Abstract
The E2F family is conserved from C. elegans to mammals with some family members having transcription activation functions and others having repressor functions1, 2. Whereas C. elegans3 and Drosophila melanogaster4, 5 have a single E2F activator and repressor proteins, mammals evolved to have at least three activator and five repressor proteins1, 2, 6. Why such genetic complexity evolved in mammals is not known. To begin to evaluate this genetic complexity, we targeted the inactivation of the entire subset of activators, E2f1, E2f2, E2f3a and E2f3b, singly or in combination in mice. We demonstrate that E2f3a is sufficient to support mouse embryonic and postnatal development. Remarkably, expression of E2f3b or E2f1 from the E2f3a locus (E2f3a3bki; E2f3a1ki) suppressed all the postnatal phenotypes associated with the inactivation of E2f3a. We conclude that there is significant functional redundancy among activators and that the specific requirement for E2f3a during postnatal development is dictated by regulatory sequences governing its selective spatiotemporal expression and not by its intrinsic protein functions. These findings provide a molecular basis for the observed specificity among E2F activators during development.
Keywords: E2F3a, E2F3b, Rb, development, proliferation, transcription and apoptosis
Since the identification of the founding E2F family member, E2f17, two distinct genes in lower eukaryotes and eight genes in higher eukaryotes have been identified to encode the signature DNA binding domain that endow these transcription factors with E2F classification1, 2, 6. Among the mammalian E2F activator subset, the E2f3 gene has emerged as the critical family member involved in the control of cell proliferation and development8, 9. The E2f3 locus was originally thought to encode a single DNA binding activity, but was later shown to drive the expression of two related isoforms, E2f3a and E2f3b, from two distinct promoters10. Given the critical link between the E2f3 locus and the control of cell proliferation, we used homologous recombination to individually disrupt its two isoforms in mice and rigorously evaluate how their functions are integrated with that of other E2F activators. The inactivation of E2f3a or E2f3b was achieved by targeting exon 1a or 1b sequences, respectively, using LoxP-cre technology (Fig. 1a). Mice deleted for either exon 1a or exon 1b were identified by Southern blot and genomic PCR analysis (Fig. 1b). Specific ablation of E2f3a or E2f3b was confirmed by Western blot assays using total E2F3-specific antibodies (Fig. 1c).
Figure 1.

Generation of E2f3a and E2f3b knockout mice. a. Partial exon/intron structure of the mouse E2f3 gene. The schematic illustrates the E2f3 locus with two separate promoters driving the expression of E2f3a and E2f3b. The two transcription start sites are indicated by bent arrows. The targeting vectors and the final E2f3a and E2f3b targeted alleles are shown on the left and right, respectively. The solid bar represents a HindIII-BamHI fragment used as the Southern probe. The LoxP sites are indicated as solid triangles; PCR primers are indicated by arrows. b. Southern blot (top panels) and PCR genotyping assays (bottom panels) were performed on MEFs with the indicated genotypes. c. Western blot analysis using E2F3-specific antibodies was performed on lysates derived from MEFs with the indicated genotypes. d. Micrograph of one month-old mice with the indicated genotypes. e. Genotypic analysis of offspring derived from E2f3+/−, E2f3a+/− and E2f3b+/− intercrosses (5th generation FVB). Fisher exact probability test was performed on each genetic group in comparison to the wild type group; highly significant results are indicated by an (a). f. Body weights of E2f3+/+ (grey), E2f3a−/− (red) and E2f3b−/− (blue) mice at the indicated ages; n, number of animals measured for each genotype at each age. * indicates the significant p-value. Student t-test was performed on each genetic group in comparison to the wild type group.
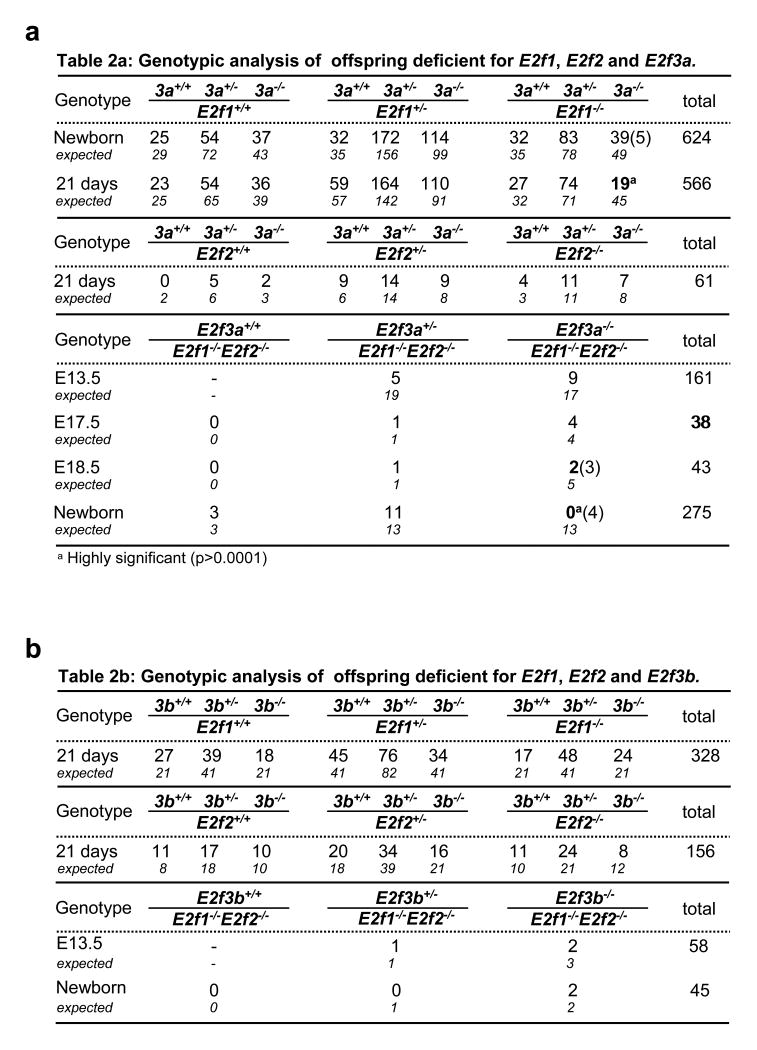
It was previously shown that inactivation of both E2f3a and E2f3b (E2f3−/−) in mice with a mixed strain background yielded offspring that developed rather normally8, 9, but we show here that breeding these mice into a pure strain background (∼98% pure) resulted in embryonic lethality (Fig. 1e and Supplementary Fig. 1). Intercrossing E2f3+/− mice of different pure backgrounds restored viability of E2f3−/− mice, albeit with some observed strain-specific biases (Supplementary Fig. 1). In contrast, the ablation of individual E2f3 isoforms (E2f3a−/− or E2f3b−/−) in a pure strain background yielded live pups at the expected Mendelian frequency (Fig. 1d-f). These results suggest that E2f3a and E2f3b have redundant functions during embryonic development. Because of the functional plasticity that exists among E2F family members8, 11–13, we examined the consequences of inactivating E2f3a or E2f3b in combination with the other two known E2F activators, E2f1 and E2f2. From the intercrosses described in Fig. 2a-b, we obtained pups doubly deleted for every possible combination of E2f1, E2f2, E2f3a or E2f1, E2f2, E2f3b at or near the expected ratios. Embryos with the two possible triple knockout genotypes (tko; E2f1−/− E2f2−/− E2f3a−/− and E2f1−/− E2f2−/− E2f3b−/−) were also obtained at late stages of embryonic development. Given that ablation of all four of these E2fs in mixed strain background result in early embryonic lethality8 (and GL unpublished data), our current findings demonstrate and that expression of at least one of the two E2f3 isoforms is necessary and sufficient to support fetal development in the absence of other E2f activators.
