Abstract
Neuroimaging has greatly enhanced the cognitive neuroscience understanding of the human brain and its variation across individuals (neurodiversity) in both health and disease. Such progress has not yet, however, propelled changes in educational or medical practices that improve people’s lives. We review neuroimaging findings in which initial brain measures (neuromarkers) are correlated with or predict future (1) education, learning, and performance in children and adults; (2) criminality; (3) health-related behaviors; and (4) responses to pharmacological or behavioral treatments. Neuromarkers often provide better predictions (neuroprognosis), alone or in combination with other measures, than traditional behavioral measures. With further advances in study designs and analyses, neuromarkers may offer opportunities to personalize educational and clinical practices that lead to better outcomes for people.
Keywords: prediction, neuroimaging
Noninvasive neuroimaging has provided remarkable new insights into human brain structure and function in both health and disease. For over a century, understanding the human brain depended upon naturally occurring brain injuries or unexpected consequences of neurosurgeries. From clinical cases such as Leborgne, Phineas Gage, H.M., and commissurotomy patients, we gleaned insights, respectively, into the roles of left prefrontal cortex in language (Broca, 1861), ventral prefrontal cortex in decision-making and social behavior (Harlow, 1868/1974), the medial temporal lobe in memory (Scoville and Milner, 1957), and functional asymmetries between the cerebral hemispheres (Gazzaniga, 1970). Noninvasive neuroimaging has permitted a second wave of discoveries about the brain that has expanded the horizon of human neuroscience, with examination of typical functions across many domains of the human mind, from perception and cognition to emotion, social and moral thought, and economic decision-making. Further, such imaging has offered the first compelling evidence that neuropsychiatric and neurodevelopmental disorders reflect fundamental differences in brain structure and function. Uniquely, neuroimaging has revealed not only universal principles of functional brain organization, but also neurodiversity: how such brain functions vary across people in relation to age, sex, personality, culture, and genetics. Here, we review progress in a novel application of neuroimaging, the use of such measureable neurodiversity to predict future human behavior. Such prediction may constitute a humanitarian and pragmatic contribution of human cognitive neuroscience to society, but this contribution will require rigorous science and also ethical considerations.
Neuroscientists, psychologists, and physicians are contemplating how human neuroimaging may inform basic and clinical research. For basic research, there is discussion about whether neuroimaging has informed cognitive theories beyond the mapping of psychological functions to neural networks (e.g., Mather et al., 2013). For clinical research, it is noteworthy that the 2013 revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), the defining document of diagnosis from the American Psychiatric Association, was little, if at all, influenced by the over 15,000 magnetic resonance imaging (MRI) studies of psychiatric disorders listed in PubMed (and this does not include studies using other methods, such as electroencephalography (EEG), magnetoencephalography (MEG), or positron emission tomography (PET)). Remarkable advances in genetics have also had little practical influence as yet on diagnosis or treatment of psychiatric disorders. Because psychiatric disorders are known to be heritable, and because these disorders must have a brain basis, it is likely that progress in genetics and neuroimaging will illuminate such disorders in the long run. Here, we will consider how neuroimaging may contribute to helping people in the nearer future.
This review focuses on structural and functional neuroimaging and considers findings in which an initial brain measure (a neuromarker) is associated with a future behavioral outcome. Some studies relate neuromarkers to individual differences in later perceptual or cognitive performance among typical or healthy people, and have relevance for education and training. Other studies relate neuromarkers to individual differences among patients with a given diagnosis to future clinical status or response to treatment (neuroprognosis), and have relevance for neuropsychiatric disorders.
Such correlational or predictive studies differ from other kinds of studies in two main ways. First, in the case of group studies (e.g., comparison of patient and control groups), neuroimaging differences are most pronounced when there is greater homogeneity of a brain measure within each group, so that groups are statistically separable. Conversely, greater heterogeneity of a brain measure within a group is more likely to yield neuromarkers that correlate with variable outcomes. Second, for studies that examine response to treatment, individual differences may delineate not the neural systems most affected by the disorder, but rather heterogeneity among patients in the neural systems that are most important and variable for how a treatment yields benefits. For example, if a behavioral therapy helps a patient with a disorder to learn how to regulate thoughts or emotions, then the neuromarkers associated with treatment response may be in neural networks that support such learning rather than in networks related to the etiology or progression of the disorder.
Neuroimaging Measures
Noninvasive neuroimaging measures provide indices of human brain structure and function that vary in their strengths and limitations. This review focuses on measures that maximize spatial information, specifically MRI-derived measures. Brain structure can be quantified by measuring volumes, thickness, or density (voxel-based morphometry or VBM). Microstructural properties of white-matter pathways can be characterized by diffusion tensor imaging (DTI). Brain functions can be quantified via functional MRI (fMRI) by activation studies that correlate experimental conditions or behavioral performance with neural activity as indexed by changes in blood oxygenation-level dependent (BOLD) signals. During a resting state, with no task or stimuli, there are spontaneous fluctuations in functionally related brain regions that correlate with one another, and the patterns of these correlations may reveal intrinsic functional relations of brain regions (Biswal et al., 1995). Resting-state fMRI, EEG, and MEG can elucidate these networks. Because it measures hemodynamic response, fMRI is inherently poor in temporal resolution, whereas EEG and MEG provide high temporal resolution (at the loss of spatial resolution).
For applications in education or medicine, there is a trade-off between measures that are task-dependent (activation fMRI, MEG, and EEG) versus measures that are task-independent (structural MRI and DTI, and fMRI, MEG, and EEG resting-state). On the one hand, tasks can selectively invoke brain responses to salient stimuli (e.g., to print in children with reading difficulty, or to sad facial expressions in depression). The advantage of this approach is that tasks and stimuli can be tailored to specifically assay salient mental operations. On the other hand, such tasks demand participant performance that can result in behavioral confounds, vary in design from study to study, and have not typically been developed to maximize reliability of measurement. In contrast, structural and resting-state measures can be acquired in a consistent fashion, have promise for reliability (e.g., Shehzad et al., 2009; Wonderlick et al., 2009; Vollmar et al., 2010), can accommodate a broad range of participants (including infants), and are independent of task performance in the scanner.
Analytic Approaches: From Correlation to Individualized Prediction
An ultimate goal of the use of neuromarkers for neuroprognosis is to perform individualized predictions of educational or health outcomes. Most studies to date have related variation in baseline brain measures to variation in subsequent outcomes. Given that such analyses hinge on knowledge of the outcomes, such analyses could be described more as postdiction than prediction (Whelan and Garavan, 2013). Yet, if neuromarkers are to become useful in practice, they must predict outcomes for new individuals based on models developed previously with other individuals. A cognitive neuroscience of prediction, therefore, needs to build on theory and methods that allow for effective creation, evaluation, and selection of prediction models (Pereira et al., 2009).
The term prediction is used in three different ways in relevant research. First, prediction can refer to a correlation between two contemporaneous values, such as height predicting weight. Second, prediction can refer to the correlation of one variable in a group at an initial time-point to another variable in the same group at a future time-point (an in-sample correlation). Third, prediction can refer to a generalizable model that applies to out-of-sample individuals. All studies reviewed here relate an initial brain measure to a future behavioral outcome, and the term correlation refers to in-sample findings, and the term prediction refers to out-of-sample generalizations.
Such research can be conceptualized as comprising three stages beginning with within-sample correlations to discover relations of interest, progressing to predictive analyses in which predictions for individuals are derived from data from other in-sample individuals, and culminating in predictive analyses in which a model from one sample is used to predict outcomes in an independent sample (Figure 1). Each stage requires more participants, so that prior stages may justify larger-scale studies. The vast majority of findings to date are correlational (61 of the 72 reviewed here), but some studies reported predictive analyses (with only one study having fully independent samples) (Table).
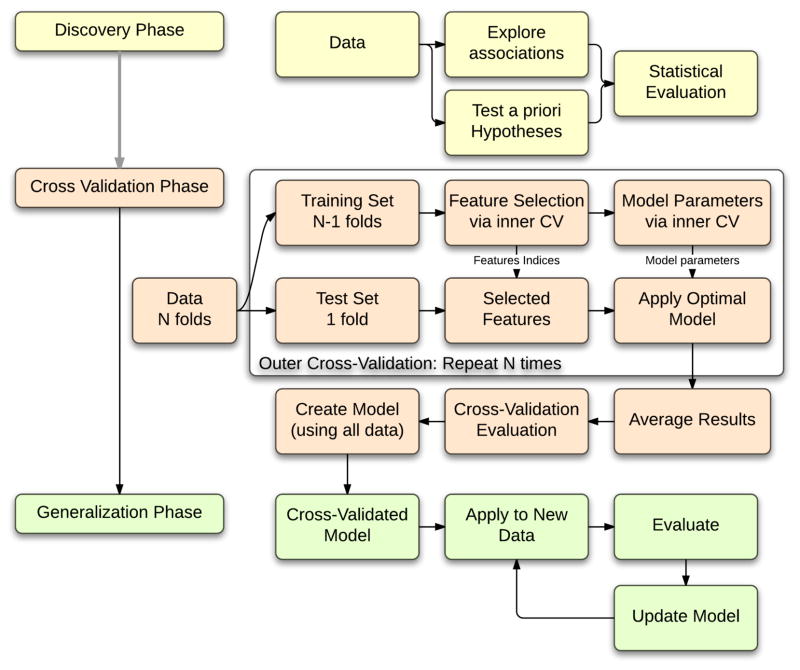
Figure 1. Three stages of predictive model identification.
1) Discovery Phase. Explore and evaluate associations between baseline neuromarkers and behavioral outcomes. 2) Cross-Validation Phase. A cross-validation routine is used to separate data into training and test sets. The model is built using training data and tested on out-of-sample test data. Upon successful evaluation of the performance of the model and features, all data are used to build a prediction model. 3) Generalization Phase. A prediction model built via cross-validation is applied to a new data set. The new data are then used to update the model.
Table.
