Abstract
Skeletal muscle tissue is one of the main sites where glucose uptake occurs in response to insulin. The glucose transporter type-4 (GLUT4) is primarily responsible for the insulin-stimulated increase in glucose uptake. Upon insulin stimulation, GLUT4 is recruited from intracellular reserves to the plasma membrane. The molecular mechanisms that regulate the translocation of GLUT4 to the sarcolemma remain to be fully identified. Here, we demonstrate that GLUT4 is localized to perinuclear stores that contain flotillin-1, a marker of lipid rafts, in skeletal muscle cells. Stimulation with insulin for 10 min results in the translocation of flotillin-1/GLUT4-containing domains to the plasma membrane in a PI3K- and PKCζ-dependent manner. We also demonstrate that caveolin-3, a marker of caveolae, is required for the insulin receptor-mediated activation of the PI3K-dependent pathway, which occurs 2 min after insulin stimulation. In fact, we demonstrate that lack of caveolin-3 significantly reduces insulin-stimulated glucose uptake in caveolin-3 null myotubes by inhibiting both PI3K and Akt, as well as the movement of GLUT4 to the plasma membrane. Interestingly, caveolin-3 moves away from the plasma membrane toward the cytoplasm 5 min after insulin stimulation and temporarily interacts with flotillin-1/GLUT4-containing domains before they reach the sarcolemma, with the consequent movement of the insulin receptor from caveolin-3-containing domains to flotillin-1-containing domains. Such translocation temporally matches the insulin-stimulated movement of Cbl and CrkII in flotillin-1/GLUT4-containing domains, as well as the activation of the GDP-GTP exchange factor C3G. Disruption of flotillin-1-based domains prevents the activation of C3G, movement of GLUT4 to the sarcolemma, and glucose uptake in response to insulin. Thus, the activation of the Cbl/C3G/TC10-dependent pathway, which occurs before flotillin-1/GLUT4-containing domains reach the plasma membrane, is flotillin-1 mediated and follows the activation of the PI3K-mediated signaling. Taken together, these results indicate that flotillin-1 and caveolin-3 may regulate muscle energy metabolism through the spatial and temporal segregation of key components of the insulin signaling.
Keywords: caveolae, glucose transporter, insulin signaling, detergent-resistant microdomains
Mammals are able to maintain glucose homeostasis under the most different conditions. A disturbance in this balance may lead to diseases such as type 2 diabetes (1). Insulin is the primary hormone responsible for maintenance of plasma glucose within a narrow physiological range. Such effect is achieved by inhibiting glucose output in the liver and stimulating glucose uptake in muscle and fat tissue. Glucose transport across the plasma membrane is a critical step for glucose metabolism in muscle and fat cells (2, 3). GLUT4 is the predominant glucose transporter in these cells. Glucose uptake occurs after insulin stimulation, when GLUT4 is recruited from intracellular stores to the plasma membrane (4, 5).
At least two signaling pathways are required for the translocation of GLUT4 to the plasma membrane by insulin in adipocytes. In the first one, activation of the insulin receptor by insulin results in phosphorylation of the insulin receptor substrate (IRS) proteins, which phosphorylate and activate phosphatidylinositol 3-kinase (PI3K), with the consequent production of the polyphosphoinositide phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 serves as an allosteric regulator of the phosphoinositide-dependent kinase (PDK). PDK phosphorylates and activates Akt, as well as atypical protein kinase C isoforms, which stimulate GLUT4 translocation (6–8).
A separate pathway has been recently demonstrated to promote GLUT4 translocation upon insulin stimulation in adipocytes. A different pool of insulin receptor can phosphorylate APS and Cbl (9, 10). Cbl interacts with Cbl-associated protein (CAP) (11), which can also bind flotillin-1, a marker of lipid rafts (12). This interaction is responsible for the localization of Cbl into lipid rafts after insulin stimulation (13). CrkII, which binds phosphorylated Cbl, is then recruited to lipid rafts. CrkII binds to the GDP-GTP exchange factor C3G, which can catalyze the exchange of GDP to GTP on the lipid raft-associated protein TC10, a member of the Rho family of small GTP binding proteins (14, 15). TC10 can then act on unknown downstream signaling molecules to stimulate the movement of GLUT4 to the plasma membrane. Although the action of insulin on glucose uptake has become more documented, gaps remain in our understanding of the precise molecular mechanisms underlying such event.
Caveolae are 50–100 nm triton-insoluble/cholesterol-enriched invaginations of the plasma membrane. Caveolins are the structural protein components of caveolar membranes. Caveolins act as scaffolding proteins to concentrate and functionally regulate signaling molecules (16–22). The caveolin gene family consists of three members: caveolin-1, caveolin-2, and caveolin-3 (18, 19, 23). Caveolin-1 and caveolin-2 are coexpressed in many cell types, including adipocytes, endothelial cells, epithelial cells, and fibroblasts (24). In contrast, caveolin-3 expression is essentially restricted to skeletal and smooth muscle cells, as well as cardiac myocytes (25–33).
Direct interaction with caveolin-1 results in the inhibition of a number of signaling molecules, such as G-protein-α subunit, Ras, nitric oxide synthase (NOS), protein kinase C (PKC), and protein kinase A (PKA) (17, 22, 25, 31–40). Several independent lines of evidence indicate that caveolin-1 may possess antiproliferative properties (41–45). Consistent with this idea, we have previously demonstrated that overexpression of caveolin-1 is sufficient to arrest mouse embryonic fibroblasts (MEFs) in the G0/G1 phase of the cell cycle, reduce their proliferative lifespan, and promote premature cellular senescence through a p53/p21-dependent pathway (46, 47). However, caveolin-1 is also capable of promoting certain signaling pathways. In fact, caveolin-1 has been shown to stimulate the estrogen receptor and the insulin receptor signaling (48, 49).
Caveolin-3 plays a key role in skeletal muscle tissue. Overexpression of caveolin-3 in skeletal muscle cells induces a Duchenne-like muscular dystrophy phenotype through down-regulation of dystrophin and its associated glycoproteins (29). In addition, we found that high levels of caveolin-3 inhibit myoblast fusion in culture (50). Lack of caveolin-3 is also associated to a myopathic phenotype. In fact, caveolin-3 null mice show a loss of caveolae in skeletal muscle tissue, changes in the microdomain distribution of the dystrophin-glycoprotein complex, T-tubule abnormalities, and mild myopathic defects (30).
Flotillin-1 was originally identified as a membrane protein resident in cholesterol-enriched domains (51). Flotillin-1 is highly expressed in adipocytes, as well as diaphragm, lung, heart, and brain (51). If expressed in insect cells, flotillin-1 can form caveolae-like intracellular vesicles (52). Although flotillin-1 has been shown to co-immunoprecipitate with caveolin-1 (52), whether flotillin-1 contributes to the organization of caveolar membranes has not been properly addressed yet (i.e., by immunogold electron microscopy). Because flotillin-1 is found in triton-insoluble/cholesterol-enriched domains in cells that do not express caveolin (53, 54), flotillin-1 is believed to represent a new class of structural component of non-caveolar detergent-resistant microdomains (DRMs).
Within the past decade, data have emerged that implicate caveolin-1 in the signaling events linking insulin action to glucose uptake in adipocytes. However, while biochemical data suggest that GLUT4 moves to caveolin-1-enriched membrane domains after insulin stimulation in adipocytes (37, 55), immunoelectron microscopy data have not supported the insulin-stimulated movement of GLUT4 to caveolae (56, 57). Caveolin-3 has also been shown to play a role in insulin signaling in muscle tissue. In fact, caveolin-3 null mice displayed insulin resistance, as demonstrated by decreased glucose uptake in skeletal muscles, impaired glucose tolerance test performance, and increases in serum lipids (58). Consistent with these data, Lisanti and colleagues (59) have recently shown increased adiposity, postprandial hyperinsulinemia, whole-body insulin resistance, whole-body glucose intolerance, and decreases in insulin-stimulated whole-body glucose uptake and whole-body glycogen synthesis in caveolin-3 null mice. In both studies, however, possible defects of GLUT4 trafficking were not investigated.
Although caveolin-3 appears to be important in glucose metabolism in skeletal muscle, the signaling pathways modulated by caveolin-3 upon insulin stimulation remain to be fully identified. In addition, the role of caveolin-3, as well as flotillin-1, in GLUT4 translocation in skeletal muscle is fully unexplored. Here, we investigated the hypothesis that flotillin-1 and caveolin-3 coordinately modulate the insulin-stimulated signaling cascades that lead to the translocation of GLUT4 to the plasma membrane in skeletal muscle cells.
MATERIALS AND METHODS
Materials
Antibodies and their sources were as follows: anti-flotillin-1 IgG (mouse mAb 18), anti-caveolin-3 IgG (mouse mAb 26), and anti-phosphotyrosine IgG (mouse mAb PY20) were from Becton-Dickinson Biosciences (San Jose, CA). Anti-GLUT4 IgG (rabbit pAb) was a generous gift of Dr. Samuel Cushman, National Institutes of Health. Anti-c-Myc IgG (rabbit pAb A-14) and anti-insulin-Rβ IgG (rabbit pAb C-19) were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-IRS1 IgG (rabbit pAb) and anti-p85 IgG (rabbit pAb) were from Upstate Cell Signaling Solutions (Waltham, MA). Anti-Akt IgG (rabbit pAb) and anti-phospho-Akt (Ser 473) IgG (rabbit pAb) were from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit secondary antibodies were from Pierce (Rockford, IL). Insulin, sodium orthovanadate, and wortmannin were from Sigma (St. Louis, MO). Okadaic acid and protein kinase Cζ pseudosubstrate inhibitor (myristoylated) were from Calbiochem (La Jolla, CA). All other biochemicals used were of the highest purity available and were obtained from regular commercial sources.
