Aging-related Arterial Stiffening and Hypertension
Although the etiology of essential hypertension remains unknown, it is clear that multiple factors may contribute to the pathogenesis of hypertension. Hypertension is an outcome of the interaction of multiple genetic and environmental factors. Several epidemiological studies indicated that the incidence of arterial stiffness and hypertension and related cardiovascular disease (stroke, myocardial infarction) is higher in the aged than in the young population.1–4 The prevalence of arterial stiffening and hypertension increases with age.1, 5 Based on an epidemiological study,6 the prevalence of hypertension is more than doubled in the elderly than in the young population. More than two-thirds of individuals after age 65 years suffer from hypertension according to the Seventh Report of the Joint National Committee (JNC-7).7 Therefore, it is generally believed that hypertension is an aging disorder. In recent years, metabolic syndrome and hypertension are increasingly seen in the middle-aged and young populations. In these sub-populations, insulin resistance and overproduction of adipokines impair endothelial and heart function leading to early and accelerated cardiovascular aging. It was reported that premature aging (progeria) is associated with accelerated vascular stiffening or vascular aging.8
Aging is defined as the age-related decline in physiological function essential for survival and fertility. Cardiovascular aging is an important factor that determines lifespan. The wall of large conduit arteries, especially aorta, thicken and lose elasticity over time and this process results in an increase in pulse wave velocity (PWV), an important and reliable measure of arterial stiffness. The increased arterial stiffness, whatever its underlying causes, would reduce the reservoir/buffering function of the conduit arteries near the heart and increase PWV, both of which increase systolic and pulse pressure. Therefore, aging-related hypertension is characterized by a significant increase in systolic blood pressure with no change or even a decrease in diastolic blood pressure, namely isolated systolic hypertension (ISH). Age is an important determinant of PWV.1 Arterial stiffening is an independent predictor of cardiovascular (CV) outcomes such as myocardial infarction, cognitive decline in aging, stroke, and kidney diseases.9–12 In a longitudinal community-based cohort study conducted in Framingham, Massachusetts, Kaess et al showed that increased aortic stiffness and augmentation are associated with higher risk of incident hypertension.13 However, initial blood pressure is not independently associated with the risk of progressive aortic stiffening. Therefore, arterial stiffness predicts an increase in systolic blood pressure and incident hypertension.13 These observations indicate a close relationship between aortic stiffening and the development of hypertension in human subjects. A recent report indicates that arterial stiffening precedes the development of hypertension in an animal model of high fat diet-induced hypertension.14 Current antihypertensive drugs were mainly designed to reduce peripheral resistance and are not adequate to alter the pathological process of vascular stiffening or even selectively reduce systolic BP in isolated systolic hypertension (ISH). This review updates the recent advances in the mechanism of aging-related arterial stiffening and hypertension based on the papers published in Hypertension in the last 2–3 years.