Publications cited in this article that used cross-validation techniques to measure out-of-sample prediction error
| Publication | Sample Size | Application | Cross-validation Method | Learning Model |
|---|---|---|---|---|
| Whelan et al., 2014 | 271 | Future adolescent alcohol misuse | 10-Fold | Logistic Regression with Elastic Net |
| Hoeft et al., 2007 | 64 | Future reading skills | LOO | Linear Regression |
| Ullman et al., 2014 | 62 | Future working memory capacity | Leave One Out (LOO) | nu-SVR |
| Ball et al., 2013 | 48 | Response to CBT in GAD, PD | Out of bag | Random forest |
| Doehrmann et al., 2013 | 39 | SAD | Stratified K-Fold | Linear Regression |
| Hoeft et al., 2011 | 25 | Future reading gains in dyslexia | LOO | Linear SVC |
| Supekar et al., 2013 | 24 | Response to math tutoring | 4-Fold | Linear Regression |
| Falk et al., 2010 | 20 | Persuasion-induced behavior change | 2-Fold | Linear Regression |
| Siegle et al., 2012 | 17 (Cohort 1) 20 (Cohort 2) |
Response to CBT for depression | Out of bag | Random forest |
| Bach et al., 2013 | 17 | Future reading skills | LOO | Discriminant analysis |
| Costafreda et al., 2009 | 16 | Response to CBT for depression | LOO | PCA + linear SVM |
CBT = cognitive behavioral therapy; GAD = generalized anxiety disorder; PD = panic disorder; SAD = social anxiety disorder
The major limitation with correlational analyses reporting the significance of the overall fit of linear or multiple regression models to a dataset is that findings are tied to the outcome for a particular group. From a predictive modeling standpoint, the error from this fit is typically termed the training error, while the error on an unseen dataset would be called the test or generalization or prediction error. Training error is always an underestimate of the test error. The quality of a model can be evaluated by measuring its test error; minimizing this error is the goal of building prediction models. One way to decompose test error is to describe it as a sum of training error and optimism (Efron, 2004). Optimism is the difference between the test error, which is always higher, and the training error.
The most common approach for reducing optimism is to use a validation set in which some data are set aside to estimate the test error. In many studies of brain imaging, this limits the amount of data available for training because of small sample sizes. A common approach is to use cross-validation in which one divides a dataset into a number of folds. One fold is held aside as a test set and data from the remaining folds (training data) are used to train the model. This model is then applied to the test set and the model error is calculated. This procedure is repeated by considering each fold as a test set. The average error across the test folds is reported as the generalization error. If the number of folds equal the number of data points, then only one data point is held out for testing and this is known as Leave One Out Cross-Validation. In general, this approach is unbiased but typically has high variance in prediction error (Kohavi, 1995; Rao et al., 2008).
Another practical approach is to randomly split data into training and test sets (e.g., 10% of the data are in the test set). The splitting is repeated several times. On each iteration, a model is fit on the training data and tested on the test data. This results in a distribution of prediction errors that can provide a confidence interval for a given application. However, such procedures can still lead to increased optimism if models are chosen or their parameters are tuned after peeking at the test results (Koban et al., 2013). Selecting models and their parameters from cross-validation on the training data can reduce such optimism. The training data itself can be subjected to cross-validation and subdivided into training and test sets to determine which model is best suited for the training data. This procedure is called “nested cross-validation”. Because different models may be selected for each cross-validation split, the most selected model might be considered to be the “best” model. A variety of learning models coupled with cross-validation has been used in brain imaging. These range from linear, multiple, and logistic regression models to approaches such as support vector machines (SVM, Vapnik, 1999; or LASSO, Tibshirani, 1996), relevance vector machines (RVM, Bishop and Tipping, 2000), and Random Forests (Breiman, 2001).
The difference between the amount of variability accounted for by within-sample correlations and out-of-sample predictions is rarely reported. Two within-sample correlational studies (Aharoni et al., 2013; Demos et al., 2012) were re-analyzed by a different investigator (Poldrack, personal communication), and the outcome variance accounted for by the generalizable model was far smaller than that for the within-sample correlation (but see Aharoni et al., 2014). Although in most cases the predictive model results in a more conservative outcome than the correlational model, the difference varies across datasets. In all cases, however, predictive analyses will be necessary to translate correlational observations into educational or clinical practice.
Future Learning and Cognitive Performance in Adults
Variation in initial neuromarkers has been associated with subsequent learning or cognitive performance, and in most cases these variations occurred in the neural networks associated with the kind of learning. Larger volumes of the striatum correlated with superior video game skill learning (Erickson et al., 2010). This correlation was specific to the dorsal striatum volume, did not extend to the hippocampus or ventral striatum, and accounted for 23% of the variance in learning. The importance of the striatum for such skill learning is consistent with evidence that lesions of the striatum impair skill learning (e.g., Heindel et al., 1989). Superior word learning correlated with DTI measures of the left arcuate fasciculus, a white-matter pathway connecting major left-hemisphere language regions (Lopez-Barroso et al., 2013).
Brain differences in language-related neural systems have also been related to variation in learning novel speech distinctions not present in a person’s native language. Superior learning was associated with anatomical differences, specifically greater asymmetry (left > right) in parietal-lobe volumes and higher white-matter density in left Heschl’s gyrus (Golestani et al., 2002, 2007). Larger anatomical structures in the language-dominant left hemisphere may support the rapid temporal processing needed to learn novel auditory distinctions that occur critically in the first 30–50 ms of nonnative language sounds. Resting-state functional connectivity has also been associated with variation in auditory language learning. Better learners of a nonnative speech contrast exhibited greater functional connectivity (correlation) than poor learners between inferior frontal and parietal regions thought to be major components of the left-hemisphere language system (Ventura-Campos et al., 2013; other related studies reviewed in Zatorre, 2013).
Neuromarkers have also correlated with musical and visual learning. For auditory learning of microtonal pitch discrimination (with intervals smaller than typically used in musical scales), individuals who at baseline exhibited higher slopes of fMRI activation in bilateral auditory cortex to pitch-interval size exhibited greater learning over a two-week training period (Zatorre et al., 2012). The higher slope of activation may reflect a finer-grained cortical encoding of pitch information that potentiates more rapid learning during training. People who were better at learning to make fine visual discriminations had, at baseline, stronger functional connectivity within portions of visual cortex and between visual cortex and prefrontal association areas (Baldassarre et al., 2012). These regions were also a subset of the regions that were activated by the discrimination task itself, suggesting that initial individual differences within the task-evoked neural networks encouraged or discouraged effective learning.
The above studies examined variation in learning across individuals, but individuals also vary across time in their performance and learning. Two fMRI studies exploited natural fluctuations in resting-state BOLD signals in an attempt to distinguish brain states within an individual that were associated with superior or inferior performance on vigilance and learning tasks. In both studies, stimulus presentation was triggered via real-time fMRI when BOLD signals in relevant brain regions were hypothesized to be in optimal or suboptimal states. In the vigilance task, an individual had better vigilance (faster reaction times) for the appearance of an unpredictable visual target when, before the appearance of the target, BOLD signal was high in the supplementary motor area (a region associated with motor planning) and low in components of the default-mode network (a network that is more active during rest than most tasks and that has been associated with internal self-reflection rather than external perceptual attention) (Hinds et al., 2013). In the memory task, an individual exhibited superior learning of scenes when BOLD signals were lower before the appearance of a scene in the posterior parahippocampal cortex, a region that is selectively responsive to scenes (Yoo et al., 2012). Thus, brain states could be identified that predicted whether an individual was ready to be vigilant or ready to learn.
Future Learning and Education in Children
Reading and mathematics are the two foundations of education, and accordingly the focus of school curriculum from elementary school through high school. The first major education experience for children is learning to read in early school years, after which they use those reading skills to learn all other subjects. Some children (5–17%) have developmental dyslexia, which is a persistent difficulty in learning to read that is not explained by sensory, cognitive, or motivational factors or lack of adequate reading instruction (Shaywitz, 1998) and that is highly heritable (Pennington et al., 1996). The best understood psychological cause of dyslexia is a weakness in phonological awareness, the understanding that spoken words are composed of discrete sounds (phonemes) that can be mapped onto letters or syllables (graphemes) (Bradley and Bryant, 1978), although several other putative causes have been identified (reviewed in Gabrieli, 2009).
Brain measures in infants have correlated with future success or failure in language and reading years before explicit reading instruction. Event-related potentials (ERPs), which are time-locked changes in electrical activity measured with EEG scalp electrodes, have revealed risk for future language and reading difficulties in newborns within hours or days of birth. These studies typically involve infants from families with a history of language or reading difficulty so as to increase the proportion of infants who will progress to language and reading difficulty. ERP responses to speech sounds within 36 hours of birth discriminated with over 81% accuracy those infants who would go on to become dyslexic at age 8 (Molfese, 2000). Newborns, tested within a week of birth, had ERP’s in response to speech sounds that correlated with language scores at ages 2.5, 3.5, and 5 years of age (Guttorm et al., 2005).
Some studies have reported that neuroimaging measures enhance or outperform traditional behavioral measures in forecasting children’s reading abilities in future months and years. One study examined how children ages 8–12, identified by their teachers as struggling readers, fared from the beginning to the end of a school year in single-word decoding skills (the ability to read aloud pseudowords on the basis of phoneme-grapheme mapping rules) (Hoeft et al., 2007). At the beginning of the school year, these children were evaluated with over a dozen behavioral measures of reading and reading-related skills, an fMRI task requiring rhyme judgments for pairs of printed words, and a voxel-based morphometry (VBM) analysis of anatomic grey and white matter densities. The beginning-of-the-year behavioral measures accounted for 65% of the variance in end-of-year scores, and the brain measures accounted for 57% of that variance. The combination of behavioral and brain measures accounted for a significantly better 81% of the variance, demonstrating enhanced forecasting of student reading skills across a school year.
