Abstract
Introduction
Malignant pleural mesothelioma (MPM) is a deadly disease with poor prognosis and few treatment options. We characterized and elucidate the roles of C-MYC and PVT1 involved in the pathogenesis of MPM.
Methods
We used siRNA-mediated knockdown in MPM cell lines to determine the effect of C-MYC and PVT1 abrogation on MPM cells undergoing apoptosis, proliferation, and cisplatin sensitivity. We also characterized the expression of microRNAs (miRNAs) spanning the PVT1 region in MPM cell lines. Copy number analysis was measured by quantitative PCR and fluorescence in situ hybridization.
Results
Copy number analysis revealed copy number gains (CNGs) in chromosomal region 8q24 in six of twelve MPM cell lines. MicroRNA analysis showed high miR-1204 expression in MSTO-211H cell lines with ≥4 copies of PVT1. Knockdown by siRNA showed increased PARP-C levels in MSTO-211H transfected with siPVT1 but not in cells transfected with siC-MYC. C-MYC and PVT1 knockdown reduced cell proliferation and increased sensitivity to cisplatin. Analysis of the expression of apoptosis-related genes in the MSTO-211H cell line suggested that C-MYC maintains a balance between pro-apoptotic and anti-apoptotic gene expression, whereas PVT1 and to a lesser extent miR-1204, upregulate pro-apoptotic genes and downregulate anti-apoptotic genes. FISH analysis of MPM tumor specimens showed a high frequency of both CNGs (11/75) and trisomy (three copies; 11/75) for the C-MYC locus.
Conclusion
Our results suggest that C-MYC and PVT1 copy number gain promotes a malignant phenotype of MPM, with C-MYC CNG stimulating cell proliferation and PVT1 both stimulating proliferation and inhibiting apoptosis.
Keywords: C-MYC, PVT1, 8q24 chromosomal region, Malignant pleural mesothelioma
Malignant pleural mesothelioma (MPM) is a highly aggressive cancer with a poor prognosis 1. MPM’s incidence in the United States has increased in recent years, 2000–3000 people are diagnosed with the disease annually 2, 3. MPM predominantly affects men who have been exposed to asbestos in an occupational setting 4–8. The risk of developing the disease increases with age (median age at diagnosis is 72 years; range, 45 to 85 years). MPM’s three major histologic subtypes are epithelioid, biphasic, and sarcomatoid 1. Epithelioid tumors are the most common and have the best prognosis of the three subtypes. However, all types are very difficult to treat and have a median overall survival duration ranging from 9 to 17 months 9, with an overall 2-year survival rate of only 20% 10. There is therefore a great need to identify new therapeutic targets and develop more effective therapies for patients with MPM.
A potential therapeutic target for MPM and one of the most common chromosomal amplification sites in cancer tissues is the 8q24 region which contains the genes C-MYC and PVT1 11–13. C-MYC encodes a transcription factor that regulates the expression of multiple genes involved in cellular responses such as growth, proliferation, apoptosis, and differentiation 14–16. Deregulated amplification and expression of the MYC locus occurs in ~30% of human cancers, including colon, prostate and breast carcinomas, and has been associated with poor prognosis 11, 17, 18. PVT1 is a candidate oncogene located adjacent to the MYC locus on chromosomal region 8q24 18–20. PVT1 has been shown to act as a non-coding RNA with many alternatively spliced isoforms 12, 21. The PVT1 locus has recently been found to contain a cluster of at least six microRNAs (miRNAs) (miR-1204, -1205, -1206, -1207-3p, -1207-5p, and -1208) that span the PVT1 region, adding further complexity to the locus 12, 21. PVT1 copy number gains (CNGs) and PVT1 overexpression both have been implicated in the pathophysiology of many tumors, including breast and ovarian cancers and acute myeloid leukemia 19, 22. Additionally, PVT1 alteration has been shown to contribute to tumor survival and chemoresistance 22, 23. However, the roles that MYC, PVT1, and miRNAs contained in the 8q24 chromosomal region play in MPM remain unclear. We therefore sought to elucidate these roles and the specific mechanisms of action of C-MYC and PVT1 involved in the pathogenesis of MPM.
In the present study, we characterized the molecular abnormalities found in the 8q24 locus in MPM cell lines and in specimens from surgically resected MPMs. Characterized the miRNA (miR-1204, -1205, 1206, -1207 3p, 1207-5p, and -1208) expression in MPM cell lines. We also determined the biological impact of siRNA-mediated C-MYC and PVT1 abrogation on MPM cellular processes such as apoptosis, cell proliferation, and response to cisplatin and then determined the effect of C-MYC, PVT1, and miR-1204 knockdown on the expression levels of apoptosis related genes. Finally, we studied C-MYC and PVT1 copy number and gene expression in MPM tumor specimens.
MATERIALS AND METHODS
Tumors Specimen and Cell Lines
From the tissue bank at The University of Texas MD Anderson Cancer Center, we obtained archived frozen and formalin-fixed, paraffin-embedded (FFPE) tissues for patients who had undergone surgical resection for MPM. We randomly selected 75 MPM samples of different histologic subtypes (37 epithelioid, 26 biphasic, 12 sarcomatoid) for analysis. Detailed clinical and pathologic information on the patients is presented in Supplementary Table 1. The study protocol was approved by the MD Anderson institutional review board. Of the 12 MPM cell lines used in this study, five (H2452, MET-5A, H2052, H28 and MSTO-211H) were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 (Cellgro Mediatech, Manassas, VA), and seven (HCT-4012, Meso, HP3, HP5, HP7, HP9 and HP10) were acquired from Dr. Harvey Pass (New York University, New York, NY) and cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Cellgro Mediatech, Manassas, VA). All media formulations included 10% fetal bovine serum (FBS) and antibiotics (Sigma-Aldrich, St. Louis, MO). All MPM cell lines had been tested for absence of mycoplasma using Universal Mycoplasma Detection Kit according to manufacturer’s instructions (ATCC, Manassas, VA) and cells were authenticated at UTMDACC Core Facility.
Isolation of DNA and Copy Number Profiling
DNA was extracted from cell lines using DNAzol Reagent (Life Technologies, Grand Island, NY) and whole-genome single nucleotide polymorphism (SNP) array profiling was performed using Affymetrix SNP 6.0 chips (Agilent Technologies, Santa Clara, CA) in five MPM cell lines. Copy number gains (CNGs) were identified using the SNP-Fast Adaptive States Segmentation Technique 2 algorithm in Nexus 5.1 software (BioDiscovery, Hawthorne, CA) with the significance threshold for segmentation setting at p < 5 × 10-7. CNGs were defined with log2 ratio values of 0.2, and two or more than two CNGs were defined by log2 ratio values of 0.7.
