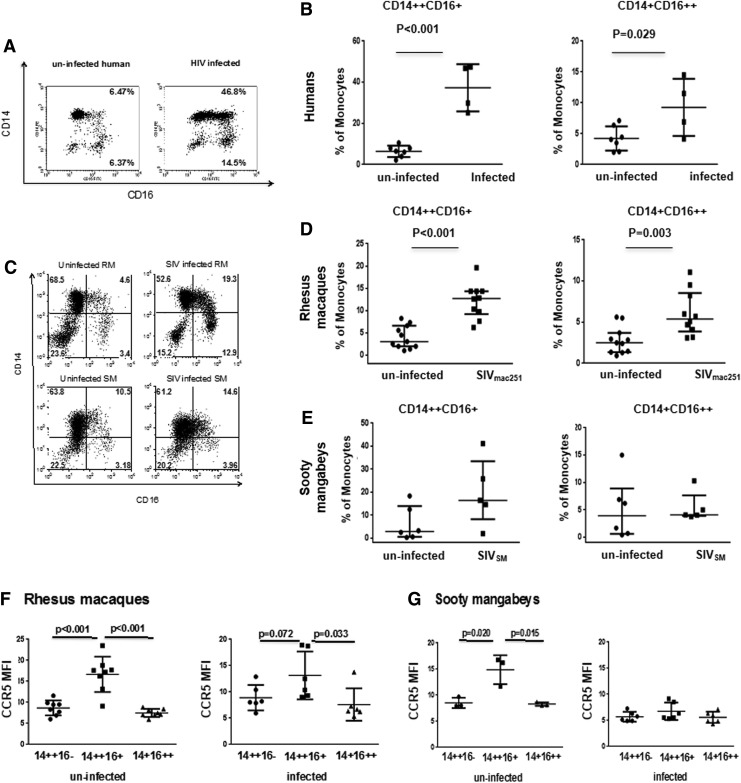
In HIV-infected individuals, both the CD14++CD16+ and the CD14+CD16++ monocyte subsets are enriched.20–22 We investigated whether infection with pathogenic and nonpathogenic lentiviruses affected these cell subsets by performing a cross-sectional analysis in Rhesus macaques (RM) infected or not with the pathogenic SIVmac251, as well as in sooty mangabeys (SM) infected or not with the nonpathogenic SIVSM (Tables 1 and 2). In parallel, we also studied these monocyte subsets in six uninfected and six HIV-1-infected individuals (Table 3). In HIV-infected patients, there was a significant increase in both the CD14++CD16+ and CD14+CD16++ subsets (Fig. 1A and B). Similarly, SIVmac251-infected macaques had a significantly higher number of both CD14++CD16+ (intermediate) and CD14+CD16++ (nonclassical) monocytes in blood than naive macaques (Fig. 1C and D) (p<0.001 and p=0.003, respectively). Interestingly, however, in SIVSM -infected or uninfected sooty mangabeys, the frequencies of either the CD14++CD16+ or the CD14+CD16++ monocyte population did not differ significantly (Fig. 1E).
FIG. 1.
Differential expression of CD14 and CD16 on the surface of peripheral blood monocytes in Rhesus macaques, sooty mangabeys, and humans. Representative flow cytometric analysis of CD14+CD16+ monocyte in one healthy volunteer and an HIV-infected individual (A). Mean percentage of CD14++CD16+ and CD14+CD16++ in uninfected and HIV-infected individuals (C). Flow cytometric density plots characteristic for healthy and SIVmac251-infected Rhesus macaques and sooty mangabeys (B). Three distinct monocyte subsets were delineated by lines that were drawn based on fluorescence-minus-one (FMO) controls: classical CD14++CD16−, I—intermediate CD14++CD16+, and NC—nonclassical CD14+CD16++. Mean frequency of CD14++CD16+ and CD14+CD16++ monocyte subsets in healthy and SIVmac251-infected Rhesus macaques (D) and SIVSM-infected and uninfected sooty mangabeys (E). Plots represent means while whiskers represent standard deviation (SD). Statistically significant differences between groups are indicated. Mean fluorescence intensity (MFI) of CCR5 expression in different monocyte subsets in Rhesus macaques (RM) (F), uninfected sooty mangabeys, and SIVSM-infected sooty mangabeys (SM) (G). Plots represent means while whiskers represent standard deviation. Statistically significant differences between groups are indicated. The paired t-test was used in the statistical analysis in B, C, D, E, and G.