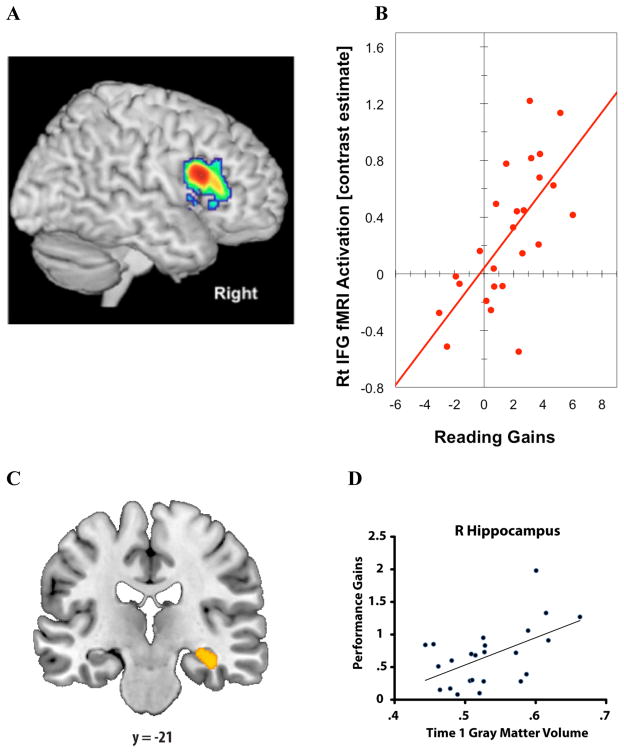
Among children with dyslexia, there is considerable variation in the degree to which individual children do or do not compensate for their reading difficulty by closing the gap between their actual and age-expected reading skills. A longitudinal study of older children (mean age of 14 years) examined how behavioral measures (17 tests of reading and reading-related skills), fMRI activation for a word-rhyming task, and DTI indices of white-matter organization predicted which children, over the next 2.5 years, would compensate or persist in their reading difficulty (Hoeft et al., 2011). None of the standard behavioral measures correlated with future reading gains, but the brain measures did yield such correlations (Figure 2). In combination, greater activation in right prefrontal cortex (a region not typically engaged for reading single words at this age) and greater white-matter organization of the right superior longitudinal fasciculus predicted with 72% accuracy whether a child would be in the compensated or persistent group. Multivoxel pattern analysis (MVPA) of whole-brain fMRI activation, a data-driven pattern classification analysis, yielded over 90% accuracy in classifying whether a dyslexic child at baseline would belong to the compensating or persistent group 2.5 years later.
Figure 2. Functional and Structural Brain Measures Predicting Educational Outcomes.
(A–B) fMRI predictor of reading gains in dyslexia. (A) Greater activation for a phonological task in right inferior frontal gyrus (Rt IFG) predicted (B) greater gains in reading 2.5 years later in dyslexic children; each red circle is an individual (based on Hoeft et al., 2011). (C–D) MRI predictor of math tutoring gains in students. (C) Greater grey-matter volume of right (R) hippocampus predicted (D) greater performance gains in students after 8 weeks of tutoring; each blue circle is an individual (from Supekar, 2013).
Longitudinal studies have also found neuromarkers associated with future reading skills in children who were not selected on the basis of family history or reading difficulty. In a 5-year longitudinal study, an auditory ERP measure (hemispheric lateralization of late mismatch negativity) in pre-reading kindergartners significantly improved the forecasting of future reading performance in 2nd, 3rd, and 5th grades in combination with pre-reading skills (Maurer et al., 2009). Only the ERP measure (and not any behavioral measure) correlated with future reading performance in 5th grade. A visual ERP study with pre-reading kindergartners also reported that the combination of behavioral measures and both ERP and fMRI responses to print explained up to 88% of the variance in 2nd grade reading ability (Bach et al., 2013). These studies suggest that neuromarkers in pre-reading kindergartners may enhance the identification of children who will struggle to read even before reading instruction begins in school. This is important because current reading interventions are most effective in young, beginning readers, and effective intervention prior to reading failure may not only be more effective, but also spare children the sense of failure that often accompanies early struggles in reading.
In older typical readers ages 9–15, fMRI activations in response to a word-rhyming task was associated with nonword reading skill up to 6 years in the future, with the specific locations of activations depending upon the child’s age (McNorgan et al., 2011). In younger children, greater activation in brain regions associated with phonological recoding (e.g., inferior frontal gyrus) was associated with greater future reading skill, whereas for older children, greater activation in brain regions associated with orthographic analysis of print (e.g., fusiform gyrus) was associated with lesser future gains. These findings underscore how different developmental stages of learning to read, perhaps transitioning from a younger gaining of skill in single word decoding (print-to-sound correspondence) to an older mastering of fluent visual analysis of connected print, may invoke relatively different components of the brain’s reading circuitry.
There is also considerable variation in how well children can learn a second language. For native Chinese speakers around age 10, greater activation in response to English words and nonwords in left fusiform gyrus and left caudate correlated with superior English word reading levels a year later (Tan et al., 2011). The putative visual word form area (VWFA), which is highly responsive to learned print, is located in the left fusiform gyrus (Dehaene and Cohen, 2011). The leftward lateralization of neuromarkers may have been related to properties of alphabetic languages such as English, because there is evidence that variation in microstructural properties of right-hemisphere white-matter pathways correlated with initial learning of Mandarin Chinese in young adults (Qi et al., 2014). The rightward lateralization of neuromarkers in native English speakers associated with future successful initial language learning may reflect the tonal and visuo-spatial properties, respectively, of spoken and written Mandarin Chinese. Thus, neuromarkers correlated with second-language learning may vary depending on the kinds of mental resources needed to learn different kinds of languages.
Mathematical problem solving skills are the foundations of later performance in science and engineering. Academic skill in arithmetic relies on multiple cognitive processes, including working memory, the mental processes that support the maintenance and manipulation of goal-relevant information over brief time periods (reviewed in Raghubar et al., 2010). In a longitudinal study, children ages 6–16 underwent behavioral testing (working memory, reasoning, and arithmetical abilities) and fMRI while performing a visuospatial working memory task (Dumontheil and Klingberg, 2012). Neuroimaging analyses focused on the intra-parietal sulcus (IPS), a brain region associated with both visuospatial working memory and numerical representation. The working memory and reasoning measures were independent predictors of arithmetical performance two years later. The magnitude of visuospatial working-memory activation in left IPS also predicted future arithmetical performance. Combining the neuroimaging and behavioral data more than doubled the accuracy of predicting future mathematical ability compared to use of only behavioral data.
The future growth of working memory ability in the same age range has also been better predicted by a combination of neuroimaging and behavioral measures than behavioral measures alone (Ullman et al., 2014). Interestingly, whereas current working memory capacity correlated with activation in frontal and parietal regions, future capacity was best predicted by structural and functional measures of the basal ganglia and thalamus. Specifically, greater activation in the caudate and thalamus and greater fractional anisotropy (FA) of surrounding white matter as measured by DTI predicted future growth in working memory over the next two years.
There is increasing interest in improving the effectiveness of learning through teaching that takes into account variation among students. One study examined whether neuromarkers could identify which children would benefit from a math-tutoring program for 3rd graders (ages 8–9) that encouraged students to shift from counting to fact retrieval as a basis for arithmetic problem-solving strategy (Supekar et al., 2013). Individual differences in how much students benefitted from the tutoring program did not correlate with baseline behavioral scores on tests of intelligence (IQ), working memory, or mathematical abilities. Conversely, at baseline, greater right hippocampal volume and resting-state intrinsic functional connectivity between right hippocampus and prefrontal and striatal regions correlated with future performance improvements (Figure 2).
Future Criminality
The criminal justice system is rife with demands for predictions of future behaviors as judgments are made about bail, sentencing, and parole. The demonstrated inaccuracy of expert clinical judgments (Monahan, 1981) has motivated the use of an actuarial approach that estimates risk for future antisocial behavior based on characteristics such as age, sex, criminal history, and drug use (e.g. Yang et al., 2010). Building on evidence that impulsivity (behavioral disinhibition) is a major risk factor for recidivism, brain activations to an impulse-control task (go/no-go task) were examined in 96 male offenders who were then followed longitudinally (Aharoni et al., 2013). The likelihood that an offender would be rearrested over a 4-year period doubled if at baseline the offender had low activation in the anterior cingulate cortex, a region associated with cognitive control and especially the resolution of cognitive conflict. Whereas the correlation between baseline brain activation and future rearrest was significant, there was no or weaker correlations for other predictors (age, scores on a psychopathy checklist, lifetime substance abuse, or behavioral error rate on the scanner task).
Future Health
Studies have examined whether neuromarkers are related to future health-related behaviors, such as alcohol abuse, drug abuse, or unhealthy eating. Alcohol use by underage drinkers is an important public health problem because such use in adolescents is risky and also associated with life-long alcoholism. Heavy or binge drinking is the primary source of preventable morbidity and mortality for the more than 6 million American college students (Wechsler et al., 2002). Early onset of alcohol use by age 12 is associated with numerous undesirable outcomes in adolescence (Gruber et al., 1996), and initiation of drinking before age 15, versus after age 20, quadruples the likelihood of alcoholism (Grant and Dawson, 1997).
In a longitudinal study, 12–14 year-olds with little or no history of substance abuse performed a go/no-go task of response inhibition while undergoing fMRI (Norman et al., 2011). About four years later, these adolescents were divided into two groups who did or did not transition to heavy use of alcohol. Widespread reductions in baseline activation, including in prefrontal and anterior cingulate cortices, were found in adolescents who later transitioned to heavy alcohol use relative to those who did not. Among adolescents ages 16–19 with an ongoing history of substance use disorders, those who exhibited less prefrontal and greater parietal activation on the same task had higher levels of substance use over the following 18 months (Mahmood et al., 2013). Overall, the findings suggest that a relative weakness in the recruitment of anterior brain regions that are most associated with cognitive control of behavior may be a predisposition for early alcohol use or sustained substance abuse.
Adolescents who exhibited greater activation in response to monetary rewards in the basal ganglia were more likely to engage in substance use (alcohol and drugs) a year later (Stice et al., 2013). In contrast, those who were already using substances at baseline exhibited lesser activation in the basal ganglia at baseline. These findings indicate that reward systems of the basal ganglia are also involved in substance abuse, but that brain measures of future risk for substance use may be quite different than brain measures reflecting the consequence of current use of substances.
