Abstract
Bioassay-guided fractionation was conducted on a CHCl3-soluble extract of the stem bark of Alstonia angustifolia (Apocynaceae) collected in Vietnam using the HT-29 human colon cancer cell line, and led to the isolation of a new sarpagine-type indole alkaloid (1), together with nine known alkaloids, including four macroline-derived alkaloids (2–5), a sarpagine-type alkaloid (6), and four macroline-pleiocarpamine bisindole alkaloids (7–10). The structure of the new compound (1) was determined on the basis of spectroscopic data interpretation. Compounds 1–10 were evaluated in vitro for their NF-κB (p65) inhibitory activity against the Hela cells in an ELISA assay. The new sarpagine alkaloid, N(4)-methyltalpinine (1), was found to show significant NF-κB inhibitory activity (ED50 = 1.2 µM). Furthermore, all the isolates (1–10) were evaluated in vitro for their antileishmanial activity, and compounds (1–4, 6 and 8–10) exhibited leishmaniacidal activity against promastigotes of Leishmania mexicana.
Keywords: Alstonia angustifolia (Apocynaceae), stem bark, sarpagine-type alkaloid, macroline-type alkaloid, macroline-pleiocarpamine bisindole alkaloid, cytotoxicity, NF-κB (p65) inhibitory activity, antileishmanial activity
1. Introduction
Species of Alstonia (Apocynaceae), a genus of evergreen trees and shrubs, are mainly distributed in the tropical and subtropical areas of Africa, Central America, southeast Asia, Polynesia and Australia, with most of these in the Malesian region (Perry and Metzger, 1980). Many Alstonia species are commercial timbers, and some species are also used as traditional medicines for the treatment of cough, ulcer, dysentery, fever, malaria, sore throats, toothache, rheumatism, and snake bites (Perry and Metzger, 1980; Sidiyasa, 1998; Gadekar, et al., 2010). Alstonia angustifolia Wall. ex A.DC., a small to medium-sized tree, with the common name “Red-leafed Pulai”, is native to Indonesia, Malaysia, the Philippines, and Vietnam. In Malaysia, the leaves of A. angustifolia are applied externally to the spleen area for the treatment of remittent fever, and the bark is used as an antimalarial remedy [Flora Singapura, 2014; Slik JWF (2009 onwards), Plants of Southeast Asia., 2014]. According to previous phytochemical investigations on Alstonia species, the effects described in traditional medicinal usage are probably due to the presence of indole alkaloids, which have been reported to be the major secondary metabolites of these plants (Hamaker and Cook, 1994; Osorio et al., 2008). Thus far, more