Figure 2.
Genotypic analysis of embryos and offspring deficient for various combinations of activating E2fs. a. Table 2a: Genotypic analysis of offspring deficient for E2f1, E2f2 and E2f3a (3a). b. Table 2b: Genotypic analysis of offspring deficient for E2f1, E2f2 and E2f3b (3b). Numbers in parantheses represent dead embryos/pups. c. Growth curves of E2f1−/− E2f2−/− E2f3a−/− (red) and litter mate control MEFs (grey). Four independent primary MEF lines of each genotype were plated in duplicate and counted every day for six days; a representative experiment is presented in (c). A Generalized Linear Model (GLM) was used to study the association between cell proliferation and group combining the data from all four experiments. The p-value shown in the graph corresponds to the main “group effect” (see Supplementary Methods). d. Growth curves of E2f1−/− E2f2−/− E2f3b−/− (blue) and litter mate control MEFs (grey). Two independent primary MEF lines of each genotype were plated in duplicate and counted every day for six days; the entire experiment was performed twice and a representative experiment is presented in (d). A Generalized Linear Model (GLM) was used to study the association between cell proliferation and group, combining the data from all four experiments. The p-value shown in the graph corresponds to the main “group effect”.
The lack of any obvious proliferative defect in isoform-specific or tko embryos prompted us to examine proliferation more closely in fibroblasts derived from these embryos. To this end, we generated mouse embryo fibroblasts (MEF) from E13.5 E2f3a−/−, E2f3b−/−, E2f1−/− E2f2−/− E2f3a−/− and E2f1−/− E2f2−/− E2f3b−/− embryos and found that all four groups of mutant MEFs had the capacity to proliferate, albeit slightly slower than littermate-derived control MEFs (Fig. 2c-d, Supplementary Fig. 2 and data not shown). Given our previous results showing that a combined deficiency in E2f1, E2f2, E2f3a and E2f3b completely abrogates proliferation of MEFs9, these results suggest a critical and redundant role for the two E2f3 isoforms in the control of proliferation. It would thus appear that expression of either E2f3 isoform is sufficient to support cell proliferation as well as embryonic development.
Next we evaluated the role of E2f activators in postnatal development. Mice deficient for each E2f3 isoform appeared externally normal, were fertile and lived a normal life-span (Fig. 1d, Supplementary Fig. 3 and data not shown). We did note, however, that older E2f3a−/− mice had less white adipose tissue (WAT) deposition than aged-matched E2f3b−/− or wild type mice, lending to their thinner appearance (Fig. 1f and data not shown). Given the observed functional redundancy among E2Fs during embryonic development8, 11, 14, we reasoned that loss of additional E2F activators might accentuate the rather mild age-dependent phenotype in E2f3a−/− mice. Both E2f1−/− and E2f2−/− mice were previously shown to have a relatively normal life span, but with age these mice developed hematopoietic related complications15–19. As tabulated in Fig. 2a, E2f2−/− E2f3a−/− offspring were born at the expected frequency, had normal birth weights and matured without any obvious additional defects. Newborn E2f1−/− E2f3a−/− pups were also of normal weight and appearance, but by their third week of life the proliferative index in most tissues was significantly reduced (Supplementary Fig. 4b) and mice became severely runted (3a-c). The weight of most organs was reduced in double knockout (dko) animals, but this decrease was generally proportional to the decrease in their total body weight (Supplementary Fig. 4a). Gross inspection, however, revealed a total absence of inguinal and subdermal fat (Fig. 3d-e), an observation that was subsequently extended to all WAT deposits in the body. In contrast, brown adipose tissue (BAT) was relatively unaffected. Analysis of food intake and fecal secretion ruled out a defect in eating behavior or fat absorption as a cause for the absence of WAT (Supplementary Fig. 4c). Serum triglycerol levels as well as leptin, a major homeostatic regulator of lipid metabolism, were significantly lower in dko mice than in control animals (Supplementary Fig. 4d), suggesting a more direct role of E2F3a function in lipid metabolism. Not surprisingly most E2f1−/− E2f3a−/− pups died within their first month of life, and the few mice that lived longer were severely incapacitated and could be kept alive only by intensive husbandry (Fig. 3b).