The largest study of future adolescent misuse of alcohol followed nearly 700 adolescents and collected detailed histories, personality measures, genetic information, structural and functional MRI data, and cognitive performance measures (Whelan et al., 2014). FMRI tasks examined inhibitory control, reward processing, and facial expressions of emotion. In 271 of these adolescents, a multi-domain analysis was used to predict future binge drinking. The most robust brain predictors of future binge drinking came from right precentral and bilateral superior frontal gyri, with contributions from several structural (gray matter volume) and functional (inhibitory control and reward outcome) features. In the predictive model, these brain measures were coupled with life events, personality measures, and an anxiety sensitivity subscale of the substance-use risk profile scale. Any one feature in isolation had only a modest influence on prediction, and many of the features predicting future misuse were different from the features dissociating groups of binge drinkers and non-binge drinkers. Such a study highlights the multiple causal factors for substance abuse, as well as the scale of data needed to predict the future unfolding of such multifaceted processes.
Healthy eating so as to avoid or reduce obesity is also a major public health concern. Neuroimaging studies have reported that fMRI activations in response to food-related pictures forecast future changes in body mass index (BMI) over the next six months to one year. Two studies examined the relation between baseline fMRI activations and weight gain over the following year in girls identified as having body image concerns. Activations in response to palatable food occurred in brain regions associated with reward anticipation (e.g., regions of basal ganglia) or reward valuation (e.g., orbitofrontal cortex). In one case, dopamine-related genetic variation interacted with blunted brain activation to correlate with elevated risk for future weight gain (Stice et al., 2010). In another case, lateral orbitofrontal cortical activation during initial orientation to appetizing food cues correlated with future increases in BMI over a 1-year period (but behavioral patterns of response did not correlate with BMI increases) (Yokum et al., 2011).
Another study demonstrated the specificity of brain activations to food cues in relation to future weight gain (Demos et al., 2012). Women arriving at college saw pictures of food, sexual scenes, and control pictures during fMRI. At baseline and again towards the end of the school year, the women’s weights and self-reports of sexual behavior were measured. Greater initial response in the reward-responsive nucleus accumbens for food pictures correlated with greater BMI gains 6 months later, whereas greater initial response to sexual scenes correlated with greater sexual desire and more sexual experiences 6 months later.
In a related study, college age-women participated in an fMRI study of brain responses to pictures of foods and for response inhibition on a go/no-go task, followed by experience sampling via smartphone. Over the course of one week, they were periodically asked to report their desire to eat food, attempts to resist the temptation to eat, and whether or not and how much they actually ate (Lopez et al., 2014). Greater nucleus accumbens activation to food pictures correlated with greater desires for food, more likelihood to give in to the temptation to eat, and larger amounts eaten. Greater activation of the inferior frontal gyrus during response inhibition was associated with reduced surrender to temptation and less eating. Overall, these studies suggest that an interplay between response to food cues that occurs in reward-sensitive striatal and orbitofrontal regions and response in cognitive control regions of the lateral prefrontal cortex contributes to future healthy or unhealthy eating patterns.
Another health-related behavior is the use of sunscreens for protection against sunburn and some forms of skin cancer. In one study, participants saw slides communicating the importance and proper application of sunscreen (Falk et al., 2010). Participants also reported recent use of, intentions to use, and attitudes toward sunscreen. Greater activation in medial prefrontal cortex, a brain region associated with self-reference, correlated with changes in the use of sunscreen as measured by an unexpected self-report one week later. Brain measures accounted for about 25% of the change in use of sunscreen above and beyond self-reported changes in attitudes and intentions following presentation of the health information during scanning. Activation in medial prefrontal cortex may broadly represent value, because the magnitude of activation in that brain region (and the striatum) in response to individual consumer goods was associated with subsequent preferences for choosing those goods (Levy et al., 2011).
Some studies have examined how brain function at one point in time correlates with mental health outcomes at future time points, independent of treatment. For example, greater amygdala activation to emotional facial expressions among patients with depression correlated with reduced symptoms of depression 6 month later, controlling for initial depression severity and medication status (Canli et al., 2005). In a memory paradigm with negative pictures, greater baseline activation for successfully recalled pictures in posterior cingulate cortex and medial prefrontal cortex correlated with greater improvement in depressive symptoms 18 months later (Foland-Ross et al., 2014).
Future Response to Treatment
Biomarkers in general, and neuromarkers in particular, are not used currently to predict treatment response for neuropsychiatric disorders despite considerable evidence that any specific pharmacological or behavioral treatment is likely to be effective for some patients but ineffective for a considerable number of other patients. Measurement of treatment efficacy varies, but it typically involves a patient report or clinician observation, often via a structured interview or questionnaire. A highly effective treatment results in remission, the absence of symptoms, or in a substantial response, defined as an outcome in which the patient remains somewhat symptomatic but is much improved (Frank et al., 1991).
Across many neuropsychiatric diagnoses, remission or substantial response occurs in about 50% of patients for a given therapy. For depression, cognitive behavioral therapy (CBT) is effective in 40–60% of patients (Hollon et al., 2002) and selective serotonin reuptake inhibitors are effective in 40–60% of patients, although many patients who fail to respond to an initial treatment will respond to another treatment or combination of treatments (Souery et al., 2006). Similar 40–60% success rates for a given pharmacological or behavioral treatment have been reported for generalized anxiety (Pollack et al., 2003), social anxiety disorder (Otto et al., 2000), and ADHD (Wender, 1998; Biederman et al., 2010). This variability in treatment response, which is not understood and not simply a consequence of disease severity, suggests that there are clinically important neurobiological differences among patients sharing a diagnostic label such that a specific treatment will be effective for some but not other patients.
To a remarkable degree, there is an absence of evidence about which treatment is likely to be effective for a particular patient. Although patients often do benefit from a second or third sort of attempted treatment, there is considerable human and economic cost for delaying effective treatment for patients and families who are often in crisis. The idea of personalized medicine, that people vary in their response to treatments and that more effective medicine can be practiced by knowing which treatment is most likely to benefit a particular patient, has been associated often with genetics. It seem plausible, however, that quantitative brain measures may also reveal variation among patients that provides an evidence-based rationale for what treatment is most likely to help a particular patient among currently available treatments.
Future Response to Pharmacological Treatment
Over 20 studies of depression have reported that pre-treatment neuroimaging measures can correlate with or predict clinical improvement following pharmacological treatment (reviewed in Pizzagalli et al., 2011, Fu et al., 2013). In one study, prior to treatment there was reduced subgenual anterior cingulate cortex (ACC) metabolism measured by PET in patients who subsequently responded poorly to medicine relative to either healthy controls or patients who responded well to medication and who exhibited greater-than-normal metabolism (Mayberg et al., 1997). No clinical measure, such as depression severity, or behavioral measure, such as cognitive performance, distinguished the patients who would or would not respond to treatment. The subgenual ACC (Brodmann area 25) is especially salient for depression because it has been shown to be functionally and structurally atypical in depression (Drevets et al., 1997), and been a target for deep brain stimulation treatment of depression (Mayberg et al., 2005).
A meta-analysis of 20 studies on depression supported the conclusion that increased baseline activation of ACC, extending into orbitofrontal cortex, was associated with better treatment response, but that decreased activation of insula and striatum was also associated with better treatment response (Fu et al., 2013). In an fMRI activation study in which patients viewed faces with sad facial expressions of varying intensity, a machine learning approach (SVM and leave-one-out cross-validation) identified patients who would have remission with 71% sensitivity/86% specificity (Costafreda et al., 2009). There is also evidence that structural brain measures at baseline were associated with treatment outcome. Across studies, worse response to treatment has been associated with decreased grey matter volume in left dorsolateral prefrontal cortex and also in right hippocampus (Fu et al., 2013). Finally, repetitive transcranial magnetic stimulation (rTMS) is a less common treatment for depression, but resting-state functional connectivity measures have been associated with clinical response to such treatment (Salomons et al., 2013). Higher cortico-cortical connectivity and lower cortico-thalamic, cortico-striatal, and cortico-limbic connectivity at baseline were associated with better treatment response.
In an open-label study examining the efficacy of treating generalized anxiety disorder with venlafaxine, patients viewed faces with fearful or neutral expressions. Greater activations for fearful relative to neutral faces in rostral ACC and lesser activations for fearful relative to neutral faces in left amygdala both correlated with greater clinical improvement (Whalen et al., 2008). These correlations occurred despite no activation differences in the rostral ACC or amygdala either between patients and controls or between pre-treatment and post-treatment in the patients, who did improve clinically in response to treatment. Such a finding underscores the idea that neuromarkers that are associated with treatment response need not reflect the same functions as those related to etiology.
Future Response to Behavioral Treatment
Perhaps the best validated kind of behavioral treatment for neuropsychiatric disorders is cognitive behavioral therapy (CBT), which meta-analyses indicate to be effective for many disorders, including depression, generalized anxiety disorder, panic disorder, and social anxiety disorder (e.g., Butler et al., 2006; Hofmann et al., 2012). Multiple studies have reported that CBT is similarly effective as pharmacological treatments for depression (DeRubeis et al., 2005), generalized anxiety disorder (Mitte, 2005), pediatric anxiety (Walkup et al., 2008), and social anxiety disorder (Heimberg et al., 1998). For disorders that are treated primarily with medications, CBT has been shown to enhance clinical outcome relative to other augmentations for obsessive-compulsive disorder (OCD) (Simpson et al., 2013) and schizophrenia (Grant et al., 2012).