Metabolic Syndrome Contributes to Aging-related Arterial Stiffening and Hypertension
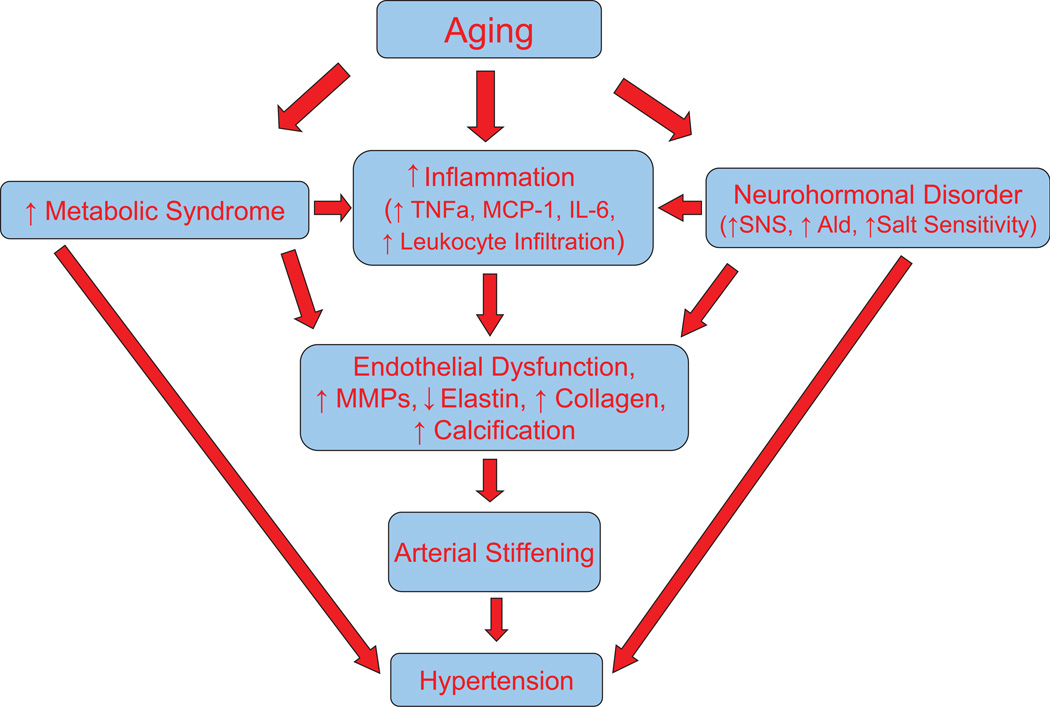
With aging, the prevalence of metabolic syndrome, an important risk factor for cardiovascular disease, is increased although the underlying mechanism is not fully understood. Metabolic syndrome is defined by three or more of the following characteristics: abnormal obesity, dyslipidemia, hypertension, insulin resistance, and hyperglycemia. Age induces an increase in visceral fat and circulating leptin which is associated with a significant increase in blood pressure.15 Numerous studies indicated that metabolic syndrome promotes arterial stiffening and accelerates vascular aging and development of hypertension in humans.5, 16–18 A recent report showed that body fat is associated with reduced arterial stiffening until middle age,19 indicating that the vascular system may be capable of adapting to obesity and that an adverse association between body fat and arterial stiffening only appears in later life. Therefore, aging may promote metabolic syndrome-induced arterial stiffening (Fig. 1). A longitudinal study showed that clustering of metabolic syndrome is associated with maladaptive carotid remodeling and stiffening.17 Notably, a recovery from the metabolic syndrome restored carotid properties to normal levels, indicating that metabolic syndrome-induced arterial remodeling and stiffening is reversible.17 In an animal model of diet-induced obesity, normalization of the metabolic state by weight loss leads to a return of arterial stiffness and high blood pressure to normal,14 emphasizing that metabolic syndrome is a potential interventional target for arterial stiffening and hypertension. Hyperglycemia and dyslipidemia cause vascular endothelial dysfunction and oxidative stress which activates extracellular matrix metalloproteinases (MMPs) leading to vascular remodeling and arterial stiffening.
Figure 1. A diagram of mechanistic link of aging, arterial stiffening and hypertension.
SNS, sympathetic nervous system; Ald, aldosterone; MMP, matrix metalloproteinase; MCP-1, Monocyte chemotactic protein-1; TNFα, tumor necrosis factor; IL-6, interleukin-6;
Arterial stiffening, at least, reflects gradual fragmentation and loss of elastin fibers and accumulation of stiffer collagen fibers in the media of large arteries, and occurs independently of atherosclerosis. The arterial compliance is determined by the ratio of elastin and collagen. Aging is associated with a decreased ratio of elastin/collagen which is due to, in part, an enhanced degradation of elastin and/or increased accumulation of stiffer collagen. Elastin degradation is associated with progressive aortic stiffening and all-cause mortality.20 Upregulation of MMPs may be involved in aging-associated elastin fragmentation and collagen deposition (Fig. 1).21 Inhibition of MMPs results in preservation of intact elastin fibers, abolishes collagen deposition, and blunts an age-associated increase in arterial blood pressure.21 Higher serum levels of MMP2, cathepsin S, and elastin-derived peptides are independently associated with baseline PWV and changes in arterial stiffness in patients with chronic kidney disease.20 A recent study indicated that the expression and activity of MMP2 may be regulated by calpain-1.2 Overexpression of calpain-1 activates MMP2 resulting in elastin degradation and increased production of collagen I and III.2 Calpain-1 also induces TGFβ1/Smad signaling and alkaline phosphatase activation and increases total calcium content but reduces the expression of calcification inhibitors, osteopontin, and osteonectin, in cultured SMCs in vitro and arterial rings ex vivo. The cross-talk of calpain-1 and MMP2 leads to secretion of active MMP2 which remodels extracellular matrix via increasing collagen deposition and vascular calcification,2 resulting in increased arterial stiffness. Calpain-1 levels are upregulated with aging within human aortic intima, establishing it as a potential molecular candidate to attenuate age-associated extracellular matrix remodeling and hypertension.