To further characterize the differences between the RM and SM, we analyzed the mean level of expression of the chemokine receptor CCR5, which is used by both HIV and SIV for entry, on monocyte subsets of uninfected or infected RM and SM. CCR5 expression was significantly higher in the CD14++CD16+ populations in uninfected RM and SM (Fig. 1F and G). However, the difference was less apparent in the context of the infection by both lentiviruses. The expression of CCR5 was 3-fold lower in the CD14++CD16+ populations of SIVSM-infected sooty mangabeys (Fig. 1G), which is consistent with the previous findings.35,36
In vitro GC treatment of macaque and human PBMCs decreases the frequency of the CD16+ monocytes
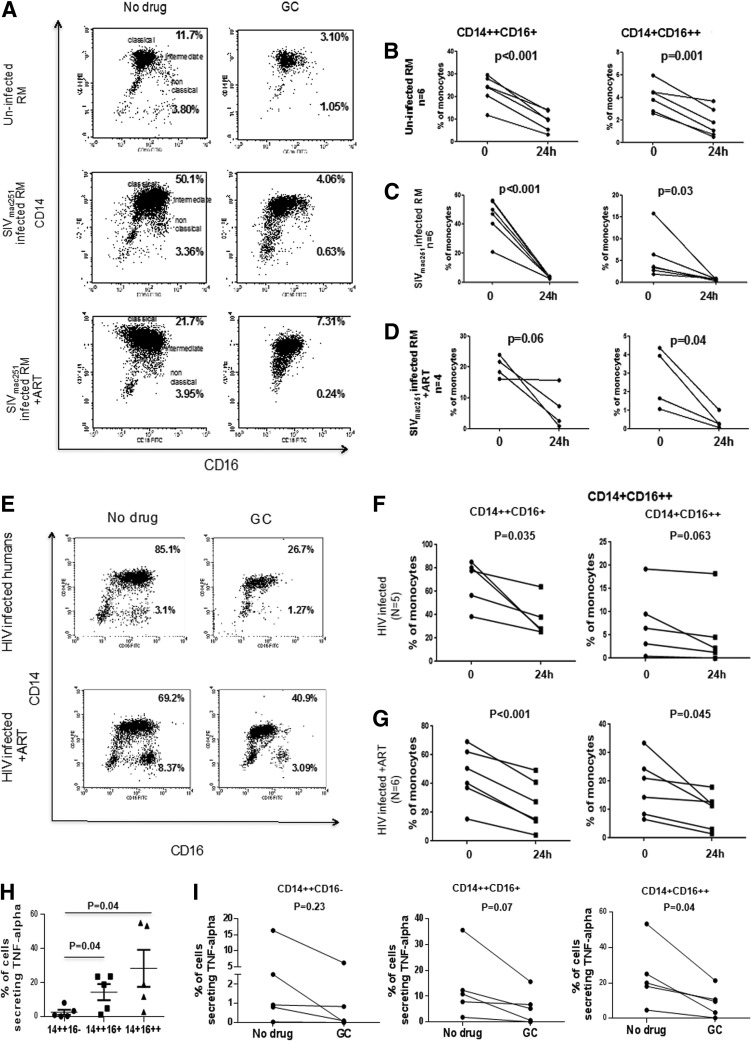
We next assessed the in vitro impact of GC treatment on monocyte subsets in PBMCs obtained from uninfected or from SIVmac251-infected macaques and HIV-infected humans, respectively. PBMCs were cultured for 24 h with GC and the monocyte subsets were enumerated in the presence or absence of the drug. The frequencies of CD14++CD16+ monocytes in cultured samples (even unstimulated) are usually higher than that found in ex vivo samples from the same host27 and GC responsiveness in nonhuman primates has been reported to be less pronounced than in humans.37 In vitro treatment with GC decreased the frequency of CD14++CD16+ monocytes (intermediate) and the change was significant for the CD14+CD16++ monocyte population (nonclassical) in the PBMCs of uninfected macaques (Fig. 2B), as well as those infected with SIVmac251 (Fig. 2C), including the four animals treated with ART (Fig. 2D). Similarly, in vitro GC treatment of PBMCs from 10 HIV-infected individuals treated or not with ART (Table 3) resulted in a significant decrease in the frequency of intermediate and of nonclassical monocytes (Fig. 2E–G). The effect of GC treatment in vitro is thought to be due to the death of monocytes due to the activation of caspase 3.27,29 Moreover, we confirmed conditions settings that both CD14++CD16+ and CD14+CD16++ monocytes secrete TNF-α (Fig. 2H). Interestingly, we observed that in vitro GC treatment significantly decreased TNF-α release only in the CD14+CD16++ monocytes (Fig. 2I).
