Abstract
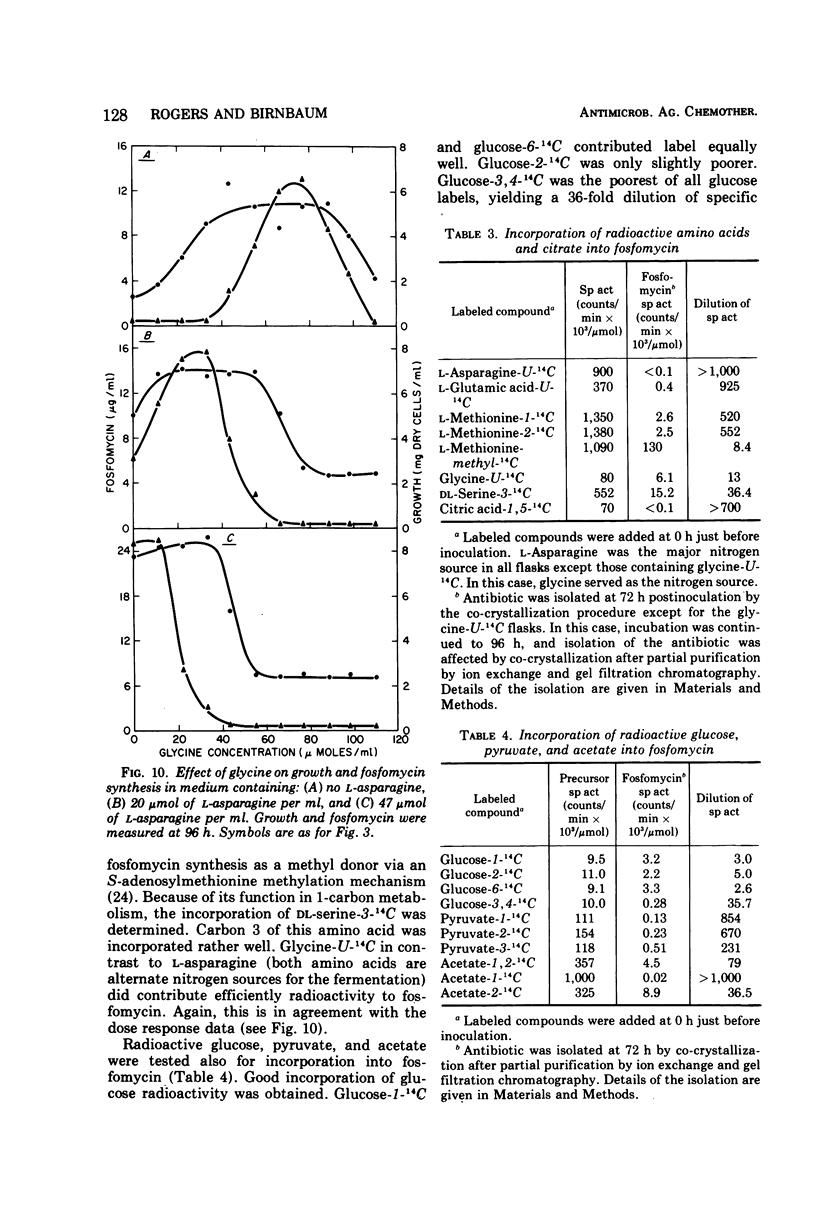
The antibiotic fosfomycin was produced as a secondary metabolite in a glucose-asparagine medium containing citrate, l-methionine, and l-glutamate. The citrate requirement for antibiotic synthesis was related to its requirement for growth. In contrast, l-methionine and l-glutamate caused a marked stimulation of fosfomycin production and had no effect on growth. l-Methionine had to be added early to effect maximal antibiotic synthesis later in the fermentation. The l-glutamate requirement was not specific, since several tricarboxylic acid cycle intermediates could replace this amino acid. l-Asparagine was the most effective nitrogen source for growth and production of fosfomycin. Glycine, an alternate nitrogen source, supported fosfomycin synthesis only when added in excess of that needed for growth. Cobalt and inorganic phosphate were required also for antibiotic production at concentrations exceeding those supporting maximal growth. Radioactive incorporation studies showed that the methyl carbon of methionine was the precursor of the methyl of fosfomycin. Carbon 1 of fosfomycin was derived from glucose carbons 1 and 6, whereas glucose-2-14C labeled fosfomycin carbon 2. Radioactivity from acetate-2-14C was distributed equally between fosfomycin carbons 1 and 2. No incorporation of acetate-1-14C, asparagine-U-14C, citrate-1,5-14C, or glutamate-U-14C occurred. The labeling pattern of fosfomycin carbons 1 and 2 was similar to that found in 2-aminoethylphosphonate from Tetrahymena.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argoudelis A. D., Eble T. E., Fox J. A., Mason D. J. Studies on the biosynthesis of lincomycin. IV. The origin of methyl groups. Biochemistry. 1969 Aug;8(8):3408–3411. doi: 10.1021/bi00836a040. [DOI] [PubMed] [Google Scholar]
- Chaiet L., Miller T. W., Goegelman R. T., Kempf A. J., Wolf F. J. Phosphonomycin: isolation from fermentation sources. J Antibiot (Tokyo) 1970 Jul;23(7):336–347. doi: 10.7164/antibiotics.23.336. [DOI] [PubMed] [Google Scholar]
- Christensen B. G., Leanza W. J., Beattie T. R., Patchett A. A., Arison B. H., Ormond R. E., Kuehl F. A., Jr, Albers-Schonberg G., Jardetzky O. Phosphonomycin: structure and synthesis. Science. 1969 Oct 3;166(3901):123–125. doi: 10.1126/science.166.3901.123. [DOI] [PubMed] [Google Scholar]
- Claridge C. A., Rossomano V. Z., Buono N. S., Gourevitch A., Lein J. Influence of cobalt on fermentative methylation. Appl Microbiol. 1966 Mar;14(2):280–283. doi: 10.1128/am.14.2.280-283.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness D. R. Bacterial growth on aminoalkylphosphonic acids. J Bacteriol. 1966 Sep;92(3):623–627. doi: 10.1128/jb.92.3.623-627.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendlin D., Stapley E. O., Jackson M., Wallick H., Miller A. K., Wolf F. J., Miller T. W., Chaiet L., Kahan F. M., Foltz E. L. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969 Oct 3;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- Holden J. T., Van Balgooy J. N., Kittredge J. S. Transport of aminophosphonic acids in Lactobacillus plantarum and Streptococcus faecalis. J Bacteriol. 1968 Oct;96(4):950–957. doi: 10.1128/jb.96.4.950-957.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M. Biosynthesis of 2-aminoethylphosphonic acid in cell-free preparations from Tetrahymena. Biochim Biophys Acta. 1972 Jan 28;261(1):102–113. doi: 10.1016/0304-4165(72)90319-4. [DOI] [PubMed] [Google Scholar]
