Abstract
Study Objectives:
Maintaining adequate serum levels of vitamin D may be important for sleep duration and quality; however, these associations are not well understood. We examined whether levels of serum 25(OH)D are associated with objective measures of sleep in older men.
Setting and Participants:
Cross-sectional study within a large cohort of community-dwelling older men, the MrOS study.
Interventions:
Among 3,048 men age 68 years or older, we measured total serum vitamin D. Objective estimates of nightly total sleep time, sleep efficiency, and wake time after sleep onset (WASO) were obtained using wrist actigraphy worn for an average of 5 consecutive 24-h periods.
Results:
16.4% of this study population had low levels of vitamin D (< 20.3 ng/mL 25(OH)D). Lower serum vitamin D levels were associated with a higher odds of short (< 5 h) sleep duration, (odds ratio [OR] for the highest (≥ 40.06 ng/mL) versus lowest (< 20.3 ng/mL) quartile of 25(OH)D, 2.15; 95 % confidence interval (CI), 1.21–3.79; Ptrend = 0.004) as well as increased odds of actigraphy-measured sleep efficiency of less than 70% (OR, 1.45; 95% CI, 0.97–2.18; Ptrend = 0.004), after controlling for age, clinic, season, comorbidities, body mass index, and physical and cognitive function. Lower vitamin D levels were also associated with increased WASO in age-adjusted, but not multivariable adjusted models
Conclusions:
Among older men, low levels of total serum 25(OH)D are associated with poorer sleep including short sleep duration and lower sleep efficiency. These findings, if confirmed by others, suggest a potential role for vitamin D in maintaining healthy sleep.
Citation:
Massa J, Stone KL, Wei EK, Harrison SL, Barrett-Connor E, Lane NE, Paudel M, Redline S, Ancoli-Israel S, Orwoll E, Schernhammer E. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: the MrOS Sleep Study. SLEEP 2015;38(2):251–257.
Keywords: vitamin D, sleep, cohort, elderly
INTRODUCTION
The National Sleep Counsel reports over 50% of the elderly population in the United States have troublesome sleep disturbances1; however, there is some debate over this estimate.2–5 These reports may have a significant impact on the health of the elderly as sleep disturbances have been associated with poor health indicators and with increased risk of morbidity and mortality.1,6 The prevalence of sleep disturbances increases with aging. Although there are multiple other potential risk factors for poor sleep, associations of age-related decreases in vitamin D with sleep disturbances have not been systematically evaluated.6,7
Vitamin D deficiency is common among the elderly because as skin ages it cannot synthesize vitamin D from sunlight as efficiently as younger skin.8 Further, lifestyle changes in developed countries have lead people to spend more time indoors; therefore exposure to the sun is limited, leading to potentially even greater health consequences in an older population.9–11 Lastly, circulating vitamin D and sleep characteristics in older adults have both been reported to vary by race/ethnicity.12–14 Poor sleep outcomes appear to be more prevalent among black men compared to white men,12 which may be due to an association with a high prevalence of vitamin D deficiency among African Americans.15
Previous studies in humans have suggested an important role of vitamin D for a number of different health outcomes including a decreased risk of colorectal cancer and multiple sclerosis and increased risk of reduced bone health and brain cancer.16,17 Interest in the role of Vitamin D in the promotion of sleep quality has recently increased due to results of animals studies that found vitamin D receptors in areas of the brainstem that regulate sleep.18–20 Studies which have compared brain regions associated with sleep-wake cycles and vitamin D target neurons in the diencephalon and other brainstem nuclei, suggest vitamin D has direct effects on the initiation and maintenance of sleep.21
Few studies have examined associations between serum vitamin D levels and sleep outcomes. A two-year uncontrolled trial of vitamin D supplementation and neurological issues in 1,500 patients with neurological problems reported, in secondary analyses, improved sleep patterns when patients achieved serum 25(OH)D levels of 60–80 ng/mL.22 A small case-control study of 28 vitamin D-deplete US veterans who were supplemented with either 1,200 IU/daily or 50,000 IU/weekly vitamin D to assess possible associations with pain, found in a secondary analysis that supplementation increased subjective measures of sleep duration (pre to post supplementation 4.6 to 5.3 hours; P = 0.019) and sleep efficiency (pre to post supplementation 59.8 to 66.6%; P = 0.012).23 A third investigation in 81 sleep clinic patients found that those with serum 25(OH)D ≥ 20 ng/mL had less subjective daytime sleepiness as measured by the Epworth Sleepiness Scale score (r = 0.45, P < 0.05).24 These studies were small, uncontrolled, or relied on subjective measures of sleep outcomes and therefore leave questions about an association between serum 25(OH)D and sleep including the optimal serum 25(OH)D levels needed for proper sleep.