FIG. 2.
Effects of ex vivo treatment with glucocorticoid on phenotype and function of CD14++CD16+ and CD14+CD16++ monocytes. (A) Representative flow cytometric dot plots in monocyte subsets following 24-h GC culture of samples derived from uninfected RM (upper panel), SIVmac251-infected RM (middle panel), and SIVmac251-infected RM treated with antiretroviral therapy (ART) (lower panel). Data are derived from samples cultured for 24 h in the absence (left) or presence (right) of glucocorticoid (GC). The numerical value refers to the frequencies of CD14++CD16+ monocytes (upper right) and CD14+CD16++ monocytes (lower right). Changes in the percentage of monocyte subsets in ex vivo PBMCs from uninfected RM (B), SIV chronically infected RM (C), and ART-treated RM (D) following in vitro culture with GC for 24 h. Representing flow analysis of monocyte subsets in peripheral blood mononuclear cells (PBMCs) from an HIV-infected (top) and ART-treated (lower) individual untreated (left) and GC treated (right) (E). Change in the frequency of CD14++CD16+ex vivo in HIV-infected (F) and ART-treated (G) individuals incubated with GC for 24 h. Assessment of tumor necrosis factor (TNF)-α secretion by different monocyte subsets derived from uninfected humans following in vitro lipopolysaccharide (LPS) stimulation (H). Changes in the percentage of monocyte subsets secreting TNF-α following in vitro culture with GC for 24 h (I). The paired t-test was used in the statistical analysis.
Short-term in vivo treatment of macaques with high doses of GC decreases the frequency of both CD14++CD16+ and CD14+CD16++ monocytes
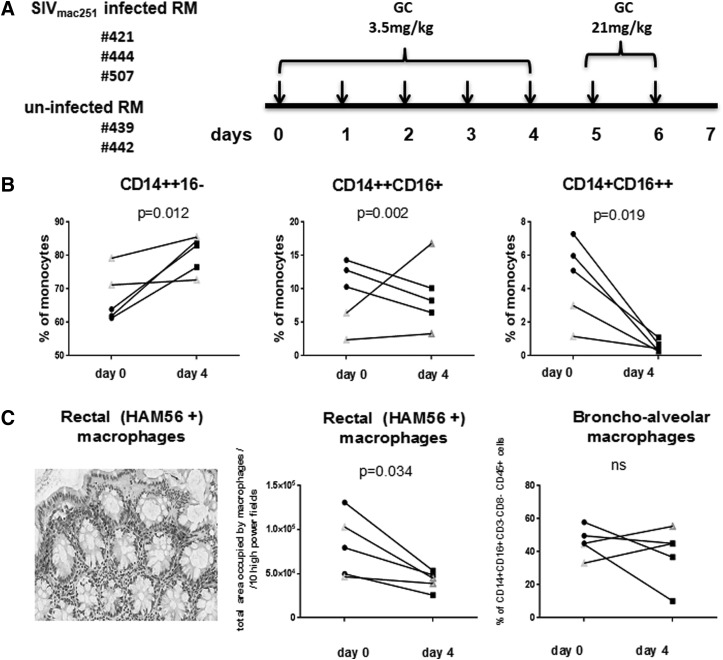
Since the CD16+ monocyte population was decreased by in vitro GC treatment, we tested whether the same effect could be observed after in vivo treatment with GC of SIVmac251-infected or uninfected Rhesus macaques. In this exploratory study, we analyzed three macaques infected with SIVmac251 (421, 444, and 507) and two that had been exposed to SIVmac251 but remained uninfected (439 and 442) (Table 1). We treated them with GC at a dose of 3.5 mg/kg of body weight for 4 consecutive days and then with 21 mg/kg for 2 additional days (Fig. 3A). By day 4 of GC treatment, there was an increase in the percentage of classical monocytes and a