Figure 3.
E2f1 and E2f3a are essential for postnatal development. a. Micrograph of E2f1+/+ E2f3a+/+, E2f1−/− E2f3a−/−, E2f1+/+ E2f3b+/+ and E2f1−/− E2f3b−/− 30 day-old mice. b. Survival graph of mice with the indicated genotypes over a period of one year. c. Body weights of mice with the indicated genotypes at the indicated ages; (E2f1+/+ E2f3+/+), represents mice that were pooled from the E2f1+/+ E2f3a+/+ and E2f1+/+ E2f3b+/+ genetic groups; n, number of animals measured for each genotype. Student t-test was performed on each genetic group in comparison to the wild type group. d. Micrograph of E2f1+/+ E2f3a+/+ and E2f1−/− E2f3a−/− 30 day-old mice with an open abdominal cavity; Inguinal white adipose tissue (WAT) in the wild type mouse is indicated by the white arrows; note the absence of WAT in the mutant mouse. e. H&E staining tissue sections of 21- day old (unless indicated) E2f1+/+ E2f3a+/+ and E2f1−/− E2f3a−/− mice. Top left: skin sections; note the complete absence of WAT beneath the skin of the mutant mouse. Middle left: adrenal gland sections; note the reduction of the adrenal cortex in dko mutant mice. (m), medulla; (c), cortex. Bottom left: ovary sections; note the presence of follicles (f) in both genetic groups but the poor development of corpus lutea (cl) in dko mutant female mice (six months of age). Top right: proximal tibia sections; note the disorganization of nuclei in the growth plate (gr pl) of dko mutant mice; (bm), bone marrow. Middle right: lung sections; note the reduction of alveolar branching in dko mutant mice. Bottom right: testis sections; note the reduction in testis size and lack of spermatocytes. Higher magnification images for each tissue are presented in the bottom panels.
Pathological analysis revealed additional organ defects that could account for the growth retardation, general unhealthy disposition and high morbidity of E2f1−/− E2f3a−/− mice. For example, by 21 days of age, the growth plates in long bones of dko mice were severely dysplastic with many cells having mega nuclei that contributed to poor development of trabeculae (Fig. 3e). This was accompanied by a significant decrease in circulating GH and IGF-1 levels and a relative increase in brain/body weight ratio (Supplementary Fig. 4a and Fig. 4e), providing an interesting parallel to GH deficiency in humans, which causes dwarfism and mainly affects the growth of limbs but not the skull. Moreover, the outer cortex of their adrenal glands lacked a functional zona fasciculata and their lungs were also abnormal, with a decrease in the branching of the epithelium (Fig. 3e). Young mutant males exhibited severe testicular hypoplasia and females had hypoplastic ovaries that lacked a well-developed corpora lutea (Fig. 3e). As a result the few mice that lived past two months of age were infertile. We conclude that multi-organ failure resulted in the general deterioration of health and early death of E2f1−/− E2f3a−/− mice. These results illustrate the critical and redundant roles of E2f1 and E2f3a during postnatal development.
In contrast to E2f1−/−E2f3a−/− mice, E2f1−/− E2f3b−/− and E2f2−/− E2f3b−/− progeny developed normally through puberty and adulthood and had a normal life span (Fig. 3b). While young E2f1−/− E2f3b−/− mice were slightly smaller than wild type controls (Fig. 3a and 3c), thorough histological examination failed to detect any of the mutant phenotypes observed in E2f1−/−E2f3a−/− animals, including in WAT, bone, adrenal glands, lungs, and gonads (data not shown). These observations suggest important differences between E2f3a and E2f3b during postnatal development. Analysis of E2f1−/− E2f2−/− E2f3a−/− and E2f1−/− E2f2−/− E2f3b−/− tko animals also revealed significant differences between the contribution of E2f3a and E2f3b towards postnatal development. While both sets of tko embryos could be carried to late stages of gestation, E2f1−/− E2f2−/− E2f3a−/− embryos died perinatally and E2f1−/− E2f2−/− E2f3b−/− pups lived well into adulthood, albeit their body weight was somewhat reduced (Fig. 2a-b and data not shown). These findings demonstrate that mouse development can proceed in the presence of a single E2f activator, E2f3a.
The different roles of E2f3a and E2f3b during postnatal development could reflect differences in the function of their gene products or differences in the control of their expression20–22. To differentiate between these possibilities we used homologous recombination in mouse ES cells to replace the coding sequence of exon 1a with the coding sequence of exon 1b (E2f3a3bki; Fig. 4a top panel). We were careful to avoid the inadvertent perturbation of regulatory regions that govern expression from the E2f3a locus by leaving the 5′ untranslated region and the 3′ splicing junction of exon 1 intact (Fig. 4b). This strategy resulted in the synthesis of an E2f3a3bki mRNA that was expressed with a similar cell cycle profile as endogenous E2f3a and other known E2F-targets, without affecting the expression of endogenous E2f3b (Fig. 4d). Strikingly, both E2f3a3bki/3bki as well as E2f1−/− E2f3a3bki/3bki mice had normal body weight, WAT deposition, bone structure and growth, adrenal gland morphology and function, as well as normal lung and gonad morphology (Fig. 4h, 4j, Supplementary Fig. 5 and data not shown). We suggest that the differential requirement for E2f3a and E2f3b during postnatal development is not based on intrinsic differences between E2F3a and E2F3b protein functions, but rather on differences between how their loci dictate their respective expression.
Figure 4.