Generation of conditionally immortalized skeletal muscle cells
Conditionally immortalized skeletal muscle cells expressing normal levels of endogenous caveolin-3 and lacking caveolin-3 expression were derived from the “immortomouse” as described previously (50, 60). The “immortomouse” is a transgenic mouse that harbors a temperature-sensitive SV40 large T-antigene gene (tsA58) under the control of an interferon-γ sensitive promoter (H-2Kb). Cells derived from this mouse proliferate under permissive conditions (33°C in the presence of γ-interferon) and differentiate under non-permissive conditions (37°C in the absence of γ-interferon). Several cell lines derived from different tissues have been successfully generated in the past using this mouse model, including skeletal muscle cells (61–65). Briefly, 1-month-old mice (~15 g) were killed, and hind limb muscles (gastrocnemius and soleus muscles) were isolated from both the left and right legs and collected in a Petri dish containing PBS + penicillin and streptomycin. The muscle was minced to very fine pieces and transferred to a tube containing 0.5% collagenase type II (Life Technologies) and 1% dispase (Life Technologies) diluted in PBS + penicillin and streptomycin. Muscle was incubated for 30 min at 37°C and mixed during the incubation. The supernatant was collected and an equal volume of complete medium was added (Ham’s F-10 with 20% FBS). Sample was filtered through a cell strainer and centrifuged at 900 g for 10 min. The pellet, containing skeletal muscle cells, was resuspended in complete medium. Then, cells were subjected to five rounds of preplating. Cells were passed under permissive conditions for no longer then 5–7 days: Ham’s F-10 with 20% FBS in the presence of γ-interferon (50 U/ml) at 33°C (50). Differentiation to multinucleated myotubes was achieved by growing the cells under non-permissive conditions in differentiation medium: DMEM with 2% horse serum in the absence of γ-interferon at 37°C.
Immunoblot analysis
Cells were collected in boiling sample buffer. Cellular proteins were resolved by SDS-PAGE (12.5% acrylamide; 7.5% for probing with IRS1 antibodies) and transferred to BA83 nitrocellulose membranes (Schleicher and Schuell, Keene, NH). Blots were incubated for 2 h in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.2% Tween 20) containing 2% powdered skim milk and 1% bovine serum albumin (BSA). After three washes with TBST, membranes were incubated for 2 h with the primary antibody and for 1 h with horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG. Bound antibodies were detected using an ECL detection kit (Pierce, Rockford, IL).
Preparation of DRMs
Cells were scraped into 2 ml of Mes-buffered saline containing 1% (vol/vol) Triton X-100. Homogenization was carried out with 10 strokes of a loose fitting Dounce homogenizer. The homogenate was adjusted to 40% sucrose by the addition of 2 ml of 80% sucrose prepared in Mes-buffered saline and placed at the bottom of an ultracentrifuge tube. A 5–30% linear sucrose gradient was formed above the homogenate and centrifuged at 45,000 rpm for 16–20 h in a SW60 rotor (Beckman Coulter, Fullerton, CA). A light-scattering band confined to the 15–20% sucrose region was observed that contained endogenous flotillin-1 and caveolin-3 but excluded most of other cellular proteins. From the top of each gradient, 1 ml gradient fractions were collected to yield a total of 11 fractions. Fractions 4–6, representing DRMs, and fractions 9–11, representing non-DRMs, were pooled together. An equal amount of protein from each of the two groups was separated by SDS-PAGE and subjected to immunoblot analysis.
Immunofluorescence
Cells grown on glass coverslips were washed three times with PBS and fixed for 30 min at room temperature with 2% paraformaldehyde in PBS. Fixed cells were rinsed with PBS and permeabilized with 0.1% Triton X-100, 0.2% bovine serum albumin for 10 min. Cells were then treated with 25 mM NH4Cl in PBS for 10 min at room temperature to quench free aldehyde groups. Cells were rinsed with PBS and incubated with the primary antibody (diluted in PBS with 0.1% Triton X-100, 0.2% bovine serum albumin) for 1 h at room temperature. After three washes with PBS (10 min each), cells were incubated with the secondary antibody for 1 h at room temperature: lissamine rhodamine B sulfonyl chloride-conjugated goat anti-rabbit antibody (5 μg/ml) and/or fluorescein isothiocyanate-conjugated goat anti-mouse antibody (5 μg/ml). Finally, cells were washed three times with PBS (10 min each wash) and slides were mounted with slow-Fade antifade reagent (Molecular Probes, Eugene, OR) and observed using a Zeiss confocal microscope (LSM 5 Pascal). The localization of flotillin-1/GLUT4 at the sarcolemma or perinuclear compartments was determined by analyzing 100 randomly selected myotubes, before and after insulin stimulation, from three independent experiments. Data were expressed as percentage of cell number (see Results).
Insulin treatment
Cells were serum starved for 4 h. Cells were incubated for the indicated period of time with 160 nM insulin in serum-free medium. Cells were then processed for immunofluorescence, co-immunoprecipitation, and for the isolation of DRMs as indicated.
Co-immunoprecipitation
Cells were washed twice with PBS and lysed for 30 min at 4°C in a buffer containing 10 mM Tris, pH 8.0, 0.15 M NaCl, 5 mM EDTA, 1% Triton X-100, and 60 mM octyl glucoside (cells were lysed in the following buffer for IRS-1/p85 co-immunoprecipitations: 137 mM NaCl, 20 mM Tris, pH 7.5, 1 mM MgCl2, 1 mM CaCl2, 1% NP-40, 10% glycerol, 100 μM sodium orthovanadate, 100 μg/ml okadaic acid). Samples were precleared for 1 h at 4°C using protein A-Sepharose (20 μl; slurry, 1:1) and subjected to overnight immunoprecipitation at 4°C using the intended antibody in the presence of protein A-Sepharose (30 μl; slurry, 1:1). As an internal control, immunoprecipitations with unrelated IgG were performed (unpublished data). After three washes with the immunoprecipitation buffer, samples were separated by SDS-PAGE (12.5% acrylamide) and transferred to nitrocellulose. Blots were then probed with the intended antibody.
Small interfering RNA
Knockdown of flotillin-1 expression was achieved by transfection of cells with mall interfering RNA (siRNA) duplexes. The target sequence was as follows: 5′-AGCAAATCCAGAGGATCTCTC-3′. Scrambled siRNA was used as a control. Undifferentiated cells were transfected with siRNA using lipofectamine. After 24 h, cells were differentiated for 3 days and cell lysates subjected to immunoblot analysis or immunoprecipitation assays.
Glucose uptake
Skeletal muscle cells were cultured in 12 well plates and serum starved for 4 h at 37°C. Cells were then incubated in Krebs-Ringer phopshate buffer (128 mM NaCl, 4.7 mM KCl, 1.25 mM MgCl2, 0.65 mM CaCl2, and 0.1 M sodium phosphate buffer pH 7.8) for 30 min at 37°C. Vehicle or 160 nM insulin and cold 2-deoxy-glucose (0.02 mM) were added for 10 min. 3H-2-deoxy-glucose (1 μCi/ml) was added during the last 5 min of incubation in the presence or absence of insulin. Cells were then transferred to ice and washed three times with cold PBS. Cells were subsequently solubilized in 0.1% SDS, and the 3H content was determined by scintillation counting. Experimental conditions were performed in triplicate each of three independent experiments.
RESULTS
GLUT4 is enriched into flotillin-1-containing membranes and forms a stable complex with flotillin-1 in skeletal muscle cells
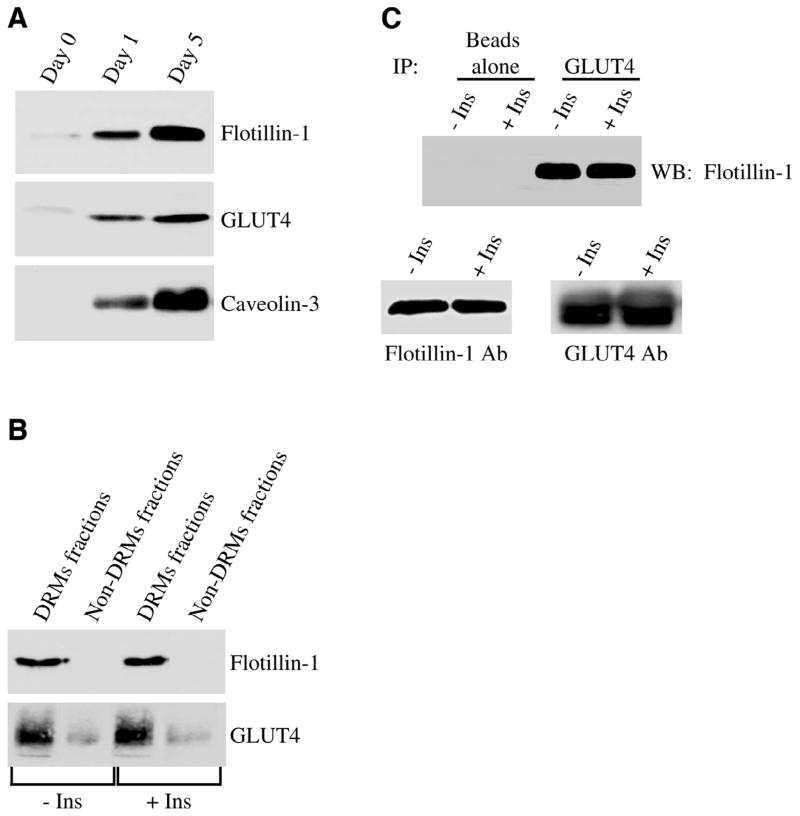
To investigate the possible role of flotillin-1 in the recruiting of GLUT4 from intracellular stores to the plasma membrane, we employed conditionally immortalized skeletal muscle cells that we have previously characterized (50, 60). We evaluated first the expression levels of flotillin-1 and GLUT4 in undifferentiated myoblasts and differentiated myotubes. Figure 1A shows that both proteins are expressed at very low levels in myoblasts, while their expression is up-regulated in differentiated myotubes. Caveolin-3, a marker of terminal differentiation, is expressed only in myotubes (Fig. 1A). We next evaluated whether both flotillin-1 and GLUT4 are enriched into DRMs and insulin affects their localization in differentiated myotubes. Both flotillin-1 and GLUT4 were found in DRMs in skeletal muscle cells, and insulin did not change their localization in these domains (Fig. 1B). This result was confirmed in Fig. 1C, where we demonstrate that flotillin-1 and GLUT4 were part of the same protein complex, as shown by the ability of GLUT4 antibodies to co-immunoprecipitate flotillin-1. Insulin did not affect the interaction between the two proteins (Fig. 1C).
