Abstract
Background
Michigan's BioTrust for Health, a public health research biobank comprised of residual dried bloodspot (DBS) cards from newborn screening contains over 4 million samples collected without written consent. Participant-centric initiatives (PCI) are IT tools that hold great promise to address the consent challenges in biobank research.
Methods
Working with Private Access Inc., a pioneer in patient-centric web solutions, we created and pilot tested a dynamic informed consent simulation, paired with an educational website, focusing on consent for research utilizing the DBS in Michigan's BioTrust for Health.
Results
Out of 187 pilot testers recruited in two groups, 137 completed the consent simulation and exit survey. Over 50% indicated willingness to set up an account if the simulation went live and willingness to recommend it to others. Participants raised concerns about the process of ID verification and appeared to have little experience with sharing health information online.
Conclusions
Applying online, dynamic approaches to address the consent challenges raised by biobanks with legacy sample collections should be explored given the positive reaction to our pilot and the strong preference for active consent. Balancing security and privacy with accessibility and ease of use will continue to be a challenge.
Keywords: Biobank, Public health, informed consent, online, Participant-Centric Initiatives, dynamic consent
BACKGROUND
Collections of biospecimens in large population biobanks are increasingly valuable for health and scientific research. When collection has preceded informed consent – as is the case for retrospective biobanks - significant questions related to informed consent follow [1,2,3,4,5,6,7]. In some cases, de-identification has been proposed as a mechanism for reducing risk such that written, informed consent is not necessary. Opt-out procedures maximize ongoing participation, but face ethical questions about their ability to allow participants autonomy if participants are unaware of their participation [8,9,10]. Even when consent is obtained, retrospective biobanks face the challenge – common to all types of biobanks - of whether consenting participants can be truly informed in the process of opting into unknown, future research. [8,9,10,11]
Michigan is home to a state biobank comprising residual dried bloodspots (DBS) left over from newborn screening, including some 4 million “retrospective” DBS collected from a generation of Michiganders before written consent policies were in place [12,13]. The incorporation of Michigan's BioTrust for Health (BIoTrust) in 2010 was significant in the history of NBS biobanking in the US as it was a first-of-its-kind effort to bring the research “goldmine” of ~4 million DBS samples to the research community to promote and stimulate new research [13,14]. The grandfathered collection of DBS, collected between July 1984 and May 2010 without written consent were combined in this biobank with new DBS, which are now added to the research pool only with written parental consent [13]. Thus the biobank has a dual consent policy for secondary research uses of DBS, an opt-in, broad, parental-proxy consent for new spots and an opt-out option for those who did not have the opt-in process available to them. This raises the issues noted above to “opt out” approaches.
In light of the consent challenges facing the BioTrust and other retrospective biobanks, online and dynamic approaches to solving the problem of consent for biobanking are gaining attention and adherents [15,16,17,18,19,20]. We simulated an online, dynamic tool designed to simultaneously educate Michiganders and record tiered consent preferences for participation in the BioTrust. This study presents user experiences with the online tool that was designed to be easy to implement, publicly accessible, and that meets the ethical goals of informed consent.
Our efforts build upon the call for participant-centric initiatives (PCIs), IT tools designed to place patients and research participants at the center of health and research decision-making [17,21,22,23,46]. These initiatives, often referred to as “dynamic consent” models, seek to implement “new methods for consent and for exercising choice over the use of samples and information in response to changing research needs while not hampering research with burdensome practices” [17,24]. Even though the model appears promising on many fronts, efforts to implement dynamic consent for biobanking have been limited. The EnCoRe (Ensuring Consent and Revocation) Project and Oxford Radcliffe Biobank in the UK have undertaken such a project and are in the testing phase of a dynamic consent tool [9,17,20,23,46]. Our PCI approach to consent for the BioTrust represents a first for DBS biobanking in the US.
In a rapidly advancing health information economy, online tools could be used to establish an access point for public education and communication regarding the BioTrust, particularly among parents and adult “retrospective” participants [25]. Hypothetically, donors (or parents of minors) could use an online consent portal to register consent or dissent for DBS research use. With an asynchronous consent process, parents and participants who never had an opportunity to consent at the time of collection could register their preferences, at their convenience and outside of normal interactions in a clinic, hospital or health department. Furthermore, an online platform would be flexible enough to enable participants to personalize options (tiered consent) and choose to amend consent preferences on an ongoing basis [26]. Ideally, such a system would be incorporated into the existing data architecture of the biobank. For example, individual consent preferences could become a searchable data “tag” associated with each sample's digital record. In the long-term, individuals whose parents initially provided consent to participate in the BioTrust would also have a point of access upon reaching the age of majority. Individuals who prefer a more active role in biobank participation could be given greater opportunity to engage as partners in the research enterprise and those who prefer a one-time interaction would be afforded a simple and convenient way to register their preference once and for all [27].
In partnership with Private Access ™, we created and tested an education portal and consent simulation for a large population biobank. Private Access is recognized in this field as innovating “matchmaking” between rare disease patients and researchers [23]. Shelton [16] presents the perspective of the CEO of Private Access on the significance of this particular project and on the broader field of patient/participant-centered approaches to privacy and consent in health research, arguing that properly configured online consent will be a technological solution that respects individuals privacy wishes and simultaneously facilitates and strengthens the research enterprise.
Michigan's approach to, and experience with, implementing DBS biobanking has broad implications [28]. A third of U.S. states retain DBS for long-term use [29], and commonalities in the structure and design of the BioTrust with large population biobanks in general make the Michigan experience relevant and instructive to the broader biobanking and NBS communities [12,13,25,30,31]. Public opinion research demonstrates that individuals prefer to give consent for research use of their de-identified biospecimens [1,32,33,34,35] and, although the fate of proposed changes to the Common Rule are uncertain, the call for broad, written consent for research on de-identified biospecimens collected prospectively was a key element in the proposal [36]. The BioTrust's policies and implementation offers a kind of test case for this consent model since its dual collection, dual consent model comprises DBS incorporated under the proposed conditions as well as the old.
In what follows, we present data collected from our pilot test of this online, dynamic consent simulation and education platform. Specifically, this paper focuses on measures of user experience and satisfaction with the simulation and predictors (both demographic and user experience based) of the likelihood of setting up an account if it were a live system.