than 60 indole alkaloids, with the majority being macroline-type derivatives, have been isolated and identified from A. angustifolia species (Ghedira et al., 1988; Hu, et al., 1989; Kam and Choo, 2004; Tan, et al., 2011; Tan, et al., 2013), and some of these compounds have been reported to possess antiprotozoal properties (Wright et al., 1992) and activities against multidrug-resistant in KB cells (Tan et al., 2011).
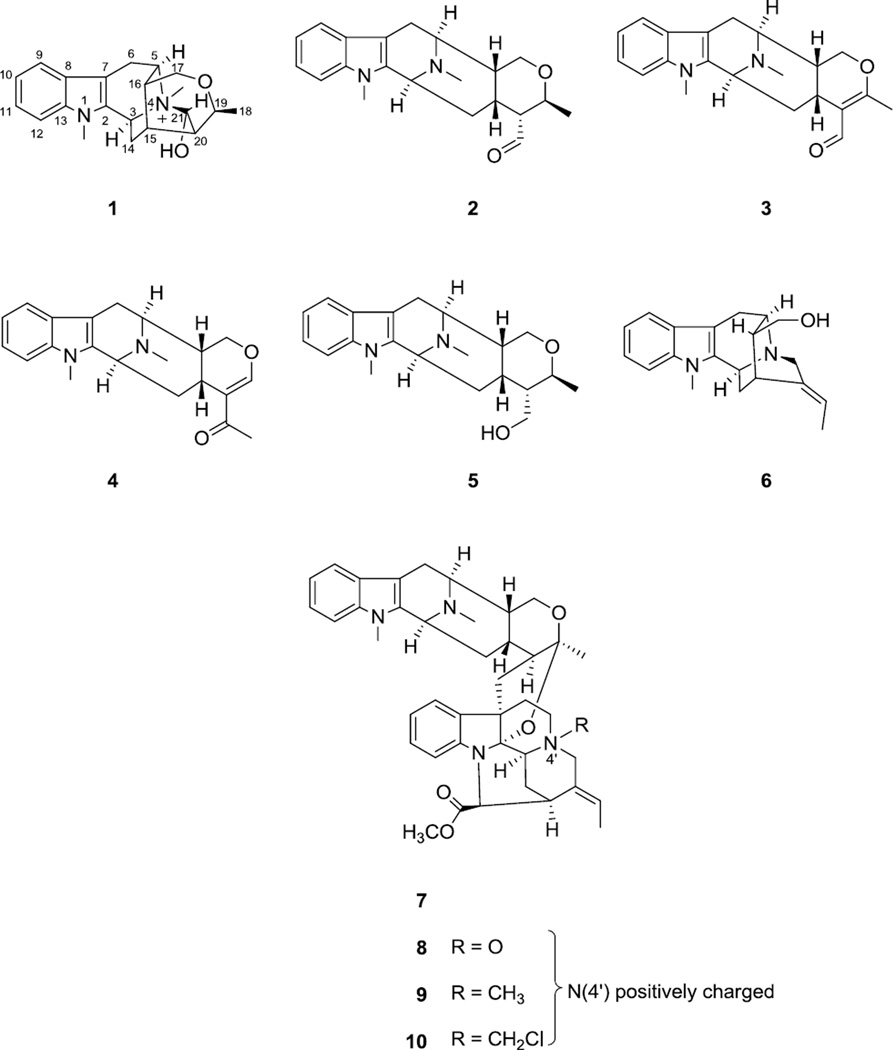
As part of our continuing efforts to discover naturally occurring biologically active agents from plants, a chloroform-soluble partition of a methanol extract from the stem bark of A. angustifolia collected in Vietnam was found to show cytotoxicity against the HT-29 human colon cancer cell line, with an ED50 value of 12.0 µg/mL, and thus was fractionated by bioactivity-guided isolation using this assay. The present investigation led to the isolation and identification of a new sarpagine-type indole alkaloid (1), together with nine known alkaloids (Fig. 1), including four macroline derived alkaloids, 21-deoxy-N(4)-methyl-21-oxo-N(4),21-secotalpinine [N(4)-methyl-N(4),21-secotalpinine, 2) (Kam et al., 2004)], alstonerinal (3) (Kam et al., 1999), alstonerine (4) (Bi et al., 1994), macrocarpine B (5) (Kam et al., 2004), a sarpagine-type alkaloid affinisine (6) (Zocoler et al., 2005), as well as four macroline-pleiocarpamine bisindole alkaloids, villalstonine (7) (Ghedira et al., 1988), villalstonine N(4)-oxide (8) (Ghedira et al., 1988), villalstonidine D (9) (Tan et al., 2013) and villalstonidine E (10) (Tan et al., 2013).
Figure 1.
Structures of compounds 1–10.
Although the crude extracts of two other Alstonia species, A. macrophylla and A. congensis, have been reported to show pronounced antileishmanial activeties (Camacho, et al., 2003; Lumpu et al., 2013), and certain alkaloids isolated from A. angustifolia have been tested for their antiamoebic and antiplasmodial activities (Wright et al., 1992), to this date the leishmanicidal activities of alkaloids from Alstonia species have not been evaluated. Thus, besides the human cancer cell line and NF-κB inhibitory bioassays employed to evaluate the cytotoxic and NF-κB inhibitory activities of the principles from this plant, all the isolates obtained in the present study were also screened for their potential leishmanicidal activities against promastigotes of Leishmania mexicana.
2. Results and discussion
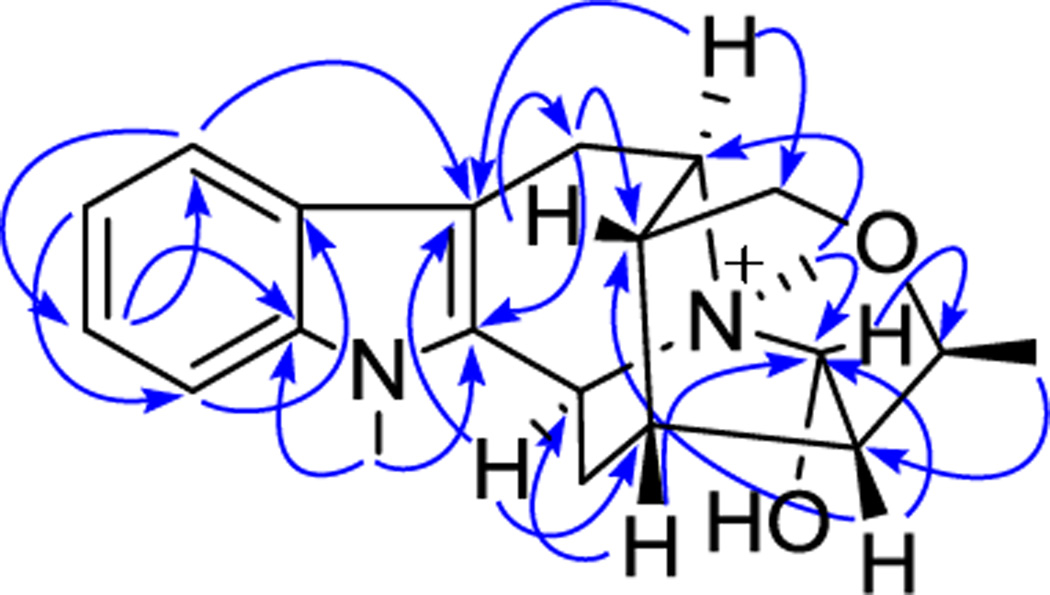
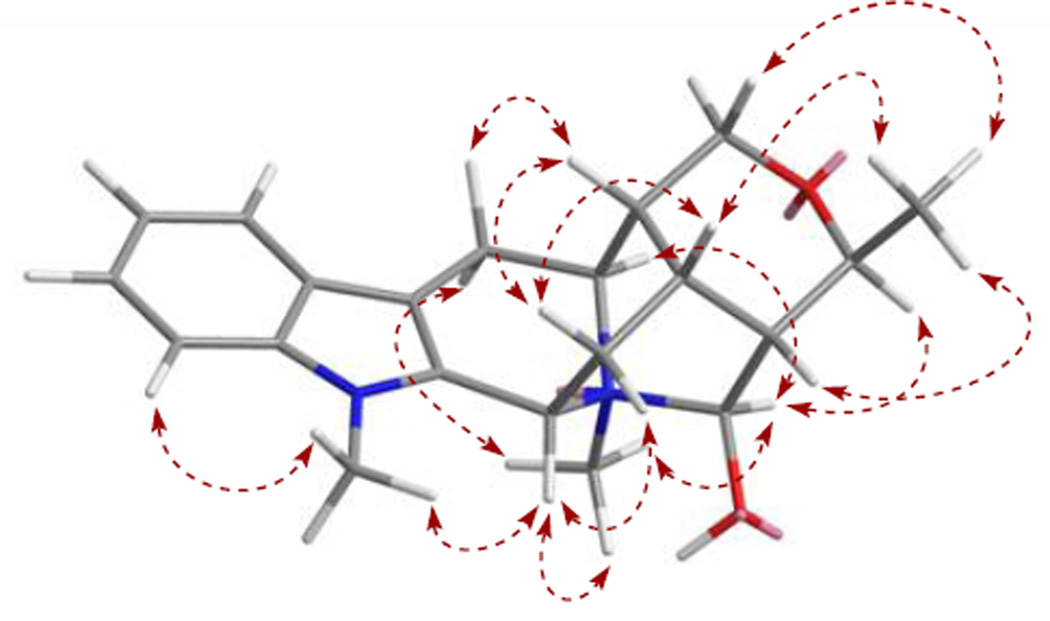