Inflammation Mediates Aging-related Arterial Stiffening and Hypertension
Inflammation is increased with aging. An increase in inflammatory cytokines and chemokines leads to infiltrations of T cells and macrophages which could cause tissue injury. Overactive sympathetic nervous system causes inflammatory responses. Aging is associated with elevated sympathetic nervous activity22 which may contribute to increased inflammation in aging.23 Aldosterone is known to cause inflammatory responses and T cell infiltration. Aging is associated with dysregulation of aldosterone24 which may play a role in aging-related inflammation. It has been reported that metabolic syndrome is linked to increased inflammation (Fig. 1).25–26 Obesity is associated with chronic inflammatory responses including abnormal adipokine production, increased release of proinflammatory cytokines and chemokines, and macrophage and lymphocyte infiltration.26 These inflammatory responses in adipose tissue may contribute to the pathogenesis of obesity and obesity-induced vascular endothelial dysfunction and arterial stiffening. T regulatory lymphocytes suppress inflammatory responses and attenuate hypertension-associated organ damage. It was reported that high fructose diet-induced metabolic syndrome decreased the number and function of the T regulatory lymphocytes,27 which may promote metabolic syndrome-associated arterial stiffening and hypertension.
The mechanistic role of immune activation and inflammation in the pathogenesis of hypertension is increasingly appreciated based on a recent comprehensive review.28 Several reports indicate that autoimmunity and inflammation may be associated with hypertension.28–29 Anti-CD20 antibody treatment prevents the development of hypertension in a mouse model of lupus.29 Anti-CD20 antibody blunts the upregulation of TNFα and MCP-1 in renal cortices and attenuates the increase in circulating T cells in mice with lupus, contributing to the antihypertensive effect of anti-CD20.29 This study also suggests a new and interesting role of autoantibody in regulating cytokine release and T cell migration although the mechanistic link remains to be resolved. Cold exposure causes pulmonary hypertension which is preceded by the upregulation of TNFα in pulmonary arteries and lungs.30 RNAi inhibition of TNFα prevents infiltration of T cells and macrophages and attenuates cold-induced pulmonary hypertension and pulmonary arterial remodeling,30 suggesting that TNFα is a critical cytokine that initiates cold-induced pulmonary vascular damage. It has been shown that T cell infiltration and inflammation are involved in angiotensin II-induced hypertension and vascular dysfunction.28 Genetic deletion of T cells (RAG−/−) prevents angiotensin II-induced hypertension, suggesting an important role of T cells in this model of hypertension.28 Inflammation and mechanical stretch promote collagen deposition and arterial stiffening via activation of p38 mitogen-activated protein kinase.31 A recent study showed that cardiotrophin 1 (CT-1), a cytokine of the interleukin-6 family, may be involved in aging-related arterial stiffening.32 CT-1 is a potent fibrotic factor in heart, vessels and kidneys.33 Genetic deletion of CT-1 leads to decreased arterial fibrosis, stiffness, and senescence and increases lifespan (longevity) in aging mice likely via downregulation of apoptosis and inflammatory pathways.32 Therefore, inflammation may play a critical role in aging-related vascular remodeling, arterial stiffening and hypertension (Fig. 1) although the underlying mechanism remains to be determined.