Figure 1.
Flotillin-1 and GLUT4 are part of same protein complex in skeletal muscle cells. A) Immunoblot analysis. Skeletal muscle cells were left undifferentiated or differentiated for different periods of time (1 and 5 days). Cell lysates were then subjected to immunoblot analysis with anti-flotillin-1, anti-GLUT4, and anti-caveolin-3 IgGs. B) Sucrose density gradient centrifugation. Skeletal muscle cells were differentiated for 5 days, treated with or without insulin (160 nM for 10 min), and detergent-resistant microdomains (DRMs fractions) were separated from the bulk of cellular membranes and cytosolic proteins (Non-DRMs fractions) by equilibrium sucrose density gradient centrifugation. Expression of flotillin-1 and GLUT4 was examined by imunoblotting analysis using specific antibody probes. C) Co-immunoprecipitation. Skeletal muscle cells were differentiated for 5 days and treated with or without 160 nM insulin for 10 min. Cell lysates were immunoprecipitated with anti-GLUT4 IgGs. Immunoprecipitates were subjected to Western blotting analysis using an antibody probe specific for flotillin-1. Immunoprecipitation with beads alone was used as an internal control. Insulin did not change either flotillin-1 or GLUT4 total protein expression (lower panels).
GLUT4 moves together with flotillin-1 from intracellular stores to the plasma membrane upon insulin stimulation in skeletal muscle cells through PI3K- and PKCζ-dependent pathways
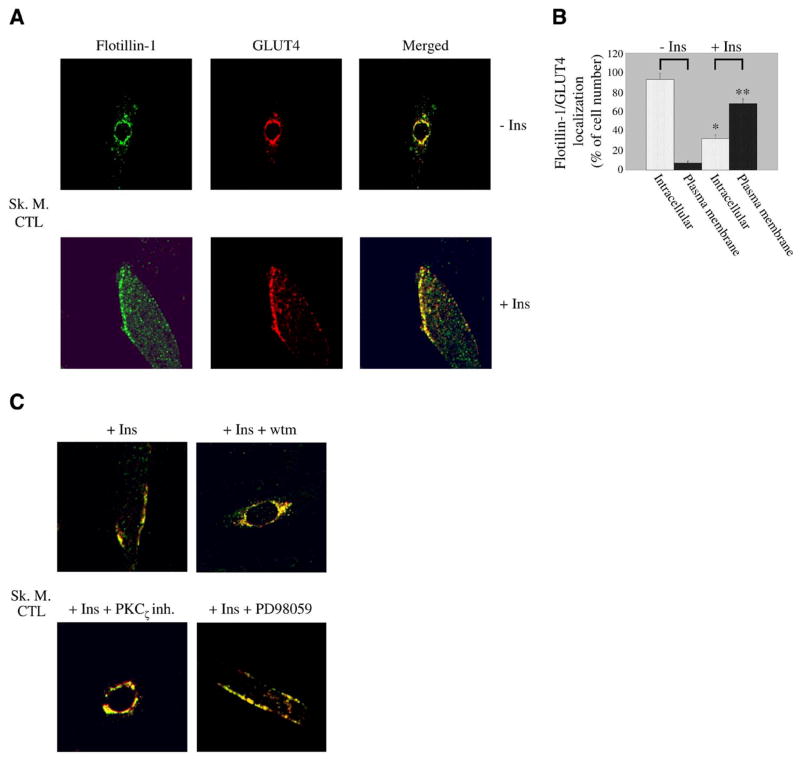
Differentiated skeletal muscle cells were subjected to immunofluorescent labeling using antibody probes against flotillin-1 and GLUT4. Figure 2A, upper panels, illustrates that the two proteins are colocalized to a perinuclear compartment in the absence of insulin. After insulin stimulation (160 nM for 10 min), flotillin-1 together with GLUT4 moved to the sarcolemma (Fig. 2A, lower panels). Quantification of flotillin-1/GLUT4 localization before and after insulin stimulation is shown in Fig. 2B. The translocation from a perinuclear compartment to the plasma membrane was PI3K- and PKCζ-dependent, as shown by the perinuclear localization of flotillin-1 and GLUT4 after insulin stimulation in the presence of wortmannin and protein kinase Cζ pseudosubstrate inhibitor, respectively (Fig. 2C). These effects were specific as demonstrated by the inability of PD98059, an inhibitor of the p42/44 MAP kinase pathway, to block the translocation of flotillin-1 and GLUT4 to the sarcolemma after insulin stimulation (Fig. 2C).
Figure 2.
Flotillin-1 and GLUT4 move to the sarcolemma in response to insulin in a PI3K/PKCζ-dependent fashion in skeletal muscle cells. A) Immunofluorescence. Differentiated myotubes were left untreated or treated with insulin (160 nM for 10 min). Localization of flotillin-1 and GLUT4 was examined using anti-flotillin-1 and anti-GLUT4 IgGs followed by incubation with fluorescent secondary antibodies. B) Quantification. Quantification of localization of flotillin-1 and GLUT4 in skeletal muscle cells before and after stimulation with insulin. Before insulin stimulation, 93 ± 6% of cells expressed flotillin-1/GLUT4 around the nucleus and only 7 ± 1.5% at the sarcolemma. After insulin stimulation, flotillin-1/GLUT4 was found in perinuclear compartments in 32 ± 4% of cells and at the plasma membrane in 68 ± 5% of cells. Values are mean ± SE; n = 100; *P ≤ 0.005 (intracellular localization +Ins vs. −Ins); **P ≤ 0.005 (plasma membrane localization +Ins vs. −Ins). C) Skeletal muscle cells were differentiated for 5 days and treated with insulin (160 nM for 10 min) in the presence or absence of wortmannin (wtm), protein kinase Cζpseudoinhibitor (PKCζ inh.), and PD98059. Expression of flotillin-1 and GLUT4 was detected as in A. Green: flotillin-1; red: GLUT4; yellow: colocalization between flotillin-1 and GLUT4.
Activation of the PI3K/Akt pathway occurs within 2 min after insulin stimulation and requires caveolin-3
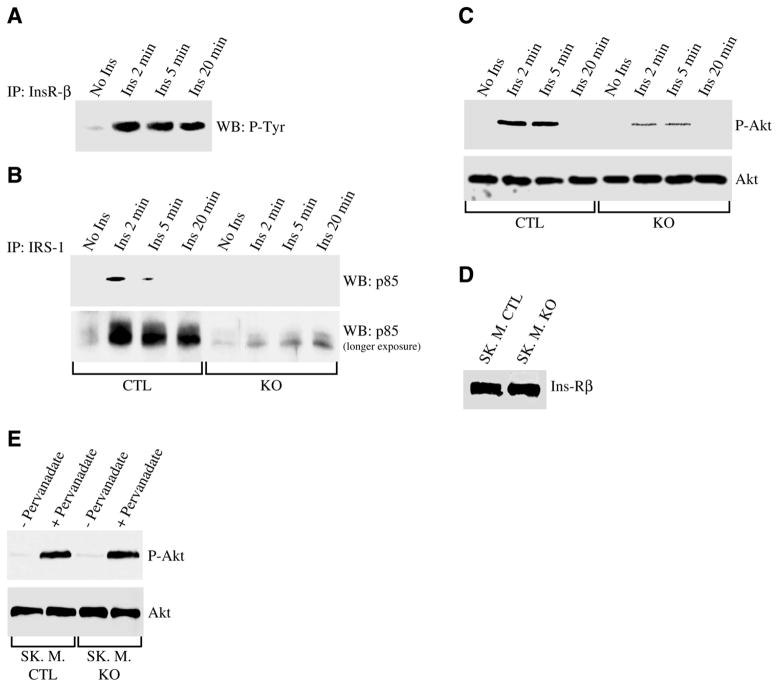
To examine the time course of the activation of the PI3K/Akt pathway, we began to evaluate the phosphorylation of the insulin receptor after insulin stimulation. As shown in Fig. 3A, phosphorylation of the insulin receptor occurred 2 min after insulin treatment. Five minutes after insulin stimulation, the activation of the receptor remained elevated (Fig. 3A). Consistent with these data, stimulation with insulin for 2 min was sufficient to activate PI3K (Fig. 3B) and Akt (Fig. 3C). Caveolin-3 expression was necessary to activate this pathway as demonstrated by the significant reduced activation of both PI3K and Akt after insulin stimulation in caveolin-3 null skeletal muscle cells (Fig. 3B, 3C).
Figure 3.
Activation of Ins-R/PI3K/Akt pathway occurs 2 min after insulin stimulation and requires caveolin-3 expression. Control (A, B, C) and caveolin-3 null (B, C) skeletal muscle cells were differentiated for 5 days and treated with or without insulin (160 nM) for 2, 5, and 20 min. Cell lysates were immunoprecipitated with anti-insulin receptor-β IgGs (A) and IRS-1 IgGs (B), and immunoprecipitates were subjected to immunoblot analysis using specific antibody probes against phospho-tyrosine proteins (A), and p85 (B). In addition, total cell lysates were separated by SDS-PAGE and subjected to Western blotting analysis using anti-phospho-Akt and Akt IgGs (C). Total IRS-1 and p85 protein expression was not affected by insulin (data not shown). Moreover, total cell lysates from control and caveolin-3 null myotubes were subjected to immunoblot analysis with anti-Ins-Rβ IgGs (D). Finally, control and caveolin-3 null myotubes were left untreated or treated with 10 μM pervanadate for 15 min, and cell lysates subjected to immunoblot analysis using anti-phospho-Akt and Akt IgGs (E).