decrease in the frequency of the CD14++CD16+ and CD14+CD16+ subsets in all three SIVmac251-infected macaques (Fig. 3B). In the infected SIVmac251 macaques that had undetectable plasma viral RNA, GC also decreased the percentage of CD14+CD16++ monocytes in both animals but not CD14++CD16+ monocytes (Fig. 3B). We extended our analysis to enumerate tissue macrophage by immunohistochemistry on both rectal and bronchoalveolar samples collected from all animals before the start of treatment and compared them with samples collected at the time of necropsy (day 7). At the end of high-dose GC treatment, we observed a significant decrease (p=0.034) in the total number of macrophage in rectal tissue, but not in bronchoalveolar lavage (Fig. 3C), despite the high dose treatment with GC.
FIG. 3.
In vivo treatment of macaques with a high dose of GC. Schematic representation of the first study. (A) Three SIVmac251-infected RM and two uninfected RMs were treated for 4 days with 3.5 mg/kg GC and the remaining 2 days with 21 mg/kg GC. After 7 days, animals were sacrificed. Frequencies of CD14++CD16−, CD14++CD16+, or/and CD14+CD16++ monocytes before and after 4 days of GC (B) treatment. Filled symbols represent SIV-infected macaques. Open symbols represent uninfected macaques. The p-values were calculated for SIVmac251-infected animals (n=3). Detection of rectal macrophage by staining with HAM56 antibody (left panel) (C). Analysis of total area occupied by HAM56-positive rectal macrophage per 10 high-power fields before and after GC treatment (middle panel) (C). Frequencies of CD14+CD16+CD3−CD8−CD45+ bronchoalveolar macrophage before and after GC treatment (right panel). (C) Filled symbols represent SIVmac251-infected macaques; open symbols represent uninfected macaques. The paired t-test was used in the statistical analysis.
We assessed the effect of short-term GC treatment on T cell activation, T cell number, and plasma virus levels after 4 days of GC treatment. No significant changes in the frequency of CD4+ and CD8+ T cells or in the expression of Ki67, CD25, and FoxP3-expressing cells were seen in peripheral blood, regardless of infection (data not shown). Similarly, we found no significant changes in plasma virus levels following 4 days or 6 days of GC treatment (data not shown).