To our knowledge, our study is the first to explore the association of total circulating levels of vitamin D and objective sleep measures. Therefore, to test the hypothesis that lower 25(OH)D levels are cross-sectionally associated with poorer sleep in older men, we measured 25(OH)D in a cohort of 3,048 community-dwelling men aged 65 and older enrolled in the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. Objective assessments of sleep disturbances were obtained using wrist actigraphy for an average of 5.2 consecutive nights.
METHODS
Study Population
Participants were initially recruited for the Osteoporotic Fractures in Men (MrOS) Study.25 During the baseline examination from 2000 to 2002, 5,994 community-dwelling men ≥ 65 years were enrolled at 6 clinical centers in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California.26 In order to participate, men needed to be able to walk without the assistance of another person, and must not have had a bilateral hip replacement.
The MrOS Sleep Study, an ancillary study of the parent MrOS cohort, was conducted between December 2003 and March 2005 and recruited 3,135 of MrOS participants (exceeding the goal of 3,000 participants) for a comprehensive sleep assessment. Of the 3,135 participants, 3,048 had serum vitamin D measured. There were 87 participants who did not have sufficient serum for this assay and 3 participants who had out of range data and therefore had their values set to missing, leaving a total of 3,045. Among these men, 79 did not have actigraphy. Thus for this analysis, we included a total of 2,966 participants.
The study protocols were approved by institutional review boards at each of the participating sites, and all study participants provided written informed consent.
Serum Vitamin D Assays
During the in-clinic examination for the Sleep Study visit, participants provided a fasting serum sample, which was centrally stored at −70°C, and remained frozen until thawed for performing the Vitamin D assays. Concentrations of 25(OH) vitamin D2 and 25(OH) vitamin D3 were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (ThermoFisher Scientific, Franklin, MA and Applied Biosystems-MDS Sciex, Foster City, CA) at the Mayo Clinic Reference Laboratories (Singh RJ, PhD, Mayo Clinic Laboratory, Rochester, MN). Using 3 different target markers as quality controls for each assay, inter-assay CVs for 25(OH) vitamin D3 were 9.7% at 9.0 IUs, 7.5% at 29 IUs, and 5.8% at 76 IUs. For 25(OH) vitamin D2, CVs were 11.2% at 11 IUs, 8.5% at 28 IUs, and 7.7% at 74 IUs. We used total 25(OH) vitamin D for our primary analyses, combining 25(OH) vitamin D2 and 25(OH) vitamin D3.
Sleep Assessment
At the Sleep Visit, participants were instructed to wear the Sleep-watch-O (Ambulatory Monitoring, Inc) actigraph on their wrist for 5 consecutive 24-h periods. The actigraph, which measures acceleration using a piezoelectric biomorph-ceramic cantilevered beam, was worn on the wrist of the non-dominant hand. Average duration of use (standard deviation [SD]) was 5.2 (0.9) 24-h periods. Data were collected continuously and stored in 1-min epochs. The digital integration mode of analysis, which sums the absolute level of acceleration on a second by second basis over the epoch, was used to quantify the amount of movement in each minute from which sleep-wake status could be inferred. Each participant completed a morning log, which was used to edit the actigraph data. Action W-2 software (Ambulatory Monitoring, Inc.) was used to analyze the raw data, and the University of California San Diego sleep scoring algorithm was used to determine sleep/wake status.27,28 The following sleep parameters were calculated: mean nightly total sleep time (TST; from sleep onset to final awakening), sleep efficiency (percent of time sleeping while in bed), and minutes of wake after sleep onset (WASO; wake from sleep onset to the end of the last sleep episode while in-bed).