Neurohumoral Dysfunction Plays a Role in Aging-related Vascular Dysfunction and Hypertension
The autonomic nervous system is critical in the regulation of peripheral resistance and blood pressure. Metabolic syndrome is associated with increased sympathetic overactivity.34 Insulin resistance and dysregulated adipokine production in obesity-related metabolic syndrome leads to sympathetic activation contributing to metabolic syndrome-induced hypertension.34 The sympathetic nervous activity is positively associated with arterial blood pressure in women after menopause.22 The autonomic support of arterial blood pressure is greater in older women than in young women. The sympathetic nerve activity is elevated in older women which contributes to the increased incidence of hypertension in aging women.22. The aging-associated increase in sympathetic activity is likely due to blunted baroreflex sensitivity. Large artery stiffness (carotid artery and aortic arch) is associated with an age-related decrease in sympathetic baroreflex sensitivity in elderly men and women.35 This is mainly due to changes in the mechanical property (e.g., loss of elasticity) of the large barosensory arteries as a result of stiffening. The diminished sympathetic baroreflex sensitivity in the elderly leads to sympathetic activation and increased prevalence of hypertension in the aged population (Fig. 1).35 Increased inflammation in brain stem (e.g., NTS) is causally associated with neurogenic hypertension.36 It was reported that senescence-associated brain structural and functional deterioration may also contribute to aging-related autonomic dysfunction (e.g., elevated sympathetic activity) and hypertension.37
It is known that salt sensitivity and vascular endothelial dysfunction are increased in the aging population (Fig. 1). Salt intake is one of the main environmental factors contributing to the development of hypertension. Salt sensitivity is more common in the old than in the young population. The relationship of age and salt sensitivity appears to be stronger in hypertensive than in normotensive individuals.38 Recent studies suggested that overactivation of the innate or adaptive immune system leads to renal inflammation which contributes to salt sensitivity.28 Functional T cells are required for the development of Dahl salt-sensitive hypertension.39 Inhibition of T cell and macrophage infiltration in kidneys attenuates high salt-induced hypertension in mice with haplodeficiency of an anti-aging gene klotho.40 Therefore, aging-related salt sensitivity may be mediated by increased inflammation. Interestingly, the vascular endothelial expression of sodium channel (EnNaC) increases with age.41 Spironolactone and amiloride decreased EnNaC abundance and prevents endothelial stiffening in aging mice.41 Therefore, upregulation of EnNaC mediates aging-associated endothelial salt sensitivity which contributes to vascular stiffening in aging. Aging is also associated with dysregulation of aldosterone and its receptors24 which impair vascular function leading to vascular remodeling and hypertension.
Aging-related endothelial dysfunction and the resultant vascular remodeling and stiffening may be due to decreased nitric oxide (NO) bioavailability because of eNOS dysfunction and eNOS uncoupling.42–44 Short-term treatment with a lipid-lowering agent fenofibrate, improves endothelium-dependent vasodilation in older adults by reducing oxidative stress and increasing eNOS.43 A decrease in NO leads to decreased intracellular cGMP levels contributing to arterial remodeling. Upregulation of phosphodiesterases that decomposes cGMP also decreases intracellular cGMP levels. Inhibition of phosphodiesterase 5 increases bioavailability of intracellular cGMP which results in vasodilation and attenuation of hypertension and kidney damage.45 It was recently reported that upregulation of PDE1C may be involved in cold-induced pulmonary hypertension and pulmonary arterial remodeling.46 Inhibition of PDE1 eliminated cold-induced macrophage infiltration, NADPH oxidase activation, and superoxide production and reversed pulmonary arterial remodeling.46