Treatment with moderate doses of GC decreased the frequency of CD14+CD16++ subsets and did not affect SIV DNA levels in tissues of SIV-infected macaques on ART
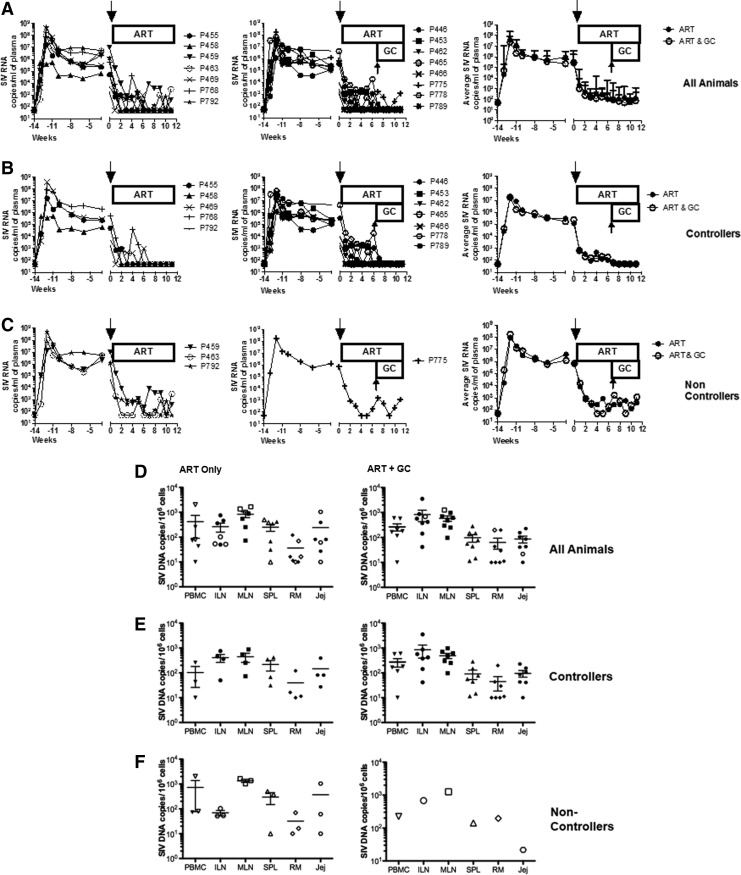
To assess the in vivo effect of GC at doses typically used in the clinic, we investigated whether a prolonged, moderate dose GC treatment of SIV-positive Rhesus macaques affected the frequency of proinflammatory monocytes and the viral reservoir. We used 15 SIVmac251 chronically infected macaques on ART (Table 2). Eight of these animals were also treated with GC at the moderate dose of 2 mg/kg of body weight for 41 consecutive days (Fig. 4A). ART resulted in a significant decrease in the frequency of classical CD14++CD16− monocytes in blood (Fig. 4B) and an unexpected significant increase in the intermediate CD14++CD16+ subset, leaving unaltered the nonclassical CD4+CD16++ monocyte subset. The GC treatment significantly reduced only the nonclassical (CD14+CD16++) subpopulation (Fig. 4C). To assess whether GC treatment affected plasma viral RNA levels or viral DNA in tissue, the animals treated with ART with or without GC were separated according to their responsiveness to ART in “controller” that had undetectable viral RNA (<50 copies) in plasma after 8 weeks of ART treatment (Fig. 5A and B) and “noncontroller” that had detectable virus in plasma during the same interval (Fig. 5C). GC treatment did not significantly change plasma virus levels in all animals (Fig. 5A) or in “controllers” and “noncontrollers” (Fig. 5B and C). The total number of CD4+ or CD8+ T cells or of B cells was also not affected by GC treatment (Supplementary Figs. S1, S2, and S3, respectively; Supplementary Data are available online at www.liebertpub.com/aid). Analysis of SIV DNA levels in PBMCs, iliac or mesenteric lymph nodes, spleen, and rectal mucosa did not reveal differences among the ART plus GC-treated versus the ART only group, indicating that prolonged treatment with moderate doses of GC did not affect the SIV-DNA levels in tissues (Fig. 5D, E, and F).
FIG. 5.
Effects of glucocorticoid treatment on plasma and cell-associated virus level in the blood and tissues of ART and ART plus GC-treated animals. SIV viral RNA copies per ml in the plasma of animals treated with ART with or without GC for all animals (A). Animals with viral RNA levels below the level of detection (<50 copies/ml), below 8 at 12 weeks, were designated as controllers (B) and animals with detectable viral RNA levels (>50 copies/ml) were designated as noncontrollers (C). Cell-associated SIV DNA virus levels in PBMCs, lymph nodes, spleen, jejunum, and rectum in ART-treated animals (left) compared to ART animals treated with GC (right) (D, E, F).