Compound 1 was obtained as a light brownish resin, and gave a positive test for Dragendorff’s reagent. In the UV spectrum, absorptions at 219, 269, 282 and 290 nm are corresponding to an indole chromophore (Houlihan, 1972). The molecular formula of compound 1 was determined as C21H27N2O2 based on the [M]+ ion peak at m/z 339.2059 (calcd 339.2073) in the HRESIMS. In the 1H NMR spectrum (Table 1), proton signals at δH 7.53 (br d, J = 7.9, H-9), 7.12 (br t, J = 7.8, H-10), 7.25 (br t, J = 7.7, H-11) and 7.43 (br d, J = 8.2, H-12), were recognized belonging to the aromatic ring of the indole moiety. Also observed were two nitrogen-attached methyl groups at δH 3.73 and 3.07 (each 3H, s, N1-CH3 and N4-CH3), and an alkyl methyl group at δH 1.38 (3H, d, J = 6.8 Hz, H-18). In addition to the protons mentioned above, another 13 proton signals remained to be assigned in the 1H NMR spectrum, and were ascribed to three methylene groups [δH 3.36 (dd, J = 17.4 and 5.3 Hz) and 3.09 (d, J = 16.6 Hz), each 1 H, H-6α and H-6β; 2.47 (brt, J = 12.2 Hz) and 1.98 (ddd, J = 13.2, 5.0 and 1.8 Hz), each 1 H, H-14α and H-14β; 3.54 (dd, J = 11.9, 2.1 Hz) and 3.80 (d, J = 11.7 Hz), each 1 H, H-17α and H-17β], and seven methine groups [δH 4.99 (d, J = 10.7 Hz, H-3), 4.15 (q, J = 6.8 Hz, H-19), 3.91 (t, J = 5.4 Hz, H-5), 2.36 (brs, H-15), 1.77 (brs, H-16), 2.04 (brs, H-20), 4.95 (d, J = 1.9 Hz, H-21), each 1H]. The 13C NMR spectrum (Table 1) displayed a total of 21 carbon resonances, which were classified from the DEPT and HSQC spectra as three methyl carbon signals (including two nitrogen-attached methyls and one alkyl methyl), three methylene carbon signals (including one oxygenated methylene and two alkyl methylenes), seven methine carbon signals (including two N-vicinal methines, a oxygenated methine, a hemiaminal methine, and three alkyl methines), as well as eight sp2 carbon signals (including four tertiary carbons and four quaternary carbons) of the indole moiety. By studying the COSY and TOCSY spectra, a large spin system that conjugated with the indole unit comprising three methylene (C-6, C-14 and C-17), seven methine (C-3, C-5, C-15, C-16, C-19, C-20, C-21) and one methyl (C-18) groups was established. In the HMBC spectrum, the proton signal of the methyl group attached on N4 (δH 3.07, s, 3H), exhibiting a strong correlation with a hemiaminal methine carbon (δC 98.4, C-21), suggested that C-21 is connected with N-(4) in compound 1 to form a ring containing a carbinolamine functionality. Based on above NMR spectroscopic analysis, compound 1 was found to resemble closely