Reduced activation of the PI3K/Akt pathway in caveolin-3 null cells was not due to reduced expression of the insulin receptor (Fig. 3D). In addition, the insulin receptor signaling was successfully activated by the insulin mimetic pervanadate in both control and caveolin-3 null cells, as demonstrated by comparable expression of phosphorylated Akt (Fig. 3E). Taken together, these results suggest that lack of caveolin-3 may prevent the ability of the insulin receptor to activate downstream signaling partners (PI3K and Akt) only when the receptor is activated by insulin and not when the insulin receptor is activated independently of insulin (i.e., by insulin mimetics).
Lack of caveolin-3 reduces glucose uptake by inhibiting the movement of GLUT4 to the sarcolemma and p38-mediated activation of GLUT4
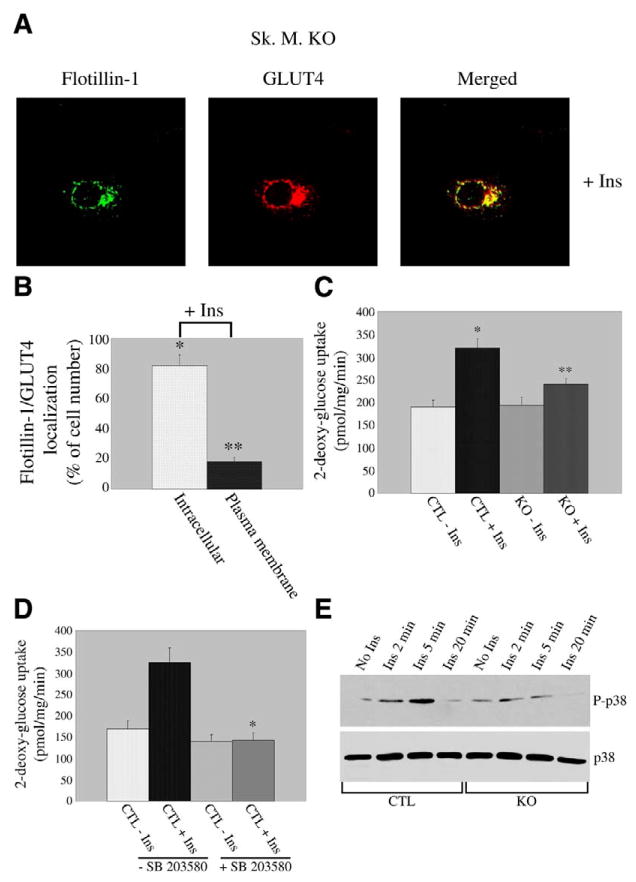
To further investigate the specific role of caveolin-3 in insulin signaling, we examined the effect of lack of caveolin-3 on insulin-stimulated GLUT4 translocation and glucose uptake by employing skeletal muscle cells derived from caveolin-3 null mice. We demonstrate in Fig. 4A that the ability of flotillin-1/GLUT4 domains to reach the plasma membrane after insulin stimulation was significantly compromised. In fact, while flotillin-1 and GLUT4 reached the sarcolemma in 68 ± 5% of control cells after insulin stimulation (Fig. 2A and B), flotillin-1/GLUT4-containing domains were found at the plasma membrane only in 18±3% of caveolin-3 null cells (Fig. 4A and B). Both flotillin-1 and GLUT4 remained in a perinuclear region in the majority of caveolin-3 null cells (82±7%) upon insulin stimulation (Fig. 4A and B), in contrast to control cells (32±4%, Fig. 2A and B). In support of this finding, caveolin-3 null cells showed a drastic inhibition (~68% vs. control cells) of insulin-mediated glucose uptake (Fig. 4C). In contrast, basal glucose uptake was not affected (Fig. 4C).
Figure 4.
GLUT4 translocation and glucose uptake are inhibited in caveolin-3 null cells. A) Immunofluorescence. Caveolin-3 null cells were differentiated for five days and treated with 160 nM insulin for 10 min. Cells were then incubated with flotillin-1 and GLUT4 IgGs. Expression of flotillin-1 and GLUT4 was evaluated using fluorescent secondary antibodies. Green: flotillin-1; red: GLUT4; yellow indicates colocalization between flotillin-1 and GLUT4. B) Quantification. Quantification of localization of flotillin-1 and GLUT4 in caveolin-3 null myotubes after insulin stimulation. Flotillin-1/GLUT4 remained localized to perinuclear compartments in 82 ± 7% of cells and moved to sarcolemma in 18 ±3 % of cells after insulin stimulation. Values are mean ± SE; n = 100; *P ≤ 0.005 (intracellular localization KO+Ins vs. CTL+Ins; Fig. 2B); **P ≤0.005 (plasma membrane localization KO+Ins vs. CTL+Ins (Fig. 2B). C) Glucose uptake. Skeletal muscle cells derived from control and caveolin-3 null mice were differentiated for 5 days and treated with or without insulin (160 nM) for 10 min. 3H-2-deoxy-glucose (1 μCi/ml) was added during the last 5 min of incubation. Cells were subsequently solubilized and the 3H content determined by scintillation counting. Values are mean ± SE; n = 9; *P ≤0.005 (CTL+Ins vs. CTL–Ins); **P ≤0.01 (KO+Ins vs. CTL+Ins). D) Glucose uptake. Control skeletal muscle cells were differentiated for 5 days and treated with or without insulin (160 nM for 10 min), in the presence or absence of the p38 MAP kinase inhibitor SB203580 (SB203580 was also added to cells during serum starvation for 4 h). Glucose uptake was determined as described in C. Values are mean ± SE; n = 9; *P ≤0.005 (CTL+Ins+SB vs. CTL+Ins–SB). E) Immunoblot analysis. Differentiated control and caveolin-3 null myotubes were left untreated or treated with insulin (160 nM) for different periods of time (2, 5, and 20 min). Cell lysates were then subjected to immunoblot analysis using an antibody probe specific for the phosphorylated form of p38 MAP kinase. Immunoblot analysis with anti-p38 MAP kinase IgGs (lower panel), which recognize total p38 MAP kinase, indicates that total p38 MAP kinase protein expression was not modified by insulin treatment.
The p38 MAP kinase pathway is believed to play a role in the insulin-mediated activation of GLUT4 (66–68). Consistent with this data, we found that the p38 MAP kinase inhibitor SB203580 dramatically prevented insulin-stimulated glucose uptake in control skeletal muscle cells (Fig. 4D). Interestingly, activation of p38 MAP kinase by insulin was significantly inhibited in myotubes lacking caveolin-3 (Fig. 4E). This result contributes to explain the reduced insulin-mediated glucose uptake that we observed in caveolin-3 null cells.
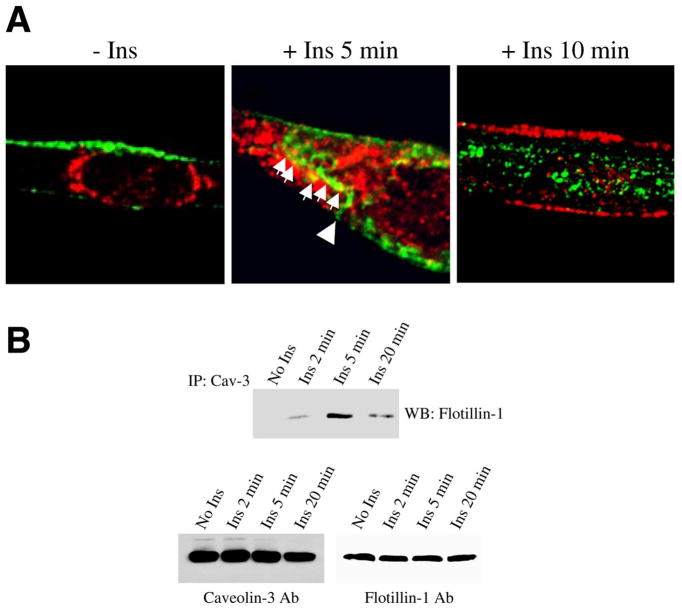
Caveolin-3 moves from the plasma membrane toward the cytoplasm upon insulin stimulation and temporarily interacts with flotillin-1/GLUT4-containing domains
Caveolin-3 is localized at the sarcolemma of differentiated myotubes (50, 60). As we have shown in Fig. 2A that flotillin-1, together with GLUT4, moved to the plasma membrane in response to insulin, we asked whether the localization of caveolin-3 was affected by insulin in differentiated myotubes. We show in Fig. 5A that caveolin-3 moved from the sarcolemma (left panel) to the cytoplasm (right panel) upon insulin stimulation (160 nM for 10 min), as GLUT4 moved from perinuclear compartments (left panel) to the plasma membrane (right panel). Interestingly, caveolin-3 and GLUT4 colocalized in the cytoplasm when the cells were treated with insulin for 5 min, suggesting that caveolin-3-containing domains, as they move away from the sarcolemma (see arrowhead in Fig. 5A, middle panel), may temporarily interact with flotillin-1/GLUT4-containing domains, before they reach the sarcolemma (see arrows in Fig. 5A, middle panel). In support of these findings, we show in Fig. 5B that caveolin-3 and flotillin-1 did not interact in the absence of insulin and that their interaction peaked 5 min after insulin stimulation. Their interaction returned to basal levels after 20 min (Fig. 5B).
Figure 5.
Caveolin-3 moves away from the sarcolemma and interacts with GLUT4 after insulin stimulation. A) Skeletal muscle cells were differentiated for 5 days. Cells were left untreated (left panel) and treated with insulin (160 nM) for 5 (middle panel) and 10 (right panel) min. Cells were then incubated with antibody probes specific for caveolin-3 and GLUT4, followed by incubations with fluorescent secondary antibodies. Green: caveolin-3; red: GLUT4; yellow indicates colocalization between caveolin-3 and GLUT4. B) Differentiated myotubes were left untreated or treated with 160 nM insulin for different periods of time (2, 5, and 20 min). Cell lysates were immunoprecipitated with anti caveolin-3 IgGs. Immunoprecipitates were then subjected to immunoblot analysis with an antibody probe specific for flotillin-1. Insulin did not change caveolin-3 and flotillin-1 total protein expression (lower panels).