Aging-related Genes and Hypertension: A Perspective
Aging is an independent risk factor for arterial stiffness and hypertension.1–5 Arterial stiffening and hypertension are aging-related disorders. Therefore, it would be interesting to assess if anti-aging treatment slows and attenuates vascular dysfunction and hypertension. Klotho gene was identified as an aging-suppressor gene which extends lifespan when overexpressed and shortens lifespan when disrupted.47 In humans, the prevalence of hypertension increases with age while the klotho level declines with age after the age of 40 years. High levels of klotho are independently associated with lower likelihood of having hypertension and related cardiovascular disease. The development and progression of genetic hypertension is also age-dependent in spontaneous hypertensive rats (SHRs). In SHRs, klotho gene expression was downregulated while blood pressure is elevated.48 Interestingly, in vivo expression of klotho gene prevented progression of hypertension and abolished kidney damages in SHRs,48 suggesting that klotho may be involved in the pathogenesis of spontaneous hypertension. In vivo expression of klotho abolished the downregulation of IL-10, an anti-inflammatory cytokine, and the upregulation of Nox2 expression, NADPH oxidase activity, and superoxide production in aortas and kidneys of SHRs.48 These findings suggest that anti-aging gene klotho protects against cardiovascular aging via inhibition of inflammation and oxidative stress. It seems equally important to assess if disruption of anti-aging gene klotho affects blood pressure. In a recent study, Zhou et al demonstrated that haplodeficiency of klotho gene (+/−) causes spontaneous and persistent hypertension in mice.40 Blood pressure starts to elevate spontaneously around 16–17 weeks of age. Interestingly, the high salt (HS) intake further increases BP and exacerbates hypertension in KL(+/−) mice, indicating that KL deficiency elicited salt-sensitive hypertension.40 The HS loading causes inflammation as evidenced by increased expression of MCP-1 and infiltration of macrophages and T cells in kidneys in KL(+/−) mice. Blockade of CC chemokine receptor 2 (CCR2) abolishes the HS-induced increase in BP in KL(+/−) mice,40 suggesting an important role of monocyte chemotaxis in klotho deficiency-induced salt-sensitive hypertension. It would be interesting to assess of klotho deficiency may be involved in aging-related arterial stiffening.
Since the mechanism of aging-related arterial stiffening and hypertension include metabolic syndrome, inflammation, and neurohormonal dysfunction (Fig. 1), effective control of these aging-accelerating factors would benefit the cardiovascular system and extend the lifespan. Some other aging-related genes such as sirtuins (e.g., Sirt1 demethylase) and mTOR (mammalian target of rapamycin) have been shown to be involved in the regulation of vascular endothelial function and lifespan. Their roles in the development of arterial stiffening and hypertension need to be investigated.
Acknowledgments
Source of Funding
This work was supported by the National institute of Health (NIH), R01 HL105302, HL102074, HL118558, HL116863, and DK 093403.
Footnotes
Disclosures
None.
References
- 1.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, Lakatta EG. Calpain-1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age-associated aortic wall calcification and fibrosis. Hypertension. 2012;60:1192–1199. doi: 10.1161/HYPERTENSIONAHA.112.196840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puntmann VO, Nagel E, Hughes AD, Gebker R, Gaddum N, Chowienczyk P, Jahnke C, Mirelis J, Schnackenburg B, Paetsch I, Fleck E. Gender-specific differences in myocardial deformation and aortic stiffness at rest and dobutamine stress. Hypertension. 2012;59:712–718. doi: 10.1161/HYPERTENSIONAHA.111.183335. [DOI] [PubMed] [Google Scholar]