Several neuroimaging studies have reported that pre-treatment neuroimaging measures correlate with or predict the magnitude of clinical improvement following CBT in unipolar major depression, schizophrenia, and social anxiety disorder. The initial study relating pre-treatment brain function to clinical efficacy of CBT occurred in 14 unmedicated patients with depression who viewed emotionally negative words prior to treatment. Both less sustained activation in subgenual ACC and more sustained activation in amygdala were associated with greater improvement in response to CBT (Siegle et al., 2006). The finding that less sustained activation in subgenual ACC was associated with better future response to CBT was replicated and extended in a larger study of patients with depression (Siegle et al., 2012). This study is noteworthy in its use of a model generated from one cohort being used to predict the outcomes of an independent cohort.
For patients with schizophrenia being treated pharmacologically, about half respond beneficially to additional CBT treatment (e.g., Wykes et al., 2008). In one set of overlapping studies, patients receiving CBT exhibited clinical improvements relative to patients who did not receive CBT (Kumari et al., 2009; Premkumar, et al., 2009; Kumari et al., 2011). The magnitude of clinical benefit among the patients receiving 6–8 months of CBT correlated with both baseline functional and structural measures. Patients who exhibited stronger activation in dorsolateral prefrontal cortex (DLPFC) during performance of a working memory task, and who exhibited stronger DLPFC-cerebellar functional connectivity in the most demanding condition of the task, derived greater benefit from CBT (Kumari et al., 2009). In another fMRI study, patients read aloud single words, heard either their own or another person’s voice that was or was not distorted, and then judged whether they had heard their own voice or that of another (Kumari et al., 2010). Across several contrasts, greater activation in left inferior frontal gyrus and lesser inferior parietal and medial prefrontal deactivation were associated with greater CBT benefit. Greater engagement of prefrontal regions in patients who benefitted more from CBT may be related to regulatory processes that can support effective CBT. There has also been some evidence for separable neuromarkers related to CBT response for positive symptoms (excess or distorted normal functions such as hallucinations or delusions) versus negative symptoms (diminished normal functions such as apathy or withdrawal) (Premkumar et al., 2009). Importantly, baseline symptom severity did not correlate with CBT response, so that the neuromarkers provided measures associated with future CBT benefit that were not clinically evident at baseline.
Current behavioral measures poorly predict treatment outcome in social anxiety disorder, another disorder often treated with CBT. Prior to CBT, patients viewed angry versus neutral faces or negative versus neutral scenes during fMRI (Doehrmann et al., 2013). Consistent with the social nature of this anxiety disorder, activations in response to scenes were not associated with treatment outcome, but activations to angry relative to neutral faces were associated with CBT outcome. Greater activations in higher-order visual cortices were predictive of superior treatment outcome (Figure 3). Initial greater clinical severity accounted for about 12% of the variance in treatment outcome, whereas the combination of baseline neuroimaging and clinical severity accounted for about 40% of the outcome variance. Similar findings were observed at a less conservative statistical threshold in prefrontal cortices, and it is possible that the relations between prefrontal and higher-order visuo-perceptual cortices may support or constrain the self-regulatory processes that are taught in CBT (i.e., that these results revealed variation in the neural mechanisms that support CBT response, rather than those of social anxiety disorder).
Figure 3. Functional Brain Measure Predicting A Clinical Outcome.
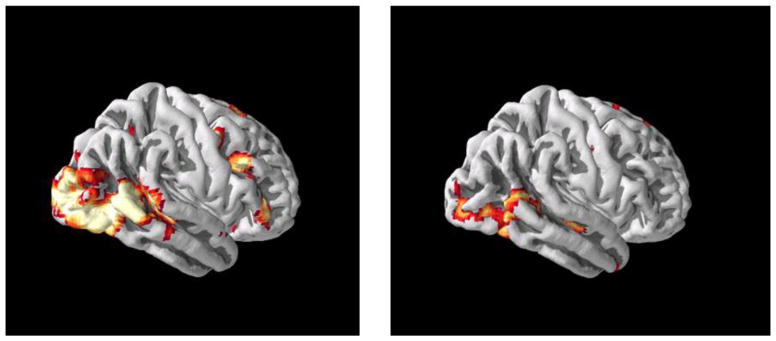
Prior to treatment, patients with social anxiety disorder who exhibited greater posterior activation (left panel) for angry relative to neutral facial expressions had better clinical response to cognitive behavioral therapy (CBT) than patients who exhibited lesser activation (right panel) (based on Doehrmann et al., 2013).
A study of patients with generalized anxiety disorder or panic disorder aimed to develop measures that might be sensitive to single-subject responses to treatment (Ball et al., 2013). Patients saw negative scenes and either maintained or reduced (via reappraisal) their emotional response to the scenes. A random forest classification was used to identify brain regions in which activations best predicted treatment outcome; there were greater activations for responders than non-responders in hippocampus during the maintenance of negative images, and in anterior insula, superior temporal, supramarginal, and superior frontal gyri during reappraisal of negative images. The neuroimaging measures yielded superior accuracy, sensitivity, and specificity in identifying individual patients as future responders or non-responders to treatment than did clinical or demographic variables. This study provides an example of data-driven analyses that are predictive even though the specific patterns of activation are not readily interpretable in a cognitive neuroscience framework.
For OCD, structural measures at baseline have been associated with variability in response to exposure therapy (Fullana et al., 2014). Thinner cortex in left rostral ACC at baseline was associated with better responses to therapy. This same region was thinner overall in patients than controls, so greater differences from controls were associated with better outcomes. The neuroanatomical locus is similar to that observed often in studies of depression outcomes, which raises the possibility that similar neural mechanisms may support behavioral therapies across diagnoses.
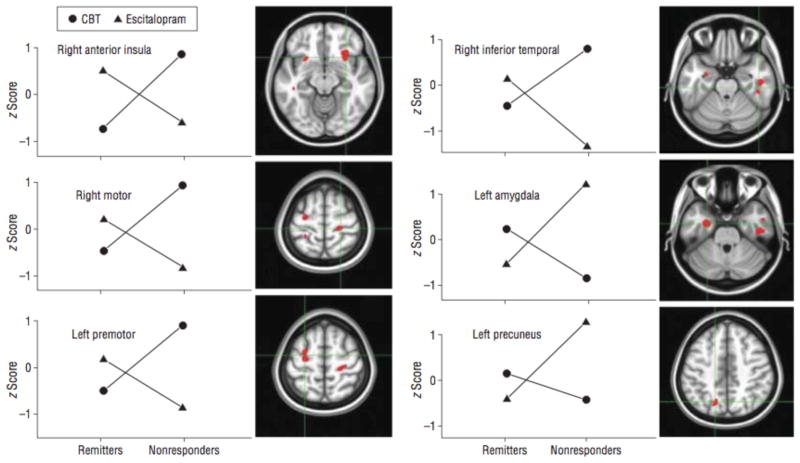
The above studies examined the relations between pre-treatment neuromarkers and one kind of treatment, such as CBT, or a medication, or rTMS. The relevant choice that must be made by a patient or physician, however, is not whether to pursue one kind of treatment, but rather to select among alternative treatments. Therefore, an important and practical goal is to examine whether there are differential predictors of effectiveness for alternative treatments. One study employed PET imaging prior to patients being randomly assigned to a medication (escitalopram oxalate) or CBT to treat their depression (McGrath et al., 2013b). Six limbic and cortical regions showed a differential response to the two treatments, with right anterior insula hypometabolism correlating with future remission to CBT (and poor medication response), and right anterior insula hypermetabolism correlating with future remission to medication (but a poor response to CBT) (Figure 4). Subgenual ACC metabolism was higher in patients who failed to respond to either treatment than in patients who remitted from depression (McGrath et al., 2013a).
Figure 4. Treatment-Specific Biomarker Candidates for Treatment of Depression.
Mean regional activity values for remitters and nonresponders segregated by treatment (either Escitalopram given as escitalopram oxalate or cognitive behavioral therapy (CBT)) are plotted for the 6 regions showing a significant treatment × outcome analysis of variance interaction effect. Regional metabolic activity values are displayed as region/whole-brain metabolism converted to z scores. From McGrath et al., 2013b.
Another study with a small number of pediatric patients with generalized anxiety also found correlations between pretreatment brain functions and treatment outcomes, but did not find differences between behavioral (CBT) and pharmacological (fluoxetine) treatments. Greater baseline activation of the amygdala to negative facial expressions was associated with better symptom improvement regardless of treatment type (McClure et al., 2007).
Future Relapse for Alcohol, Drug Addiction, Smoking, and Diet
Alcoholism, drug addiction, smoking, and obesity are major public health problems that share a similar treatment aim, namely abstinence from a substance that is harmful to the brain and body. Several studies have examined relations between neuromarkers and whether individuals abstain successfully or relapse into their health problems. Generally, these studies examined patients who recently became abstinent at the initiation or termination of a treatment program, and then followed these patients over weeks or months to learn which patients continued to abstain versus those who relapsed. Improved identification of risk for relapse could support individualized treatment approaches that vary for those at minimal or maximal risk for relapse.
At least 60% of patients who seek treatment for an alcohol-use disorder relapse within 6 months following treatment (Maisto et al., 2006; Udo et al., 2009), and there have been several studies in which baseline brain measures are associated with future abstinence versus relapse. In two studies of recently abstinent patients, greater activation of the basal ganglia (putamen), ACC, and medial prefrontal cortex in response to alcohol-associated visual stimuli was associated with greater likelihood of relapse 3 weeks or 3 months later (Braus et al., 2001; Grusser et al., 2004). Other measures, such as self-reported intensity of craving, history of intake, or duration of abstinence before scanning, were not associated with likelihood of relapse. Both anatomic (Rando et al., 2011) and regional cerebral blood flow (Noel et al., 2002) studies reported that baseline measures of the medial prefrontal cortex were associated with likelihood of relapse. Similarly, patients who relapsed exhibited reduced volumes of medial and/or lateral prefrontal cortex (Durazzo et al., 2011; Cardenas et al., 2011) and reduced white-matter FA in frontal regions (Sorg et al., 2012) relative to patients who sustained abstinence. Broadly, greater reward response to alcohol-related stimuli and lesser strength in cognitive control regions were related to relapse.