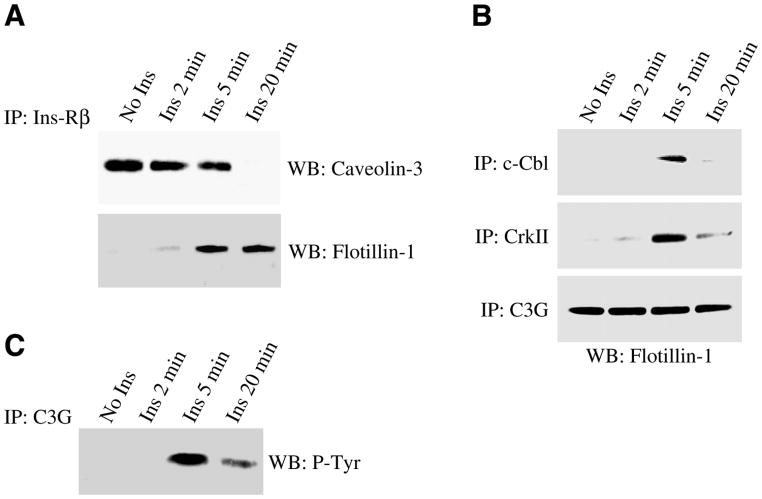
Insulin promotes the movement of the insulin receptor from caveolin-3-containing domains to flotillin-1-containing domains after 5 min
The insulin receptor has been previously shown to co-immunoprecipitate with caveolin-3 in skeletal muscle tissue (58). As we observed interaction between caveolin-3-containing domains and flotillin-1/GLUT4-containing domains 5 min after insulin stimulation, we next asked whether the insulin receptor is part of the same protein complex with caveolin-3 before insulin stimulation, and can interact with flotillin-1 after insulin stimulation. Toward this end, cell lysates from differentiated myotubes were immunoprecipitated with anti-insulin receptor IgGs, and immunoprecipitates subjected to immunoblot analysis with antibody probes specific for caveolin-3 and flotillin-1. Figure 6A illustrates that the insulin receptor was mainly localized in caveolin-3-containing domains in the absence of insulin. After insulin stimulation, the insulin receptor moved from caveolin-3-containing domains to flotillin-1-containing domains (Fig. 6A). The fact that the interaction between the insulin receptor and flotillin-1 begins after 5 min is consistent with the data of Fig. 5B, where we demonstrated that the interaction between caveolin-3-containing domains and flotillin-1-containing domains is maximal 5 min after insulin treatment.
Figure 6.
Activation of the Ins-R/Cbl/C3G pathway occurs 5 min after insulin stimulation. Skeletal muscle cells were differentiated for 5 days and treated with or without insulin (160 nM) for 2, 5, and 20 min. Cell lysates were immunoprecipitated with anti-Ins-Rβ (A), anti-Cbl IgGs (B), anti-CrkII IgGs (B), and anti-C3G IgGs (B, C), and immunoprecipitates subjected to immunoblot analysis using anti-caveolin-3 IgGs (A), anti-flotillin-1 IgGs (A, B), and anti-phosphotyrosine IgGs (C). Total caveolin-3, flotillin-1, Cbl, CrkII, and C3G protein expression was not affected by insulin (data not shown).
The movement of Cbl and CrkII to flotillin-1-containing domains, as well as the activation of C3G, occurs 5 min after insulin stimulation
As we have shown in Fig. 6A that the insulin receptor moved from caveolin-3-containing domains to flotillin-1-containing domains 5 min after insulin stimulation, and flotillin-1 is known to directly bind the Cbl-associated protein CAP in adipocytes (12), we next asked whether flotillin-1 mediates the insulin stimulated activation of the Cbl/C3G/TC10 pathway in skeletal muscle cells. Differentiated myotubes were immunoprecipitated using specific antibody probes directed against Cbl, CrkII, and C3G. Immunoprecipitates were then subjected to immunoblot analysis using anti-flotillin-1 IgGs. Figure 6B shows that both Cbl and CrkII temporarily moved into flotillin-1-containing domains 5 min after insulin stimulation. In contrast, the GDP/GTP exchange factor C3G was located in flotillin-1-containing domains before and after insulin stimulation (Fig. 6B). However, activation of C3G occurred 5 min after insulin treatment (Fig. 6C), which temporally matched the translocation of the insulin receptor, Cbl, and CrkII into flotillin-1-containing domains. Taken together, these data suggest that flotillin-1 may mediate the activation of the Cbl/C3G/TC10 pathway 5 min after insulin treatment and that activation of this pathway follows the activation of the PI3K/Akt-dependent pathway, which occurs 2 min after stimulation with insulin.
Disruption of flotillin-1-containing domains prevents the activation of C3G, movement of GLUT4 to the sarcolemma, and glucose uptake in response to insulin
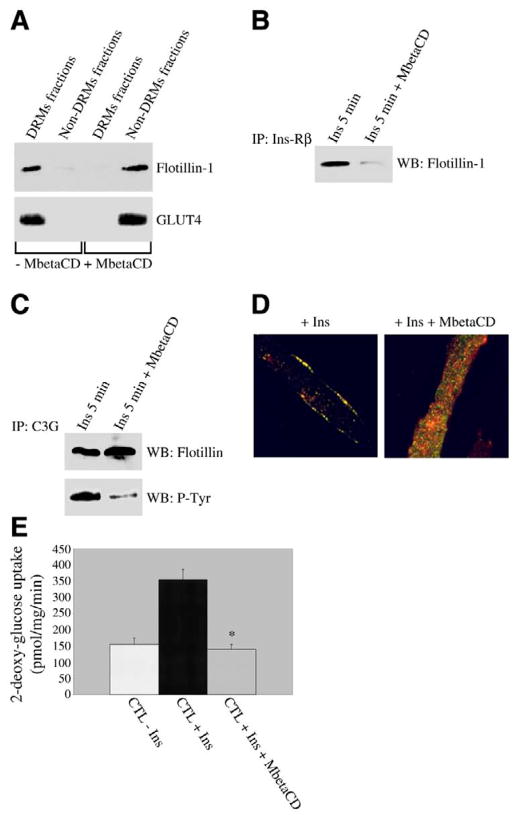
To confirm a major role of flotillin-1 in the activation of the Cbl/C3G/TC10 pathway and GLUT4 translocation after insulin stimulation, we disrupted flotillin-1-containing domains using the cholesterol-sequestering agent methyl-β-cyclodextrin (MbetaCD). Figure 7A shows that we successfully disrupted flotillin-1-containing domains, as demonstrated by the exclusion of both flotillin-1 and GLUT4 from DRMs in differentiated myotubes stimulated with insulin upon treatment with MbetaCD. Treatment with MbetaCD also prevented the interaction between the insulin receptor and flotillin-1 5 min after insulin stimulation (Fig. 7B). Consistent with the idea that the insulin-promoted translocation of the insulin receptor to flotillin-1-containing domains is required for the consequent activation of the Cbl/C3G/TC10 pathway, we show in Fig. 7C that the insulin-stimulated activation of C3G is dramatically inhibited by the treatment with MbetaCD.
Figure 7.
Disruption of flotillin-1-containing domains prevents activation of C3G, movement of GLUT4 to the sarcolemma, and glucose uptake after insulin treatment. A) Sucrose density gradient centrifugation. Skeletal muscle cells were differentiated for 5 days and treated with or without 10 mM methyl-meta-cyclodextrin (MbetaCD) for 1 h before insulin stimulation (160 nM for 10 min) in the presence or absence of MbetaCD. Detergent-resistant microdomains (DRMs fractions) were separated from the bulk of cellular membranes and cytosolic proteins (Non-DRMs fractions) by equilibrium sucrose density gradient centrifugation. Expression of flotillin-1 and GLUT4 was examined by imunoblotting analysis using specific antibody probes. B, C) Coimmunoprecipitation. Skeletal muscle cells were differentiated for 5 days and treated with or without 10 mM methyl-meta-cyclodextrin (MbetaCD) for 1 h before insulin stimulation (160 nM for 5 min) in the presence or absence of MbetaCD. Cell lysates were immunoprecipitated with anti-Ins-Rβ IgGs (B) and C3G IgGs (C). Immunoprecipitates were then subjected to Western blotting analysis with anti-flotillin-1 IgGs (B, C) and anti-phosphotyrosine IgGs (C). MbetaCD did not change total InsR-β, flotillin-1, and C3G protein expression (data not shown). D) Immunofluorescence. Skeletal muscle cells were differentiated for 5 days and treated with or without 10 mM methyl-meta-cyclodextrin (MbetaCD) for 1 h before insulin stimulation (160 nM for 10 min) in the presence or absence of MbetaCD. Cells were then incubated with flotillin-1 and GLUT4 IgGs. Expression of flotillin-1 and GLUT4 was evaluated using fluorescent secondary antibodies. Green: flotillin-1; red: GLUT4. E) Glucose uptake. Control skeletal muscle cells were differentiated for 5 days and treated with or without 10 mM methyl-meta-cyclodextrin (MbetaCD) for 1 h before insulin stimulation (160 nM for 10 min) in the presence or absence of MbetaCD. 3H-2-deoxy-glucose (1 μCi/ml) was added during the last 5 min of incubation. Cells were subsequently solubilized, and 3H content was determined by scintillation counting. Values are mean ± SE; n = 9; *P ≤ 0.005 (CTL+Ins+MbetaCD vs. CTL+Ins).
Interestingly, disruption of flotillin-1-containing domains with MbetaCD prevented the translocation of both flotillin-1 and GLUT4 to the sarcolemma in response to insulin (Fig. 7D). In support of these data, insulin-stimulated glucose uptake was prevented by the treatment with MbetaCD (Fig. 7E).