to a known sarpagine-type derived indole alkaloid, talpinine (Naranjo et al., 1972; Yu and Cook, 1998). Talpinine was oringinally isolated from the stem bark of Pleiocarpa talbotii (Apocynaceae), with its structure confirmed by enantiospecific synthesis. Although the 1H and 13C NMR data of talpinine have been reported (Yu and Cook, 1998), the full assigments are not available so far. Furthermore, the planar structure of 1 was confirmed by HMBC correlations of H-5 with C-3, C-7 and C-17, H-6 with C-2 and C-16, H-14 with C-2, C-16 and C-20, H-15 with C-21, H-16 with C-6 and C-20, H-17 with C-5, C-15 and C-19, H-18 with C-20, H-19 with C-21, H-20 with C-15 and C-21, as well as H-21 with C-3 and C-19. The relative stereo configurations at the stereogenic centers of 1 were established based on the important NOE correlations, including H-16 with H-15 and H-6β, H-15 with H-18, H-20 and H-17β, H-5 with H-6α, H-17α, and N(4)-CH3, H-3 with N(1)-CH3 and N(4)-CH3, as well as H-21 with H-5, H-19 and N(4)-CH3, consistent with those of talpinine (Fig. 2.). In addition, an energy-minimized model was generated by Chem3D Ultro 10.0 based on a presumed configuration, which matched well with all the observed key NOESY correlations (Fig. 3.). Thus, the structure of compound 1 was deduced as N(4)-methyltalpinine, which is a new sarpagine-type alkaloid.
Table 1.
1H and 13C NMR Spectroscopic Data of Compound 1a
| position | 2 | |
|---|---|---|
| δH, mult (J in Hz)b | δCc | |
| 2 | 134.2 | |
| 3 | 4.99, d (10.7) | 53.1 |
| 5 | 3.91, t (5.4) | 61.3 |
| 6β | 3.09, d (16.6) | 24.7 |
| 6α | 3.36, dd (17.4, 5.3) | |
| 7 | 101.7 | |
| 8 | 127.3 | |
| 9 | 7.53, d (7.9) | 119.5 |
| 10 | 7.12, t (7.8) | 121.0 |
| 11 | 7.25, t (7.7) | 123.7 |
| 12 | 7.43, d (8.2) | 110.6 |
| 13 | 139.7 | |
| 14β | 1.98, ddd(13.2, 5.0, 1.8) | 32.4 |
| 14α | 2.47, brt (12.2) | |
| 15 | 2.36, brs | 22.7 |
| 16 | 1.77, brs | 38.2 |
| 17β | 3.80, d (11.7) | 63.1 |
| 17α | 3.54, dd (11.9, 2.1) | |
| 18 | 1.38, d (6.8) | 15.7 |
| 19 | 4.15, q (6.8) | 72.7 |
| 20 | 2.04, brs | 48.2 |
| 21 | 4.95, d (1.9) | 98.4 |
| N1-CH3 | 3.73, s | 29.9 |
| N4-CH3 | 3.07, s | 43.5 |
Obtained in CD3OD. Assignments are based on 1H-1H COSY, HSQC, and HMBC spectroscopic data.
Measured at 400 MHz with residual signals of CD3OD at δ 3.31 ppm used as reference.
Measured at 100 MHz with residual signals of CD3OD at δ 49.0 ppm used as reference.
Figure 2.
Key HMBC (→) correlations observed for compound 1.
Figure 3.
Preferred conformation and selected NOESY (↔) correlations observed for compound 1.