Covariates
All other covariate data were collected at the time of the sleep visit and included questions on medical history, smoking (never, past, current), alcohol intake (usual drinks per week), and medication and supplement use. Body weight, used to calculate body mass index ([BMI] body mass divided by square of height), was measured using a digital scale and height using a wall-mounted Harpenden stadiometer. Cognitive function was assessed by having participants complete both the Modified Mini-Mental Status (MMSE) Examination29 and Part B of the Trail Making Test.30 One component of neuromuscular function was assessed by walking speed, which was determined by timing completion of a 6 m walking course performed at the participant's usual walking speed. Quality of life was assessed by the modified version of the Medical Outcomes Study 12-item short form (SF-12)31 and physical activity was evaluated by the Physical Activity Scale for the Elderly (PASE).32 Race and age were assessed at an earlier visit (mean 3.4 years [SD 0.5]) prior to the Sleep exam Participants were asked to bring all prescription and non-prescription medications used within the 30 days preceding the clinic sleep visit. If a participant forgot to bring one or more medications, a clinic staff member was responsible for obtaining this information over the telephone or at a return visit. All medications were entered into an electronic database, verified by pill bottle examination, and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).33
Participants were also asked to provide a urine sample at their first sleep visit to assess their creatinine levels. Creatinine as-says were conducted at Oregon Veterans administration Clinical Lab using a Roche COBAS Integra 6000 c501 automated analyzer (Roche Diagnostics Corp., Indianapolis, IN). The analyzer was calibrated daily in the clinical laboratory. Standard settings were used. Each sample was stored in a −70°C freezer and then thawed at room temperature for the assay. The mean (SD) creatinine levels were 90.28 (1.28) (mg/dL) with a CV% of 1.4.
Statistical Methods
Cut-points of serum 25(OH)D (< 20.3, 20.3–30.04, 30.05–40.05, and ≥ 40.06 ng/mL) were considered a priori based on clinical definitions in the literature which correspond well to most definitions of deficient, insufficient, adequate guidelines as well as levels found to be associated with risk of multiple health outcomes.34–38 We identified potential confounders of the serum 25(OH)D and sleep outcome relationship by performing a backward selection process, using variables that varied significantly with a P value of < 0.05 across categories of serum 25(OH)D and keeping these variables in the fully adjusted multivariable models if they have a P value < 0.10 after adjustment for the other covariates. Characteristics of participants across categories of total serum vitamin D are presented in Table 1. For our main analysis (Tables 2–4), we used linear and logistic regression models to evaluate the association between categories of serum vitamin D and (1) total sleep time, (2) sleep efficiency, and (3) WASO. We considered these sleep variables as both continuous and dichotomized outcomes, using cut-points that are well established in the literature and have been used to evaluate outcomes in similarly aged cohorts.39–43 We evaluated the association between serum vitamin D and total sleep time by comparing groups with < 5 versus ≥ 5 hours. We used ≥ 70 (percent of time asleep) as the cut-point for sleep efficiency and ≥ 90 min as the cut-point for WASO.
Table 1.
Baseline and sleep characteristics by categories of total serum 25(OH)D among 2,966 men participating in the MrOS Sleep Study.