- 4.Rosano C, Watson N, Chang Y, Newman AB, Aizenstein HJ, Du Y, Venkatraman V, Harris TB, Barinas-Mitchell E, Sutton-Tyrrell K. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension. 2013;61:160–165. doi: 10.1161/HYPERTENSIONAHA.112.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira I, van de Laar RJ, Prins MH, Twisk JW, Stehouwer CD. Carotid stiffness in young adults: a life-course analysis of its early determinants: the Amsterdam Growth and Health Longitudinal Study. Hypertension. 2012;59:54–61. doi: 10.1161/HYPERTENSIONAHA.110.156109. [DOI] [PubMed] [Google Scholar]
- 6.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 8.Gerhard-Herman M, Smoot LB, Wake N, Kieran MW, Kleinman ME, Miller DT, Schwartzman A, Giobbie-Hurder A, Neuberg D, Gordon LB. Mechanisms of premature vascular aging in children with hutchinson-gilford progeria syndrome. Hypertension. 2012;59:92–97. doi: 10.1161/HYPERTENSIONAHA.111.180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P, Laurent S, Boutouyrie P, Briet M. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–1457. doi: 10.1161/HYPERTENSIONAHA.112.197210. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension. 2013;62:542–549. doi: 10.1161/HYPERTENSIONAHA.113.01318. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58:839–846. doi: 10.1161/HYPERTENSIONAHA.111.177469. [DOI] [PubMed] [Google Scholar]
- 12.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–119. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension. 2013;61:828–834. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safar ME, Thomas F, Blacher J, Nzietchueng R, Bureau JM, Pannier B, Benetos A. Metabolic syndrome and age-related progression of aortic stiffness. J Am Coll Cardiol. 2006;47:72–75. doi: 10.1016/j.jacc.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira I, Beijers HJ, Schouten F, Smulders YM, Twisk JW, Stehouwer CD. Clustering of metabolic syndrome traits is associated with maladaptive carotid remodeling and stiffening: a 6-year longitudinal study. Hypertension. 2012;60:542–549. doi: 10.1161/HYPERTENSIONAHA.112.194738. [DOI] [PubMed] [Google Scholar]
- 18.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61:943–947. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corden B, Keenan NG, de Marvao AS, Dawes TJ, Decesare A, Diamond T, Durighel G, Hughes AD, Cook SA, O'Regan DP. Body fat is associated with reduced aortic stiffness until middle age. Hypertension. 2013;61:1322–1327. doi: 10.1161/HYPERTENSIONAHA.113.01177. [DOI] [PubMed] [Google Scholar]
- 20.Smith ER, Tomlinson LA, Ford ML, McMahon LP, Rajkumar C, Holt SG. Elastin degradation is associated with progressive aortic stiffening and all-cause mortality in predialysis chronic kidney disease. Hypertension. 2012;59:973–978. doi: 10.1161/HYPERTENSIONAHA.111.187807. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Zhang J, Telljohann R, Jiang L, Wu J, Monticone RE, Kapoor K, Talan M, Lakatta EG. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–466. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, Joyner MJ. Aging enhances autonomic support of blood pressure in women. Hypertension. 2014;63:303–308. doi: 10.1161/HYPERTENSIONAHA.113.02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, Li W, Santisteban MM, Yuan W, Febo M, Mocco J, Feng Y, Scott E, Baekey DM, Raizada MK. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63:542–550. doi: 10.1161/HYPERTENSIONAHA.113.02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JM, Underwood PC, Ferri C, Hopkins PN, Williams GH, Adler GK, Vaidya A. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension. 2014;63:1205–1211. doi: 10.1161/HYPERTENSIONAHA.114.03231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostan R, Bucci L, Cevenini E, Palmas MG, Pini E, Scurti M, Vescovini R, Caruso C, Mari D, Vitale G, Franceschi C, Monti D. Metabolic syndrome in the offspring of centenarians: focus on prevalence, components, and adipokines. Age (Dordr) 2013;35:1995–2007. doi: 10.1007/s11357-012-9483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuentes E, Fuentes F, Vilahur G, Badimon L, Palomo I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators Inflamm. 2013;2013:136584. doi: 10.1155/2013/136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leibowitz A, Rehman A, Paradis P, Schiffrin EL. Role of T regulatory lymphocytes in the pathogenesis of high-fructose diet-induced metabolic syndrome. Hypertension. 2013;61:1316–1321. doi: 10.1161/HYPERTENSIONAHA.111.203521. [DOI] [PubMed] [Google Scholar]