Compounds 1–10 obtained in the present study were evaluated against the HT-29 human colon cancer cell line for their cytotoxicity, as summarized in Table 2. Among these compounds, a monomeric macroline indole alkaloid, alstonerial (3), together with two macroline–pleiocarpamine type bisindole alkaloids, villalstonine (7) and villalstonidine E (10), were found to exhibit cytotoxicity against the HT-29 cells, with ED50 values of 8.6, 8.0 and 6.5 µM, respectively.
Table 2.
Bioactivity Evaluation of Compounds Isolated from A. angustiforlia
| Compound | Bioassay | ||
|---|---|---|---|
| HT-29a | NF-κB (p65)a |
L. mexicana promastigotesb |
|
| 1 | >20 | 1.2 | 183.3 |
| 2 | >20 | >20 | 57.8 |
| 3 | 8.6 | >20 | 104.7 |
| 4 | >20 | >20 | 145.4 |
| 5 | NT | >20 | >200 |
| 6 | >20 | >20 | 138 |
| 7 | 8.0 | >20 | >200 |
| 8 | >20 | >20 | 80.3 |
| 9 | >20 | >20 | 120.4 |
| 10 | 6.5 | >20 | 78 |
| Paclitaxelc | 0.006 | - | - |
| Rocaglamided | 0.005 | 0.08 | - |
| Amphotericin Be | 0.09 | ||
Results are expressed as ED50 values (µM); Compounds with ED50 > 20 µM were considered inactive.
Results are expressed as IC50 values (µM); Compounds with IC50 >200 µM are considered to be inactive.
Used as a positive control substance for HT-29 cell line.
Used as a positive control substance for NF-κB (p65) cell line.
Used as a positive control substance for L. mexicana promastigote assay.
In the present investigation, all the isolates were also evaluated for their NF-κB (p65) inhibitory activity against Hela cells in an enzyme-based Elisa assay. Only the new compound, N(4)-methyltalpinine (1), was found to show significant NF-kB (p65) inhibitory activity, with an ED50 value of 1.2 µM, which implied that the four ring system containing a carbinolamine feature formed by the junction of N(4) and C-21 in 1 is important for the observed activity. To the best of our knowledge, N(4)-methyltalpinine (1) is the first member of the sarpagine-type indole alkaloids reported to have NF-κB inhibitory activity.
As mentioned before, several alkaloids isolated from A. angustifolia have been evaluated for their antiprotozoal activities against Entamoeba histolytica and Plasmodium falciparum in vitro (Wright et al., 1992). In this study, the bisindole alkaloids evaluated, were found to possess significant activity against both protozoal species, but were less potent than the positive controls, and the monomeric alkaloids were all considerably less active than the dimers tested. Most of the compounds (1–4, 6, 8–10) obtained in present study were found to possess leishmanicidal activities against promastigotes of L. mexicana (Table 2). Based on the present results, the antileishmanial activities exhibited by the dimeric- and monomeric indole alkaloids did not show obvious differences. Of the four bisindole alkaloids (7–10), villalstonine (7) was inactive against promastigotes of L. mexicana, while its derivatives (8–10), all possessing a quaternary ammonium cation at N(4'), were found to be active against the same parasites. This is the first time that pure indole alkaloids isolated from Alstonia species have been evaluated for their antileishmanial activities.
3. Experimental
3.1 General experimental procedures
Optical rotations were measured on a Perkin-Elmer 343 automatic polarimeter (PerkinElmer, Waltham, MA). UV spectra were obtained on a Hitachi U-2910 spectrophotometer (Hitachi, Tokyo, Japan). NMR spectroscopic data were recorded at room temperature on a Bruker Avance DRX-400 or a Bruker Avance-300 spectrometer (Bruker, Billerica, MA), using standard Bruker pulse sequences, and the data were processed using MestReNova 6.0 software (Mestrelab Research SL, Santiago de Compostela, Spain). High-resolution electrospray ionization mass spectra (HRESIMS) were performed on a Micromass Q-Tof™ II (Micromass, Wythenshawe, UK) mass spectrometer operated in the positive-ion mode, with sodium iodide being used for mass calibration. Column chromatography was carried out with silica gel (230–400 mesh; Sorbent Technologies, Atlanta, GA). Analytical TLC was conducted on precoated 250 µm thickness silica gel UV254 aluminum-backed plates (Sorbent Technologies). Waters Xbridge® (4.6 × 150 mm), semi-preparative (10 × 150 mm), and preparative (19 × 150 mm) C18 (5 µm) columns were used for analytical, semi-preparative and preparative HPLC, respectively, as conducted on a Waters system comprised of a 600 controller, a 717 Plus autosampler, and a 2487 dual wavelength absorbance detector. 205 nm and 254 nm were selected as UV detection wavelength.