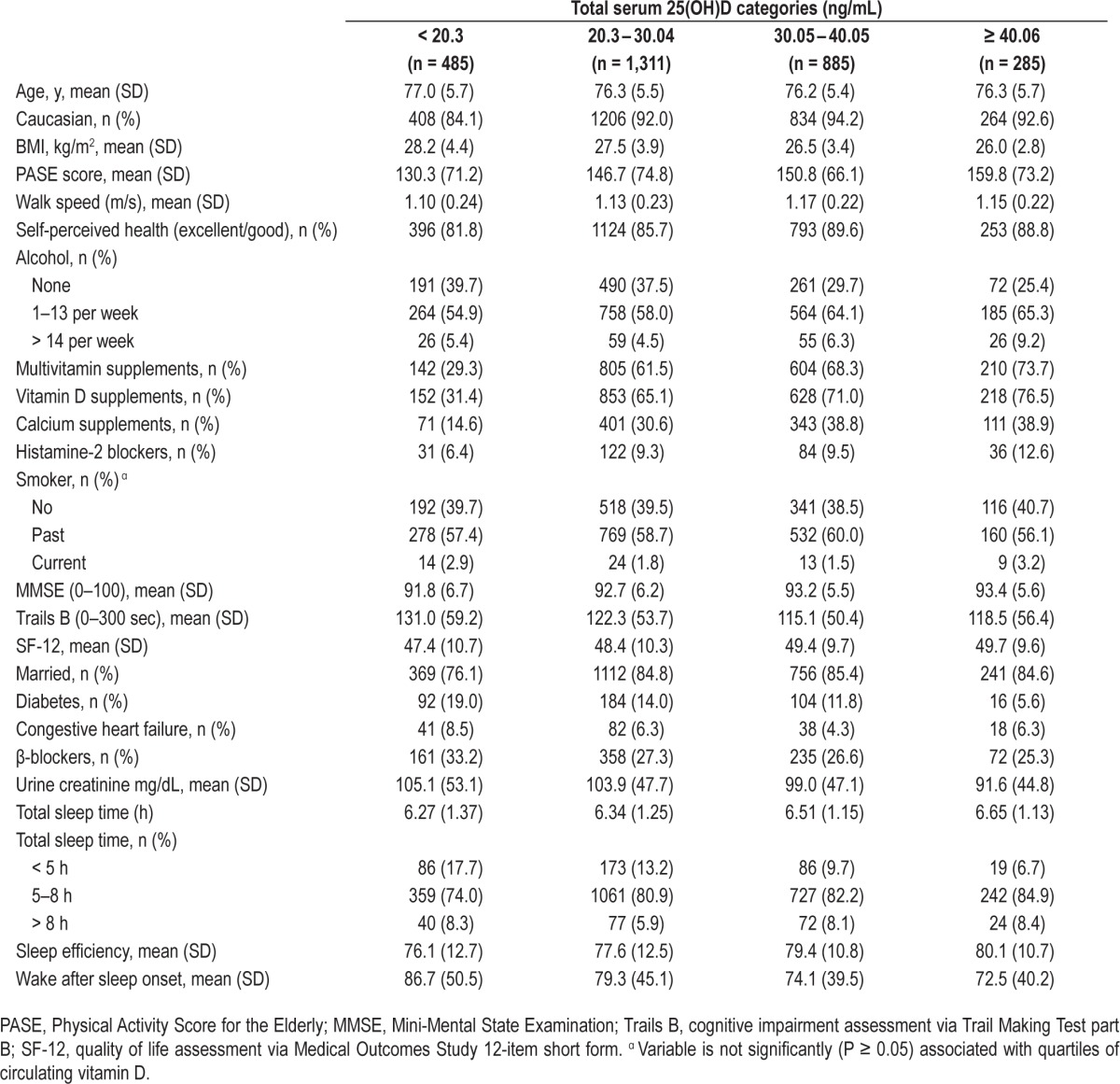
Table 2.
Multivariable odds ratios for total sleep time < 5 hours (compared to ≥ 5 hours) by total serum 25(OH) vitamin D categories in the MrOS Sleep Cohort.
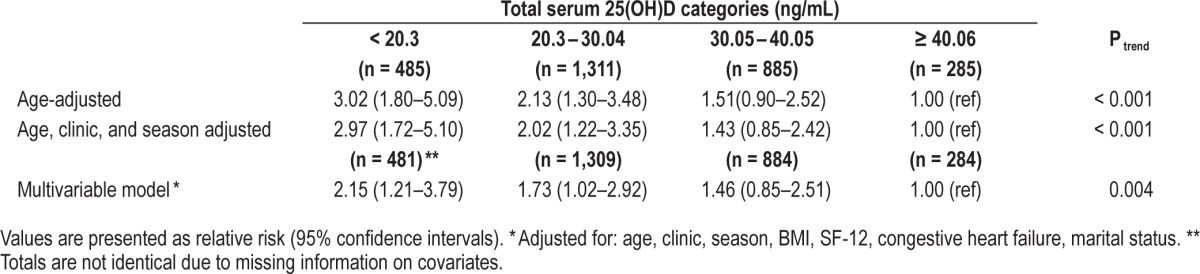
Table 3.
Multivariable odds ratios for sleep efficiency < 70 (compared to ≥ 70) by total serum 25(OH) vitamin D levels in the MrOS Sleep Cohort.