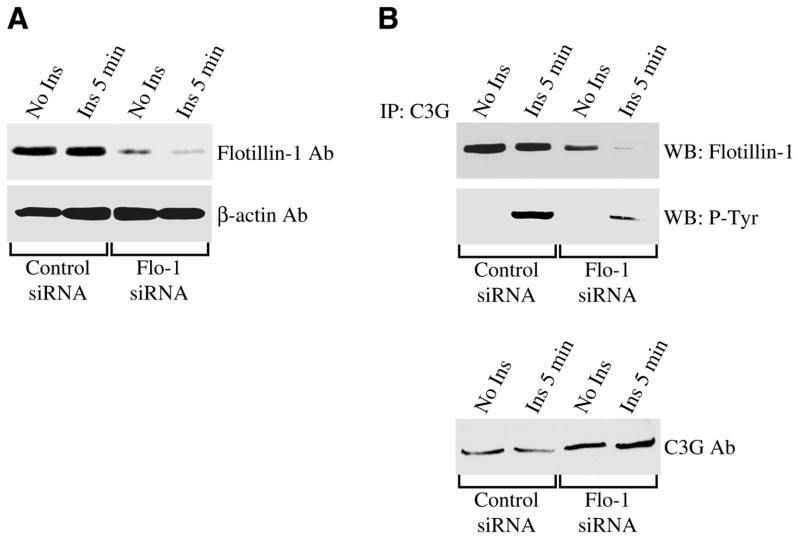
To further demonstrate the direct role of flotillin-1 in the activation of the Cbl/C3G/TC10 pathway, activation of C3G was investigated after endogenous flotillin-1 expression was down-regulated by siRNA. Figure 8A illustrates that flotillin-1 expression was reduced by ~90% in myotubes transfected with siRNA directed against flotillin-1. Down-regulation of flotillin-1 expression resulted in reduced interaction between C3G and flotillin-1 (Fig. 8B, upper panel). In addition, reduced flotillin-1 levels were sufficient to dramatically inhibit the activation of C3G in response to insulin, as demonstrated by reduced phosphorylation of C3G in flotillin-1 siRNA-transfected cells, as compared with control siRNA-transfected myotubes (Fig. 8B, middle panel). Low level of phosphorylated C3G was not the result of reduced total C3G protein expression in flotillin-1 siRNA-transfected cells (Fig. 8B, lower panel). In contrast, C3G expression was up-regulated in cells where flotillin-1 expression was reduced by siRNA, which may represent a compensatory mechanism adopted, unsuccessfully, by the cells to overcome reduced C3G activation due to lack of flotillin-1.
Figure 8.
Down-regulation of flotillin-1 expression prevents insulin-stimulated activation of C3G. A) Undifferentiated myoblasts were transfected with flotillin-1 siRNA (Flo-1 siRNA). Transfection with scrambled siRNA was used as a control. Cells were then differentiated to multinucleated myotubes for 3 days and cell lysates subjected to immunoblot analysis using an antibody probe specific for flotillin-1. Immunoblot analysis with anti-β-actin IgGs was done as an internal control. B) Cells were transfected and differentiated as in A. Cells were then treated with or without insulin (160 nM) for 5 min. Cell lysates were immunoprecipitated with anti-C3G IgGs and immunoprecipitates subjected to immunoblot analysis using anti-flotillin-1 IgGs and anti-phosphotyrosine IgGs. Total C3G protein expression is shown in the lower panel.
DISCUSSION
The localization of flotillin-1 within the cell is intriguing. Liu and colleagues (69) have shown that while flotillin-1 is localized at the plasma membrane in CHO, HepG2, and HeLa cells, it has a Golgi-like intracellular distribution in COS-1 cells, and an intracellular granular-like staining in MDCK cells and pre-adipocytes. Interestingly, flotillin-1 moved from intracellular granular structures in pre-adipocytes to the plasma membrane in differentiated adipocytes (69). They concluded that flotillin-1 can be found both at the plasma membrane and in intracellular structures and that its movement from intracellular compartments to the plasma membrane may be promoted by specific signaling mechanisms. In support of this hypothesis, we have shown in the present study that flotillin-1 is localized to perinuclear domains in skeletal muscle cells under basal conditions and that insulin promoted the movement of flotillin-1 to the plasma membrane.
GLUT4 is found in intracellular stores before moving to the plasma membrane in response to insulin. However, the precise nature of these stores remains to be determined. We demonstrate here for the first time that GLUT4 localizes to flotillin-1-containing domains, which are concentrated in perinuclear regions in skeletal muscle cells under basal conditions. After insulin stimulation, GLUT4, together with flotillin-1, moves to the sarcolemma where glucose uptake takes place. The fact that flotillin-1 was found in perinuclear regions in differentiated skeletal muscle cells, but at the plasma membrane in adipocytes (69), under basal conditions, suggests that the role of flotillin-1 in targeting GLUT4 to the plasma membrane after insulin stimulation may differ between muscle and fat tissue. The key role of flotillin-1-containing domains in the translocation of GLUT4 to the sarcolemma in skeletal muscle cells after insulin stimulation is demonstrated by lack of GLUT4 staining at the plasma membrane and glucose uptake when flotillin-1-based domains are disrupted by a cholesterol-sequestering agent.
Activation of the Ins-R/PI3K pathway occurs after insulin stimulation. However, the dynamic of its activation in skeletal muscle cells remains to be fully explored. We show here that the insulin receptor, PI3K, and Akt are all activated within 2 min after insulin stimulation in skeletal muscle cells. Expression of caveolin-3 is necessary for the activation of this pathway, as demonstrated by reduced activation of both PI3K and Akt in cells lacking caveolin-3 expression. These results are consistent with data showing that caveolin-3 co-immunoprecipitates with the insulin receptor (58) and that caveolin-3 can stimulate the phosphorylation of IRS-1 in vivo if co-transfected with the insulin receptor (70). We conclude that the activation of the Ins-R/PI3K pathway occurs in caveolar membranes in skeletal muscle cells and is an early event.
In addition to Akt, PI3K can also activate atypical PKC after insulin stimulation (71, 72). We demonstrate that activation of PKCζ is required to mediate the signaling pathway that leads to the translocation of flotillin-1/GLUT4-containing domains to the plasma membrane in response to insulin, as shown by the perinuclear localization of both flotillin-1 and GLUT4 after insulin stimulation in the presence of protein kinase Cζ pseudosubstrate inhibitor. Consistent with this data, we show that wortmannin, a PI3K inhibitor, produced an identical effect.
If the caveolin-3-mediated activation of the PI3K pathway is indeed required for the trafficking of flotillin-1/GLUT4-containing domains away from a perinuclear compartment toward the sarcolemma, this step would be inhibited in cells lacking caveolin-3. Interestingly, our results show that flotillin-1/GLUT4-containing domains remain localized to a perinuclear compartment after insulin stimulation in caveolin-3 null myotubes. Consistent with this result, we demonstrate that insulin-stimulated glucose uptake is impaired in skeletal muscle cells lacking caveolin-3. The fact that insulin-stimulated glucose uptake is not completely prevented in caveolin-3 null myotubes is consistent with the marginal activation of both PI3K (Fig. 3B) and Akt (Fig. 3C), as well as the residual movement of GLUT4 to the sarcolemma (Fig. 4B) observed in these cells upon treatment with insulin.
Activation of the Cbl/C3G/TC10 pathway is required, in addition to the PI3K/Akt pathway, for the movement of GLUT4 to the plasma membrane in response to insulin in adipocytes. The role of this pathway in the modulation of GLUT4 translocation in skeletal muscle cells remains to be fully understood. We show here that c-Cbl and CrkII transiently move into flotillin-1-containing domains 5 min after insulin stimulation in differentiated myotubes. In contrast, the GDP/GTP exchange factor C3G, which activates the Rho-family GTPase TC10, co-immunoprecipitates with flotillin-1 before and after insulin treatment. Phosphorylation has been previously shown to activate C3G (73–76). Interestingly, phosphorylation of C3G transiently occurs 5 min after stimulation with insulin. Flotillin-1-containing domains are required for the activation of this pathway as shown by the dramatically reduced activation of C3G upon insulin stimulation when flotillin-1-based domains are disrupted by either a cholesterol-sequestering agent or flotillin-1 siRNA. Taken together, these results indicate that activation of the Cbl/C3G/TC10 pathway occurs in flotillin-1-based domains in skeletal muscle cells and is a late event, i.e., occurs 5 min after insulin stimulation.
How are the two pathways activated? It is believed that two separate pools of insulin receptor mediate the activation of the PI3K/Akt and Cbl/C3G/TC10 pathways in adipocytes. Our results show that caveolin-3 moves away from the plasma membrane upon insulin stimulation and temporarily interacts with flotillin-1/GLUT4-containing domains 5 min after insulin stimulation, before they reach the sarcolemma, in skeletal muscle cells. In addition, we show that the insulin receptor moves from caveolin-3-containing domains to flotillin-1-containing domains 5 min after insulin treatment, which temporally matches the activation of C3G. Taken together, our results suggest for the first time that the same pool of insulin receptor may activate both pathways in a two-step event that is spatially and temporally regulated by caveolin-3 and flotillin-1 in skeletal muscle cells (Fig. 9). Within 2 min after insulin stimulation, the insulin receptor activates the PI3K-dependent pathway in caveolar membrane (Step I); after 5 min, the insulin receptor moves from caveolin-3-based domains to flotillin-1-based domains where it activates the Cbl/C3G/TC10 pathway (Step II). To our knowledge, this is the first example of functional cooperation between two different types of DRMs in the regulation of the same signaling pathway within the same cell type.