METHODS
Working with Private Access Inc., we designed, developed and pilot tested a simulated online consent /permissions portal for the Michigan BioTrust for Health, with a particular focus on consenting “donors” and parental proxies to the retrospective collection of DBS. Before recruiting participants, we launched an educational website, explorebiobanking.org, which served as the starting point for pilot testers who were instructed to browse educational content related to the BioTrust and prompted to consent to participate in the Private Access study. Key educational components included short animated videos and an FAQ page.1
Recruitment: Two Pilot-Test Groups
Research participants were recruited to pilot test the Private Access consent portal in two groups. Group 1 was comprised of Michigan citizens recruited with the help of six community-based partners who had previously collaborated in our efforts to engage Michiganders about the BioTrust by co-hosting community meetings on the topic in Flint, Grand Rapids, Jackson, Detroit, and Petosky (Northern MI) [37].
To aid in recruitment, community partners were given promotional posters and trifold fliers along with a stack of postcards containing instructions for at-home completion of the simulation and a unique identifying code so that we could track the recruitment rates around the state. Recruits were asked to complete the process within a two-month time frame. A total of 385 recruitment postcards were distributed among the community partner organizations (Table 1). Due to a lower-than-expected participation rate, we decided to focus a second recruitment effort on a group that, we hypothesized, would be particularly willing to utilize an online consent portal, namely college-aged students (~18-22). Additionally, this group would likely comprise a number of individuals who themselves were “donors” to the BioTrust collection of DBS.
Table 1.
Pilot test enrollment
| Total Enrolled | Recruitment cards distributed | Recruitment rate % | ||
|---|---|---|---|---|
| Group 1: Community member participants | 92 | 385 | 24.1 | |
| Participants by community | Flint | 25 | 50 | 50 |
| Detroit | 13 | 55 | 23.6 | |
| Grand Rapids | 24 | 100 | 24 | |
| Jackson | 10 | 60 | 16.6 | |
| Dearborn/Dearborn Heights | 9 | 60 | 15 | |
| Other | 11 | n/a | n/a | |
| Petoskey | 0 | 60 | 0 | |
| Group 2: UM Students | 95 | n/a | n/a | |
Group 2 was therefore comprised of students from the University of Michigan, Ann Arbor campus who were informed of the study via on-campus flyers and in-person recruitment to complete the simulation in computer labs on campus.
All participants completed an IRB-approved online consent and were compensated with $25 for their time. Table 1 presents a summary of the recruitment data for each group and Table 2 summarizes demographic characteristics of each group. In addition, Table 2 presents summary demographic information for participants who attended the 10 community meetings we held in the state of Michigan on this issue for the sake of comparison.
Table 2.
Community meeting & pilot test participant demographics
| Community Meeting Participants n=393 | Pilot Test Group 1 n=92 | Pilot Test Group 2 n=95 | Group 1 vs. Group 2 χ2 or Fisher's Exact p-value | |||
|---|---|---|---|---|---|---|
| Gender % | Female | 75.0 | 64.8 | 50.0 | 0.030 | |
| Race/Ethnicity % | White not Hispanic | 16.0 | 19.6 | 53.7 | <0.001 | |
| African-American | 34.0 | 38.0 | 9.5 | |||
| Asian or Pacific Islander | 21.0 | 29.3 | 25.3 | |||
| Other | Arab or Arab-American | 12.0 | 9.8 | 1.0 | ||
| Native American | 3.0 | 0.0 | 1.0 | |||
| Hispanic | 11.0 | 0.0 | 3.2 | |||
| NR | 3.0 | 3.3 | 6.3 | |||
| Education % | <12 years | 30.0 | 5.6 | 0.0 | <0.001 | |
| 12-15 years | 50.0 | 57.8 | 83.2 | |||
| >15 years | 20.0 | 34.4 | 15.8 | |||
| Age % | <25 | 25.0 | 37.0 | 95.8 | <0.001 | |
| 26-35 | 15.0 | 15.2 | 3.2 | |||
| 36-45 | 21.0 | 15.2 | 0.0 | |||
| 46-55 | 18.0 | 20.7 | 0.0 | |||
| >55 | 21.0 | 12.0 | 0.0 | |||
| Has donated blood to the Red Cross or for medical use % | 34.0 | 33.7 | 48.4 | 0.039 | ||
| Self-rated health % | Excellent | 12.9 | 22.8 | 24.2 | 0.130 | |
| Very good | 44.6 | 50.0 | 60.0 | |||
| Fair | 37.7 | 26.1 | 14.7 | |||
| Not so hot | 4.7 | 1.1 | 0.0 | |||
1 Group 1 is comprised of participants recruited through various community organizations
2 Group 2 is comprised of students on the University of Michigan, Ann Arbor campus
3 Computed with “No Responses” dropped (Group 1 n=89, Group 2 n=92)
Private Access Features: Account Setup, Select-a-Guide, User Preferences Matrix
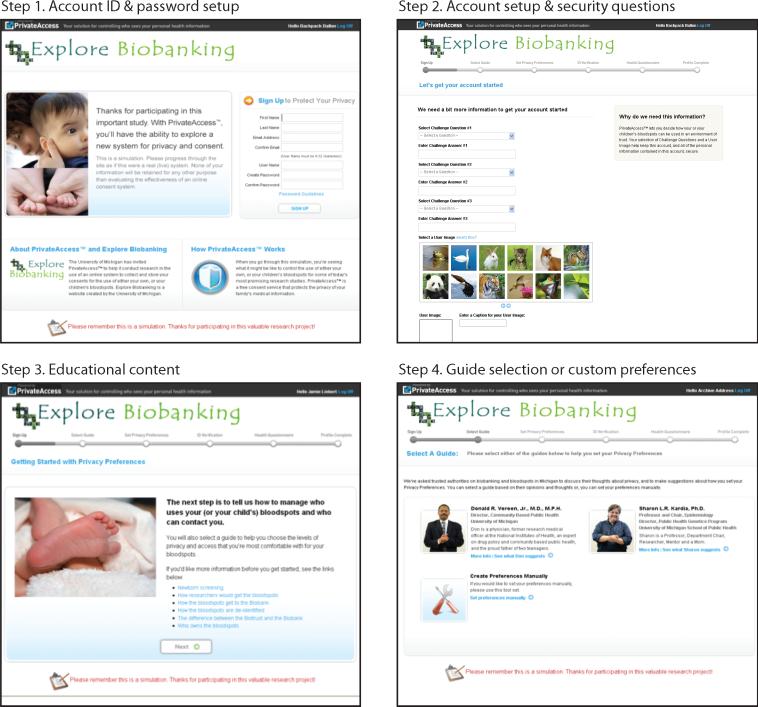
Figure 1 illustrates the step-by step process through the simulation. Participants were instructed to create a user profile that included giving basic information (e.g. name, contact information, etc.), creating a user name, password, site-key and establishing three security questions. After the account setup, users were shown a sample user agreement and given the opportunity to review basic information about the BioTrust through links to content from explorebiobanking.org.