- 28.Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension. 2013;62:226–230. doi: 10.1161/HYPERTENSIONAHA.113.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathis KW, Wallace K, Flynn ER, Maric-Bilkan C, LaMarca B, Ryan MJ. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension. 2014;64:792–800. doi: 10.1161/HYPERTENSIONAHA.114.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crosswhite P, Chen K, Sun Z. AAV Delivery of Tumor Necrosis Factor-alpha Short Hairpin RNA Attenuates Cold-Induced Pulmonary Hypertension and Pulmonary Arterial Remodeling. Hypertension. 2014;64:1141–1150. doi: 10.1161/HYPERTENSIONAHA.114.03791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Andres N, Calvier L, Labat C, Fay R, Diez J, Benetos A, Zannad F, Lacolley P, Rossignol P. Absence of cardiotrophin 1 is associated with decreased age-dependent arterial stiffness and increased longevity in mice. Hypertension. 2013;61:120–129. doi: 10.1161/HYPERTENSIONAHA.112.201699. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Andres N, Rousseau A, Akhtar R, Calvier L, Inigo C, Labat C, Zhao X, Cruickshank K, Diez J, Zannad F, Lacolley P, Rossignol P. Cardiotrophin 1 is involved in cardiac, vascular, and renal fibrosis and dysfunction. Hypertension. 2012;60:563–573. doi: 10.1161/HYPERTENSIONAHA.112.194407. [DOI] [PubMed] [Google Scholar]
- 34.Canale MP, Manca di Villahermosa S, Martino G, Rovella V, Noce A, De Lorenzo A, Di Daniele N. Obesity-related metabolic syndrome: mechanisms of sympathetic overactivity. Int J Endocrinol. 2013;2013:865965. doi: 10.1155/2013/865965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waki H, Hendy EB, Hindmarch CC, Gouraud S, Toward M, Kasparov S, Murphy D, Paton JF. Excessive leukotriene B4 in nucleus tractus solitarii is prohypertensive in spontaneously hypertensive rats. Hypertension. 2013;61:194–201. doi: 10.1161/HYPERTENSIONAHA.112.192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzourio C, Laurent S, Debette S. Is hypertension associated with an accelerated aging of the brain? Hypertension. 2014;63:894–903. doi: 10.1161/HYPERTENSIONAHA.113.00147. [DOI] [PubMed] [Google Scholar]
- 38.Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Chen K, Lei H, Sun Z. Klotho Gene Deficiency Causes Salt-Sensitive Hypertension via Monocyte Chemotactic Protein-1/CC Chemokine Receptor 2-Mediated Inflammation. J Am Soc Nephrol. 2014 Jun 5; doi: 10.1681/ASN.2013101033. doi. 2013101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paar M, Pavenstadt H, Kusche-Vihrog K, Druppel V, Oberleithner H, Kliche K. Endothelial sodium channels trigger endothelial salt sensitivity with aging. Hypertension. 2014;64:391–396. doi: 10.1161/HYPERTENSIONAHA.114.03348. [DOI] [PubMed] [Google Scholar]
- 42.Oelze M, Kroller-Schon S, Steven S, Lubos E, Doppler C, Hausding M, Tobias S, Brochhausen C, Li H, Torzewski M, Wenzel P, Bachschmid M, Lackner KJ, Schulz E, Munzel T, Daiber A. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 2014;63:390–396. doi: 10.1161/HYPERTENSIONAHA.113.01602. [DOI] [PubMed] [Google Scholar]
- 43.Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension. 2012;60:1517–1523. doi: 10.1161/HYPERTENSIONAHA.112.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 2014;29:250–264. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown KE, Dhaun N, Goddard J, Webb DJ. Potential therapeutic role of phosphodiesterase type 5 inhibition in hypertension and chronic kidney disease. Hypertension. 2014;63:5–11. doi: 10.1161/HYPERTENSIONAHA.113.01774. [DOI] [PubMed] [Google Scholar]
- 46.Crosswhite P, Sun Z. Inhibition of phosphodiesterase-1 attenuates cold-induced pulmonary hypertension. Hypertension. 2013;61:585–592. doi: 10.1161/HYPERTENSIONAHA.111.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]