Relapse after treatment occurs at an estimated 50% rate within a year among individuals with stimulant dependence who seek treatment (Miller, 1996). Several neuroimaging studies have reported that neuromarkers can contribute to identification of future abstinence or relapse. One group of patients with methamphetamine dependence underwent fMRI 3 or 4 weeks after cessation of drug use and near completion of a 28-day inpatient program, and were followed for about a year at which point about half of the patients had relapsed (Paulus et al., 2005). During fMRI, participants attempted to either predict where a stimulus would appear or to simply note that a stimulus had appeared. None of multiple sociodemographic, baseline symptom, or use characteristics predicted relapse, but those patients who would later relapse exhibited greater activation than those who would not relapse in multiple brain regions, including prefrontal, parietal, and insular cortices. A pattern of activation across right insular, posterior cingulate, and temporal regions correctly identified 20 of 22 patients who did not relapse, and 17 of 18 patients who did relapse. Other studies have reported correlations between baseline fMRI activations and future relapse for cocaine use after 1 week (Prisciandaro et al., 2013), after a 10-week outpatient program (and a better predictor than subjective reports of craving) (Kosten et al., 2006), and after an 8-week outpatient program (Brewer et al., 2008; Jia et al., 2011). Specific locations of activations that correlated with future relapse varied across these studies, perhaps reflecting differences in experimental paradigms, analyses, or participants.
Tobacco smoking is the leading preventable cause of death in the developed world, with one billion tobacco-related deaths projected for the 21st century (World Health Organization, 2008). Identifying smokers at high-risk for relapse could influence the design of cessation programs to fit with individual risk profiles. In one study, adult nicotine-dependent women underwent fMRI while viewing smoking-related and unrelated pictures before quitting smoking (Janes et al., 2010). The women then made an attempt to quit smoking, and pre-quit measures were related to subsequent success or failure in smoking cessation. Greater baseline activation to smoking-related pictures in the insula correlated with likelihood of future relapse. The identification of insula reactivity as a correlate of future relapse is of interest because lesions to the insula in smokers were associated with reduced smoking that was immediate and without relapse (Naqvi et al., 2007). Smokers who did not quit successfully also exhibited reduced functional connectivity between an insula-containing network and dorsolateral prefrontal cortex and dorsal ACC, suggesting a weakness in interactions between brain regions associated with smoking desires with regions associated with cognitive control. A combination of brain functional data and a behavioral task resulted in 79% accuracy in identifying smokers who would or would not abstain from smoking. Future success in quitting smoking has also been associated with grey-matter volumes in cortical and subcortical regions (Froeliger et al., 2010).
Brain measures may also help identify what sort of information presented to people aiming to quit smoking are likely to be effective. Ads aimed at encouraging people about to try to quit smoking were presented during fMRI, and relapse was followed for a month (Falk et al., 2011). Greater activation in medial prefrontal cortex at baseline was associated with successful quitting. The addition of the brain measures to other measures (self-reported intentions, self-efficacy, and ability to relate to the ads), more than doubled the accuracy of a model accounting for changes in smoking behavior. In another study with a large number of smokers, increased activation in brain regions associated with self reference, especially the medial prefrontal cortex, in response to individually tailored smoking cessation messages was associated with the probability of quitting 4 months later (Chua et al., 2011).
Healthy eating is a goal for individuals with obesity, and there is evidence that brain measures at baseline are associated with short- and long-term outcomes in a weight-loss program (Murdaugh et al., 2012). Obese individuals viewed high-calorie food versus control pictures before and after a 12-week weight-loss program, with a 9-month follow-up. Greater baseline activation in the nucleus accumbens, insula, and ACC in response to high-calorie food pictures correlated with lesser weight loss after 12 weeks. Further, less successful weight maintenance at 9 months correlated with greater post-treatment activation in insula, ventral tegmental area, and other regions. The relevant regions are associated with interoception (insula), reward (nucleus accumbens, ventral tegmental area), and cognitive control (anterior cingulate), which are all processes related to dietary decisions.
Future Response to Placebos
Positive medical responses to placebo treatments are often powerful and can rival the effectiveness of active treatments, such as medicines for depression (e.g., Walsh et al., 2002). Consequently, exploitation of placebo mechanisms may offer a safe therapeutic approach for some patients, but there is evidence for considerable variation in response to placebos (Walsh et al., 2002). Most studies of the brain basis of individual differences in placebo responses have focused on pain, in part because cortical and subcortical brain regions involved in pain have been relatively well characterized. Placebo analgesia (the positive influence of placebo on experienced pain) was related to a pattern of increased activation in several cortical control regions and decreased activation in somatosensory activation during the anticipation of pain, rather than activation during reported analgesia to pain itself (Wager et al., 2011). Patients with better future response to placebo treatments exhibited lesser resting-state functional connectivity between medial prefrontal cortex and insula during a pain-rating task (Hashmi et al., 2012). Furthermore, greater network efficiency during the resting-state was associated with better response to future psychologically mediated analgesia related to treatment for chronic knee pain (Hashmi et al., 2014). A range of other findings also indicate that functional and structural brain measures may help identify individual patients most likely to benefit from placebo treatments (reviewed by Koban et al., 2013).
Predicting Individual Futures with Neuromarkers: Hopes and Challenges
As reviewed above, neuromarkers obtained from noninvasive brain imaging have shown great promise for identifying children and adults more likely to learn well or poorly in particular domains, more likely to progress to unhealthy (or even criminal) behaviors, and more likely to respond to particular pharmacological, behavioral, or placebo treatments for many neurodevelopmental and neuropsychiatric disorders. Although the amount of scientific evidence is modest in many areas (with reading and depression having perhaps the greatest concentrations of studies to date), there are also numerous studies reporting that predictive neuromarkers either outperform or significantly enhance traditional measures of individual variability, such as self-reports, clinical rating scales, or scores on educational or neuropsychological tests. It is these kinds of studies that express both a practical and humanitarian possibility of improving lives through recognizing individual differences in brain function and structure that greatly influence the diversity of educational and clinical outcomes, and using that recognition to individually optimize educational and clinical practices.
Because of these hopes, the challenges of translating cognitive neuroscience measures into better futures for people need to be carefully identified and thoughtfully overcome. First, many of the reviewed studies were performed with relatively small samples, and in particular many of the older studies used statistical approaches that were overly liberal by current standards. Although such pioneering studies must often begin with modest resources because it is their outcomes that justify larger studies, the translation of such science to practical application now requires larger studies that can support more rigorous statistics. This is particularly true for studies of neurodiversity that focus on individual differences because there must be adequate sampling not only of a population as a whole, but also the diversity of individuals within that population. Second, studies must mature from correlations between baseline measures and clinical or educational outcomes to predictive models that apply the outcomes from one group (training set) to another group (test set) and finally to an individual. This is essential because use of such measures must operate with new individuals for whom a clinical or educational intervention is being planned. Third, few studies have integrated findings across multiple imaging modalities, even when the multiple brain measures could be made during a single MRI session. Combining multiple kinds of neuromarkers may enhance their predictive accuracy. Fourth, it will be important for future studies to involve plausible, alternative interventions (e.g., McGrath et al., 2013b) because the question is less often whether a person should receive help, but rather which kind of help is most likely to rapidly improve the person’s education, skills, or health.
Neuromarkers will be useful to the extent they outperform, alone or in combination with traditional measures, measures that are otherwise available. Indeed, multiple studies have reported such value from neuromarkers, but other studies have not examined whether the neuromarkers significantly improve predictions above and beyond readily available measures. All forms of brain and behavioral assessment improve over time, and perhaps a new behavioral assessment will outperform neuromarkers in the near or distant future. At the same time, behavioral assessments in many educational and clinical areas have been developed and maintained over many years, so it is unknown when breakthroughs might occur. Perhaps neuroimaging measures will be also useful tools in developing a new generation of brain-validated behavioral assessments that can be readily used in schools, hospitals, and medical offices. At the conceptual limit, there ought to be a strong relation between measures of mind and brain, such that a new generation of behavioral measures could capitalize on the novel insights of neuroimaging.
If neuroimaging remains necessary for optimal prediction, there could be concerns about cost and availability of MRIs or other measures. In this regard, the cost of MRI imaging in particular has raised concerns about its potential wider use. One solution for availability could be to use more transportable technologies, such as wireless EEG devices, with assessment paradigms developed through coupled MRI and EEG studies. Any economic analysis, however, ought to include the costs of current practices in which patients are often inadvertently directed to treatments that turn out to be ineffective for that patient (often around half of patients for a given treatment in many cases) or in which children must demonstrate academic failure before receiving educational intervention. The cost of a neuropsychological assessment and report for an individual child or adult, for example, often exceeds that of an MRI.
If neuromarkers are proven to enhance prediction, there will be ethical and societal issues to consider. Because of their biological nature, brain measures can be overly valued and potentially divert public and scientific interest in behavioral and social factors (Kagan, 2013). If neuromarkers become more useful, they will provoke questions about how to most ethically use predictive information to help people rather than simply select people most likely to succeed. This important concern, however, must be weighed against the questionable validity of many current practices, such as the finding that parole decisions made by experienced judges appear to be greatly influenced by the time of day and proximity to a meal at which a case is reviewed (Danziger et al., 2011), or that medical schools continue to conduct interviews for admissions despite evidence that decisions based on such interviews have no correlation with objective measures of medical school performance (DeVaul et al., 1987; Milstein et al., 1981). For in-patient treatments for substance abuse, there is little scientific justification for the prototypical 28-day treatment period. Even an imperfect predictive measure of relapse may lead to more rational treatment durations that are related to individual variation. Neuromarkers and neuroprognosis may offer practical and valuable contributions because so many current educational and medical decisions occur in the absence of scientific evidence that can guide those decisions.
The present review considered mostly studies with relatively short-term longitudinal educational and clinical outcomes, but future research may also attempt to predict longer-term outcomes. Educational and medical practices often respond to crisis, such as failure in learning to read or in coping with depression. Longer-term research may examine whether neuromarkers can help identify children at early risk, with the hopes of diverting those children away from a trajectory towards failure and crisis (such as ongoing studies attempting to identify whether infants at familial risk for autism will or will not progress to autism over the next few years (Bosl et al., 2011; Wolff et al., 2012)). Such early predictions may require novel forms of intervention (e.g., language learning remediation in 2- or 3-year-olds that minimizes their difficulty in learning to read as 5- and 6-year-olds) with the hope that such children never experience the crises as children or adults that now initiate intervention.