Copy Number Analysis
We used fluorescence in situ hybridization (FISH) and real-time quantitative PCR (q-PCR) to quantify 8q24 CNGs in MPM tumor specimens. We used directly labeled fluorescent chromosomal centromeric probes (CEP 8, SpectrumGreen) for chromosome 8 and locus-specific probes (LSI) for regions 8q24.12-q13 (C-MYC Spectrum Orange) (Vysis, Abbott Laboratories, Chicago, IL). Fluorescence in situ hybridization (FISH) was performed according to the manufacturer’s instructions. Copy number analysis was performed in 50 nuclei per tumor in at least four areas. Copy number alteration was defined as the presence of more than two gene copies per cell on average of the 50 cells. Trisomy was defined as the presence of three copy number alterations and CNG was defined the presence of at least four copies. To enrich for malignant cell content for q-PCR analysis, tumor tissues were manually microdissected for subsequent DNA extraction from FFPE tissue sections. Tumor DNA was extracted using the Pico Pure DNA Extraction Kit (Arcturus, Life Technologies, Grand Island, NY) according to the manufacturer's instructions. DNA samples with proportions of microdissected tumor cell greater than 70% were qualified for qPCR analysis. MYC and PVT1 gene copy numbers were examined by q-PCR using the comparative Ct method using the ABI 7300 real time PCR system (Applied Biosystems, Grand Island, NY). The sequences of PCR primers used to CNG was for C-MYC, 5’-TCAAGAGGTGCCACGTCTCC-3’ and 5’-TCTTGGCAGCAGGATAGTCCTT-3’ (flanking exon 3), for PVT1, 5’-ACAGTGATCTTCAGTGGTCTGG-3’ and 5’-CGTGTGTCATTCCAGTGCAT-3’ (flanking exon 2). Each PCR was carried out using Power SYBR Green PCR Master Mix (Applied Biosystems) at 50°C for 2 minutes and 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. β-Actin was introduced as the endogenous reference gene, and TaqMan Control Human Genomic DNA (Applied Biosystems) was amplified as a standard control for calibration. MYC and PVT1 gene copy number in normal human genomic DNA was set as 2 and copy number ≥4 was considered as CNG 24. All sample and standard DNA reactions were set in triplicate to gauge reaction accuracy.
Isolation of mRNA and miRNA Analysis
Total RNA was extracted from frozen MPM tumor specimens and cell lines using TRI Reagent (Life Technologies, Grand Island, NY). Spectrophotometric analysis using Nanodrop 1000 (Nanodrop-Thermo Fisher Scientific, Waltham, MA) was used to quantify RNA, and Agilent BioAnalyzer RNA nano-chips (Agilent Technologies, Santa Clara, CA) were used to gauge RNA quality. Affymetrix U133 Plus 2.0 gene expression arrays (Affymetrix, Santa Clara, CA) were used to determine global expression levels of genes, including MYC and PVT1 in the total RNA extracted from tumor specimens. Using a High Capacity RNA-to-cDNA Kit and Taqman Gene Expression PCR Assays (Applied Biosystems, Grand Island, NY), we performed quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of the RNA extracted from cell lines to quantify MYC and PVT1 levels. The sequences of PCR primers used to gene expression was for C-MYC, 5’-CAGCTGCTTAGACGCTGGATT-3’ (flanks between exon 1 and 2), and 5’-GTAGAAATACGGCTGCACCGA-3’, for PVT1, 5’-TTACAGGCGTGTGCCACAAAGC-3’, and 5’- GCCTGTAATCCCAGCACGTTGA-3’ (flanks between exon 5 and 6). GAPDH was used as the endogenous control. TaqMan microRNA assays (Applied Biosystems, Grand Island, NY) were used to quantify the levels of miRNAs (miR-1204, -1205, -1206, -1207-3p, -1207-5p, and -1208) with U6 as the endogenous control. Standard PCR assays were performed on triplicate samples in standard cycling conditions using the ABI PRISM 7300 Sequence Detection System (Applied Biosystems Grand Island, NY). This process was used to yield relative expression levels using the 2(-∆∆CT) method with the help of ABI 7300 System SDS v1.4 software (Applied Biosystems, Grand Island, NY).
TaqMan Human Apoptosis Array
TaqMan gene expression assays were used along with the three control genes (18S, ACTB, and GAPDH) to detect the expression levels of the 93 apoptosis-related genes on a micro fluidic 384-well array (TaqMan® Array Human Apoptosis, Applied Biosystems, Grand Island, NY), using an Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems-Life Technologies, Grand Island, NY). Fold changes of gene expression in experimental samples were quantified relative to the control samples using the equation 2(-∆∆CT). Data were analyzed using Data Assist software version 3.0 (Life Technologies) and a 2.0-fold change in gene expression was defined as the threshold for up- or down-regulation.
MTS Assay
Cisplatin was purchased from Selleck Chemicals, Houston, TX. To determine the median half-maximal inhibitory concentration (i.e., IC50), cells were seeded in octuplicate at a density of 2,000 per well in 96-well plates. 24 hours later, cells were treated with increasing concentrations (0 to 30µM) of drugs. An endpoint viability assay using MTS (3-4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) assays (Promega, San Luis Obispo, CA) was performed after 72 hours of drug treatment.
siRNA Transfection and Cell Proliferation Assays
Cell lines were transfected with two gene-specific siRNA duplexes for each gene (C-MYC and PVT1) and control siRNA (Ambion, Grand Island, NY ) at a final concentration of 10 nmol/L using Lipofectamine RNAiMAX (Invitrogen, Grand Island, NY) according to the manufacturer's instructions. To verify the knockdown’s efficiency, we collected the mRNAs and protein of transfected cells and subjected them to qRT-PCR and Western blot analysis. miRNA inhibitor anti-miR-1204 was used to downregulate miR-1204 expression in MSTO-211H MPM cell line and the miRNA inhibitor negative control, at a final concentration of 50 nmol/L using Lipofectamine RNAiMAX (Invitrogen, Grand Island, NY) according to the manufacturer's instructions. miRNA inhibition by anti-miRNA was assessed by TaqMan microRNA Assays with U6 as the endogenous control (Applied Biosystems, Grand Island, NY). Proliferation assays were carried out by MTS assay according to the manufacturer’s protocols (Promega, San Luis Obispo, CA). Western blot analysis was carried out using specific antibodies against C-MYC (Santa Cruz Biotechnology, Santa Cruz, CA) and the analysis cleaved poly(ADP-ribose) polymerase (PARP-cleaved; Asp214; Cell Signaling Technology, Danvers, MA) and cleaved Caspase 3 (Cleved Casp3; Asp175; Cell Signaling Technology, Danvers, MA).