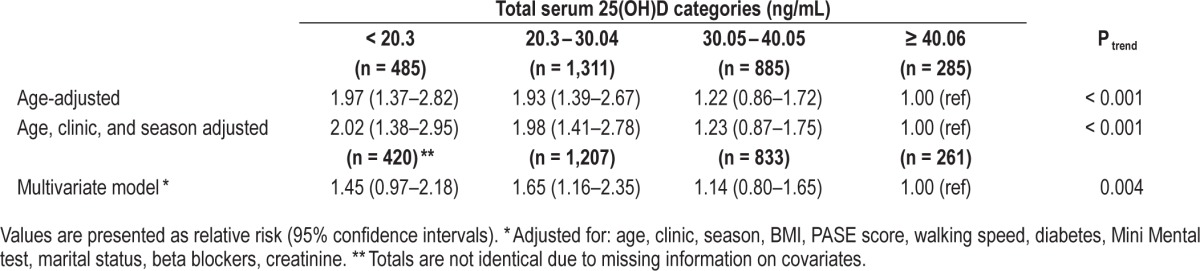
Table 4.
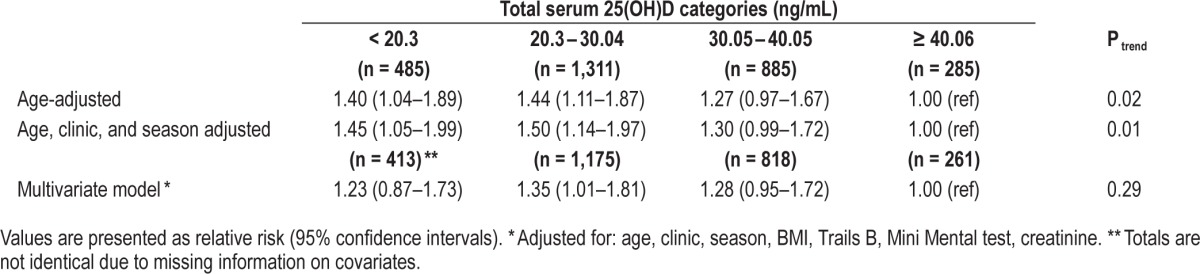
Multivariable odds ratios for wake after sleep onset ≥ 90 minutes (compared to < 90 minutes) by serum 25(OH) vitamin D levels in MrOS Sleep Cohort.
RESULTS
Of the 2,966 men included in our study, 16.4 % had a Vitamin D level < 20.3 ng/mL. Sleep disturbances were also prevalent in this cohort, with 12.3% men having a sleep duration < 5 h (mean TST in cohort was 6.4 ± 1.2), 26.3% sleep efficiency of < 70%, and 57.5% with WASO > 90 minutes.
Table 1 shows baseline demographics and sleep characteristics of men according to clinically relevant cutpoints of serum 25(OH)D. Higher serum 25(OH)D measures were associated with younger age, Caucasian race, lower BMI, higher PASE (physical activity score for the elderly), higher walking speed, less congestive heart failure, more use of vitamin D and calcium supplements, walking more often outside the home, being married, and having better objective sleep characteristics.
Vitamin D and Sleep
In age-adjusted analyses, we observed a significant, dose-dependent association between lower levels of vitamin D and higher odds of having short sleep duration, poorer sleep efficiency, and increased sleep fragmentation (all Ptrends < 0.001). These associations were slightly attenuated but persisted after further adjustment for clinic site, season, and other potential confounders. The associations between categories of 25(OH) D and odds of short sleep duration are presented in Table 2. In fully adjusted models, we observed that, compared with men in the highest vitamin D category (≥ 40.06 ng/mL), men in the 2 lowest serum 25(OH)D categories (< 30.05 ng/mL) had odds of 1.7 to 2.2 increase in short sleep duration (OR = 2.15, 95% CI: 1.21–3.79; OR for 20.3–30.04 ng/mL vs. ≥ 40.06 ng/mL: 1.73, 95% CI: 1.02–2.92; Ptrend < 0.004; Table 2). We also found a significant association between lower categories of serum 25(OH) D and shorter sleep duration when sleep duration was considered as a continuous outcome (Ptrend = 0.02).