Fig 1.
Private Access Consent Simulation
As users proceeded through the stages of the simulation, they were prompted to either input data or shown prompts where data would be required if it were a live system. In cases where users were shown a hypothetical data request or privacy enhancement, they were asked to share whether or not they would be comfortable providing the information requested; open comment fields in these instances enabled us to collect feedback on these specific elements of the simulation.
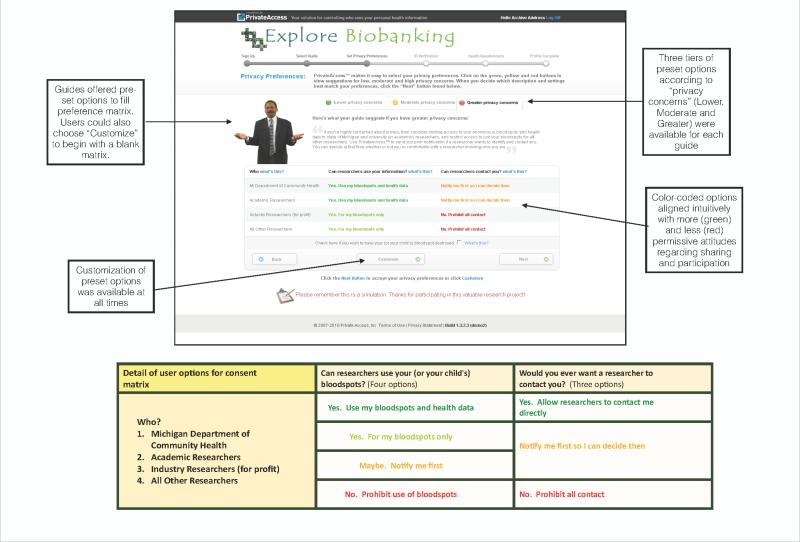
After the initial set-up, users reached the main activity of setting tiered consent and contact preferences. Users had the option to either set their consent and contact preferences manually or to consider and, if they wished, follow advice from one of two available “guides,” real-life public health experts whose brief biographies and photographs “personalized” their relationship to public health and biobanking. The guides offered preset selections for the 2x4 preference matrix, comprised of two key questions and four categories of researchers, with settings corresponding to tiered privacy concerns. Additionally, users could toggle among three completely preset matrices according to whether the user had greater, moderate or lower privacy concerns using color-coded tabs. Figure 2 presents an annotated screen shot of this central element of the consent simulation. After the matrix choices were set, participants were prompted to confirm their selections before continuing on to an identity verification step.
Fig 2.
Private Access Consent Preferences Matrix
ID Verification
One of the challenges associated with online systems for managing personal health data is verifying that the individual who is using the online tool to manage health information is in fact the individual they claim to be. In developing our simulation, we were cognizant that the state of Michigan created the requirement that individuals verify their identity with a copy of their state-issued ID in order to successfully opt out of the BioTrust. We worked with Private Access to determine what appropriate and available mechanisms for online ID verification could be incorporated into the simulation. In addition to the creation of a user name and password, a two-factor authentication was added that drew on publicly available data sources (banking history, mortgage records & state of issuance of social security number). Each user was presented with three questions about their banking activity and the state of origin of their social security number. In a live system, if the user answered all three questions correctly, then they would be deemed to be the individual they claimed to be and their consent preferences would thus be validated.
Since our users were going through the process as a simulation, we only showed them a sample ID verification page, after they completed the consent matrix, and asked: “Would you answer these questions or questions like these?” Participants who indicated ‘no’ were further asked: “If no, how would you suggest verifying identification?”
Final Steps
Upon completion of the ID verification, users were given the option of testing out a family health history creation tool (not discussed in this paper) and then directed to the exit survey (hosted on Survey Monkey). Thus three main data sources were available for analysis from each group, (1) a pre-survey, (2) preferences and information entered during the consent simulation and (3) an exit survey.
Analytical Approach
Descriptive statistics were generated for six demographic variables: gender, race/ethnicity, age, altruism, and self-rated health. Because altruistic values are highly prevalent among blood donors [43], we employed a measure of altruism defined as whether the participant self-identified as a blood donor. This measure replicated a question used to gauge respondents altruistic beliefs and practices in a study evaluating veterans' attitudes toward participating in biobank research [44] and in a study evaluating public attitudes about a proposed large-scale national cohort study of genes and environment [45]. Chi-square and Fisher's exact tests were then used to ascertain significant differences in demographic characteristics between the Community and UM groups (Table 2). We similarly compared demographic characteristics of those who completed the simulation versus those who did not (Table 2b).
We developed a two-step logistic regression model to identify predictors of whether an individual would be likely to set up an account based on responses to the exit survey question: “If this were real (live) instead of a simulation, would you set up a Private Access account to manage permissions for use of your or your child's bloodspots?” using demographic variables, user experience variables and participant preferences regarding consent and contact. User experience variables included: overall experience, impressions of length of time to completion and ease of navigation, likelihood to recommend the service to others, reasons for not using an online personal health record service (e.g., Google Health), preference for privacy settings (i.e., set by guide or customized), and willingness to respond to ID verification questions. Consent and contact preferences were measured by responses to two questions about whether an individual would like to be asked each time their DBS would be used in research and whether parents should be able to decline having their baby's blood stored for use in research. We first conducted univariable analysis using each of the predictors that we hypothesized would be associated with the likelihood a participant would set up a Private Access account. Based on the results of the univariable logistic regression models, we used α <.10 as the criterion for inclusion and exclusion in our multivariable modeling of this outcome.