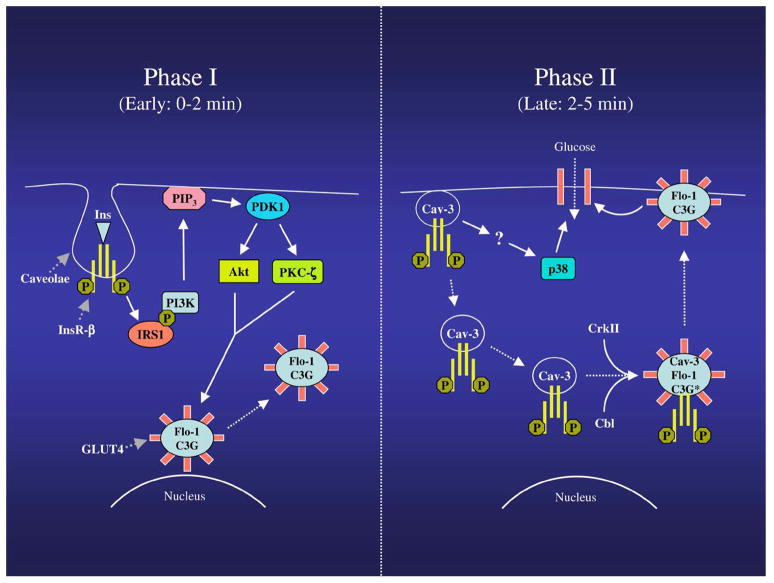
Figure 9.
Schematic diagram summarizing the modulation of GLUT4 translocation by flotillin-1 and caveolin-3. Phase I (0–2 min): Upon insulin stimulation, the insulin receptor, which is localized into caveolar membranes, activates IRS1, which phosphorylates and activates PI3K, with consequent production of PIP3. PIP3 serves as an allosteric regulator of PDK. PDK phosphorylates and activates Akt, as well as PKCζ, which stimulate the movement of flotillin-1/GLUT4-containing domains from a perinuclear compartment toward the plasma membrane. Caveolin-3 expression is necessary for the activation of both PI3K and Akt. Phase II (2–5 min): Caveolin-3-containing domains move from the sarcolemma to the cytoplasm, where they interact with flotillin-1/GLUT4-containing domains. Insulin receptor now moves from caveolin-3-containing domains to flotillin-1/GLUT4-containing domains, where it promotes recruiting of Cbl, and CrkII, as well as activation of C3G (indicated as C3G*). Flotillin-1 expression is necessary for activation of C3G. Activation of flotillin-1/Cbl/C3G-dependent, but PI3K-independent, pathway finalizes the movement of flotillin-1/GLUT4-containing domains to the plasma membrane, where glucose uptake takes place. Caveolin-3 expression is also required for insulin-dependent activation of p38 MAP kinase, which is necessary for glucose uptake.
Thus, the spatial and temporal compartmentalization of signaling molecules by caveolin-3 and flotillin-1 might play a key role in providing specificity in the trafficking/docking/fusion of GLUT4-containing vesicles. More precisely, the early caveolin-3-mediated activation of the PI3K pathway may be important for the trafficking of flotillin-1/GLUT4-containing domains away from a perinuclear compartment and toward the sarcolemma. Subsequently, the late flotillin-1-mediated activation of the Cbl/C3G/TC10 pathway may serve for the final docking and fusion of GLUT4 vesicles to the plasma membrane. Given the fact that insulin is not the only stimulus able to activate the PI3K pathway, the existence of a second insulin-initiated pathway, i.e., the Cbl/C3G/TC10 pathway, which is PI3K-independent, may provide a safeguard mechanism to avoid unregulated glucose uptake when cells are under the influence of stimuli which activate PI3K in an insulin receptor-independent manner.
Movement to the plasma membrane and activation of GLUT4 is believed to be modulated by two separate mechanisms. p38 MAP kinase has been proposed as a possible positive regulator of GLUT4 activity, but not translocation, in response to insulin (66–68). We show here that p38 MAP kinase is activated by insulin in control myotubes and that inhibition of this pathway with the specific SB203580 inhibitor totally prevents glucose uptake. In addition, we show that the activation of the p38 MAP kinase pathway by insulin is significantly inhibited in caveolin-3 null myotubes, where glucose uptake is impaired. Thus, we conclude that 1) activation of the p38 MAP kinase pathway is necessary for glucose uptake, 2) caveolin-3 expression positively regulates the insulin-mediated activation of p38 MAP kinase, and 3) the reduced activation of p38 MAP kinase detected in caveolin-3 null cells may contribute to explain the reduced glucose uptake observed in the absence of caveolin-3.
Proteins that bind GLUT4 could also play a role in promoting GLUT4 activity. For example, an inhibitor may be removed from GLUT4 after insulin stimulation, as suggested by previous studies showing that the cytosolic C terminus of GLUT4 is more accessible to antibodies upon insulin stimulation than it is before insulin stimulation (77, 78). Interestingly, caveolins are known to directly bind numerous signaling molecules. Thus, it is possible to speculate that the temporary interaction between caveolin-3-containing domains and flotillin-1/GLUT4-containing domains may result not only in the translocation of the insulin receptor from the former to the latter but also in the movement of a potential inhibitor of GLUT4 from flotillin-1/GLUT4-containing domains to caveolin-3-containing domains, leading to GLUT4 activation. However, additional experiments are necessary to directly prove this hypothesis.
Acknowledgments
This work was supported by grants from the National Institutes of Health and the American Heart Association (to F. Galbiati). We thank Dr. Samuel Cushman for kindly providing a polyclonal antibody probe directed against GLUT4.
References
- 1.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- 3.Charron MJ, Brosius FC, III, Alper SL, Lodish HF. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci USA. 1989;86:2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czech MP, Buxton JM. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J Biol Chem. 1993;268:9187–9190. [PubMed] [Google Scholar]
- 5.Satoh S, Nishimura H, Clark AE, Kozka IJ, Vannucci SJ, Simpson IA, Quon MJ, Cushman SW, Holman GD. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J Biol Chem. 1993;268:17820–17829. [PubMed] [Google Scholar]
- 6.Sharma PM, Egawa K, Huang Y, Martin JL, Huvar I, Boss GR, Olefsky JM. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J Biol Chem. 1998;273:18528–18537. doi: 10.1074/jbc.273.29.18528. [DOI] [PubMed] [Google Scholar]
- 7.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 8.Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed Z, Smith BJ, Kotani K, Wilden P, Pillay TS. APS, an adapter protein with a PH and SH2 domain, is a substrate for the insulin receptor kinase. Biochem J. 1999;341:665–668. [PMC free article] [PubMed] [Google Scholar]
- 10.Ribon V, Saltiel AR. Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem J. 1997;324:839–845. doi: 10.1042/bj3240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribon V, Printen JA, Hoffman NG, Kay BK, Saltiel AR. A novel, multifuntional c-Cbl binding protein in insulin receptor signaling in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:872–879. doi: 10.1128/mcb.18.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura A, Baumann CA, Chiang SH, Saltiel AR. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc Natl Acad Sci USA. 2001;98:9098–9103. doi: 10.1073/pnas.151252898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumann CA, Ribon V, Kanzaki M, Thurmond DC, Mora S, Shigematsu S, Bickel PE, Pessin JE, Saltiel AR. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- 14.Drivas G, Shih A, Coutavas E, Rush M, D’Eustachio P. Characterization of four novel ras-like genes expressed in a human teratocarcinoma cell line. Mol Cell Biol. 1990;10:1793–1798. doi: 10.1128/mcb.10.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang SH, Baumann CA, Kanzaki M, Thurmond DC, Watson RT, Neudauer CL, Macara IG, Pessin JE, Saltiel AR. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature. 2001;410:944–948. doi: 10.1038/35073608. [DOI] [PubMed] [Google Scholar]
- 16.Lisanti MP, Scherer P, Tang ZL, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: A signalling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 17.Couet J, Li S, Okamoto T, Scherer PS, Lisanti MP. Molecular and cellular biology of caveolae: Paradoxes and plasticities. Trends Cardiovasc Med. 1997;7:103–110. doi: 10.1016/S1050-1738(97)00001-7. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, A family of scaffolding proteins for organizing “pre-assembled signaling complexes” at the plasma membrane. J Biol Chem, (Mini-review) 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 19.Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song KS, Li S, Okamoto T, Quilliam L, Sargiacomo M, Lisanti MP. Copurification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent free purification of caveolae membranes. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 21.Song KS, Sargiacomo M, Galbiati F, Parenti M, Lisanti MP. Targeting of a G alpha subunit (Gi1 alpha) and c-Src tyrosine kinase to caveolae membranes: Clarifying the role of N-myristoylation. Cell Mol Biol (Noisy-Le-Grand) 1997;43:293–303. [PubMed] [Google Scholar]
- 22.Sargiacomo M, Scherer PE, Tang ZL, Casanova JE, Lisanti MP. In vitro phosphorylation of caveolin-rich membrane domains: Identification of an associated serine kinase activity as a casein kinase II-like enzyme. Oncogene. 1994;9:2589–2595. [PubMed] [Google Scholar]
- 23.Tang ZL, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- 24.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 25.Song KS, Scherer PE, Tang ZL, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 26.Minetti C, Sotogia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco P, Egeo A, Donati MA, Volonté D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nature Genetics. 1998;18:365–368. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- 27.Galbiati F, Volonte D, Minetti C, Chu JB, Lisanti MP. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the golgi complex. J Biol Chem. 1999;274:25632–25641. doi: 10.1074/jbc.274.36.25632. [DOI] [PubMed] [Google Scholar]
- 28.Galbiati F, Volonte D, Minetti C, Bregman DB, Lisanti MP. Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 Undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of lgmd-1c mutants and rescues wild-type caveolin-3. J Biol Chem. 2000;275:37702–37711. doi: 10.1074/jbc.M006657200. [DOI] [PubMed] [Google Scholar]