Statistical Analysis
Data obtained from cell culture assays and from the qRT-PCR and Western blot analyses were summarized using descriptive and inferential statistics accompanied by graphs of relative expression created using the Prism software program (version 5.0; GraphPad Software, La Jolla, CA). DNA copy number analysis using Affymetrix SNP6.0 microarray chip was conducted on Nexus 5.1 software (BioDiscovery, Hawthorne, CA). Analysis clinical and demographic data for the patients whose archived tumor samples were analyzed using the chi-square, Fisher exact, Wilcoxon rank-sum, and Kruskal-Wallis tests. Overall survival (OS) and recurrence-free survival (RFS) distributions were estimated using the Kaplan-Meier method, compared between groups of patients using the log-rank test. Cox proportional hazard models were used to calculate OS duration data and which was defined as the time from surgery to the patient’s death or last contact, and for RFS duration, which was defined as the time from surgery to tumor recurrence or the patient’s last contact.
RESULTS
8q24 Locus amplification and C-MYC and PVT1 Expression in MPM Cell Lines
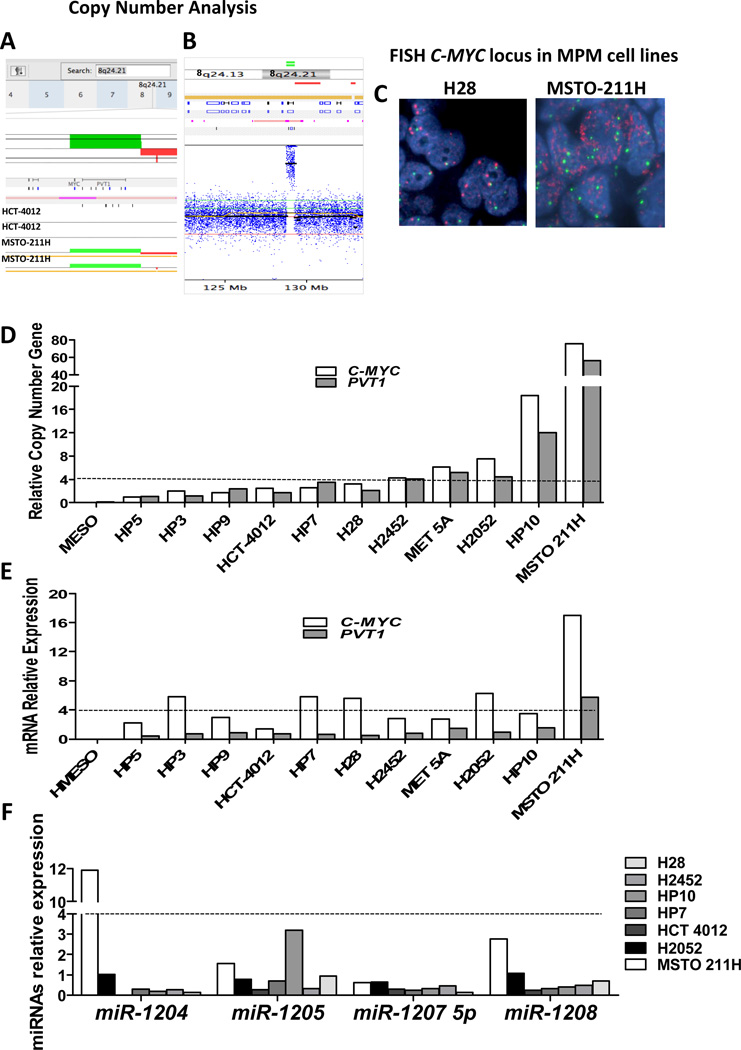
We performed global SNP/copy number analysis using Affymetrix SNP 6.0 chips in four MPM cell lines (H28, MSTO-211H, H2052, and H2452) and, for comparison, in the control normal cell line HCC-4012 (a telomerase-transformed pleural mesothelial cell line). DNA copy number analysis revealed CNG of chromosomal region 8q24, which contains both the C-MYC and PVT1 genes, in three of the five MPM cell lines (MSTO-211H, H2052, and H2452) relative to the control cell line (HCC-4012; Fig. 1A and 1B). In order to confirm our finding of CNG of the C-MYC locus in MPM cell lines, we performed FISH and q-PCR on 12 MPM cell lines. FISH analysis revealed a relatively high frequency of CNG defined as ≥ 4 copies of the C-MYC locus (6/12, 50%; Fig. 1C). Similarly, q-PCR analysis of genomic DNA showed a high frequency (5/12, 42%) of C-MYC CNG, also defined as ≥4 copies (Fig. 1D). We also used q-PCR to quantify CNG for the PVT1 gene, again detecting a high frequency (5/12, 42%) of PVT1 CNG (Fig. 1D). Interestingly, C-MYC and PVT1 CNGs were always detected in the same cell lines (H2452, MET 5A, H2052, HP10, and MSTO-211H). Of these cell lines, only HP10 and MSTO-211H had amplification for C-MYC (>15 copies).
Figure 1.
C-MYC locus amplification is observed in malignant pleural mesothelioma (MPM) cell lines but not in normal mesothelial cell lines. A, SNP/Copy number analysis by Nexus 5.1 software showed amplification (represented as green bars) of chromosomal region 8q24.21, which contains the oncogene C-MYC. Duplicate samples of MPM cell line MSTO-211H were compared to normal cell line HCC-4012. B, Copy number analysis by Nexus 5.1 software showed C-MYC locus amplification in a representative sample of the MSTO-211H cell line. C, Representative examples of C-MYC copy number examined by FISH in MPM cell lines. (a) An H28 specimen shows no copy number gain (CNG). (b) An MSTO-211H specimen shows CNG. Red signals represent the C-MYC gene probe, whereas green signals represent the internal control probe (magnification 1,000×). D, q-PCR analysis of C-MYC and PVT1 CNG in MPM cell lines. E, Relative expression of C-MYC and PVT1 mRNA by qRT-PCR in MPM cell lines showed increased levels (i.e., ≥4-fold changes) of C-MYC gene expression in five MPM cell lines (HP3, HP7, H28, H2052, and MSTO-211H) and only the MSTO-211H cell line showed an increased level of PVT1 gene expression (≥4-fold change). F, Relative expression of miRNAs by qRT-PCR in MPM cell lines showed expression of miR-1204, -1205, -1207-5p, and -1208 in all seven MPM cell lines expression of miR-1204, -1205, -1207-5p, and -1208 in all seven MPM cell lines and not detect miR-1206 or miR-1207-3p expression in any of the cell lines tested.