Expression of E2f3b or E2f1 from the E2f3a locus suppresses phenotypes due to loss of E2f3a. a. Targeting strategies for replacement of E2f3a with E2f3b (E2f3a3bki; top schematic) or E2f3a with E2f1 (E2f3a1ki; bottom schematic). Relevant exons are labeled; (E2f1) E2f1 ORF. LoxP sites are indicated as solid triangles; PCR genotyping primers are indicated by thin arrows; primers for measuring the expression of E2f3a3bki and E2f3b alleles by real-time (RT) PCR are indicated by thick arrows. Note that RT primer 1 spans a region that is complementary to exon 1a and exon 1b, as indicated by the red/black colored arrow. b, c. Southern blot (top panels) and PCR (bottom panels) genotyping of genomic DNA derived from livers of one month-old E2f3a3bki (b) and E2f3a1ki (c) mice. d. Real-time PCR gene expression analysis of the E2f3b (left panel) and E2f3a3bki (right panel) alleles in E2f3a+/+ (grey square) or E2f3a+/3bki (red/blue square) MEFs. Primers used for each allele are indicated (for primer sequence information see Supplementary Fig. 6). Note that RT primers used to detect E2f3a3bki also detect endogenous E2f3b allele since the 3′ portion of RT primer 1 is complementary with exon 1b sequences. e. Western blot analysis of E2F1 protein in proliferating MEFs with the indicated genotypes; α-tubulin was detected as a loading control. (*) indicates a non-specific band. f. Western blot analysis of E2F1 proteins in serum stimulated quiescent MEFs having the indicated genotypes; (hrs) indicates the time when cells were harvested post-stimulation. g. Western blot analysis of E2F3a and E2F3b proteins (E2F3 Sc-878) in proliferating MEFs with the indicated genotypes. (*) indicates a non-specific band. h. Body weights of six month-old E2f3a+/+, E2f3a−/−, E2f3a3bki/3bki and E2f3a1ki/1ki mice; n, number of animals measured for each genotype. Student t-test was performed for each genetic group in comparison with labeled group; (*) indicates significant p-values. i. Micrograph of one month-old E2f3a+/+, E2f1−/− E2f3a−/− and E2f1−/− E2f3a1ki/1ki mice. j. H&E staining of tissue sections of skin and bone from 21 day-old mice with the indicated genotypes. Higher magnification images of each tissue are presented in the bottom panels. (WAT), white adipose tissue; (epi), epidermis; (s mus), skeletal muscle; (gr pl), growth plate; (bm), bone marrow.
The above results prompted us to take a similar genetic strategy to determine whether a more distantly related family member, E2f1, could also substitute for E2f3a. Introduction of the murine E2f1 coding sequence into exon 1a of the E2f3a locus (Fig. 4a bottom panel and Fig. 4c) resulted in the cell cycle-dependent expression of E2F1 protein (Fig. 4e-f) without affecting expression from the nearby E2f3b locus (Fig. 4g). Remarkably, E2f3a1ki/1ki as well as E2f1−/− E2f3a1ki/1ki mice lacked any of the phenotypes caused by a deficiency in E2f3a and lived to old age (Fig. 4h-j, Supplementary Fig. 5 and data not shown). Together, these findings suggest that the specific role of E2f3a in postnatal development is largely predicated by regulatory sequences governing its spatiotemporal expression pattern. However, we can not rule out specific functions for individual E2fs in tissues that are not critical for development and that were not analyzed here. These observations provide a molecular basis for the observed specificity among E2f family members during development.
Many reasons have been provided to explain why mammals have an expanded E2F family when worms and flies have a single E2F activator and a single repressor. Extensive genetic and biochemical analysis using cell culture systems has demonstrated that different E2F activators interact with specific cofactors and elicit different biological responses23–25. These studies inspired the view that the complexity of the mammalian E2F family affords a more complex and presumably better equipped organism to develop, but in vivo evidence in support of this hypothesis has been lacking. The in vivo analyses presented here provide new perspectives to this old problem. Our current findings suggest that biological processes regulated by E2F during development in worms, flies and mammals are more alike than previously anticipated since, like in worms and flies, a single E2f activator (E2f3a) is sufficient to support development in the mouse. The surprising observation that E2F3a protein function during postnatal development can be substituted equally well by either the structurally related E2F3b or the more distantly related E2F1 proteins highlights the critical nature of E2f3a regulatory sequences for postnatal development. It would appear that beyond broadening gene expression patterns, the mammalian genome has gained little developmental currency by the acquisition of additional E2f family members. It remains possible that the evolution of multiple E2fs in mammals might represent an adaptation that could serve, beyond simply broadening expression patterns, to meet the challenges faced by aging animals reared in their natural habitat.
Methods
Construction of E2f3a, E2f3b, E2f3a3bki and E2f3a1ki targeting vectors
The E2f3a knockout vector contained a neo cassette flanked by LoxP sites, which was inserted into the HaeII restriction site 108 bp upstream of the E2f3a starting codon. An additional LoxP site was inserted into the HindIII site ∼300 bp downstream of exon 1a. The two arms of homology used for recombination included a total 3.1 kb HindIII-EcoRI fragment upstream of the neo cassette and a 900 bp fragment downstream of the 3′ LoxP site. For the E2f3b knockout targeting vector, the same LoxP–flanked neo cassette was inserted ∼ 800 bp upstream of exon 1b and an additional LoxP site was inserted ∼1.8 kb downstream of exon 1b. The two arms of homology used for recombination included a 1 kb HindIII-HindIII fragment upstream of the neo cassette and a 9 kb EcoRI-XhoI fragment downstream of the 3′ LoxP site. The E2f3a3bkiand E2f3a1ki knockin constructs were identical to the E2f3a knockout construct described above with the following exceptions. For the E2f3a3bki construct, the exon 1b open reading frame (ORF) was replaced with the exon 1a ORF. For the E2f3a1ki construct, the E2f1 ORF carrying its own termination codon was inserted downstream of the first ATG in exon 1a. All the final targeting vectors were confirmed by direct DNA sequencing.