- Hornemann U., Speedie M. K., Hurley L. H., Floss H. G. Demonstration of a C-methylating enzyme in cell free extracts of indolmycin-producing Streptomyces griseus. Biochem Biophys Res Commun. 1970 May 22;39(4):594–599. doi: 10.1016/0006-291x(70)90245-7. [DOI] [PubMed] [Google Scholar]
- Jackson M., Stapley E. O. Phosphonomycin. II. Fermentation studies. Antimicrob Agents Chemother (Bethesda) 1969;9:291–296. [PubMed] [Google Scholar]
- KORNBLATT J. A., BERNATH P., KATZ J. THE DETERMINATION OF SPECIFIC ACTIVITY OF BAC14O3 BY LIQUID SCINTILLATION ASSAY. Int J Appl Radiat Isot. 1964 Apr;15:191–194. doi: 10.1016/0020-708x(64)90065-1. [DOI] [PubMed] [Google Scholar]
- Kochi H., Kikuchi G. Reactions of glycine synthesis and glycine cleavage catalyzed by extracts of Arthrobacter globiformis grown on glycine. Arch Biochem Biophys. 1969 Jul;132(2):359–369. doi: 10.1016/0003-9861(69)90377-4. [DOI] [PubMed] [Google Scholar]
- Liang C. R., Rosenberg H. On the distribution and biosynthesis of 2-aminoethylphosphonate in two terrestrial molluscs. Comp Biochem Physiol. 1968 May;25(2):673–681. doi: 10.1016/0010-406x(68)90377-0. [DOI] [PubMed] [Google Scholar]
- Liang C. R., Rosenberg H. The biosynthesis of the carbon-phosphorus bond in Tetrahymena. Biochim Biophys Acta. 1968 Mar 11;156(2):437–439. doi: 10.1016/0304-4165(68)90283-3. [DOI] [PubMed] [Google Scholar]
- Origuchi M., Kittredge J. S., Roberts E. Biosynthesis of 2-aminoethylphosphonic acid in Tetrahymena. Biochim Biophys Acta. 1968 Aug 6;165(1):164–166. doi: 10.1016/0304-4165(68)90200-6. [DOI] [PubMed] [Google Scholar]
- PHARES E. F. Degradation of labeled propionic and acetic acids. Arch Biochem Biophys. 1951 Sep;33(2):173–178. doi: 10.1016/0003-9861(51)90094-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., La Nauze J. M. The metabolism of phosphonates by microorganisms. The transport of aminoethylphosphonic acid in Bacillus cereus. Biochim Biophys Acta. 1967 Jun 13;141(1):79–90. doi: 10.1016/0304-4165(67)90247-4. [DOI] [PubMed] [Google Scholar]
- Shafer H., VandenHeuvel W. J., Ormond R., Kuehl F. A., Wolf F. J. Characterization of phosphonomycin by microchromatographic and related techniques. J Chromatogr. 1970 Oct 7;52(1):111–117. doi: 10.1016/s0021-9673(01)96550-1. [DOI] [PubMed] [Google Scholar]
- Stapley E. O., Hendlin D., Mata J. M., Jackson M., Wallick H., Hernandez S., Mochales S., Currie S. A., Miller R. M. Phosphonomycin. I. Discovery and in vitro biological characterization. Antimicrob Agents Chemother (Bethesda) 1969;9:284–290. [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Weissbach H. Escherichia coli B N5-methyltetrahydrofolate-homocysteine methyltransferase: sequential formation of bound methylcobalamin with S-adenosyl-L-methionine and N5-methyltetrahydrofolate. Arch Biochem Biophys. 1969 Feb;129(2):728–744. doi: 10.1016/0003-9861(69)90234-3. [DOI] [PubMed] [Google Scholar]
- Trebst A., Geike F. Zur Biosynthese von Phosphonoaminosäuren. Die Veteilung der Radioaktivität in Aminoäthylphosphonsaure nach Einbau von positions-markierter Glucose durch Tetrahymena. Z Naturforsch B. 1967 Sep;22(9):989–991. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Warren W. A. Biosynthesis of phosphonic acids in Tetrahymena. Biochim Biophys Acta. 1968 Mar 11;156(2):340–346. doi: 10.1016/0304-4165(68)90263-8. [DOI] [PubMed] [Google Scholar]