We observed evidence of a linear trend across categories of vitamin D and odds of poorer sleep efficiency in fully adjusted models (Ptrend = 0.004; sleep efficiency < 70% vs. ≥ 70%; MV OR for < 20.3 ng/mL vs. ≥ 40.06 ng/mL: 1.45, 95% CI: 0.97–2.18; OR for 20.3–30.04 ng/mL vs. ≥ 40.06 ng/mL: 1.65 95% CI: 1.16–2.35; Table 3). The relationship between lower serum 25(OH)D and lower sleep efficiency was also significant when sleep efficiency was analyzed as a continuous variable (Ptrend = 0.04).
In age-adjusted as well as age, clinic, and season-adjusted models, we observed a significant association between low levels of vitamin D and WASO ≥ 90 min (OR for < 20.3 ng/mL vs. ≥ 40.06 ng/mL: 1.45, 95% CI: 1.05–1.99; Table 4). However, the association was attenuated and no longer statistically significant after multivariable adjustment, although categories 1–3 all suggested greater risk compared to category 4, and the 20.3–30.04 ng/mL serum 25(OH)D category (category 2) compared to the highest was statistically significant (relative risk: 1.35, 95% CI: 1.01–1.81; Table 4). In addition, there was a significant relationship between low categories of serum 25(OH) D and greater WASO when WASO was considered as a continuous outcome (Ptrend = 0.04).
We performed additional analyses stratified by “Caucasian” and “other race” and found similar trends with increasing odds of short sleep time and worse sleep efficiency with lower circulating vitamin D in both groups, although numbers were small in “other race”(data not shown). There was no significant difference in the association between circulating vitamin D and sleep duration or sleep efficiency between “Caucasian” and “other race” (P for interaction = 0.57).
DISCUSSION
In this large study of older community-dwelling men, we found a significant trend with lower total serum 25(OH)D being associated with shorter sleep duration, as well as a 2-fold higher odds of shorter sleep duration among men in the lowest category of total serum 25(OH)D, < 20.3 ng/mL, as compared to the highest, ≥ 40.06 ng/mL. We also observed a significant trend of lower serum 25(OH)D being associated with poorer sleep efficiency. We did not observe an association between total serum 25(OH)D and WASO after adjustment for multiple confounders.
The mechanisms by which vitamin D could affect sleep are not yet clear. In animal studies, nuclear concentrations of the vitamin D hormone-target neurons have been found in specific areas of the brain and spinal cord, some of which are thought to play a role in sleep including: anterior and posterior hypothalamus, substantia nigra, midbrain central gray, raphe nuclei, and the nucleus reticularis pontis oralis and caudalis.18–20,44 Sim ilar findings were reported in a study of immunohistochemical investigations with antibodies to vitamin D receptor proteins, which found evidence for target neurons in the same regions of the brainstem and hypothalamus.45 The presence of vitamin D target neurons in these regions of the brainstem that affect sleep suggests vitamin D may mediate an individual's sleep.
Past investigations in humans into vitamin D and sleep, although small and limited in number, have also reported improved sleep outcomes with higher levels of supplemental vitamin D. A recent uncontrolled clinical trial of vitamin D supplements in individuals with neurological complaints and sleep problems found maintaining vitamin D levels of 60–80 ng/mL over months resulted in normal sleep patterns.22 Huang et al. conducted a small study where they supplemented individuals with either 1,200 IU/day or 50,000 IU/week of vitamin D and found improved sleep latency (P = 0.019) and increased sleep duration (P = 0.012).23 Finally, investigators measured serum 25(OH)D in a study of sleep clinic patients and found a significant correlation between low levels of vitamin D and increase in day time sleepiness.24
Similarly, our study found that the lowest category of total serum 25(OH)D, compared to the highest, was related to shorter sleep duration and lower sleep efficiency. However, our study did not find an independent association between waking after sleep onset (WASO) and categories of serum 25(OH)D levels. Although none of the previous studies assessed these sleep outcomes specifically, four did suggest overall sleep improvement with higher serum 25(OH)D,22,23,46,47 and one with dietary intake of vitamin D.48 Of note, most of the participants in our study who were in the highest category of serum 25(OH)D did not have serum levels as high as those associated with improved sleep in the Gominak study (60–80 ng/mL).22 In our study, the median serum 25(OH)D in the highest category was only 44 ng/mL. Hence it is possible that higher levels of serum 25(OH) D in our study would have exhibited stronger associations with WASO.