To determine whether C-MYC and PVT1 expression levels were also elevated in MPM cell lines, we analyzed mRNA expression using qRT-PCR of both genes on 12 MPM cell lines. qRT-PCR analysis showed increased levels (i.e., ≥4-fold changes) of C-MYC gene expression in five MPM cell lines (HP3, HP7, H28, H2052, and MSTO-211H). For the PVT1 gene, only the MSTO-211H cell line with CNG for PVT1 showed an increased level of gene expression (≥4-fold change) (Fig. 1E). We did not detect increased levels of PVT1 gene expression in the other MPM cell lines that had CNG for PVT1.
miRNA Expression in the PVT1 region in MPM Cell Lines
PVT1 encodes a non-coding RNA and is a host gene for the following miRNAs several miRNAs, including: miR-1204, -1205, -1206, -1207-3p, -1207-5p, and -1208. We screened a panel of seven MPM cell lines for expression of miR-1204, -1205, -1206, -1207-3p, -1207-5p and -1208 in the PVT1 region using qRT-PCR. We detected expression of miR-1204, -1205, -1207-5p, and -1208 in all seven MPM cell lines (Fig. 1F and Supplementary Table 2). We did not detect miR-1206 or miR-1207-3p expression in any of the cell lines tested. Interestingly, the MSTO-211H cell line with PVT1 CNG showed elevated expression of miR-1204 (≥ 4 fold change) and miR-1208 (≥ 2 fold change). Additionally, we detected a relatively high expression of miR-1205 in HP10 (≥ 3 fold change).
The Roles of C-MYC and PVT1 in Oncogenesis
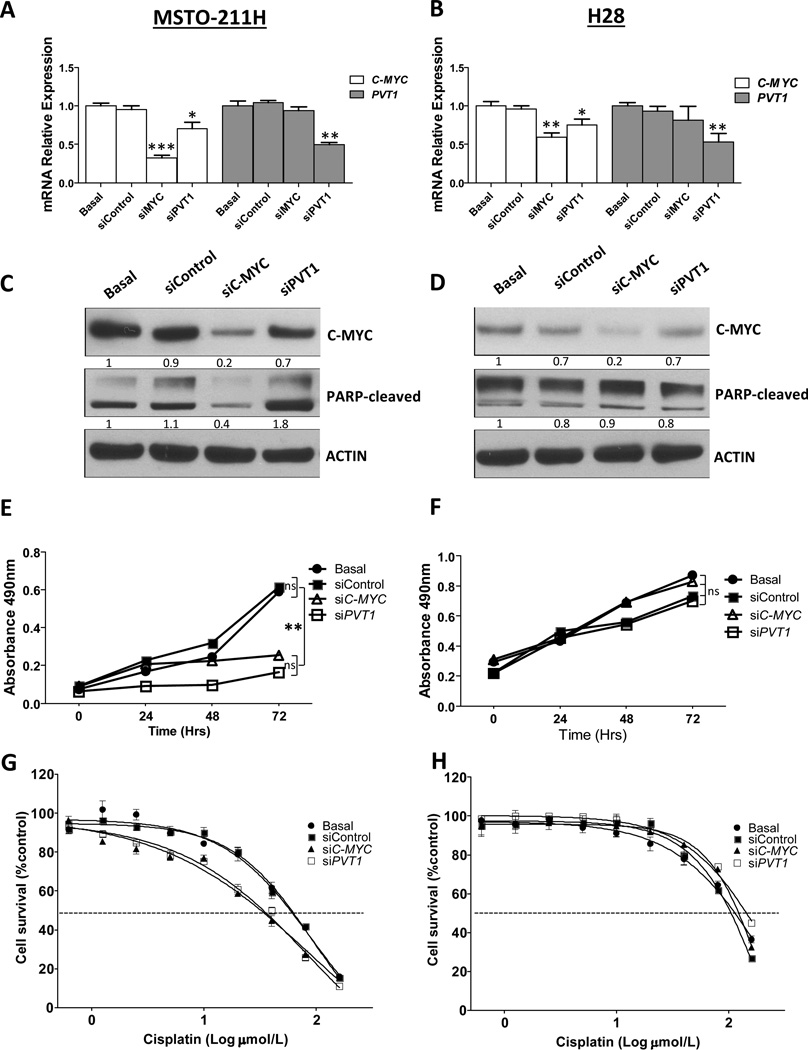
To examine the effects of abrogation of C-MYC and PVT1 function in MPM cell lines, we knocked down C-MYC and PVT1 in the MSTO-211H and H28 MPM cell lines using specific siRNA. Cell lines were selected for their numbers of C-MYC and PVT1 CNG (>10 copies and <4 copies, respectively). In both cell lines, qRT-PCR analysis showed that C-MYC and PVT1 knockdown significantly decreased mRNA expression relative to control siRNA-transfected and non-transfected cells (Fig. 2A and 2B). We first assessed the cytotoxic effects of C-MYC and PVT1 knockdown on MPM cell lines by inducing apoptosis (Fig 2C and 2D). We found that PVT1 knockdown increased PARP-cleaved and cleaved Caspase 3 expression in the MSTO-211H cell line relative to control siRNA-transfected and nontransfected cells 72 hours after transfection (Fig. 2C and Supplementary Fig 2). After transfection of siC-MYC, we observed a decrease, rather than an increase, in PARP-cleaved levels (Fig. 2C). We did not detect changes in PARP-cleaved levels under any treatment of the H28 cell line (Fig. 2D). Interestingly, we found that C-MYC knockdown by siRNA induced a decrease in PVT1 expression in the MSTO-211H and H28 cell lines, suggesting that C-MYC might regulate PVT1 expression in the MSTO-211H cell lines (Fig. 2A and B).
Figure 2.
Knockdown of C-MYC and PVT1 by siRNA decreased expression of C-MYC and PVT1, increased sensitivity to cisplatin, and reduced cell proliferation in malignant plural mesothelioma (MPM) cell lines. A, qRT-PCR analisys of C-MYC and PVT1 expression showed decreased C-MYC and PVT1 expression after knockdown of C-MYC and PVT1 by siRNAs in the MSTO-211H cell line. B, H28 MPM cell lines showed decreased C-MYC and PVT1 expression after knockdown of C-MYC and PVT1 by siRNAs. ***p < 0.001, ** p < 0.01, * p < 0.05. C, Western blot analysis of C-MYC and PARP-C expression after knockdown of C-MYC and PVT1 in MST0-211H cell lines showed decreased expression of C-MYC and increased PARP-C levels. D, Knockdown of C-MYC and PVT1 by siRNAs in H28 MPM cell lines showed decreased expression of C-MYC, and not detect changes in PARP-C levels when knocked down C-MYC and PVT1 in the H28 cell line E, Knockdown of C-MYC and PVT1 significantly reduced cell proliferation in MSTO-211H. **p < 0.01. F, However, proliferation was not reduced in H28 MPM cell lines. G, Knockdown of C-MYC and PVT1 by siRNA caused a 1.9-fold (p < 0.05) and 1.7-fold (p < 0.05) decrease in the cisplatin IC50 in MSTO-211H cell lines. H, However, knockdown did not cause cisplatin IC50 to decreasein H28 MPM cell lines. MPM cells subjected to gene-specific siRNA experiments were compared with control siRNA-transfected and nontransfected cells.