RESULTS
Results from the pilot tests are presented in two main sections: Recruitment and User Experience.
I. RECRUITMENT - DEMOGRAPHIC CHARACTERISTICS OF GROUPS
Table 2 summarizes the demographic characteristics of each group and further shows the participant demographics from the first research arm of this project, namely a series of 10 community meetings (n=393) held in the state of Michigan [37]. As compared with the community meetings, there was a notable improvement in the gender balance among pilot testers in both groups. Community group demographics were more closely aligned with the distribution of participants in our earlier community meetings across race/ethnicity, age and education variables than the student group.
A statistically significant difference was detected between the two pilot test groups along several demographic variables: gender (p=.03), race/ethnicity (p<.001), education (p<.001), age (p<.001) and an indicator of altruism, (viz. participant has donated blood to the Red Cross for medical use) (p=0.039).
II. USER EXPERIENCE
Overall Participant Experience
Pilot testers’ overall experience with Private Access was mostly positive or neutral, with relatively small numbers of participants indicating negative experiences in both groups (Table 3). Among community group participants 64.3% indicated a “very” or “somewhat” positive experience with the site overall, whereas 68.7% of the student group held this view.
Table 3.
Summary of participant experiences and preferences by cohort affiliation
| Group 1 n=56 % | Group 2 n=86 % | Group 1 vs. group 2 p-value | |||
|---|---|---|---|---|---|
| User Experience Factors | Would set up account (Y) | Yes | 58.9 | 52.3 | 0.638 |
| No/Not Sure | 41.1 | 47.7 | |||
| Overall experience | Somewhat or Very positive | 64.3 | 68.7 | 0.855 | |
| Neutral | 26.8 | 25.6 | |||
| Somewhat or Very negative | 9.0 | 5.9 | |||
| Impression of length of time to completion | It took less time than I thought it would | 42.9 | 17.4 | 0.003 | |
| It took about the right amount of time | 35.7 | 44.2 | |||
| It took too long but it was worthwhile | 16.1 | 17.4 | |||
| It took too long | 5.4 | 20.9 | |||
| Navigation impression | Fairly or Very easy | 82.1 | 89.5 | 0.287 | |
| Some parts easy; some parts more difficult | 10.7 | 8.1 | |||
| Somewhat or Very difficult | 7.1 | 2.4 | |||
| Would recommend service to others | Somewhat or Very likely | 82.2 | 77.9 | 0.541 | |
| Somewhat or Very unlikely | 17.9 | 22.1 | |||
| Reasons for not using a personal health record service | I don't know how this would work | 40 | 51.8 | 0.084 | |
| I don't have regular access to the internet | 2 | 1.9 | |||
| I don't want my medical record on the web | 50 | 30.6 | |||
| Other, please specify | 8 | 16.5 | |||
| Guided or Custom settings preference | n=92 | n=95 | |||
| Custom preferences | 36.9 | 90.5 | <0.005 | ||
| Guided selections | 63 | 9.5 | |||
| ID verification: “Would you answer these questions or questions like these?” | n=56 | n=87 | 0.005 | ||
| Yes | 82 | 59.8 | |||
| No | 17.9 | 40.2 | |||
| Contact and consent preferences | Ask me each time my DBS would be used for research | Agree/strongly agree | 85.7 | 58.1 | 0.001 |
| Disagree/strongly disagree | 14.3 | 41.9 | |||
| n=56 | n=82 | ||||
| Parents should be able to decline having their baby's blood stored fo use in research | Agree/strongly agree | 96.4 | 95.1 | 0.712 | |
| Disagree/strongly disagree | 3.6 | 4.9 |
1Group 1 is comprised of participants recruited through various community organizations
2Group 2 is comprised of students from the University of Michigan, Ann Arbor campus
Time to Completion
A significant difference between the groups emerged with their impressions of the time it took to complete the simulation (p=.003). While 42.9% of community group participants indicated that it was less time consuming than they had anticipated, only 17.4% of the students found this to be the case. At the other end of the spectrum, 20.9% of the student participants found that the simulation took too long, while only 5.4% of community group participants registered this view. Well over a third of each group (35.7%, 44.2%) indicated that the process took about the right amount of time.
Ease of Navigation
Users in both groups overwhelmingly indicated that the navigation through the simulation was either “very” or “fairly” easy: 82.1% (community groups) and 89.5% (student group) (Table 3). Differences between the groups were not statistically significant for this measure of user experience.
Recommend vs. Use
Willingness to recommend Private Access to others exceeded personal interest in setting up an account for both groups, but group affiliation was not significant for either question. The student group was almost evenly divided on the likelihood that they would set up an account, with 52.3% of the participants answering “yes” and an equally divided dissenting opinion between 22.1% saying “no” and 25.6% selecting “not sure” (Table 3). Yet, for this group, 77.9% were either “very” or “somewhat” likely to recommend Private Access to others.
Community group participants were slightly more enthusiastic about creating an account for themselves, with 58.9% saying “yes”, only 16.1% selecting “no” and 25% registering a “not sure.” This group also demonstrated greater enthusiasm for recommending the service to others, with 82.2% of participants indicating that they would be “very” or “somewhat” likely to recommend it.
Comfort/Experience with Online Health Information Services
A mere 3.6% of community participants and 2.3% of UM student respondents indicated that they had experience using online systems to manage and access their health information. A follow up question queried reasons why participants did not utilize such a service. Half of community participants indicated that they would not want their medical record on the web and 40% cited a lack of understanding of how such a service would work. Fewer participants in the student group indicated concern about having their medical record on the web (30.6%), though a greater number (51.8%) indicated that they did not use a web-based service because they “did not know how this would work.”
User Actions: Guides and Prompts
Community group participants were far more inclined than students to avail themselves of preset options suggested by the guides (63% vs. 9.5%) (Table 3). A full 90.5% of the student group opted to set custom preferences rather than follow the advice of the guides. This difference between groups was significant (p<.001).
User Actions: ID Verification
Pilot testers from the community groups were generally more comfortable with the idea of answering ID verification questions, with 82% saying ‘yes’, while the students registered more skepticism with only 59.8% saying yes to such an approach to ID verification. This difference in group preference proved significant upon further analysis (p=.005) (Table 3).