Discussion
ART in humans targets a combination of essential steps in HIV replication, including reverse transcription, integration, protease cleavage, as well as viral entry and has profoundly changed the clinical outcome of HIV infection.38 Nevertheless, prolonged ART fails to eradicate HIV. Indeed, upon ART interruption, virus replication and infection of new cells resume within weeks,39,40 demonstrating a residual viral reservoir that cannot be eradicated by ART. CD16-positive monocytes may contribute to the HIV cellular reservoir because they are permissive to HIV infection in vitro and are more resistant than CD4+ T cells to virus-induced cell death. Immune activation is a hallmark of HIV infection and proinflammatory CD16-positive monocytes can release cytokines11 that, in turn, contribute to the maintenance of immune activation. Recently, nonclassical CD16++ monocytes collected from HIV-infected patients were described as a predominant source of TNF-α release following stimulation with LPS.41 A similar pattern of TNF-α secretion was confirmed in our study. One of the pathogenic consequences of HIV infection is the translocation of microbial products such as LPS from the gastrointestinal tract to the blood.42 Thus, CD16++ monocytes by responding to LPS may play an important part in chronic generalized immune activation, which is associated with disease progression during HIV infection. Consistent with CD16++ monocytes playing a role in the pathogenesis of lentiviral infections, we observed a significant expansion of CD14++CD16+ and CD14+CD16++ monocytes in the highly pathogenic SIV infection of Rhesus macaques. In sharp contrast, no significant difference in CD16++ monocytes was observed in nonpathogenic SIV infection of sooty mangabeys. The contribution of CD16-positive monocytes to HIV/SIV pathogenesis may not be limited to their ability to trigger and maintain immune activation, but also include their ability to migrate into tissues, pass the blood–brain barrier, and, possibly, to differentiate into long-lived HIV-infected macrophages.43
CD16+ monocytes may be involved in the pathogenesis of HIV infection as well as other inflammatory disorders.13–18 CD16-positive monocytes constitute more mature stages of monocyte development and they share many phenotypic features with macrophages. In the current study, we wished to target CD16-positive monocytes by GC treatment not only to deplete this proinflammatory and prothrombotic subset,44 but also to decrease tissue macrophage-associated SIV-DNA that may contribute to the persistence of the viral reservoir. CD16+ monocytes may also play a role in the pathogenesis of other inflammatory disorders.13–18
We demonstrated that suboptimal antiretroviral treatment of macaques decreases the size of the circulating subset of classical monocytes. We also found that short-term, high-dose GC in vivo treatment resulted in a significant decrease in both subsets of intermediate and nonclassical monocytes and macrophage in tissues even in the absence of ART. Thus, GC might potentially be used in transient and high doses to purge CD16-positive monocytes and to therefore assess their contribution, if any, to the maintenance of the HIV reservoir in vivo. In the present study, the small number of animals precluded our ability to definitively address the question. The decrease in CD16-positive monocytes was not significant when GC was used at lower doses for longer periods of time in combination with ART. Thus, low-dose GC administration, as opposed to in vitro stimulation or short-term in vivo treatment with higher doses of GC, does not result in a significant depletion of all subsets of CD16-positive monocytes and therefore may not be a useful tool to address the contribution of monocytes to the maintenance of the HIV reservoir. Further studies will be necessary to establish an effective, yet feasible, regimen of GC treatment that may target both nonclassical and intermediate monocytes. It is possible that the lack of decrease in SIV-DNA is due to the inability of GC to deplete the intermediate CD16-positive monocytes. Repeated high doses of GC, at tolerable intervals, may be necessary to affect the total SIV/HIV-DNA burden in the body. Indeed, it is possible that a high tissue macrophage turnover is necessary to observe a sizable change in virus DNA. The use of GC treatment could be envisioned as an auxiliary to other approaches aimed at HIV eradication. To this aim the macaque model may be a very useful tool, providing that the best suppression of viral replication is obtained with ART.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, DARE collaboratory Grant P30AI073961 from NIAID, grant R37A1-6698 to Guido Silvestri and Grant PS10D1107 to Yerkes National Primate Research center. We thank Teresa Habina for editorial assistance, Hue Chung and Ranajit Pal for the viral SIV DNA measurement, and Dr. Alan Landay for useful discussions. Marcin Moniuszko was supported by a grant from the Polish National Science Center (NN401530240). FTC and PMPA was a generous gift from Gilead Science, Inc. and Raltegrevir was a generous gift from Merck and Co.