We additionally investigated the effect of C-MYC and PVT1 knockdown by siRNA on cell proliferation. We found significant proliferation inhibition in the MSTO-211H cell line when knocked down C-MYC and PVT1 (Fig. 2E), relative to the control siRNA-transfected and nontransfected cells (Fig. 2E). Proliferation was more strongly inhibited when we knocked down PVT1 than when we knocked down C-MYC in the MSTO-211H cell line. Inhibition of cell proliferation was not observed in the H28 cell line when knocked down C-MYC and PVT1 (Fig. 2F).
We then evaluated the effect of C-MYC and PVT1 knockdown by siRNA on the MPM cell lines’ sensitivity to cisplatin. Upon treatment with siC-MYC and siPVT1, the MSTO-211H cell lines were significantly more sensitive to cisplatin in vitro (Fig. 2G; p < 0.05), than were the control siRNA-transfected and non-transfected cells. This increased cisplatin sensitivity was not observed in H28 cells transfected with siC-MYC and siPVT1 (Fig. 2H). These findings suggest that depletion of C-MYC and PVT1 significantly contributes to increases in the sensitivity of MPM cell lines with 8q24 amplification in response to cisplatin.
We also analyzed the effect on cell proliferation and cisplatin sensitivity of miR-1204 downregulation by anti-miR-1204 in MSTO-211H MPM cell lines. qRT-PCR showed that anti-miR-1204 significantly decreased miR-1204 expression relative to control cells transfected with control anti-miRNA and non-transfected cells (Supplementary Fig. 1A). However, we found no significant differences between cells treated with anti-miR-1204, control cells transfected with control anti-miRNA, and non-transfected cells in terms of cisplatin sensitivity (Supplementary Fig. 1B) and proliferation (Supplementary Fig. 1C). These finding suggest that changes in apoptosis and cisplatin sensitivity produced by PVT1 are not mediated by miR-1204; rather, these PVT1-driven changes may be mediated, either individually or jointly, by other miRNAs spanning the PVT1 region.
Overall, our in vitro analysis strongly supports our hypothesis that 8q24 amplification can contribute to different oncogenic functions in MPM cancer cells. Specifically, CNGs of C-MYC stimulates cell proliferation, and CNGs of PVT1 stimulates proliferation and inhibits apoptosis.
Regulation of Apoptosis-Related Gene Expression by C-MYC and PVT1
Our in vitro findings demonstrate that PVT1 knockdown increases PARP-cleaved and cleaved Caspase 3 expression in the MSTO-211H cell line. In contrast, after C-MYC knockdown, we observed a decreased PARP-cleaved expression. The effects of C-MYC and PVT1 knockdown suggest that although both genes regulate apoptosis, they do so by alternative means. To understand how these genes are orchestrated to regulate apoptosis, we used the TaqMan Human Apoptosis Array to study the expression of 93 genes involved in apoptosis in two MPM cell lines with C-MYC and PVT1 CNGs; MSTO-211H cell line transfected with siC-MYC, siPVT1, and anti-miR-1204 and HP10 cell line transfected with siC-MYC and siPVT1.
Table 1 and supplementary Table 3 depicts changes in the expression levels of apoptosis-related genes after knockdown of C-MYC, PVT1, and miR-1204 (≥ 2.0-fold change of expression). Knockdown of C-MYC in the MSTO-211H cell line reveal seven genes that were upregulated (LTB, BIRC7, BCL2L14, PYCARD, BCL2A1, FASLG, and BIRC2) and three genes that were downregulated (NFKB1, BBC3, and TNFRSF21) relative to nontransfected cells or cells transfected with scrambled siRNA (control). Of the upregulated genes, four have known apoptotic functions (LTB, BCL2L14, PYCARD, and FASLG) and the remaining three (BIRC7, BCL2A1, and BIRC2) have known anti-apoptotic function. Among the downregulated genes, one gene (NFKB1) has anti-apoptotic function and two (BBC3 and TNFRSF21) have pro-apoptotic function. PVT1 knockdown upregulated five genes (LTB, BCL2L14, PYCAR, FASLG, and TNFRSF1B) and downregulated six genes (BCL2L1, BCL2, ICEBERG, CASP5, TNF, and BIRC8) relative to the expression levels of nontransfected cells or cells transfected with scrambled siRNA (i.e., controls). All genes whose expression was enhanced by PVT1 knockdown have pro-apoptotic function. Four of the downregulated genes (BCL2L1, BCL2, ICEBERG, and BIRC8) have anti-apoptotic function, whereas the other two downregulated genes (CASP5 and TNF) have pro-apoptotic function. Knockdown of C-MYC and PVT1 in the HP10 cell line revealed a similar tendency of genes upregulated and downregulated, but less pronounced. Interestingly, one of the most highly upregulated genes in HP10 cell line was LTB gene also upregulated in MSTO-211H cell line after C-MYC and PVT1 knockdown.
Table 1.
Gene Expression of Apoptosis-related Genes After Knockdown C-MYC, PVT1 and miR-1204 in MSTO-211H Cell Lines
| Fold Change | |||||
|---|---|---|---|---|---|
| Gene Name | siC-MYC | siPVT1 | Anti-miR-1204 | Gene Description | Function |
| Genes upregulated | |||||
| LTB | 17.56 | 7.31 | 5.25 | Lymphotoxin beta | Pro-apoptotic |
| BIRC7 | 9.96 | −1.61 | 8.83 | Baculoviral IAP repeat-containing protein 7 | Anti-apoptotic |
| BCL2L14 | 9.43 | 4.35 | 6.57 | BCL2 like protein 14 | Pro-apoptotic |
| PYCARD | 9.59 | −4.06 | 9.92 | PYD and card domain-containing protein | Pro-apoptotic |
| BCL2A1 | 3.79 | −1.23 | 1.41 | BCL2 -related protein A1 | Anti-apoptotic |
| FASLG | 3.29 | 3.03 | 1.27 | FAS ligand | Pro-apoptotic |
| BIRC2 | 2.93 | 1.66 | 2.22 | Baculoviral IAP repeat-containing protein 2 | Anti-apoptotic |
| TNFRSF1B | 0.89 | 2.12 | 1.50 | Tumor necrosis factor receptor superfamily member 1B | Pro-apoptotic |
| Genes downregulated | |||||
| NFKB1 | −2.15 | 1.49 | 1.01 | Nuclear factor NF-kappa-B p105 subunit | Anti-apoptotic |
| BBC3 | −2.20 | −1.17 | 1.01 | BCL2 binding component 3 | Pro-apoptotic |
| TNFRSF21 | −2.50 | 1.21 | −1.75 | Tumor necrosis factor receptor superfamily member 21 | Pro-apoptotic |
| BCL2L1 | 1.11 | −2.02 | −1.35 | BCL2 like 1 | Anti-apoptotic |
| BCL2 | −1.47 | −2.13 | −1.05 | B-cell lymphoma 2 | Anti-apoptotic |
| ICEBERG | 1.26 | −6.95 | −5.31 | Caspase 1 inhibitor | Anti-apoptotic |
| CASP5 | −1.08 | −9.52 | −2.47 | Caspase 5, apoptosis related cysteine protease | Pro-apoptotic |
| TNF | −1.43 | −11.48 | −1.62 | Tumor necrosis factor | Pro-apoptotic |
| BIRC8 | 1.10 | −42.69 | −2.47 | Baculoviral IAP repeat-containing protein 8 | Anti-apoptotic |
Bold, ≥2.0-fold change of expression, p < 0.05
We also investigated whether miR-1204 downregulation using anti-miR-1204 in the MSTO-211H cell line regulated the expression of apoptosis-related genes. miR-1204 downregulation increased the expression of five genes (LTB, BIRC7, BCL2L14, PYCAR, and BIRC2) and decreased the expression of three genes (ICEBERG, CASP5, and BIRC8). Three of the upregulated genes (LTB, BCL2L14, and PYCAR) have pro-apoptotic function, whereas the other two upregulated genes (BIRC7 and BIRC2) are involved in anti-apoptotic function. Two of the downregulated genes (ICEBERG and BIRC8) have known anti-apoptotic function, and the other downregulated gene (CASP5) has pro-apoptotic function.