Fat absorption assay
Fat absorption assays were performed on one month-old mice at the mouse metabolic phenotyping center at the University of Cincinnati as described previously1.
Blood serum analysis
Mice at 21 days of age were sacrificed and serum was collected from blood by centrifugation at 6000xg. The serum was then stored at -80°C until further analysis. Triglycerides (TG), cholesterol (Ch) and leptin (Lep) were analyzed by the Mouse Metabolic Phenotyping Center at the University of Cincinnati. Analysis of serum growth hormone (GH) and Insulin-like growth factor 1 (IGF-1) was performed at the Vanderbilt hormone assays & analytical core services.
Generation of MEFs and cell culture conditions
Primary MEFs were isolated from E13.5 embryos using standard methods2. MEFs were cultured in DMEM with 15% fetal bovine serum (FBS). Proliferation assays were performed by plating MEFs at 1.45 x 105 cells per 60-mm dish. Duplicate plates were counted daily using a Beckton Dickson Coulter Counter and were re-plated 72 hours after at the same initial density. Four independent E2f1-/- E2f2-/- E2f3a-/- MEF lines and two independent E2f1-/- E2f2-/- E2f3b-/- MEF lines with their control littermate were used in the proliferation assays. Statistical analysis was performed by pooling all experiments.
Protein and RNA analysis
Cells were scraped from culture dishes in chilled PBS, centrifuged, and washed once with ice-cold PBS. Total protein extracts were prepared by incubating cells in RIPA extraction buffer for 30 min on ice. Total protein was then separated by SDS-PAGE and transferred to PVDF membranes. Blots were probed with antibodies specific for E2F3 (Santa Cruz sc878) or E2F1 (Santa Cruz sc193); anti-α-tubulin (Sigma T6199) was used to determine protein loading. Total RNA was extracted from MEFs using Qiagen Rneasy mini kit. Reverse transcription of 2 μg of total RNA was performed by combining 1 μl of Superscript III reverse transriptase (Invitrogen), 4 μl of 10X buffer, 0.5 μl of 100 mM oligo dT primer, 0.5 μl of 25 mM dNTPs, 1.0 μl of 0.1 M DTT, 1.0 μl of RNAse Inhibitor (Roche) and water up to a volume of 20 μl. Reactions were incubated at 50°C for 60 minutes and then diluted 5 fold with 80 μl of water. Real-time RT-PCR was performed using the BioRad iCycler PCR machine. Each PCR reaction contained 0.5 μl of cDNA template and primers at a concentration of 100 nM in a final volume of 25 μl of SYBR Green Reaction Mix (BioRad). Each PCR reaction yielded only the expected amplicon as shown by the melting-temperature profiles of the final products and by gel electrophoresis. Standard curves were generated using cDNA to determine the linear range and PCR efficiency of each primer pair. Reactions were performed in triplicate and relative amounts of cDNA were normalized to GAPDH. Primer sequences are listed in Supplementary Fig 6.
Histopathology and immunohistochemistry
Tissue samples were collected and fixed in 10% neutral formalin. 5 μm thick sections were cut and then stained for with H&E by standard protocols. Immunohistochemistry using Ki-67 specific antibodies (BD Pharmingen 550609) was performed on paraffin embedded sections.
Statistical analysis
For the proliferation assays in MEFs, a Generalized Linear Model (GLM) was used to study the association between the outcome variable and group. Time was used as a categorical variable, and the interaction “time” by *group” was also included in the model. A fixed effect “experiment” was included to take into accounts the differences (or variability) among experiments.
Supplementary Material
Figure 1 a, Partial exon/intron structure of the mouse E2f3 gene. The schematic illustrates the E2f3 locus with two separate promoters driving the expression of E2f3a and E2f3b. The two transcription start sites are indicated by bent arrows. The targeting vectors and the final E2f3a and E2f3b targeted alleles are shown on the left and right, respectively. The solid bar represents a HindIII-BamHI fragment used as the Southern probe. The loxP sites are indicated as solid triangles; PCR primers are indicated by arrows. b, Southern blot (top panels) and PCR genotyping assays (bottom panels) were performed on MEFs with the indicated genotypes. c, Western blot analysis using E2F3-specific antibodies was performed on lysates derived from MEFs with the indicated genotypes. d, Micrograph of 1-month-old mice with the indicated genotypes. e, Genotypic analysis of offspring derived from E2f3+/−, E2f3a+/− and E2f3b+/− intercrosses (fifth generation FVB). A Fisher exact probability test was performed on each genetic group in comparison to the wild-type group; highly significant results are indicated by an asterisk. f, Body weights of E2f3+/+ (grey), E2f3a−/− (red) and E2f3b−/− (blue) mice at the indicated ages; n, number of animals measured for each genotype at each age; error bars represent deviations within each genetic group. A Student's t-test was performed on each genetic group in comparison to the wild-type group. Highly significant results are indicated by an asterisk.
Figure 2 a, Genotypic analysis of offspring deficient for E2f1, E2f2 and E2f3a (3a); highly significant results are indicated by an asterisk. Numbers in parentheses represent dead embryos/pups. b, Genotypic analysis of offspring deficient for E2f1, E2f2 and E2f3b (3b). c, Growth curves of E2f1−/− E2f2−/− E2f3a−/− (red) and littermate control MEFs (grey). Four independent primary MEF lines of each genotype were plated in duplicate and counted every day for 6 days; a representative experiment is presented in c, where the error bars represent deviations between duplicate measurements within the same experiment. d, Growth curves of E2f1−/− E2f2−/− E2f3b−/− (blue) and littermate control MEFs (grey). Two independent primary MEF lines of each genotype were plated in duplicate and counted every day for 6 days; the entire experiment was performed twice and a representative experiment is presented in d, where the error bars represent deviations between duplicate measurements within the same experiment. For the cell proliferation studies in c, d, a generalized linear model (GLM) was used to study the association between cell proliferation and group behaviour, combining the data from all experiments in each genetic study. The P-value shown in the graphs corresponds to the main ‘group effect’ (see Methods).