Marcin Moniuszko performed culture experiments, provided human samples, performed FACS analysis, and prepared figures; Namal Liyanage, Melvin Doster, and Katherine McKinnon performed animal experiments, FACS analysis, and prepared figures; Charles Brown and Vanessa Hirsch performed immunohistochemistry; Monica Vaccari, Shari Gordon, Poonam Pegu, Claudio Fenizia, Robyn Parks, and Marjorie Robert-Guroff provided animal data; Robert Flisiak, Anna Grzeszczuk, and Milena Dabrowska provided human data; Danuta Lipinska and Kamil Grubczak provided human and animal data; Guido Silvestri provided sooty mangabey samples and data; Marcin Moniuszko, Genoveffa Franchini, Mario Stevenson, and Joseph McCune wrote the article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gartner S, Markovits P, Markovitz DM, et al.: The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 1986;233:215–219 [DOI] [PubMed] [Google Scholar]
- 2.Ho DD, Rota TR, and Hirsch MS: Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest 1986;77:1712–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauza CD, Galindo J, and Richman DD: Human immunodeficiency virus infection of monoblastoid cells: Cellular differentiation determines the pattern of virus replication. J Virol 1988;62:3558–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi C. and Pamer EG: Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong KL, Tai JJ, Wong WC, et al.: Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011;118:e16–e31 [DOI] [PubMed] [Google Scholar]
- 6.Zawada AM, Rogacev KS, Rotter B, et al.: SuperSAGE evidence for CD14++ CD16+ monocytes as a third monocyte subset. Blood 2011;118:e50–e61 [DOI] [PubMed] [Google Scholar]
- 7.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al.: Nomenclature of monocytes and dendritic cells in blood. Blood 2010;116:e74–e80 [DOI] [PubMed] [Google Scholar]
- 8.van Furth FR. and Cohn ZA: The origin and kinetics of mononuclear phagocytes. J Exp Med 1968;128:415–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner LM, Li W, Holmes M, et al. : Phase I trial of recombinant macrophage colony-stimulating factor and recombinant gamma-interferon: Toxicity, monocytosis, and clinical effects. Cancer Res 1994;54:4084–4090 [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock HW, Fingerle G, Strobel M, et al. : The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol 1993;23:2053–2058 [DOI] [PubMed] [Google Scholar]
- 11.Kim WK, Sun Y, Do H, et al. : Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol 2010;87:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinoza-Jimenez A, Peon AN, and Terrazas LI: Alternatively activated macrophages in types 1 and 2 diabetes. Mediators Inflamm 2012;2012:815953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hearps AC, Maisa A, Cheng WJ, et al.: HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS 2012;26:843–853 [DOI] [PubMed] [Google Scholar]
- 14.Hearps AC, Martin GE, Angelovich TA, et al.: Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012;11:867–875 [DOI] [PubMed] [Google Scholar]
- 15.Hovden H, Frederiksen JL, and Pedersen SW: Immune system alterations in amyotrophic lateral sclerosis. Acta Neurol Scand 2013;128:287–296 [DOI] [PubMed] [Google Scholar]
- 16.Madjid M. and Fatemi O: Components of the complete blood count as risk predictors for coronary heart disease: In-depth review and update. Tex Heart Inst J 2013;40:17–29 [PMC free article] [PubMed] [Google Scholar]
- 17.Terry RL, Getts DR, Deffrasnes C, et al.: Inflammatory monocytes and the pathogenesis of viral encephalitis. J Neuroinflam 2012;9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woollard KJ: Immunological aspects of atherosclerosis. Clin Sci (Lond) 2013;125:221–235 [DOI] [PubMed] [Google Scholar]
- 19.Ziegler-Heitbrock L: The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J Leukoc Biol 2007;81:584–592 [DOI] [PubMed] [Google Scholar]
- 20.Ellery PJ, Tippett E, Chiu YL, et al.: The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 2007;178:6581–6589 [DOI] [PubMed] [Google Scholar]
- 21.Han J, Wang B, Han N, et al.: CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr 2009;52:553–559 [DOI] [PubMed] [Google Scholar]