- 29.Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M, Bermudez J, Pedemonte M, Weidenheim KM, Pestell RG, et al. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci USA. 2000;97:9689–9694. doi: 10.1073/pnas.160249097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 31.Scherer PE, Tang ZL, Chun MC, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution: Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- 32.Smart E, Ying YS, Conrad P, Anderson RGW. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994;127:1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moldovan N, Heltianu C, Simionescu N, Simionescu M. Ultrastructural evidence of differential solubility in Triton X-100 of endothelial vesicles and plasma membrane. Exp Cell Res. 1995;219:309–313. doi: 10.1006/excr.1995.1233. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelilal cell caveolae via palmitoylation: implications for caveolae localization. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction of hetero-trimeric G proteins with caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Song KS, Lisanti MP. Expression and characterization of recombinant caveolin: Purification by poly-histidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J Biol Chem. 1996;271:568–573. [PubMed] [Google Scholar]
- 37.Scherer PE, Lisanti MP, Baldini G, Sargiacomo M, Corley-Mastick C, Lodish HF. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994;127:1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelman JA, Zhang XL, Galbiati F, Volonte D, Sotgia F, Pestell RG, Minetti C, Scherer PE, Okamoto T, Lisanti MP. Molecular genetics of the caveolin gene family: Implications for human cancers, diabetes, Alzheimer’s disease, and muscular dystrophy. Am J Hum Genet. 1998;63:1578–1587. doi: 10.1086/302172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galbiati F, Volonte D, Meani D, Milligan G, Lublin DM, Lisanti MP, Parenti M. The dually acylated NH2-terminal domain of gi1alpha is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. Palmitoylation of caveolin-1 is required for the recognition of dually acylated g-protein alpha subunits in vivo. J Biol Chem. 1999;274:5843–5850. doi: 10.1074/jbc.274.9.5843. [DOI] [PubMed] [Google Scholar]
- 40.Razani B, Rubin CS, Lisanti MP. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J Biol Chem. 1999;274:26353–26360. doi: 10.1074/jbc.274.37.26353. [DOI] [PubMed] [Google Scholar]
- 41.Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 42.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engelman JA, Wycoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–16381. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- 44.Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP. Reciprocal regulation of Neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for mammary tumorigenesis. J Biol Chem. 1998;273:20448–20455. doi: 10.1074/jbc.273.32.20448. [DOI] [PubMed] [Google Scholar]
- 45.Sager R, Sheng S, Anisowicz A, Sotiropoulou G, Zou Z, Stenman G, Swisshelm K, Chen Z, Hendrix MJC, Pemberton P, et al. RNA genetics of breast cancer: Maspin as a paradigm. Cold Spring Harbor Sym Quant Biol. 1994;LIX:537–546. doi: 10.1101/sqb.1994.059.01.060. [DOI] [PubMed] [Google Scholar]
- 46.Volonte D, Zhang K, Lisanti MP, Galbiati F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell. 2002;13:2502–2517. doi: 10.1091/mbc.01-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galbiati F, Volonte D, Liu J, Capozza F, Frank PG, Zhu L, Pestell RG, Lisanti MP. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell. 2001;12:2229–2244. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto M, Toya Y, Schwencke C, Lisanti MP, Myers M, Ishikawa Y. Caveolin is an activator of insulin receptor signaling. J Biol Chem. 1998;273:26962–26968. doi: 10.1074/jbc.273.41.26962. [DOI] [PubMed] [Google Scholar]
- 49.Schlegel A, Wang C, Pestell RG, Lisanti MP. Ligand-independent activation of oestrogen receptor alpha by caveolin-1. Biochem J. 2001;359:203–210. doi: 10.1042/0264-6021:3590203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volonte D, Peoples AJ, Galbiati F. Modulation of myoblast fusion by caveolin-3 in dystrophic skeletal muscle cells: Implications for Duchenne muscular dystrophy and limb-girdle muscular dystrophy-1C. Mol Biol Cell. 2003;14:4075–4088. doi: 10.1091/mbc.E03-03-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- 52.Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem. 1999;274:12702–12709. doi: 10.1074/jbc.274.18.12702. [DOI] [PubMed] [Google Scholar]
- 53.Salzer U, Prohaska R. Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood. 2001;97:1141–1143. doi: 10.1182/blood.v97.4.1141. [DOI] [PubMed] [Google Scholar]
- 54.Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- 55.Gustavsson J, Parpal S, Stralfors P. Insulin-stimulated glucose uptake involves the transition of glucose transporters to a caveolae-rich fraction within the plasma membrane: implications for type II diabetes. Mol Med. 1996;2:367–372. [PMC free article] [PubMed] [Google Scholar]
- 56.Malide D, Ramm G, Cushman SW, Slot JW. Immunoelectron microscopic evidence that GLUT4 translocation explains the stimulation of glucose transport in isolated rat white adipose cells. J Cell Sci. 2000;113:4203–4210. doi: 10.1242/jcs.113.23.4203. [DOI] [PubMed] [Google Scholar]
- 57.Voldstedlund M, Tranum-Jensen J, Vinten J. Quantitation of Na+/K(+)-ATPase and glucose transporter isoforms in rat adipocyte plasma membrane by immunogold labeling. J Membr Biol. 1993;136:63–73. doi: 10.1007/BF00241490. [DOI] [PubMed] [Google Scholar]
- 58.Oshikawa J, Otsu K, Toya Y, Tsunematsu T, Hankins R, Kawabe J, Minamisawa S, Umemura S, Hagiwara Y, Ishikawa Y. Insulin resistance in skeletal muscles of caveolin-3-null mice. Proc Natl Acad Sci USA. 2004;101:12670–12675. doi: 10.1073/pnas.0402053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capozza F, Combs TP, Cohen AW, Cho YR, Park SY, Schubert W, Williams TM, Brasaemle DL, Jelicks LA, Scherer PE, et al. Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol. 2005;288:C1317–C1331. doi: 10.1152/ajpcell.00489.2004. [DOI] [PubMed] [Google Scholar]
- 60.Volonte D, Liu Y, Galbiati F. The modulation of caveolin-1 expression controls satellite cell activation during muscle repair. FASEB J. 2005;19:237–239. doi: 10.1096/fj.04-2215fje. [DOI] [PubMed] [Google Scholar]
- 61.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanda S, Landgren E, Ljungstrom M, Claesson-Welsh L. Fibroblast growth factor receptor 1-induced differentiation of endothelial cell line established from tsA58 large T transgenic mice. Cell Growth Differ. 1996;7:383–395. [PubMed] [Google Scholar]
- 63.Ehler E, Jat PS, Noble MD, Citi S, Draeger A. Vascular smooth muscle cells of H-2Kb-tsA58 transgenic mice. Characterization of cell lines with distinct properties. Circulation. 1995;92:3289–3296. doi: 10.1161/01.cir.92.11.3289. [DOI] [PubMed] [Google Scholar]
- 64.Morgan JE, Beauchamp JR, Pagel CN, Peckham M, Ataliotis P, Jat PS, Noble MD, Farmer K, Partridge TA. Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigen: a model system for the derivation of tissue-specific and mutation-specific cell lines. Dev Biol. 1994;162:486–498. doi: 10.1006/dbio.1994.1103. [DOI] [PubMed] [Google Scholar]
- 65.Noble M, Groves AK, Ataliotis P, Ikram Z, Jat PS. The H-2KbtsA58 transgenic mouse: a new tool for the rapid generation of novel cell lines. Transgenic Res. 1995;4:215–225. doi: 10.1007/BF01969114. [DOI] [PubMed] [Google Scholar]
- 66.Somwar R, Perreault M, Kapur S, Taha C, Sweeney G, Ramlal T, Kim DY, Keen J, Cote CH, Klip A, et al. Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle: potential role in the stimulation of glucose transport. Diabetes. 2000;49:1794–1800. doi: 10.2337/diabetes.49.11.1794. [DOI] [PubMed] [Google Scholar]
- 67.Somwar R, Kim DY, Sweeney G, Huang C, Niu W, Lador C, Ramlal T, Klip A. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem J. 2001;359:639–649. doi: 10.1042/0264-6021:3590639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3-L1 adipocytes and L6 myotubes. J Biol Chem. 1999;274:10071–10078. doi: 10.1074/jbc.274.15.10071. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Deyoung SM, Zhang M, Dold LH, Saltiel AR. The stomatin/prohibitin/flotillin/HflK/C Domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J Biol Chem. 2005;280:16125–16134. doi: 10.1074/jbc.M500940200. [DOI] [PubMed] [Google Scholar]
- 70.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 72.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 73.de Jong R, van Wijk A, Heisterkamp N, Groffen J. C3G is tyrosine-phosphorylated after integrin-mediated cell adhesion in normal but not in Bcr/Abl expressing cells. Oncogene. 1998;17:2805–2810. doi: 10.1038/sj.onc.1202207. [DOI] [PubMed] [Google Scholar]
- 74.Shivakrupa R, Radha V, Sudhakar C, Swarup G. Physical and functional interaction between Hck tyrosine kinase and guanine nucleotide exchange factor C3G results in apoptosis, which is independent of C3G catalytic domain. J Biol Chem. 2003;278:52188–52194. doi: 10.1074/jbc.M310656200. [DOI] [PubMed] [Google Scholar]
- 75.Ling L, Zhu T, Lobie PE. Src-CrkII-C3G-dependent activation of Rap1 switches growth hormone-stimulated p44/42 MAP kinase and JNK/SAPK activities. J Biol Chem. 2003;278:27301–27311. doi: 10.1074/jbc.M302516200. [DOI] [PubMed] [Google Scholar]
- 76.Ichiba T, Hashimoto Y, Nakaya M, Kuraishi Y, Tanaka S, Kurata T, Mochizuki N, Matsuda M. Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J Biol Chem. 1999;274:14376–14381. doi: 10.1074/jbc.274.20.14376. [DOI] [PubMed] [Google Scholar]
- 77.Wang W, Hansen PA, Marshall BA, Holloszy JO, Mueckler M. Insulin unmasks a COOH-terminal Glut4 epitope and increases glucose transport across T-tubules in skeletal muscle. J Cell Biol. 1996;135:415–430. doi: 10.1083/jcb.135.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith RM, Charron MJ, Shah N, Lodish HF, Jarett L. Immunoelectron microscopic demonstration of insulin-stimulated translocation of glucose transporters to the plasma membrane of isolated rat adipocytes and masking of the carboxyl-terminal epitope of intracellular GLUT4. Proc Natl Acad Sci USA. 1991;88:6893–6897. doi: 10.1073/pnas.88.15.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]