Acknowledgments
We thank Cynthia Gibbs, Sara Beach, Elizabeth Norton, Allyson Mackey, Jon Walters, and Maheen Shermohammed for help with the manuscript. Writing of this paper was supported by the Poitras Center for Affective Disorders Research at the McGovern Institute for Brain Research and NIH grant R01HD067312.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharoni E, Vincent GM, Harenski CL, Calhoun VD, Sinnott-Armstrong W, Gazzaniga MS, Kiehl KA. Neuroprediction of future rearrest. Proc Natl Acad Sci USA. 2013;110:6223–6228. doi: 10.1073/pnas.1219302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni E, Mallett J, Vincent GM, Harenski CL, Calhoun VD, Sinnott-Armstrong W, Gazzaniga MS, Kiehl KA. Predictive accuracy in the neuroprediction of rearrest. Soc Neurosci. 2014;9:332–336. doi: 10.1080/17470919.2014.907201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach S, Richardson U, Brandeis D, Martin E, Brem S. Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. Neuroimage. 2013;82:605–615. doi: 10.1016/j.neuroimage.2013.05.062. [DOI] [PubMed] [Google Scholar]
- Baldassarre A, Lewis CM, Committeri G, Snyder AZ, Romani GL, Corbetta M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci USA. 2012;109:3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball TM, Stein MB, Ramsawh HJ, Campbell-Sills L, Paulus MP. Single-subject anxiety treatment outcome prediction using functional neuroimaging. Neuropsychopharmacology. 2013;39:1254–1261. doi: 10.1038/npp.2013.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Kotarski M, Spencer T. A randomized, 3-phase, 34-week, double-blind, long-term efficacy study of osmotic-release oral system-methylphenidate in adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2010;30:549–553. doi: 10.1097/JCP.0b013e3181ee84a7. [DOI] [PubMed] [Google Scholar]
- Bishop CM, Tipping ME. Variational Relevance Vector Machines. Proceedings of the Sixteenth Conference on Uncertainty in Artificial Intelligence; San Francisco, CA, USA: Morgan Kaufmann Publishers Inc; 2000. pp. 46–53. [Google Scholar]
- Biswal B, Yetkin FA, Houghton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med Sci. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011;9:18–34. doi: 10.1186/1741-7015-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley L, Bryant P. Difficulties in auditory organisation as a possible cause of reading backwardness. Nature. 1978;271:746–747. doi: 10.1038/271746a0. [DOI] [PubMed] [Google Scholar]
- Braus D, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random Forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P. Remarks on the seat of the faculty of articulate language, followed by an observation of aphemia. Some papers on the cerebral cortex. 1861:49–72. [Google Scholar]
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JD, Gotlib IH. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Durazzo TC, Gazdzinski S, Mon A, Studholme C, Meyerhoff DJ. Brain morphology at entry into treatment for alcohol dependence is related to relapse propensity. Biol Psychiatry. 2011;70:561–567. doi: 10.1016/j.biopsych.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci. 2011;14:426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Khanna A, Mourao-Miranda J, Fu CH. Neural correlates of sad faces predict clinical remission to cognitive behavioural therapy in depression. Neuroreport. 2009;20:637–641. doi: 10.1097/WNR.0b013e3283294159. [DOI] [PubMed] [Google Scholar]
- Danziger S, Levav J, Avnaim-Pesso L. Extraneous factors in judicial decisions. Proc Natl Acad Sci USA. 2011;108:6889–6892. doi: 10.1073/pnas.1018033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O’Reardon JP, Lovett ML, Gladis MM, Brown LL. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- DeVaul RA, Jervey F, Chappell JA, Caver P, Short B, O’Keefe S. Medical school performance of initially rejected students. JAMA. 1987;257:47–51. [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Triantafyllou C, Saygin ZM, Whitfield-Gabrieli S, Hofmann SG. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry. 2013;70:87–97. doi: 10.1001/2013.jamapsychiatry.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Klingberg T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereb Cortex. 2012;22:1078–1085. doi: 10.1093/cercor/bhr175. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. The estimation of prediction error. J Am Stat Assoc. 2004;99:619–632. [Google Scholar]
- Erickson KI, Boot WR, Basak C, Neider MB, Prakash RS, Voss MW, Graybiel AM, Simons DJ, Fabiani M, Gratton G, Kramer AF. Striatal volume predicts level of video game skill acquisition. Cereb Cortex. 2010;20:2522–2530. doi: 10.1093/cercor/bhp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. J Neurosci. 2010;30:8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Berkman ET, Whalen D, Lieberman MD. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psych. 2011;30:177–185. doi: 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Hamilton P, Sacchet MD, Furman DJ, Sherdell L, Gotlib IH. Activation of the medial prefrontal and posterior cingulate cortex during encoding of negative material predicts symptom worsening in major depression. NeuroReport. 2014;25:324–329. doi: 10.1097/WNR.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Kozink RV, Rose JE, Behm FM, Salley AN, McClernon FJ. Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacology. 2010;210:577–583. doi: 10.1007/s00213-010-1862-3. [DOI] [PubMed] [Google Scholar]
- Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Fullana MA, Cardoner N, Alonso P, Subirà M, López-Solà C, Pujol J, Segalàs C, Real E, Bossa M, Zacur E, et al. Brain regions related to fear extinction in obsessive-compulsive disorder and its relation to exposure therapy outcome: a morphometric study. Psychol Med. 2014;44:845–856. doi: 10.1017/S0033291713001128. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 2009;325:280–283. doi: 10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. The bisected brain. New York: Appleton-Century-Crofts; 1970. [Google Scholar]
- Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron. 2002;35:997–1010. doi: 10.1016/s0896-6273(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cereb Cortex. 2007;17:575–582. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry. 2012;69:121–127. doi: 10.1001/archgenpsychiatry.2011.129. [DOI] [PubMed] [Google Scholar]
- Gruber E, DiClemente RJ, Anderson MM, Lodico M. Early drinking onset and its association with alcohol use and problem behavior in late adolescence. Prev Med. 1996;25:293–300. doi: 10.1006/pmed.1996.0059. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PH, Poikkeus A, Eklund KM, Lyytinen P, Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 2005;41:291–303. doi: 10.1016/s0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Recovery after severe injury to the head. In: Van der Kloot WG, Walcott C, Dane B, editors. Readings in Behavior. New York: Holt, Rinehart and Winston; 1974. pp. 291–309. Original work published 1868. [Google Scholar]
- Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian AV. Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain. 2012;153:2393–2402. doi: 10.1016/j.pain.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Kong J, Spaeth R, Khan S, Kaptchuk TJ, Gollub RL. Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J Neurosci. 2014;34:3924–3936. doi: 10.1523/JNEUROSCI.3155-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, Holt CS, Welkowitz LA, Juster HR, Campeas R, Bruch MA, Cloitre M. Cognitive behavioral group therapy vs phenelzine therapy for social phobia: 12-week outcome. Arch Gen Psychiatry. 1998;55:1133–1141. doi: 10.1001/archpsyc.55.12.1133. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. J Neurosci. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds O, Thompson TW, Ghosh S, Yoo JJ, Whitfield-Gabrieli S, Triantafyllou C, Gabrieli JD. Roles of default-mode network and supplementary motor area in human vigilance performance: evidence from real-time fMRI. J Neurophys. 2013;109:1250–1258. doi: 10.1152/jn.00533.2011. [DOI] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Lyytinen H, Whitfield-Gabrieli S, Glover GH, Reiss AL, Gabrieli JD. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci USA. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JD. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognit Ther Res. 2012;36:427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, Thase ME, Markowitz JC. Treatment and prevention of depression. Psychol Sci Public Interest. 2002;3:39–77. doi: 10.1111/1529-1006.00008. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. Equal time for psychological and biological contributions to human variation. Rev Gen Psychol. 2013;17:351–357. [Google Scholar]
- Koban L, Ruzic L, Wager TD. Brain predictors of individual differences in placebo responding. In: Colloca L, Flaten MA, Meissner K, editors. Placebo and Pain: From Bench to Bedside. San Diego: Elsevier/Academic Press; 2013. [Google Scholar]
- Kohavi R. A Study of Cross-validation and Bootstrap for Accuracy Estimation and Model Selection. Proceedings of the 14th International Joint Conference on Artificial Intelligence; San Francisco, CA, USA: Morgan Kaufmann Publishers Inc; 1995. pp. 1137–1143. [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kumari V, Peters ER, Fannon D, Antonova E, Premkumar P, Anilkumar AP, Williams SC, Kuipers E. Dorsolateral prefrontal cortex activity predicts responsiveness to cognitive–behavioral therapy in schizophrenia. Biol Psychiatry. 2009;66:594–602. doi: 10.1016/j.biopsych.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Fannon D, Peters ER, Ffytche DH, Premkumar P, Raveendran V, Andrew C, Johns LC, McGuire PA, et al. Beyond dopamine: functional MRI predictors of responsiveness to cognitive behaviour therapy for psychosis. Front Behav Neurosci. 2010;4 doi: 10.3389/neuro.08.004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Peters ER, Ffytche DH, Sumich AL, Premkumar P, Anilkumar AP, Andrew C, Phillips ML, Williams SC, et al. Neural changes following cognitive behaviour therapy for psychosis: a longitudinal study. Brain. 2011;134:2396–2407. doi: 10.1093/brain/awr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Lazzaro SC, Rutledge RB, Glimcher PW. Choice from non-choice: Predicting consumer preferences from blood oxygenation level-dependent signals obtained during passive viewing. J Neurosci. 2011;31:118–125. doi: 10.1523/JNEUROSCI.3214-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RB, Hofmann W, Wagner DD, Kelley WM, Heatherton TF. Neural predictors of giving in to temptation in daily life. Psychol Sci. 2014 doi: 10.1177/0956797614531492. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barroso D, Catani M, Ripolles P, Dell’Acqua F, Rodriguez-Fornells A, de Diego-Balaguer R. Word learning is mediated by the left arcuate fasciculus. Proc Natl Acad Sci USA. 2013;110:13168–13173. doi: 10.1073/pnas.1301696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood O, Goldenberg D, Thayer R, Migliorini R, Simmons A, Tapert S. Adolescents’ fMRI activation to a response inhibition task predicts future substance use. Addict Behav. 2013;38:1435–1441. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ. Relapse in the addictive behaviors: Integration and future directions. Clin Psychol Rev. 2006;26:229–231. doi: 10.1016/j.cpr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Mather M, Cacioppo JT, Kanwisher N. Introduction to the special section 20 years of fMRI—What has it done for understanding cognition? Perspect Psychol Sci. 2013;8:41–43. doi: 10.1177/1745691612469036. [DOI] [PubMed] [Google Scholar]