3.2 Plant material
A stem bark sample of Alstonia angustifolia Wall. ex A. DC. (Apocynaceae) was collected in Nui Chua National Park, Ninh Thuan Province, Vietnam by DDS, TNN, and Vuong Tan Tu, on January 11, 2010, who also identified this plant. A voucher specimen (original collection Soejarto et al.14599; recollection on July 26, 2011 Soejarto et al. 1861) has been deposited in the John G. Searle Herbarium of the Field Museum of Natural History (under accession number FM 2294419 and 2300815, respectively), Chicago, Illinois.
3.3 Extraction and isolation
The dried and milled stem bark (340 g) of A. angustifolia was macerated overnight using MeOH at room temperature (3 × 3L). After evaporation under reduced pressure, the dried MeOH extract (50 g) was suspended in a mixture of methanol−water (9:1) solution and was then partitioned sequentially with hexane (3 × 1 L) and chloroform (3 × 1 L) to yield a CHCl3-soluble partition (20 g), which was found to be active against the HT-29 cell line (ED50 = 12.0 µg/mL). Accordingly, this fraction was subjected to separation over a Si gel column (7 × 50 cm), eluted with a gradient mixture of CH2Cl2−acetone of increasing polarity (20:1 to 1:1, then pure acetone), to yield eight fractions (F01–F08). Of the sub-fractions obtained, F01 and F03 were found to be the most active against the HT-29 cells (ED50 = 6.3 and 1.3 µg/mL, respectively). Fraction F01 (55 mg) was chromatographed on a silica gel column and eluted in a gradient manner with hexane-acetone (10:1 to pure acetone), to give three subfractions (F101–F103). Subfraction F102 which containing the major compounds was purified by HPLC (MeOH−H2O = 60:40, 0.05% triethylamine) to afford compounds 3 (2.0 mg) and 4 (4.0 mg). Part of fraction F02 (1.0 g) was subjected to passage over a Sephadex LH-20 column with elution by MeOH to give four subfractions (F201–F204). F0201 (eluted was further purified by preparative TLC (20 × 20 cm, 500 µm), developed by CHCl3–MeOH (15:1, saturated with NH3.H2O), to yield compounds 7 (Rf = 0.8; 11.0 mg), and 8 (Rf = 0.5; 2.0 mg), and the same method was applied to F202 to yield 10 (Rf = 0.4; 12.0 mg). F203 and F204 were combined as F20(34) and subjected to an open C18 column to give five subfractions. Fraction F20(34)3 was purified by HPLC with MeOH–H2O (55:45, 0.05% triethylamine) to yield compounds 6 (4.0 mg), 5 (2.0 mg) and 2 (2.0 mg). Compound 1(Rf = 0.65; 4.0 mg) and 9 (Rf = 0.6; 3.0 mg) were purified from subfractions F20(34)4 and F20(34)8, respectively, by preparative TLC (20 × 20 cm, 500 µm), using CHCl3–MeOH (10:1, saturated with NH3․H2O) as developing solvent system.
3.4 New compound information
N(4)-methyltalpinine (1): light brownish resin. [α]D23–10 (c = 0.1, MeOH); UV (MeOH) λmax (log ε) 219 (4.60), 269 (3.95), 282 (3.90) and 290 (3.86) nm; 1H and 13C NMR data shown in Table 1; HRESIMS obsd m/z 339.2059 [M]+ (calcd for C21H27O2N2, 339.2073).
3.5 Biological assay
3.5.1 Cytotoxicity assay
Human colon cancer cells (HT-29) purchased from American Type Collection were cultured in MEME medium supplemented with streptomycin (100 µg/mL), penicillin (100 units/mL), amphotericin B (Fungizone, 0.25 µg/mL) and 10% fetal bovine serum (FBS), and incubated with an atmosphere with 5% CO2 at 37 °C. Cells were trypsinized and split for subculture five days or later when they reached near-confluent state. Upon reaching about 60%–70% confluence, the medium was changed and the cells were used for test procedures one day later. After appropriate dilutions, the harvest cells were seeded in 96-well (9500 cells/190 µL) plates using complete medium, and treated with the test compounds (10 µL/well in triplicate) at various concentrations. Test samples were initially dissolved in DMSO and then diluted 10-fold with H2O2. Serial dilutions were made using 10% DMSO as the solvent. For the control groups, 10 µL of 10% DMSO were also added to each well. After two day’s incubation, the cells were fixed to the plates by 100 µL of cold 20% trichloroacetic acid (TCA) and incubated at 4 °C for 30 min. The plates were washed three times with tap water and dried overnight. The fixed cells were dyed with sulforhodamine B solution at 0.4% (w/v) in 1% acetic acid, and incubated at room temperature for 30 min. After washed three times with 1% acetic acid and allowed to air dry, the bound SRB stain was then solubilized with 10 mM unbuffered Tris base. The absorbance was read at 515 nm using a Bio-Tek µQuant microplate reader. The ED50 values of test samples with serial dilutions were calculated using non-liner regression analysis (Table curve2Dv4; AISN Software, Inc., Mapleton, OR). The cytotoxic activity of extracts, chromatographic fractions of extracts, and all pure compounds were evaluated against the HT-29 (human colon cancer) cell line, according to an established protocol (Pan et al., 2010).