As one of the few opportunities for sharing qualitative input, the responses of our testers to the open-ended follow up question are noteworthy. Out of 52 comments, 18 indicated some form of critique of the ID verification process. Typical replies focused on the personal and private nature of the information being asked, as in “Getting very personal. I don't like talking about anything that has to do with my ssn [social security number] online” and “I don't know [if I would answer the ID verification questions] but I would not answer questions about my banking history willingly on a website that has nothing to do with that...,” or “the bank account and credit profile questions seem irrelevant and suspicious.” In two additional open-ended questions on the exit survey, 7 respondents came back to their concerns about the ID verification process, for example “I didn't like the ssn parts. Difficult and unnerving,” and “the site LOOKS secure but the questions asked are very very private...” The latter quote captures the ambivalence that a number of participants expressed about their experience with the site. Many noted that it felt secure and professional, but they were still concerned about sharing such personal information online.
Likelihood Of Future Use
Over half of the pilot testers (58.9% of participants in the community group and 52.3% of UM-student participants) indicated that they would be likely to set up an account (p=0.638) (Table 3).
Logistic Regression
Based on the results of the univariable logistic regression models, we found that none of the demographic variables proved significant (p>0.10) (Table 4). Of the seven user experience factors analyzed, we found six of them to be significantly (p< 0.10) associated with the likelihood of setting up an account: 1. participants’ overall experience, 2. assessment of the length of time it took to complete the simulation, 3. impression of ease of navigation, 4. likelihood to recommend the service to others, 5. the expressed preference to not have one's medical record on the web, and 6. reaction to the ID verification process. One of the two consent and contact preferences variables was significant in the univariable analysis: participants’ reactions to the question of whether they would like be asked each time their DBS would be used in a research project (OR = 2.29; p=0.027).
Table 4.
Predictors of whether participants would set up an account (n=137)
| Univariable Odds Ratio (p-value) | Multivariable Final Model Odds Ratio (p-value) | ||||
|---|---|---|---|---|---|
| Demographic Factors | Study Cohort | UM Students group | .88 (0.728) | - | |
| Community participants group | Ref | - | |||
| Gender | Female | 1.11 (0.745) | - | ||
| Male | Ref | - | |||
| Race/Ethnicity | African-American | 2.1 (0.138) | - | ||
| Asian or Pacific Islander | .85 (0.685) | - | |||
| Other | Arab or Arab-American | 1.63 (0.470) | - | ||
| Native American | |||||
| Hispanic | |||||
| Other/NR | |||||
| White (not Hispanic) | Ref | ||||
| Education | >15 years | .94 (0.950) | - | ||
| 12-15 years | .75 (0.758) | - | |||
| <12 years | Ref | - | |||
| Age | >55 | 2.48 (0.437) | - | ||
| 46-55 | .83 (0.756) | - | |||
| 36-45 | .83 (0.853) | - | |||
| 26-35 | .99 (0.991) | - | |||
| <25 | Ref | - | |||
| User Experience Factors | Overall Experience | Somewhat or Very Positive | 21.2 (0.050) | 9.23 (0.052) | |
| Neutral (neither positive or negative) | 2.88 (0.349) | 1.70 (0.658) | |||
| Somewhat or Very negative* | Ref | Ref | |||
| Impression of length of time to completion | It took too long | 0.14 (0.003) | - | ||
| It took to long, but it was worthwhile | 0.98 (0.964) | - | |||
| It took about the right amount of time | 0.62 (0.270) | - | |||
| It took less time than I thought it would | Ref | - | |||
| Navigation impression | Somewhat or Very easy* | 6.90 (0.082) | - | ||
| Some parts easy; some more difficult | 3.57 (0.305) | - | |||
| Somewhat or Very difficult* | Ref | - | |||
| Would recommend service to others | Somewhat or very likely | 11.2 (<0.001) | 5.20 (0.010) | ||
| Somewhat or very unlikely* | Ref | Ref | |||
| Reasons for not using a personal health record service | I don't have regular access to the internet | 0.62 (0.736) | - | ||
| I don't want my medical record on the web | 0.44 (0.036) | - | |||
| Other, please specify | 0.77 (0.627) | - | |||
| I don't know how this would work | Ref | - | |||
| Guided or Custom settings preference | Preset preferences | 0.51 (0.159) | - | ||
| Custom preferences | Ref | - | |||
| ID verification: “Would you answer these questions or questions like these?” | Yes | 2.89 (0.006) | 2.34 (0.096) | ||
| No | Ref | Ref | |||
| Consent and Contact Preferences | “Ask me each time my DBS would be used for research.” | Agree/strongly agree | 2.29 (0.027) | 2.20 (0.078) | |
| Disagree/strongly disagree | Ref | Ref | |||
| “Parents should be able to decline having their baby's blood stored for use in research.” | Agree/strongly agree | .58 (0.533) | - | ||
| Disagree/strongly disagree | Ref | - | |||
Criterion for inclusion in step one: α <.10, criterion for inclusion in final model: α <.10
Based on an inclusion/exclusion criterion of α<0.10, our two-step multivariable regression models indicate that four factors stand out as particularly salient as to whether an individual is likely to set up an account. Individuals who indicated a positive overall experience were more likely than those who indicated that their experience was somewhat or very negative to set up an account (OR=9.23, p=0.052). Those that were somewhat or very likely to recommend the simulation to others (OR=5.20, p=.010) were also more likely to set up an account. A positive response to the ID verification step (indicating comfort with the proposed verification process) remained a significantly associated variable (OR= 2.34, p=0.096), as did the desire to be asked each time one's DBS would be used for research (OR=2.20, p=.078).
DISCUSSION
Overall User Experience
Overall, participants who completed the process indicated a positive experience with the Private Access informed consent simulation. The data indicate that participants’ decisions about whether or not to participate or recommend the service to others were not negatively impacted by basic design issues or simulation length. The 20% of students who found that the simulation took too long indicates that the design and ease of use expectations are greater for younger users.
Participants’ experience, and comfort level, with online systems for health records and information was quite low among both of our groups. As this was a convenience sample, we cannot generalize these rates; however, the reasons that were given for not using an online portal may be instructive to those considering implementation of online education and consent for a biobank. The large number of participants from each group who would not want their health information online indicates minimally that measures need to be taken to enhance people's sense of comfort with online health information systems, though this is less so for the student-aged population.