Additionally, previous studies in vitamin D used supplemental vitamin D to assess the relationship between D and sleep quality measures. In our study, there was a nonsignificant suggestion that our strongest associations were found in those vitamin D supplement users, which may suggest that there is something in the way the supplement works that is beneficial for sleep outcomes. Alternatively, individuals who use supplements may have other health habits that correlate with better sleep.
Data on covariates such as physical activity, BMI, and smoking status were controlled for in this study, yet it is possible that there was residual confounding due to errors in measurement of these covariates or missing covariates. It is unlikely that this potential residual confounding could explain our results because we found only small differences in magnitude of associations between the age-adjusted analyses and the multivariate analyses that controlled for these traits. Another limitation is that the actigraph records were not additionally hand scored, which could have lead to non-differential misclassification of the outcome, causing results to be biased towards the null. Lastly, the study comprised only older men; therefore, our findings may not be generalizable to young men or women. Lastly, lack of additional hand scoring of actigraphic records
In conclusion, we found that low levels of serum 25(OH)D in older men are associated with short sleep duration and poorer sleep efficiency. If vitamin D does indeed play a causal role in poorer sleep, then low levels of serum 25(OH)D may put men at risk for poor sleep. Supplementation of vitamin D in older individuals may prove to reduce the burden of poor sleep in this population. However, further studies, including trials of supplementation, are needed in order to better elucidate this relationship between serum vitamin D and sleep outcomes.
DISCLOSURE STATEMENT
This research was supported by NIH/NIA grant AG030089 entitled “The Interplay between Sleep, Melatonin, and Mortality in Older Men” (PI Eva Schernhammer). The MrOS sleep cohort is funded through NIH grant HL071194. The funding source had no role in the design or analysis of the study or in the decision to submit the manuscript for publication. The authors have indicated no financial conflicts of interest.
ACKNOWLEGMENTS
The authors are grateful to the participants of the MrOS cohort for their dedication to this study. In addition, we would like to thank the staff of the MrOS study centers for their valuable contributions.
Author contributions: Dr. Ancoli-Israel, study design, manuscript preparation; Dr. Schernhammer, funding, data analysis and interpretation, manuscript preparation; Dr. Wei, data analysis and manuscript preparation; Dr. Stone, funding, data analysis and interpretation, manuscript preparation; and Dr. Massa, data analysis and interpretation, manuscript preparation.
Footnotes
A commentary on this article appears in this issue on page 171.
REFERENCES
- 1.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Soldatos CR, Lugaresi E. Nosology and prevalence of sleep disorders. Semin Neurol. 1987;7:236–42. doi: 10.1055/s-2008-1041423. [DOI] [PubMed] [Google Scholar]
- 3.Zilli I, Ficca G, Salzarulo P. Factors involved in sleep satisfaction in the elderly. Sleep Med. 2009;10:233–9. doi: 10.1016/j.sleep.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Grandner MA, Martin JL, Patel NP, et al. Age and sleep disturbances among American men and women: data from the U.S. Behavioral Risk Factor Surveillance System. Sleep. 2012;35:395–406. doi: 10.5665/sleep.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56:181–9. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–9. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- 8.IOM (Institute of Medicine) Washington, DC: The National Academies Press; 2011. Dietary reference intakes for calcium and vitamin D. [PubMed] [Google Scholar]
- 9.Grant WB, Schwalfenberg GK, Genuis SJ, Whiting SJ. An estimate of the economic burden and premature deaths due to vitamin D deficiency in Canada. Mol Nutr Food Res. 2010;54:1172–81. doi: 10.1002/mnfr.200900420. [DOI] [PubMed] [Google Scholar]
- 10.Norman AW, Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood) 2010;235:1034–45. doi: 10.1258/ebm.2010.010014. [DOI] [PubMed] [Google Scholar]