3.5.2 Enzyme-based ELISA NF-κB Assay
The NF-κB assay was carried out according to an published protocol (Renard et al., 2001; Deng et al., 2009). In summary, a nuclear extract was prepared from HeLa cells obtained from the American Type Culture Collection. An EZ-Detect™ Transcription Factor Assay System ELISA kit (Pierce Biotechnology, Rockford, IL) was used to assess the specific binding ability of activated NF-κB to the biotinylated-consensus sequence under the presence of tested compounds. Compounds were tested at four concentration levels to obtain the inhibitory concentration. The binding activity of the p65 subunit of NF-κB was measured by detecting the chemiluminescent signal in a Fluostar Optima plate reader (BMG Labtech Inc., Durham, NC).
3.5.3 Parasite Strain and Promastigote Assay
Leishmania mexicana ((MNYC/BZ/62/M379) amastigotes expressing DSRed fluorescent protein was recovered from footpads of Balb/c infected mice. Promastigotes were obtained by in vitro culture of amastigotes in Schneider’s medium supplemented with 10% fetal calf serum (FCS, Sigma, St. Louis, MO) and incubated at 28°C. After transformation in promastigotes parasites were cultured in M199, serial passages were carried out until reach log growth phase. All work including experimental animals was approved by the Institutional Animal Care and Use Committee (IACUC) of The Ohio State University.
Promastigotes were seeded in 1×106/mL of complete M199 medium, after 48 h of treatment, live parasites were identified by flow cytometry based in their DSRED fluorescence and death parasites were identified by their lost of fluorescence. The proportion of death parasites was used to calculate IC50 values. Untreated parasites were used as a live control and Quillaja saponaria (Quillajaceae) saponin-treated parasites resulted in 100% dead parasites, as reported in the literature (Lezama-Dávila et al., 2012). Amphotericin B is used in this assay as a positive control.
Supplementary Material
Highlights.
A new indole alkaloid and nine known alkaloids were isolated from A. angustifolia.
The structure of the new compound was determined by spectroscopic data analysis.
Compounds were evaluated for their NF-κB inhibitory and antileishmanial activities.
New compound 1 showed potent NF-κB inhibitory activity.
Acknowledgement
Support from grant P01 CA125066 (to ADK) from the National Cancer Institute, NIH, Bethesda, MD, and from RC4 AI092624 (to ARS and ADK) from National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD, are acknowledged.
We thank Dr. Craig McElroy, College of Pharmacy, and Dr. Chun-hua Yuan, Campus Chemical Instrumentation Center (CCIC), The Ohio State University, for facilitating the acquisition of NMR spectra. The plant material was collected under the terms and conditions of a Memorandum of Agreement between the University of Illinois at Chicago and the Institute of Ecology and Biological Resources (IEBR) of the Vietnam Academy of Science and Technology, Hanoi, Vietnam. Thanks are expressed to the Director of Nui Chua National Park for permission, and to the Director of IEBR for overseeing the field operation in the collection of the plant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bi Y, Zhang LH, Hamaker LK, Cook JM. Enantiospecific synthesis of (−)-alstonerine and (+)-macroline as well as a partial synthesis of (+)-villalstonine. J. Am. Chem. Soc. 1994;116:9027–9041. [Google Scholar]
- Deng Y, Balunas MJ, Kim J-A, Lantvit DD, Chin Y-W, Chai H-B, Sugiarso S, Kardono LBS, Fong HHS, Pezzuto JM, Swanson SM, Carcache-Blanco EJ, Kinghorn AD. Bioactive 5,6-dihydro-alpha-pyrone derivatives from Hyptis brevipes. J. Nat. Prod. 2009;72:1165–1169. doi: 10.1021/np9001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho MdR, Phillipson JD, Croft SL, Solis PN, Marshall SJ, Ghazanfar SA. Screening of plant extracts for antiprotozoal and cytotoxic activities. J. Ethnopharmacol. 2003;89:185–191. doi: 10.1016/s0378-8741(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Flora Singapura. [accessed April 2014];2014 Further Information available at http://florasingapura.com/Alstonia-Angustifolia.php. [Google Scholar]