Recruitment
For the purposes of our pilot testing, recruitment via campuses and community groups was adequate for meeting our aims. Monetary incentives and, among community groups, help from trusted community partners to distribute recruitment postcards, helped motivate participation. On a statewide scale, a sustained effort to inform the public and promote the tool would be necessary to garner widespread participation. Relatively modest investments in outreach via social media outlets (e.g., Facebook advertising) have been shown to have potential for aiding this effort [25]. Snowball effects would likely aid an ongoing recruitment process, as evidenced by a high percentage of pilot testers who indicated they would likely recommend the platform to others (82.2% and 77.9%).
ID Verification
Given the privacy issues attending health care information, providing security and assurances of confidentiality are of utmost importance to an online system. Prior to testing, we viewed the multiple layers of security involved in the process as both an advantage of the design (greater indications of data security) and a potential source of user dissatisfaction as multiple steps can encumber the ease of use and accessibility of the process for all.
The lower rate of assent among the student group (59.8% vs. 82%) to the question of whether they would respond to the ID verification questions, along with comments shared in the simulation and exit surveys, indicate that the ID verification process had a negative impact on user experience. Helen Nissenbaum's notion of privacy as “contextual integrity” highlights the centrality of the norm of appropriateness that “dictate[s] what information about persons is appropriate, or fitting, to reveal in a particular context” [38, p.120]. Violations of this norm emerge when information from one situation is inserted into a different context [38, p.122]. Feedback from our pilot testers suggests that, for some, the integration of personal financial data into the process of identity verification for health data constituted such a violation of the expected norms of appropriateness. Private Access has recently identified a new process for ID verification that uses the camera function on a computer or smart phone to capture an image of the user's state-issued identification (e.g. drivers license, passport). Several commercial vendors (e.g Jumio, ID Checker) are already actively marketing this technology. Although Private Access has not yet implemented this approach, they believe that the familiarity that most people have with verifying their identity with government issued ID will make users more comfortable than the process that was used in our pilot [39].
As technologies for ID verification continue to evolve and as public familiarity with the need for such tools, and the assurances they provide, increases, online ID verification, whether for e-commerce, banking or health IT applications, will become a more ubiquitous feature of our digital lives. The introduction of systems that rely on biometric authentication (e.g. facial recognition, fingerprint etc.) rather than personal information gathered from a third party source, may minimize the kind of discomfort that some of our pilot testers experienced with our system. Providing privacy assurances for end-users will still have to be balanced with creating an atmosphere of trust. Determining appropriate security and validation measures that are scaled to the sensitivity of the information in question will be an important ongoing task for web-based PCIs.
Comparison of Group Outcomes
The variation in reactions and completion rates from the two participant groups suggests that a one-size fits all design approach may not be optimal for a consent portal. Given the complexity of the subject matter and the broad general population target audience, a design approach that enables users to toggle between different levels of information depth and relative ease of use may be a way to satisfy users across the full-spectrum of literacy, tech literacy, interest and motivation to utilize a dynamic consent portal.
The “at-home” testing conditions are likely more indicative of how such a portal might fare if it were launched for the general public, thus the reactions from participants in Group 1 might deserve greater weight when considering future iterations of a consent portal. The higher number of these participants who did not complete the simulation indicates that attention to the clarity and user-friendliness of the portal will be important to ensure that it has maximum efficacy. We were not able to follow up with participants who started but did not complete the process so we cannot say for sure why they did not complete it. Yet the reactions of the student group are also highly instructive for future developments as this group is in the age demographic that is most impacted by this particular project (“donors” to the BioTrust's legacy collection are currently between the ages of 4 and 30).
Retrospective Consent: Is Consent Documentation Necessary?
An ongoing debate in the field, contested in the literature and in policy making circles, is whether consent is necessary for research involving de-identified biospecimens, and if so, is it possible to obtain in a meaningful sense. Proposed changes to the Common Rule {45 CFR part 46a}, which currently excludes de-identified tissue samples from the category of human subjects research [13], would make broad, short form consent necessary for secondary research uses of biospecimens. This proposed shift is itself controversial, though it was developed in light of research demonstrating that participants in biobanks strongly prefer to give consent for the secondary use of their samples and information. Further, the view of risks to individuals posed by donating samples to biobanks, and the need for enhancing protections, may be shifting given recent studies showing how research participants can be identified from ‘anonymous’ DNA [40].
The goal of this project was to test the feasibility of creating an online portal for registering individuals’ consent preferences regarding the disposition of their (or their children's) DBS, with a particular focus on retrospective consent. With over 4 million residual DBS cards grandfathered into Michigan's biobank, the task of contacting, educating and consenting stakeholders with samples in this collection is challenging. Indeed, the waiver of consent granted to the MDCH for secondary research use of de-identified DBS was granted in consideration of the impracticability of such an effort [13].
At the same time that the waiver was granted for legacy DBS, a consent process for prospectively collected DBS was put in place, indicating recognition that, when possible, consented, educated donors are preferable to unconsented, uneducated donors when it comes to residual DBS research. The proposed change to the common rule appears to concede this point as well, although the merits of broad consent for biobanking are highly debatable [5,9,10,11,18,41]. In spite of the logistical burdens of contacting and consenting such a large population, one of the underlying rationales for conducting this project was that when it comes to donors to the legacy collection and donors to the prospective collection, the moral obligation is the same. Therefore, if a method or technology for overcoming the logistical burdens that separate the two groups could be tried, then it was minimally worth the effort, if not an ethical imperative.
Participant Centric Initiatives
The impetus for developing a web-based consent portal was the recognition that the burden of recontacting, educating and consenting these individuals could be significantly diminished if the process were online. The longer term vision was that such a portal could be integrated into the biobank's data management systems so that each legacy DBS card, when digitized, could also be tagged with the individuals consent and recontact preferences. Experimentation with online dynamic consent systems is ongoing [20,21,23,46] with notable recent projects recruiting individuals to submit genotypic and phenotypic information to web-based research biobanks with a very broad consent to perform research using these data (23andMe, Consent to Research - weconsent.us, Reg4All.org). More work will be required to determine if lessons learned from dynamic consent for prospective biobanks can apply equally to retrospective collections such as the BioTrust.