Figure 3 a, Micrograph of E2f1+/+ E2f3a+/+, E2f1−/− E2f3a−/−, E2f1+/+ E2f3b+/+ and E2f1−/− E2f3b−/− 30-day-old mice. b, Survival graph of mice with the indicated genotypes over a period of 1 year. c, Body weight of mice with the indicated genotypes and ages; E2f1+/+ E2f3+/+ represent mice that were pooled from the E2f1+/+ E2f3a+/+ and E2f1+/+ E2f3b+/+ genetic groups; n, number of animals measured for each genotype; error bars represent deviations within each genetic group. A Student's t-test was performed on each genetic group in comparison to the wild-type group. Significant and highly significant results are indicated by a single and double asterisk, respectively. d, Micrograph of E2f1+/+ E2f3a+/+ and E2f1−/− E2f3a−/− 30-day-old mice with an open abdominal cavity; Inguinal WAT in the wild-type mouse is indicated by the white arrows; note the absence of WAT in the mutant mouse. e, Haematoxylin and eosin stained tissue sections of 21-day-old (unless indicated) E2f1+/+ E2f3a+/+ and E2f1−/− E2f3a−/− mice. Top left: skin sections; note the complete absence of WAT beneath the skin of the mutant mouse. Middle left: adrenal gland sections; note the reduction of the adrenal cortex in double knockout mutant mice. Bottom left: ovary sections; note the presence of follicles (f) in both genetic groups but the poor development of corpus lutea (cl) in double knockout mutant female mice (6 months of age). Top right: proximal tibia sections; note the disorganization of nuclei in the growth plate (gr pl) of double knockout mutant mice. Middle right: lung sections; note the reduction of alveolar branching in double knockout mutant mice. Bottom right: testis sections; note the reduction in testis size and lack of spermatocytes. Scale bars, 200 μm. bm, bone marrow; c, cortex; m, medulla.
Figure 4 a, Targeting strategies for replacement of E2f3a with E2f3b (E2f3a3bki; top schematic) or E2f3a with E2f1 (E2f3a1ki; bottom schematic). Relevant exons are labelled; E2f1 indicates the E2f1 ORF. loxP sites are indicated as solid triangles; PCR genotyping primers are indicated by thin arrows; primers for measuring the expression of E2f3a3bki and E2f3b alleles by real-time (RT) PCR are indicated by thick arrows. Note that RT primer 1 spans a region that is complementary to exon 1a and exon 1b, as indicated by the red/black coloured arrow. b, c, Southern blot (top panels) and PCR (bottom panels) genotyping of genomic DNA derived from livers of 1-month-old E2f3a3bki (b) and E2f3a1ki (c) mice. d, Real-time PCR gene expression analysis of the E2f3b (left panel) and E2f3a3bki (right panel) alleles in E2f3a+/+ (grey square) or E2f3a+/3bki (red/blue square) MEFs. Primers used for each allele are indicated (for primer sequence information see Supplementary Fig. 6). Note that RT primers used to detect E2f3a3bki also detect endogenous E2f3b allele as the 3′ portion of RT primer 1 is complementary with exon 1b sequences. Error bars are derived from reactions performed in triplicate. e, Western blot analysis of E2F1 protein in proliferating MEFs with the indicated genotypes; α-tubulin was detected as a loading control. Asterisk indicates a nonspecific band. f, Western blot analysis of E2F1 proteins in serum stimulated quiescent MEFs having the indicated genotypes; ‘h’ indicates the time when cells were harvested after stimulation. g, Western blot analysis of E2F3a and E2F3b proteins (E2F3 Sc-878) in proliferating MEFs with the indicated genotypes. Asterisk indicates a nonspecific band. h, Body weights of 6-month-old E2f3a+/+, E2f3a-/-, E2f3a3bki/3bki and E2f3a1ki/1ki mice; n, number of animals measured for each genotype; error bars represent deviations within each genetic group. Student's t-tests were performed between all genetic groups and only significant pair-wise comparisons between groups are indicated by brackets (P-values). i, Micrograph of 1-month-old E2f3a+/+, E2f1−/− E2f3a−/− and E2f1−/− E2f3a1ki/1ki mice. j, Haematoxylin and eosin staining of tissue sections of skin and bone from 21-day-old mice with the indicated genotypes. Scale bars, 200 μm. bm, bone marrow; epi, epidermis; gr pl, growth plate; s mus, skeletal muscle.
Table S1: Viability of E2f3−/− mice in different strain backgrounds
Table S8: Primer sequences
Acknowledgments
We thank J. Moffitt and L. Rawahneh for histology expertise. We also thank J. Nevins, Cheryl Bock and A. Otoshi for support in the generation of the E2f3a, E2f3b, E2f3a3bki and E2f3a1ki mice and the Mouse Metabolic Phenotyping center at the University of Cincinnati for advice on the analysis of E2f1−/− E2f3a−/− mice. We are grateful to D. Guttridge, M. Ostrowski and M. Simcox for critically reading the manuscript and helpful suggestions. This work was funded by NIH grants to G.L. (R01CA85619, R01HD042619, R01CA121275, R01HD047470, P01CA097189), to L.W. (K01CA102328), DoD awards to A.d.B. (BC0300893) and J-L.C. (BC061730), and a T32 fellowship (CA106196) to R.O. G.L. is the recipient of the Pew Charitable Trusts Scholar Award and the Leukemia and Lymphoma Society Scholar Award.