- Ghedira K, Zeches-Hanrot M, Richard B, Massiot G, Le Men-Olivier L, Sevenet T, Goh SH. Alkaloids of Alstonia angustifolia. Phytochemistry. 1988;27:3955–3962. [Google Scholar]
- Gadekar R, Singour PK, Chaurasiya PK, Pawar RS, Patil UK. A potential of some medicinal plants as an antiulcer agent. Pharmacogn. Rev. 2010;4:136–146. doi: 10.4103/0973-7847.70906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker LK, Cook JM. The synthesis of macroline related sarpagine alkaloids. In: Pelletier SW, editor. Alkaloids:chemical and biological perspectives. Vol. 9. New York, NY: Elsevier Science; 1995. pp. 23–84. [Google Scholar]
- Hu W, Lan, Zhu JP, Hesse M. Indole alkaloids from Alstonia angustifolia. Planta Med. 1989;55:463–466. doi: 10.1055/s-2006-962065. [DOI] [PubMed] [Google Scholar]
- Kam TS, Choo YM. Alkaloids from Alstonia angustifolia. Phytochemistry. 2002;65:603–608. doi: 10.1016/j.phytochem.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Kam TS, Iek IH, Choo YM. Alkaloids from the stem-bark of Alstonia macrophylla. Phytochemistry. 1999;51:839–844. [Google Scholar]
- Kam TS, Choo YM, Komiyama K. Unusual spirocyclic macroline alkaloids, nitrogenous derivatives, and a cytotoxic bisindole from Alstonia. Tetrahedron. 2004;60:3957–3966. [Google Scholar]
- Lezama-Dávila CM, Isaac-Márquez AP, Kapadia G, Owens K, Oghumu S, Beverley S, Satoskar AR. Leishmanicidal activity of two naphthoquinones against Leishmania donovani. Biol. Pharm. Bull. 2012;35:1761–1764. doi: 10.1248/bpb.b12-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpu SL, Kikueta CM, Tshodi ME, Mbenza AP, Kambu OK, Mbamu BM, Cos P, Maes L, Apers S, Pieters L, Cimanga RK. Antiprotozoal screening and cytotoxicity of extracts and fractions from the leaves, stem bark and root bark of Alstonia congensis. J. Ethnopharmacol. 2013;148:724–727. doi: 10.1016/j.jep.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Osorio EJ, Robledo SM, Bastida J. In: The Alkaloids: Chemistry and Biology. Cordell GA, editor. Vol. 66. Oxford, UK: Acdemic Press; 2008. pp. 113–190. [DOI] [PubMed] [Google Scholar]
- Pan L, Kardono LBS, Riswan S, Chai H, Carcache de Blanco EJ, Pannell CM, Soejarto DD, McCloud TG, Newman DJ, Kinghorn AD. Isolation and characterization of minor analogues of silvestrol and other constituents from a large-scale recollection of Aglaia foveolata. J. Nat. Prod. 2010;73:1873–1878. doi: 10.1021/np100503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry LM, Metzger J. In Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses. Cambridge, London, MA: MIT Press; 1980. pp. 1–620. [Google Scholar]
- Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFκB. Nucleic Acids Res. 2001;29:e21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiyasa K. Blumea. Suppl. 11. Leiden, The Netherlands: Rijksherbarium/Hortus Botanicus, Leiden University; 1998. Taxonomy, Phylogeny, and Wood Anatomy of Alstonia (Apocynaceae) pp. 1–230. [Google Scholar]
- Slik JWF (2009 onwards) Plants of Southeast Asia. url. [accessed April 2014];2014 Further Information available at http://www.asianplant.net/Apocynaceae/Alstonia_angustifolia.htm. [Google Scholar]
- Tan S-J, Robinson WT, Komiyama K, Kam T-S. Macrodasine A-G, macroline indole alkaloids incorporating fused spirocyclic tetrahydrofuran-tetrahydrofuran and tetrahydrofuran-tetrahydropyran rings. Tetrahedron. 2011;67:3830–3838. [Google Scholar]
- Tan S-J, Lim K-H, Subramaniam G, Kam T-S. Macroline-sarpagine and macroline-pleiocarpamine bisindole alkaloids from Alstonia angustifolia. Phytochemistry. 2013;85:194–202. doi: 10.1016/j.phytochem.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Wright CW, Allen D, Cai Y, Phillipson JD, Said IM, Kirby GC, Warhurst DC. In vitro antiamoebic and antiplasmodial activities of alkaloids isolated from Alstonia angustifolia roots. Phytother. Res. 1992;6:121–124. [Google Scholar]
- Zocoler MA, de Oliveira AJB, Sarragiotto MH, Grzesiuk VL, Vidotti GJ. Qualitative determination of indole alkaloids of Tabernaemontana fuchsiaefolia (Apocynaceae) J. Braz. Chem. Soc. 2005;16:1372–1377. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.