There are a number of potential benefits that an online consent portal could reap for a large population public biobank such as: augmenting existing outreach and consent processes with one that is accessible to all web-connected citizens, at all hours; automating the process of curating consent preferences for the biobank manager; offering a greater measure of personal control than is typically available to participants in biobanks; enabling a multimedia education component that may improve comprehension compared to traditional paper consent [42]; facilitating ongoing contact and communication between citizens and policy makers through integrating social networking platforms into the online system and enhancing the atmosphere of transparency and ultimately building trust.
The challenges moving forward for implementing a consent PCI for biobanking will include recruitment (i.e. developing a workable strategy to get individuals to use the portal) and identity verification (striking a delicate balancing between providing assurance of identity and creating an atmosphere of unwelcome intrusion into personal details). An additional challenge for gathering consent online for large population biobanks is that the extent to which individuals would want to be “involved” in the curation of their DBS is largely unknown. Although much of the research shows that people strongly prefer to be asked permission for the use of their DBS in research, other studies suggest that there is something of a paradox at work, where people want to retain control/autonomy yet do not want to be bothered with an overly burdensome or time consuming process [27]. Can a balance be struck that facilitates a strong sense of participant autonomy and yet minimizes participant effort?
Online consent portals are promising tools for augmenting the role of participants as stewards of their own data, that may increasingly be utilized as an answer to today's problems surrounding the practicability of contacting research participants and realizing the promises of genomic data. For large population biobanks, they will not alone solve the problem of unconsented participants, yet they may be an underappreciated tool to help these research goldmines to realize their potential and maintain trust with the public.
LIMITATIONS
Several limitations of the present study are worth noting as they limit the generalizability of the results. First, the sample of pilot testers was a convenience sampling and is thus not representative of the general public. The student group, in particular, was concentrated heavily in a single age bracket, a single education bracket and were less racially and ethnically stratified than those in group 1. However, this group had a stronger presence of females and had the added benefit of addressing the subject to individuals who are highly likely to have a DBS sample in Michigan's BioTrust. The sample size for the community participant group was lower than ideal, diminishing the statistical power of the results.
We are unable to report a response rate for Group 2 given the recruitment approach (outlined above). On large college campuses, it is common for students to have many opportunities to enroll in short duration surveys and studies. As such, we followed a very typical mechanism of posting flyers in high traffic locales and opening computer labs for drop-in participation in our pilot testing. It is not possible to determine (or reasonably estimate) the number of students who might have seen these posters or noticed the drop-in opportunities.
The process was monolingual. Through consultation with our community partners, we were able to set a target enrollment for each community, and in the case of the mostly Spanish speaking community in Detroit, we determined that the absence of a Spanish language option for the web portal would make this prohibitive for participants from that community. We therefore note that future web-focused work would benefit greatly from development and testing in multi-lingual contexts.
Finally, the hypothetical nature of the simulation made it somewhat challenging to articulate to users what their participation meant. Early feedback indicated that we required a clear and unambiguous declaration on all pages that users were not setting actual consent preferences. A major step that we weren't able to assess with this pilot study was attempting to integrate these individual preferences into the data architecture of the biobank, a necessary next step that will yield invaluable knowledge about the challenges and affordances of “going live” with online consent.
CONCLUSION
By testing an online education and consent portal we found that this approach holds promise as a means for communicating with biobank participants and facilitating active participation. More than half the pilot testers stated they would be likely to set up an account, and even larger numbers indicated a likelihood to recommend the tool to others. The time to completion, about 15-20 minutes, seemed appropriate to most participants, and most participants were comfortable with the ease of navigation through the tool. Challenges included striking the proper balance between promoting a sense of privacy and security while not overburdening users with security steps or utilizing verification techniques that inadvertently put users ill at ease. Scaling such a tool to serve a large population of biobank participants would require significant efforts to make the target population largely aware of the availability of such a system and determination of how best to incorporate such a system into the data management systems of the biobank.
ACKNOWLEDGEMENTS
This work was funded by an ARRA challenge grant issued through the National Human Genome Research Institute (5RC1HG005439-02). Additional support for this work was provided by a grant from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (1R01HD067264). The authors gratefully acknowledge the community partner organizations, without whom this work would not have been possible: Community Based Organization Partners (Flint), The Asian Center (Grand Rapids), Arab Community Center for Economic and Social Services (Dearborn), Friends of Parkside (Detroit), Alliance Health (Jackson) and student participants from UM Ann Arbor. We also would like to acknowledge the leadership and staff at Private Access who were wonderful collaborators throughout this unique endeavor. Finally, we would like to thank the anonymous reviewers who made helpful and insightful suggestions for improving the manuscript.
Footnotes
Explorebiobanking.org was deactivated in 2012 and has since been replaced by a more streamlined site, mybloodspot.org, which hosts similar content, including the animations used for the pilot testing.
Disclosure
In light of any conflict his role as President and founder of Private Access, Inc. might have presented, Mr. Shelton was not involved in data collection and recruitment, testing, or analysis, all of which was conducted at the University of Michigan.
None of the other authors have a conflict of interest to declare.