We found that C-MYC knockdown affected the expression of several pro- and anti-apoptotic genes, thus maintaining a balance between these genes. PVT1 knockdown appeared to upregulate pro-apoptotic genes and downregulate anti-apoptotic genes. These results accord with our observation of an apoptotic phenotype of increased PARP-cleaved expression in the MSTO-211H cell line after PVT1 knockdown—an effect we did not observe after C-MYC knockdown.
C-MYC CNG in MPM Tumors
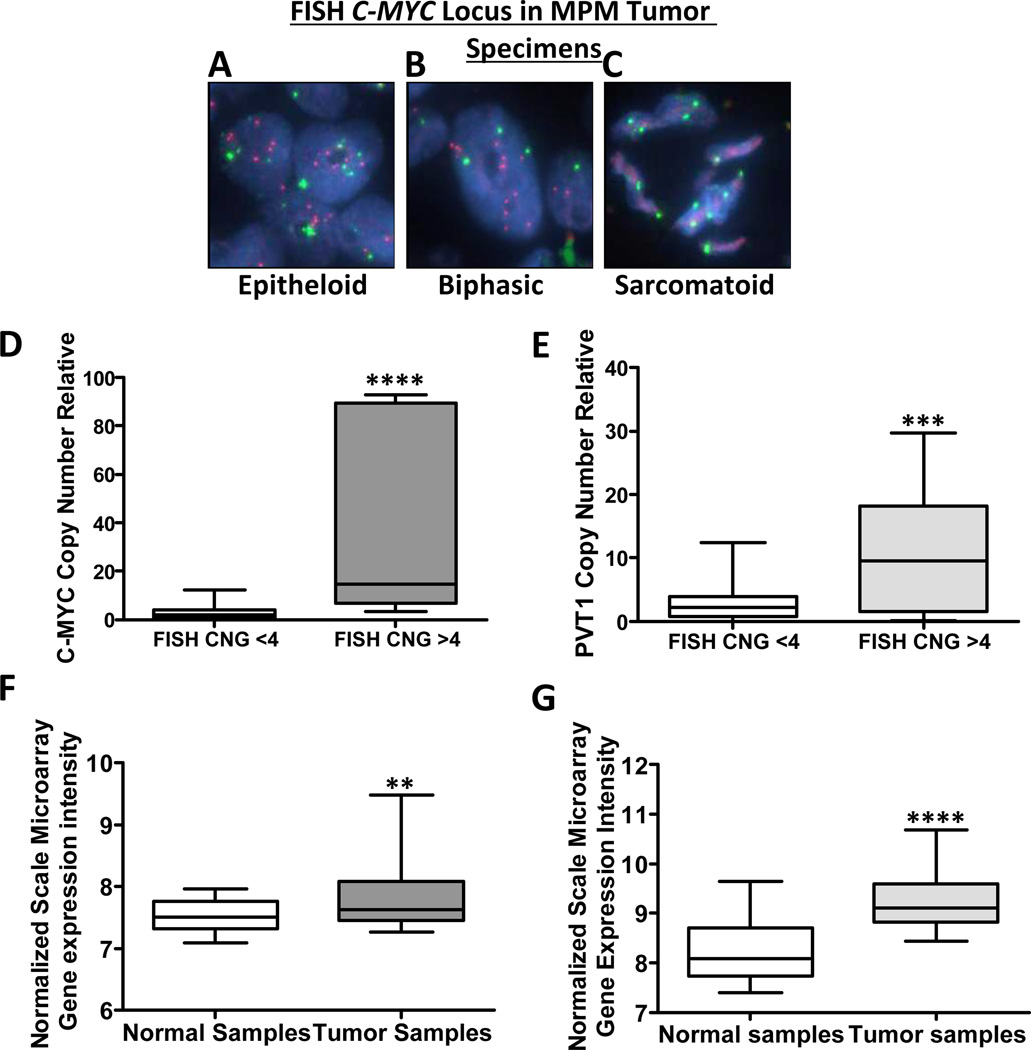
To validate the C-MYC CNG results obtained in MPM cell lines, we determined the gene copy number of the C-MYC locus in MPM tumor tissue specimens by performing FISH using the C-MYC probe on tissue microarrays (TMAs) containing 75 MPM samples from different histologic subtypes (37 epithelioid, 26 biphasic, 12 sarcomatoid). FISH analysis of TMAs revealed a high frequency of CNGs (11/75, 15%, ≥4 copies) and of trisomy (11/75, 15%, three copies) in the C-MYC locus in the MPM tumors. Interestingly, these CNGs were seen in the biphasic (6/26, 23%) and epithelioid (5/37, 13%) histotypes but not in the sarcomatoid cases (Fig. 3A, 3B, and 3C). Trisomy was seen in biphasic (6/26, 23%), epithelioid (3/37, 8%), and sarcomatoid (2/12, 17%) histotypes. Additionally, we investigated the association between C-MYC CNG detected by FISH and clinicopathologic features of resected MPM tissue specimens. We did not find any correlation between C-MYC CNGs with clinicopathologic features. After adjustment for age, tumor size, overall survival, stage, and adjuvant therapy, multivariate analysis did not show any correlation between C-MYC CNGs and patient outcome (p > 0.75).
Figure 3.