References
- 1.Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- 2.Attwooll C, Lazzerini Denchi E, Helin K. The E2F family: specific functions and overlapping interests. Embo J. 2004;23:4709–16. doi: 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceol CJ, Horvitz HR. dpl-1 DP and efl-1 E2F act with lin-35 Rb to antagonize Ras signaling in C. elegans vulval development. Mol Cell. 2001;7:461–73. doi: 10.1016/s1097-2765(01)00194-0. [DOI] [PubMed] [Google Scholar]
- 4.Dynlacht BD, Brook A, Dembski M, Yenush L, Dyson N. DNA-binding and trans-activation properties of Drosophila E2F and DP proteins. Proc Natl Acad Sci U S A. 1994;91:6359–63. doi: 10.1073/pnas.91.14.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawado T, et al. dE2F2, a novel E2F-family transcription factor in Drosophila melanogaster. Biochem Biophys Res Commun. 1998;251:409–15. doi: 10.1006/bbrc.1998.9407. [DOI] [PubMed] [Google Scholar]
- 6.DeGregori J, Johnson DG. Distinct and Overlapping Roles for E2F Family Members in Transcription, Proliferation and Apoptosis. Curr Mol Med. 2006;6:739–48. doi: 10.2174/1566524010606070739. [DOI] [PubMed] [Google Scholar]
- 7.Helin K, et al. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992;70:337–50. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, et al. The E2F1-3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–62. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 9.Humbert PO, et al. E2f3 is critical for normal cellular proliferation. Genes Dev. 2000;14:690–703. [PMC free article] [PubMed] [Google Scholar]
- 10.Leone G, et al. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol Cell Biol. 2000;20:3626–32. doi: 10.1128/mcb.20.10.3626-3632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, et al. Synergistic function of E2F7 and E2F8 is essential for cell survival and embryonic development. Dev Cell. 2008;14:62–75. doi: 10.1016/j.devcel.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giangrande PH, et al. A role for E2F6 in distinguishing G1/S- and G2/M-specific transcription. Genes Dev. 2004;18:2941–51. doi: 10.1101/gad.1239304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloud JE, et al. Mutant mouse models reveal the relative roles of E2F1 and E2F3 in vivo. Mol Cell Biol. 2002;22:2663–72. doi: 10.1128/MCB.22.8.2663-2672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaubatz S, et al. E2F4 and E2F5 play an essential role in pocket protein-mediated G1 control. Mol Cell. 2000;6:729–35. doi: 10.1016/s1097-2765(00)00071-x. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki L, et al. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–48. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 16.Murga M, et al. Mutation of E2F2 in mice causes enhanced T lymphocyte proliferation, leading to the development of autoimmunity. Immunity. 2001;15:959–70. doi: 10.1016/s1074-7613(01)00254-0. [DOI] [PubMed] [Google Scholar]
- 17.Field SJ, et al. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–61. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhu JW, et al. E2F1 and E2F2 determine thresholds for antigen-induced T-cell proliferation and suppress tumorigenesis. Mol Cell Biol. 2001;21:8547–64. doi: 10.1128/MCB.21.24.8547-8564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li FX, Zhu JW, Hogan CJ, DeGregori J. Defective gene expression, S phase progression, and maturation during hematopoiesis in E2F1/E2F2 mutant mice. Mol Cell Biol. 2003;23:3607–22. doi: 10.1128/MCB.23.10.3607-3622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opavsky R, et al. Specific tumor suppressor function for E2F2 in Myc-induced T cell lymphomagenesis. Proc Natl Acad Sci U S A. 2007;104:15400–5. doi: 10.1073/pnas.0706307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dirlam A, Spike BT, Macleod KF. Deregulated E2f-2 underlies cell cycle and maturation defects in retinoblastoma null erythroblasts. Mol Cell Biol. 2007;27:8713–28. doi: 10.1128/MCB.01118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parisi T, et al. Selective requirements for E2f3 in the development and tumorigenicity of Rb-deficient chimeric tissues. Mol Cell Biol. 2007;27:2283–93. doi: 10.1128/MCB.01854-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blais A, Dynlacht BD. E2F-associated chromatin modifiers and cell cycle control. Curr Opin Cell Biol. 2007;19:658–62. doi: 10.1016/j.ceb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giangrande PH, Hallstrom TC, Tunyaplin C, Calame K, Nevins JR. Identification of E-box factor TFE3 as a functional partner for the E2F3 transcription factor. Mol Cell Biol. 2003;23:3707–20. doi: 10.1128/MCB.23.11.3707-3720.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlisio S, Halperin T, Vidal M, Nevins JR. Interaction of YY1 with E2Fs, mediated by RYBP, provides a mechanism for specificity of E2F function. Embo J. 2002;21:5775–86. doi: 10.1093/emboj/cdf577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 a, Partial exon/intron structure of the mouse E2f3 gene. The schematic illustrates the E2f3 locus with two separate promoters driving the expression of E2f3a and E2f3b. The two transcription start sites are indicated by bent arrows. The targeting vectors and the final E2f3a and E2f3b targeted alleles are shown on the left and right, respectively. The solid bar represents a HindIII-BamHI fragment used as the Southern probe. The loxP sites are indicated as solid triangles; PCR primers are indicated by arrows. b, Southern blot (top panels) and PCR genotyping assays (bottom panels) were performed on MEFs with the indicated genotypes. c, Western blot analysis using E2F3-specific antibodies was performed on lysates derived from MEFs with the indicated genotypes. d, Micrograph of 1-month-old mice with the indicated genotypes. e, Genotypic analysis of offspring derived from E2f3+/−, E2f3a+/− and E2f3b+/− intercrosses (fifth generation FVB). A Fisher exact probability test was performed on each genetic group in comparison to the wild-type group; highly significant results are indicated by an asterisk. f, Body weights of E2f3+/+ (grey), E2f3a−/− (red) and E2f3b−/− (blue) mice at the indicated ages; n, number of animals measured for each genotype at each age; error bars represent deviations within each genetic group. A Student's t-test was performed on each genetic group in comparison to the wild-type group. Highly significant results are indicated by an asterisk.