- 11.Whiting SJ, Green TJ, Calvo MS. Vitamin D intakes in North America and Asia-Pacific countries are not sufficient to prevent vitamin D insufficiency. J Steroid Biochem Mol Biol. 2007;103:626–30. doi: 10.1016/j.jsbmb.2006.12.067. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Ancoli-Israel S, Lewis CE, Redline S, Harrison SL, Stone KL. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med. 2011;10:54–69. doi: 10.1080/15402002.2012.636276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullough ML, Weinstein SJ, Freedman DM, et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 15.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Wei MY, Giovannucci EL. Vitamin D and multiple health outcomes in the Harvard cohorts. Mol Nutr Food Res. 2010;54:1114–26. doi: 10.1002/mnfr.200900574. [DOI] [PubMed] [Google Scholar]
- 17.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9:599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 18.Musiol IM, Stumpf WE, Bidmon HJ, Heiss C, Mayerhofer A, Bartke A. Vitamin D nuclear binding to neurons of the septal, substriatal and amygdaloid area in the Siberian hamster (Phodopus sungorus) brain. Neuroscience. 1992;48:841–8. doi: 10.1016/0306-4522(92)90272-4. [DOI] [PubMed] [Google Scholar]
- 19.Stumpf WE, Bidmon HJ, Li L, et al. Nuclear receptor sites for vitamin D-soltriol in midbrain and hindbrain of Siberian hamster (Phodopus sungorus) assessed by autoradiography. Histochemistry. 1992;98:155–64. doi: 10.1007/BF00315874. [DOI] [PubMed] [Google Scholar]
- 20.Stumpf WE, O'Brien LP. 1,25 (OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry. 1987;87:393–406. doi: 10.1007/BF00496810. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi A, Eguchi N, Kimura K, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2001;98:11674–9. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79:132–5. doi: 10.1016/j.mehy.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Huang W, Shah S, Long Q, Crankshaw AK, Tangpricha V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin d supplementation. Clin J Pain. 2013;29:341–7. doi: 10.1097/AJP.0b013e318255655d. [DOI] [PubMed] [Google Scholar]
- 24.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8:693–7. doi: 10.5664/jcsm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Girardin JL, Kripke DF, Mason WJ, Elliot JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 28.Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills. 1997;85:207–16. doi: 10.2466/pms.1997.85.1.207. [DOI] [PubMed] [Google Scholar]
- 29.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 30.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–4. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 31.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–51. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 33.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 34.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 35.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–7. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 37.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr. 2008;88:1519–27. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 39.Bansil P, Kuklina EV, Merritt RK, Yoon PW. Associations between sleep disorders, sleep duration, quality of sleep, and hypertension: results from the National Health and Nutrition Examination Survey, 2005 to 2008. J Clin Hypertens (Greenwich) 2011;13:739–43. doi: 10.1111/j.1751-7176.2011.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aulmann HM, Indorf Y, Stangel W. [Changes in the thrombocyte count and density in thrombocyte concentrates during storage with reference to thrombocyte concentration and composition of the bags] Beitr Infusionsther. 1990;26:109–11. [PubMed] [Google Scholar]
- 41.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 42.Ensrud KE, Blackwell TL, Ancoli-Israel S, et al. Use of selective serotonin reuptake inhibitors and sleep disturbances in community-dwelling older women. J Am Geriatr Soc. 2006;54:1508–15. doi: 10.1111/j.1532-5415.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 43.Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stumpf WE, Sar M, Clark SA, DeLuca HF. Brain target sites for 1,25-dihydroxyvitamin D3. Science. 1982;215:1403–5. doi: 10.1126/science.6977846. [DOI] [PubMed] [Google Scholar]
- 45.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Shiue I. Low vitamin D levels in adults with longer time to fall asleep: US NHANES, 2005-2006. Int J Cardiol. 2013;168:5074–5. doi: 10.1016/j.ijcard.2013.07.195. [DOI] [PubMed] [Google Scholar]
- 47.Valtuena J, Gonzalez-Gross M, Huybrechts I, et al. Factors associated with vitamin D deficiency in European adolescents: the HELENA study. J Nutr Sci Vitaminol (Tokyo) 2013;59:161–71. doi: 10.3177/jnsv.59.161. [DOI] [PubMed] [Google Scholar]
- 48.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23:22–34. doi: 10.1111/jsr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]