Malignant plural mesothelioma (MPM) tumor tissue specimens show C-MYC and PVT1 locus–specific copy number gain (CNG) and high expression of C-MYC and PVT1. Representative examples of C-MYC CNG examined by Fluorescence in situ hybridization (FISH) in MPM tissue specimens show high frequency of CNGs. FISH analysis shows, A, CNGs in epithelioid MPM. B, CNGs in biphasic MPM. C, no CNG in sarcomatoid MPM. Red signals represent the C-MYC gene probe, whereas and green signals represent the internal control probe (magnification 1,000×). Real time quantitative PCR (q-PCR) analysis of CNG of C-MYC and PVT1 in MPM tumor specimens by compared with CNG by FISH shows a correlation between both approaches. D, The box plots depict relative CNG of C-MYC revealed by q-PCR in 11 MPM tumor cell lines with C-MYC CNG as established by FISH and 19 MPM tumor cell lines without C-MYC CNG as established by FISH. **** p < 0.0001. E, The box plots depict relative CNG of PVT1 by q-PCR in 11 MPM tumor cell lines with C-MYC CNG as established by FISH and 19 MPM tumor cell lines without C-MYC CNG as established by FISH. *** p < 0.001. Normalized mRNA expression analysis F, C-MYC and G, PVT1 transcript using Affymetrix U133 plus 2.0 chips in MPM tumor specimens compared with normal samples. **** p < 0.0001, ** p < 0.01.
To confirm the C-MYC CNG results obtained using FISH, we used q-PCR to determine gene copy numbers in 30 tumor specimens (11 with C-MYC FISH-detected CNG and 19 without C-MYC FISH-detected CNG). C-MYC CNGs (≥ 4 copies) were detected by q-PCR in 12 MPM specimens (10 with C-MYC CNG and 2 without C-MYC CNG detected on the FISH results; Fig. 3D). Additionally, we also used q-PCR to detect PVT1 CNG in the same 30 tumor specimens. PVT1 CNG was detected in 12 MPM specimens (eight with C-MYC CNG and three without C-MYC CNG based on the FISH results; Fig. 3E).
We additionally determined C-MYC and PVT1 mRNA expression levels in a subset of 55 MPM tumor specimens paired with 41 normal tissues from our database using Affymetrix U133 plus 2.0 chips. We found significant differences in C-MYC and PVT1 gene expression between the normal tissues and the tumor samples (Fig. 3F and 3G; p < 0.05).
DISCUSSION
Our results show that amplification of the 8q24 chromosomal region occurs frequently in MPM cell lines and tumor specimens. Our in vitro findings suggest that C-MYC and PVT1 CNG may promote a malignant MPM phenotype . We have demonstrated that C-MYC and PVT1 knockdown by siRNA both increased cisplatin sensitivity and reduced cell proliferation capabilities in MPM cell lines. Interestingly, induction of apoptosis (as assessed by PARP-C levels) was observed only after PVT1 knockdown and not after C-MYC knockdown. In addition, our analysis of the expression of apoptosis-related genes revealed that C-MYC helps to maintain a regulatory balance between the expression of pro- and anti-apoptotic genes, whereas PVT1 showed a trend toward upregulating pro-apoptotic genes and downregulating anti-apoptotic genes. These findings suggest the two genes have differing functions: whereas C-MYC CNG stimulates proliferation and helps to decrease sensitivity to cisplatin platinum therapy, PVT1 CNG stimulates proliferation, decreases sensitivity to platinum therapy, and suppresses apoptosis. PVT1 CNG can also contribute to the overexpression of miRNAs contained within this locus; we extended our study to include one of the most highly expressed of these miRNAs—miR-1204.
The 8q24 chromosomal region is frequently amplified in many tumors. This regional amplification leads to co-amplification of the C-MYC and PVT1 genes located in this region 19, 25. C-MYC encodes a transcription factor that regulates the expression of multiple genes involved in cellular processes such as growth, apoptosis, proliferation, and differentiation 15–17. Deregulated C-MYC expression, which has been detected in a wide variety of human tumors, promotes tumorigenesis by increasing genomic instability, induces proliferation and apoptosis, stimulates angiogenesis and migration, and promotes adaptation to a hypoxic microenvironment 17. We found C-MYC CNG to be highly frequent in MPM cell lines and tumor specimens. However, we did not find a correlation between C-MYC CNG with clinicopathologic features on the one hand, and patient outcomes on the other hand. The fact that we did not find a correlation may be due to the great aggressiveness of these tumors, which makes it difficult to obtain detailed clinical, treatment and long-term follow-up data for these patients.
C-MYC knockdown increases cisplatin sensitivity and decreases cell proliferation in MSTO-211H cell lines. Several studies have reported that MYC-mediated transcription is involved in the regulation of multiple genes, including genes involved on regulating cell cycle progression such as cyclin dependent kinases (CDK) as CDK1, CDK2, CDK4 and CDK6, among others 26–29. Interestingly, we observed that C-MYC knockdown also induces a PARP-cleaved decrease, suggesting that C-MYC CNGs could induce apoptosis in MPM. How C-MYC regulates apoptosis is a matter of continued debate. It is accepted that C-MYC triggers apoptosis and that its overexpression may help to sensitize cells to undergo apoptosis 30–32. It has been shown in vitro that the withdrawal of growth factors in cells downregulates C-MYC, inducing cell cycle arrest, whereas C-MYC overexpression rapidly induces apoptosis 32. C-MYC regulates different target genes under specific conditions and in a tissue-specific manner to regulate apoptosis 14. We have shown that C-MYC knockdown tends to keep a balance between up- and down-regulation of the expression of pro- and anti-apoptotic genes (Table 1).
PVT1 is frequently co-amplified with C-MYC in many tumors, acts as an oncogene that non-coding RNA and a cluster of at least 6 miRNAs that span the PVT1 region recently has been identified 12, 21. PVT1 has been described as an MYC activator 33 and as a downstream target of both the C-MYC and N-MYC genes 34. We found that C-MYC knockdown downregulates PVT1 in MSTO-211H and H28 cell lines, suggesting rather that C-MYC might regulate PVT1 gene expression in MPM cell lines. Additionally, PVT1 can regulate sensitivity to certain chemotherapeutic drugs (e.g., colleagues found that PVT1 inactivation increased gemcitabine sensitivity in human pancreatic cancer cells, contributing to cell survival and chemoresistance) 23. PVT1 knockdown by siRNA in MSTO-211H cell lines increases cisplatin sensitivity and PARP-cleaved levels while decreasing proliferation/viability. These finding suggest that PVT1 can act as an oncogenic non-coding RNA and that PVT1 depletion by siRNA induces a change in gene expression that enhances response to chemotherapy and induces apoptosis.