Figure 2 a, Genotypic analysis of offspring deficient for E2f1, E2f2 and E2f3a (3a); highly significant results are indicated by an asterisk. Numbers in parentheses represent dead embryos/pups. b, Genotypic analysis of offspring deficient for E2f1, E2f2 and E2f3b (3b). c, Growth curves of E2f1−/− E2f2−/− E2f3a−/− (red) and littermate control MEFs (grey). Four independent primary MEF lines of each genotype were plated in duplicate and counted every day for 6 days; a representative experiment is presented in c, where the error bars represent deviations between duplicate measurements within the same experiment. d, Growth curves of E2f1−/− E2f2−/− E2f3b−/− (blue) and littermate control MEFs (grey). Two independent primary MEF lines of each genotype were plated in duplicate and counted every day for 6 days; the entire experiment was performed twice and a representative experiment is presented in d, where the error bars represent deviations between duplicate measurements within the same experiment. For the cell proliferation studies in c, d, a generalized linear model (GLM) was used to study the association between cell proliferation and group behaviour, combining the data from all experiments in each genetic study. The P-value shown in the graphs corresponds to the main ‘group effect’ (see Methods).
Figure 3 a, Micrograph of E2f1+/+ E2f3a+/+, E2f1−/− E2f3a−/−, E2f1+/+ E2f3b+/+ and E2f1−/− E2f3b−/− 30-day-old mice. b, Survival graph of mice with the indicated genotypes over a period of 1 year. c, Body weight of mice with the indicated genotypes and ages; E2f1+/+ E2f3+/+ represent mice that were pooled from the E2f1+/+ E2f3a+/+ and E2f1+/+ E2f3b+/+ genetic groups; n, number of animals measured for each genotype; error bars represent deviations within each genetic group. A Student's t-test was performed on each genetic group in comparison to the wild-type group. Significant and highly significant results are indicated by a single and double asterisk, respectively. d, Micrograph of E2f1+/+ E2f3a+/+ and E2f1−/− E2f3a−/− 30-day-old mice with an open abdominal cavity; Inguinal WAT in the wild-type mouse is indicated by the white arrows; note the absence of WAT in the mutant mouse. e, Haematoxylin and eosin stained tissue sections of 21-day-old (unless indicated) E2f1+/+ E2f3a+/+ and E2f1−/− E2f3a−/− mice. Top left: skin sections; note the complete absence of WAT beneath the skin of the mutant mouse. Middle left: adrenal gland sections; note the reduction of the adrenal cortex in double knockout mutant mice. Bottom left: ovary sections; note the presence of follicles (f) in both genetic groups but the poor development of corpus lutea (cl) in double knockout mutant female mice (6 months of age). Top right: proximal tibia sections; note the disorganization of nuclei in the growth plate (gr pl) of double knockout mutant mice. Middle right: lung sections; note the reduction of alveolar branching in double knockout mutant mice. Bottom right: testis sections; note the reduction in testis size and lack of spermatocytes. Scale bars, 200 μm. bm, bone marrow; c, cortex; m, medulla.
Figure 4 a, Targeting strategies for replacement of E2f3a with E2f3b (E2f3a3bki; top schematic) or E2f3a with E2f1 (E2f3a1ki; bottom schematic). Relevant exons are labelled; E2f1 indicates the E2f1 ORF. loxP sites are indicated as solid triangles; PCR genotyping primers are indicated by thin arrows; primers for measuring the expression of E2f3a3bki and E2f3b alleles by real-time (RT) PCR are indicated by thick arrows. Note that RT primer 1 spans a region that is complementary to exon 1a and exon 1b, as indicated by the red/black coloured arrow. b, c, Southern blot (top panels) and PCR (bottom panels) genotyping of genomic DNA derived from livers of 1-month-old E2f3a3bki (b) and E2f3a1ki (c) mice. d, Real-time PCR gene expression analysis of the E2f3b (left panel) and E2f3a3bki (right panel) alleles in E2f3a+/+ (grey square) or E2f3a+/3bki (red/blue square) MEFs. Primers used for each allele are indicated (for primer sequence information see Supplementary Fig. 6). Note that RT primers used to detect E2f3a3bki also detect endogenous E2f3b allele as the 3′ portion of RT primer 1 is complementary with exon 1b sequences. Error bars are derived from reactions performed in triplicate. e, Western blot analysis of E2F1 protein in proliferating MEFs with the indicated genotypes; α-tubulin was detected as a loading control. Asterisk indicates a nonspecific band. f, Western blot analysis of E2F1 proteins in serum stimulated quiescent MEFs having the indicated genotypes; ‘h’ indicates the time when cells were harvested after stimulation. g, Western blot analysis of E2F3a and E2F3b proteins (E2F3 Sc-878) in proliferating MEFs with the indicated genotypes. Asterisk indicates a nonspecific band. h, Body weights of 6-month-old E2f3a+/+, E2f3a-/-, E2f3a3bki/3bki and E2f3a1ki/1ki mice; n, number of animals measured for each genotype; error bars represent deviations within each genetic group. Student's t-tests were performed between all genetic groups and only significant pair-wise comparisons between groups are indicated by brackets (P-values). i, Micrograph of 1-month-old E2f3a+/+, E2f1−/− E2f3a−/− and E2f1−/− E2f3a1ki/1ki mice. j, Haematoxylin and eosin staining of tissue sections of skin and bone from 21-day-old mice with the indicated genotypes. Scale bars, 200 μm. bm, bone marrow; epi, epidermis; gr pl, growth plate; s mus, skeletal muscle.
Table S1: Viability of E2f3−/− mice in different strain backgrounds
Table S8: Primer sequences