- 22.Jaworowski A, Kamwendo DD, Ellery P, et al.: CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis 2007;196:38–42 [DOI] [PubMed] [Google Scholar]
- 23.Ancuta P, Autissier P, Wurcel A, et al.: CD16+ monocyte-derived macrophages activate resting T cells for HIV infection by producing CCR3 and CCR4 ligands. J Immunol 2006;176:5760–5771 [DOI] [PubMed] [Google Scholar]
- 24.Lambotte O, Taoufik Y, de Goer MG, et al.: Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2000;23:114–119 [DOI] [PubMed] [Google Scholar]
- 25.Sonza S, Mutimer HP, Oelrichs R, et al.: Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS 2001;15:17–22 [DOI] [PubMed] [Google Scholar]
- 26.Zhu T, Muthui D, Holte S, et al.: Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol 2002;76:707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fingerle-Rowson G, Angstwurm M, Andreesen R, et al.: Selective depletion of CD14+CD16+ monocytes by glucocorticoid therapy. Clin Exp Immunol 1998;112:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, et al.: Enhanced frequencies of CD14++ CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin Immunol 2009;130:338–346 [DOI] [PubMed] [Google Scholar]
- 29.Dayyani F, Belge KU, Frankenberger M, et al.: Mechanism of glucocorticoid-induced depletion of human CD14+CD16+ monocytes. J Leukoc Biol 2003;74:33–39 [DOI] [PubMed] [Google Scholar]
- 30.Moniuszko M, Kowal K, Rusak M, et al.: Monocyte CD163 and CD36 expression in human whole blood and isolated mononuclear cell samples: Influence of different anticoagulants. Clin Vaccine Immunol 2006;13:704–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano JW, Williams KG, Shurtliff RN, et al.: 1997. NASBA technology: Isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol Invest 1997;26:15–28 [DOI] [PubMed] [Google Scholar]
- 32.Chung HK, Unangst T, Treece J, et al. : Development of real-time PCR assays for quantitation of simian beta retrovirus serotype-1, -2, -3, and -5 viral DNA in Asian monkeys. J Virol Methods 2008;152:91–97 [DOI] [PubMed] [Google Scholar]
- 33.Hofmann-Lehmann R, Swenerton RK, Liska V, et al.: Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: Comparison of one- versus two-enzyme systems. AIDS Res Hum Retroviruses 2000;16:1247–1257 [DOI] [PubMed] [Google Scholar]
- 34.Lee EM, Chung HK, Livesay J, et al.: Molecular methods for evaluation of virological status of nonhuman primates challenged with simian immunodeficiency or simian-human immunodeficiency viruses. J Virol Methods 2010;163:287–294 [DOI] [PubMed] [Google Scholar]
- 35.Paiardini M, Cervasi B, Reyes-Aviles E, et al.: Low levels of SIV infection in sooty mangabey central memory CD T cells are associated with limited CCR5 expression. Nat Med 2011;17:830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandrea I, Onanga R, Souquiere S, et al.: Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J Virol 2008;82:5501–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chrousos GP, Renquist D, Brandon D, et al. : Glucocorticoid hormone resistance during primate evolution: Receptor-mediated mechanisms. Proc Natl Acad Sci USA 1982;79:2036–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crum NF, Riffenburgh RH, Wegner S, et al.: Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr 2006;41:194–200 [DOI] [PubMed] [Google Scholar]
- 39.Chun TW, Engel D, Mizell SW, et al.: Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med 1999;5:651–655 [DOI] [PubMed] [Google Scholar]
- 40.Davey RT, Jr, Bhat N, Yoder C, et al. : HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA 1999;96:15109–15114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutertre CA, Amraoui S, DeRosa A, et al. : Pivotal role of M-DC8(+) monocytes from viremic HIV-infected patients in TNFalpha overproduction in response to microbial products. Blood 2012;120:2259–2268 [DOI] [PubMed] [Google Scholar]
- 42.Brenchley JM, Price DA, Schacker TW, et al.: Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 43.Williams DW, Eugenin EA, Calderon TM, et al.: Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 2012;91:401–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt PW, Shulman NS, Hayes TL, et al.: The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: A randomized trial. Blood 2013;121:4635–4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.