- Maurer U, Bucher K, Brem S, Benz R, Kranz F, Schulz E, van der Mark S, Steinhausen H, Brandeis D. Neurophysiology in preschool improves behavioral prediction of reading ability throughout primary school. Biol Psychiatry. 2009;66:341–348. doi: 10.1016/j.biopsych.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS. fMRI predicts treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2007;191:97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Dunlop BW, Holtzheimer PE, III, Craighead WE, Mayberg HS. Pretreatment brain states identify likely failures to standard treatments for depression. Biol Psychiatry. 2013a doi: 10.1016/j.biopsych.2013.12.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013b;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNorgan C, Alvarez A, Bhullar A, Gayda J, Booth JR. Prediction of reading skill several years later depends on age and brain region: implications for developmental models of reading. J Neurosci. 2011;31:9641–9648. doi: 10.1523/JNEUROSCI.0334-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR. What is a relapse? Fifty ways to leave the wagon. Addiction. 1996;91:15–28. [PubMed] [Google Scholar]
- Milstein RM, Wilkinson L, Burrow GN, Kessen W. Admission decisions and performance during medical school. J Med Educ. 1981;56:77–82. doi: 10.1097/00001888-198102000-00001. [DOI] [PubMed] [Google Scholar]
- Mitte K. Meta-analysis of cognitive-behavioral treatments for generalized anxiety disorder: a comparison with pharmacotherapy. Psychol Bull. 2005;131:785. doi: 10.1037/0033-2909.131.5.785. [DOI] [PubMed] [Google Scholar]
- Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72:238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Monahan J. Predicting violent behavior: An assessment of clinical techniques. Beverly Hills, CA: Sage Publications; 1981. [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW, III, Weller RE. fMRI reactivity to high-calorie food pictures predicts short-and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Sferrazza R, Van Der Linden M, Paternot J, Verhas M, Hanak C, Pelc I, Verbanck P. Contribution of frontal cerebral blood flow measured by (99m)Tc-Bicisate spect and executive function deficits to predicting treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2002;37:347–354. doi: 10.1093/alcalc/37.4.347. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Pollack MH, Gould RA, Worthington JJ, III, McArdle ET, Rosenbaum JF, Heimberg RG. A comparison of the efficacy of clonazepam and cognitive-behavioral group therapy for the treatment of social phobia. J Anxiety Disord. 2000;14:345–358. doi: 10.1016/s0887-6185(00)00027-x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Gilger JW. How is dyslexia transmitted? In: Chase CH, Rosen GD, Sherman GF, editors. In developmental dyslexia: Neural, cognitive, and genetic mechanisms. Baltimore: York Press; 1996. pp. 41–61. [Google Scholar]
- Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage. 2009;45:S199–209. doi: 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack MH, Meoni P, Otto MW, Hackett D. Predictors of outcome following venlafaxine extended-release treatment of DSM-IV generalized anxiety disorder: a pooled analysis of short-and long-term studies. J Clin Psychopharmacol. 2003;23:250–259. doi: 10.1097/01.jcp.0000084025.22282.84. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Fannon D, Kuipers E, Peters ER, Anilkumar AP, Simmons A, Kumari V. Structural magnetic resonance imaging predictors of responsiveness to cognitive behaviour therapy in psychosis. Schizophr Res. 2009;115:146–155. doi: 10.1016/j.schres.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Brady KT. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend. 2013;131:44–49. doi: 10.1016/j.drugalcdep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Han M, Garel K, Chen ES, Gabrieli JDE. White-matter structure in the right hemisphere predicts Mandarin Chinese learning success. J of Neurolinguistics. 2014 in press. [Google Scholar]
- Raghubar KP, Barnes MA, Hecht SA. Working memory and mathematics: A review of developmental, individual difference, and cognitive approaches. Learn Individ Differ. 2010;20:110–122. [Google Scholar]
- Rando K, Hong K, Bhagwagar Z, Li CR, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Fung G, Rosales R. On the dangers of cross-validation. An experimental evaluation. Proceedings of the 2008 SIAM International Conference on Data Mining; 2008. pp. 588–596. [Google Scholar]
- Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, Downar J. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.222. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE. Dyslexia. N Engl J Med. 1998;5:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AC, Reiss PT, Gee DG, Gotimer K, Uddin L, Lee SH, Margulies DS, Roy AK, Biswal BB, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G, Carter C, Thase M. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69:913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Foa EB, Liebowitz MR, Huppert JD, Cahill S, Maher MJ, McLean CP, Bender J, Marcus SM, Williams MT. Cognitive-behavioral therapy vs risperidone for augmenting serotonin reuptake inhibitors in obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry. 2013;70:1190–1199. doi: 10.1001/jamapsychiatry.2013.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg SF, Taylor MJ, Alhassoon OM, Gongvatana A, Theilmann RJ, Frank LR, Grant I. Frontal white matter integrity predictors of adult alcohol treatment outcome. Biol Psychiatry. 2012;71:262–268. doi: 10.1016/j.biopsych.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67:16. [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS. Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biol Psychiatry. 2013;73:869–876. doi: 10.1016/j.biopsych.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg-Lee M, Fuchs L, Menon V. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc Natl Acad Sci USA. 2013;110:8230–8235. doi: 10.1073/pnas.1222154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Chen L, Yip V, Chan AH, Yang J, Gao JH, Sio WT. Activity levels in left hemisphere caudate-fusiform circuit predict how well a second language will be learned. Proc Natl Acad Sci USA. 2011;108:2540–52544. doi: 10.1073/pnas.0909623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol. 1996;58:267–288. [Google Scholar]
- Udo T, Clifford PR, Davis CM, Maisto SA. Alcohol use post AUD treatment initiation as a predictor of later functioning. Am J Drug Alcohol Abuse. 2009;35:128–132. doi: 10.1080/00952990802707059. [DOI] [PubMed] [Google Scholar]
- Ullman H, Almeida R, Klingberg T. Structural maturation and brain activity predict future working memory capacity during childhood development. J Neurosci. 2014;34:1592–1598. doi: 10.1523/JNEUROSCI.0842-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnik VN. An overview of statistical learning theory. IEEE Trans Neural Netw. 1999;10:988–999. doi: 10.1109/72.788640. [DOI] [PubMed] [Google Scholar]
- Ventura-Campos N, Sanjuan A, Gonzalez J, Palomar-Garcia MA, Rodriguez-Pujadas A, Sebastian-Galles N, Deco G, Avila C. Spontaneous brain activity predicts learning ability of foreign sounds. J Neurosci. 2013;33:9295–9305. doi: 10.1523/JNEUROSCI.4655-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar C, O’Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, Duncan JS, Richardson MP, Koepp MJ. Identical, but not the same: intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0 T scanners. Neuroimage. 2010;51:1384–1394. doi: 10.1016/j.neuroimage.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Nelson TF, Kuo M. Underage college students’ drinking behavior, access to alcohol, and the influence of deterrence policies: Findings from the Harvard School of Public Health College Alcohol Study. J Am Coll Health. 2002;50:223–236. doi: 10.1080/07448480209595714. [DOI] [PubMed] [Google Scholar]
- Wender PH. Pharmacotherapy of attention-deficit/hyperactivity disorder in adults. J Clin Psychiatry. 1998;59:76–79. [PubMed] [Google Scholar]
- Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, Davidson RJ, Kalin NH. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Garavan H. When optimism hurts: Inflated predictions in psychiatric neuroimaging. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.014. in press. [DOI] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RA, Artiges E, Banaschewski T, Barker GJ, Bokde ALW, Buchel C, Carvalho FM, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–191. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlick JS, Ziegler DA, Hosseini-Varnamkhasti P, Locascio JJ, Bakkour A, van der Kouwe A, Triantafyllou C, Corkin S, Dickerson BC. Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage. 2009;44:1324–1333. doi: 10.1016/j.neuroimage.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic. 2008 Retrieved 6/3/2014 from http://www.who.int/tobacco/mpower/mpower_report_tobacco_crisis_2008.pdf.
- Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–537. doi: 10.1093/schbul/sbm114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Wong SC, Coid J. The efficacy of violence prediction: a meta-analytic comparison of nine risk assessment tools. Psychol Bull. 2010;136:740. doi: 10.1037/a0020473. [DOI] [PubMed] [Google Scholar]
- Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity. 2011;19:1775–1783. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JJ, Hinds O, Ofen N, Thompson TW, Whitfield-Gabrieli S, Triantafyllou C, Gabrieli JD. When the brain is prepared to learn: Enhancing human learning using real-time fMRI. Neuroimage. 2012;59:846–852. doi: 10.1016/j.neuroimage.2011.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Delhommeau K, Zarate JM. Modulation of auditory cortex response to pitch variation following training with microtonal melodies. Front Psychol. 2012;3 doi: 10.3389/fpsyg.2012.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ. Predispositions and plasticity in music and speech learning: neural correlates and implications. Science. 2013;342:585–589. doi: 10.1126/science.1238414. [DOI] [PubMed] [Google Scholar]