Additionally, by analyzing the expression of apoptosis-related genes, we demonstrated that PVT1 knockdown upregulated genes involved in pro-apoptotic functions (LTB, BCL2L14, PYCAR, FASLG, and TNFRSF1B) and downregulated genes involved in anti-apoptotic functions (BCL2L1, BCL2, ICEBERG and BIRC8). It therefore appears that PVT1 depletion favors an apoptotic phenotype. Additionally, we characterized the expression of miRNAs spanning the PVT1 region in MPM cell lines, showing different expression levels of these miRNAs in MPM cell lines and highlighting miR-1204 overexpression in MSTO-211H cell lines with PVT1 CNG. We also found that miR-1204 depletion promotes the expression of anti-apoptotic genes. This finding suggests that PVT1 CNGs, together with the action of miRNAs in this region, can contribute to survival and chemoresistance in MPM.
In summary, our findings indicate that C-MYC and PVT1 co-amplification is frequent in MPM. C-MYC and PVT1 cooperation helps to stimulate proliferation, decrease sensitivity to platinum therapy, and reduce apoptosis. Both genes also help to regulate apoptosis-related genes, with C-MYC revealing a tendency to maintain a balance between pro-apoptotic and anti-apoptotic genes, whereas PVT1 revealed a tendency to upregulate pro-apoptotic genes and downregulate anti-apoptotic genes, thereby helping to suppress apoptosis.
Supplementary Material
Acknowledgments
Grant information: This study was supported in part by grants DoD PROSPECT W81XWH-07-1-0306 and MD Anderson Cancer Center Support Grant CA016672.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
References
- 1.Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–2090. doi: 10.1200/JCO.2008.19.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weill H, Hughes JM, Churg AM. Changing trends in US mesothelioma incidence. Occup Environ Med. 2004;61:438–441. doi: 10.1136/oem.2003.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol. 1997;145:211–218. doi: 10.1093/oxfordjournals.aje.a009093. [DOI] [PubMed] [Google Scholar]
- 4.Larson T, Melnikova N, Davis SI, et al. Incidence and descriptive epidemiology of mesothelioma in the United States, 1999–2002. Int J Occup Environ Health. 2007;13:398–403. doi: 10.1179/oeh.2007.13.4.398. [DOI] [PubMed] [Google Scholar]
- 5.Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol. 2002;29:2–17. doi: 10.1053/sonc.2002.30227. [DOI] [PubMed] [Google Scholar]
- 6.Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992;34:718–721. [PubMed] [Google Scholar]
- 7.Testa JR, Carbone M, Hirvonen A, et al. A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res. 1998;58:4505–4509. [PubMed] [Google Scholar]
- 8.Carbone M, Pass HI, Rizzo P, et al. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9:1781–1790. [PubMed] [Google Scholar]
- 9.Zucali PA, Ceresoli GL, De Vincenzo F, et al. Advances in the biology of malignant pleural mesothelioma. Cancer Treat Rev. 2011;37:543–558. doi: 10.1016/j.ctrv.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Borasio P, Berruti A, Bille A, et al. Malignant pleural mesothelioma: clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg. 2008;33:307–313. doi: 10.1016/j.ejcts.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 11.Adhikary S, Peukert K, Karsunky H, et al. Miz1 is required for early embryonic development during gastrulation. Mol Cell Biol. 2003;23:7648–7657. doi: 10.1128/MCB.23.21.7648-7657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck-Engeser GB, Lum AM, Huppi K, et al. Pvt1-encoded microRNAs in oncogenesis. Retrovirology. 2008;5:4. doi: 10.1186/1742-4690-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang CV, O'Donnell KA, Zeller KI, et al. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 15.O'Donnell KA, Yu D, Zeller KI, et al. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Mol Cell Biol. 2006;26:2373–2386. doi: 10.1128/MCB.26.6.2373-2386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez PC, Frank SR, Wang L, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang CV, Kim JW, Gao P, et al. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 18.Feo S, Di Liegro C, Jones T, et al. The DNA region around the c-myc gene and its amplification in human tumour cell lines. Oncogene. 1994;9:955–961. [PubMed] [Google Scholar]
- 19.Shtivelman E, Henglein B, Groitl P, et al. Identification of a human transcription unit affected by the variant chromosomal translocations 2;8 and 8;22 of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1989;86:3257–3260. doi: 10.1073/pnas.86.9.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer KB, Maia AT, O'Reilly M, et al. A functional variant at a prostate cancer predisposition locus at 8q24 is associated with PVT1 expression. PLoS Genet. 2011;7:e1002165. doi: 10.1371/journal.pgen.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huppi K, Volfovsky N, Runfola T, et al. The identification of microRNAs in a genomically unstable region of human chromosome 8q24. Mol Cancer Res. 2008;6:212–221. doi: 10.1158/1541-7786.MCR-07-0105. [DOI] [PubMed] [Google Scholar]
- 22.Guan Y, Kuo WL, Stilwell JL, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13:5745–5755. doi: 10.1158/1078-0432.CCR-06-2882. [DOI] [PubMed] [Google Scholar]
- 23.You L, Chang D, Du HZ, et al. Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem Biophys Res Commun. 2011;407:1–6. doi: 10.1016/j.bbrc.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Yang F, Tang X, Riquelme E, et al. Increased VEGFR-2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer Res. 2011;71:5512–5521. doi: 10.1158/0008-5472.CAN-10-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storlazzi CT, Fioretos T, Paulsson K, et al. Identification of a commonly amplified 4.3 Mb region with overexpression of C8FW, but not MYC in MYC-containing double minutes in myeloid malignancies. Hum Mol Genet. 2004;13:1479–1485. doi: 10.1093/hmg/ddh164. [DOI] [PubMed] [Google Scholar]
- 26.Malumbres M. Physiological relevance of cell cycle kinases. Physiol Rev. 2011;91:973–1007. doi: 10.1152/physrev.00025.2010. [DOI] [PubMed] [Google Scholar]
- 27.Kang J, Sergio CM, Sutherland RL, et al. Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells. BMC Cancer. 2014;14:32. doi: 10.1186/1471-2407-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prall OW, Rogan EM, Musgrove EA, et al. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol Cell Biol. 1998;18:4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mateyak MK, Obaya AJ, Sedivy JM. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19:4672–4683. doi: 10.1128/mcb.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Askew DS, Ashmun RA, Simmons BC, et al. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 31.Wyllie AH, Arends MJ, Morris RG, et al. The apoptosis endonuclease and its regulation. Semin Immunol. 1992;4:389–397. [PubMed] [Google Scholar]
- 32.Evan GI, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 33.Graham M, Adams JM. Chromosome 8 breakpoint far 3' of the c-myc oncogene in a Burkitt's lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. 1986;5:2845–2851. doi: 10.1002/j.1460-2075.1986.tb04578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carramusa L, Contino F, Ferro A, et al. The PVT-1 oncogene is a Myc protein target that is overexpressed in transformed cells. J Cell Physiol. 2007;213:511–518. doi: 10.1002/jcp.21133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.