BIBLIOGRAPHY
- 1.Tarini BA, Goldenberg A, Singer D, Clark SJ, Butchart A, Davis MM. Not without my permission: Parents' willingness to permit use of newborn screening samples for research. Public Health Genomics. 2010;13(3):125–30. doi: 10.1159/000228724. doi:10.1159/000228724. [DOI] [PubMed] [Google Scholar]
- 2.Tarini BA. Storage and use of residual newborn screening blood spots: A public policy emergency. Genet Med. 2011;13(7):619–20. doi: 10.1097/GIM.0b013e31822176df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmichael M. Newborn screening: A spot of trouble. Nature. 2011;475(7355):156–158. doi: 10.1038/475156a. doi:10.1038/475156a. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell E, Anderson R, Botkin J. Policy issues and stakeholder concerns regarding the storage and use of residual newborn dried blood samples for research. PPNP. 2010;11(1):5–12. doi: 10.1177/1527154410365563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplan A. What No One Knows Cannot Hurt You: The Limits of Informed Consent in the Emerging World of Biobanking. In: Solbakk JH, Holm S, Hofmann B, editors. The Ethics of Research Biobanking. Springer; London: 2009. pp. 25–32. [Google Scholar]
- 6.Hofmann B, Solbakk JH, Holm S. Consent to Biobank Research: One Size Fits All? In: Solbakk JH, Holm S, Hofmann B, editors. The Ethics of Research Biobanking. Springer; London: 2009. pp. 3–23. [Google Scholar]
- 7.Clayton EW. Informed consent and biobanks. JLME. 2005;33(1):15–21. doi: 10.1111/j.1748-720x.2005.tb00206.x. doi:10.1111/j.1748-720X.2005.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell AV. The ethical challenges of genetic databases: Safeguarding altruism and trust. King's Law Journal. 2007;18(2):227–245. [Google Scholar]
- 9.Kaye J, Whitley EA, Kanellopoulou N, Creese S, Hughes KJ, Lund D. Dynamic consent: A solution to a perennial problem? BMJ. 2011;343 (nov01): d6900-d6900 ISSN 1756-1833. [Google Scholar]
- 10.Shickle D. The consent problem within DNA biobanks. Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences. 2006;37(3):503–519. doi: 10.1016/j.shpsc.2006.06.007. doi:10.1016/j.shpsc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Petrini C. “Broad” consent, exceptions to consent and the question of using biological samples for research purposes different from the initial collection purpose. Social Science & Medicine. 2010;(70):217–220. doi: 10.1016/j.socscimed.2009.10.004. doi:10.1016/j.socscimed.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Langbo C, Bach J, Kleyn M, Downes FP. From newborn screening to population health research: Implementation of the Michigan BioTrust for Health. Public Health Reports. 2013;128(5):377–84. doi: 10.1177/003335491312800508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mongoven A, McGee H. IRB review and public health biobanking: a case study of the Michigan BioTrust for Health. IRB: Ethics & Human Research. 2012;34(3):11–6. [PubMed] [Google Scholar]
- 14.Couzin-Frankel J. Science gold mine, ethical minefield. Science. 2009;324(5924):166–168. doi: 10.1126/science.324.5924.166. [DOI] [PubMed] [Google Scholar]
- 15.Terry SF, Shelton R, Biggers G, Baker D, Edwards K. The haystack is made of needles. Genet Test Mol Biomarkers. 2013;17(3):175–7. doi: 10.1089/gtmb.2012.1542. [DOI] [PubMed] [Google Scholar]
- 16.Shelton RH. Electronic consent channels: Preserving patient privacy without handcuffing researchers. Science Translational Medicine. 2011 Feb;3:69. doi: 10.1126/scitranslmed.3002037. DOI: 10.1126/ scitranslmed.3002037. [DOI] [PubMed] [Google Scholar]
- 17.Kaye J, Curren L, Anderson N, Edwards K, Fullerton SM, Kanellopoulou N, Lund D. From patients to partners: Participant-centric initiatives in biomedical research. Nat Rev Genet. 2012;13(5):371–376. doi: 10.1038/nrg3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinsbekk KS, Bjørn KM, Solberg B. Broad consent versus dynamic consent in biobank research: Is passive participation an ethical problem? Eur J Hum Genet. 2013;21(9):897–902. doi: 10.1038/ejhg.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maschke KJ. Alternative consent approaches for biobank research. The Lancet Oncology. 2006;7(3):193–194. doi: 10.1016/S1470-2045(06)70590-3. doi:10.1016/S1470-2045(06)70590-3. [DOI] [PubMed] [Google Scholar]
- 20.Kuehn BM. Groups experiment with digital tools for patient consent. JAMA. 2013;310(7):678–680. doi: 10.1001/jama.2013.194643. doi:10.1001/jama.2013.194643. [DOI] [PubMed] [Google Scholar]
- 21.Dixon WG, Spencer K, Williams H, Sanders C, Lund D, Whitley EA, Kaye J. A dynamic model of patient consent to sharing of medical record data. BMJ: British Medical Journal. 2014 doi: 10.1136/bmj.g1294. 348doi:10.1136/bmj.g1294. [DOI] [PubMed] [Google Scholar]
- 22.Stein DT, Terry SF. Reforming biobank consent policy: A necessary move away from broad consent toward dynamic consent. Genet Test Mol Biomarkers. 2013;17(12):855–6. doi: 10.1089/gtmb.2013.1550. [DOI] [PubMed] [Google Scholar]
- 23.Wee R. Dynamic consent in the digital age of biology. J Prim Health Care. 2013;5:259–261. [PubMed] [Google Scholar]
- 24.O'Doherty KC, Burgess MM, Edwards K, Gallagher RP, Hawkins AK, Kaye J, McCaffrey V, Winickoff DE. From consent to institutions: Designing adaptive governance for genomic biobanks. Social Science & Medicine. 2011;73(3):367–374. doi: 10.1016/j.socscimed.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 25.Platt J, Platt T, Thiel D, Kardia SLR. ‘Born in Michigan? You're in the Biobank': engaging population biobank participants through Facebook advertisements. Public Health Genomics. 2013;16(4) doi: 10.1159/000351451. DOI:10.1159/000351451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eder J, Gottweis H, Zatloukal K. IT Solutions for privacy protection in biobanking. Public Health Genomics. 2012;15:254–262. doi: 10.1159/000336663. [DOI] [PubMed] [Google Scholar]
- 27.Simon CM, L'Heureux J, Murray JC, Winokur P, Weiner G, Newbury E, Shinkunas L, Zimmerman B. Active choice but not too active: Public perspectives on biobank consent models. Genetics in Medicine. 2011;13(9):821–831. doi: 10.1097/GIM.0b013e31821d2f88. doi:10.1097/GIM.0b013e31821d2f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleck LM, Mongoven A, Marzec S. Stored blood spots: Ethical and policy challenges. Michigan State University, Institute for Public Policy and Social Research; 2008. [17 Mar 2014]. available online: ippsr.msu.edu/publications/ARBloodSpots.pdf. [Google Scholar]
- 29.Botkin JR, Goldenberg A, Rothwell E, Anderson R, Lewis M. Retention and research use of residual newborn screening bloodspots. Pediatrics. 2013;131(1):120–7. doi: 10.1542/peds.2012-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duquette D, Rafferty AP, Fussman C, Gehring J, Meyer S, Bach J. Public support for the use of newborn screening dried blood spots in health research. Public Health Genomics. 2011;14(3):143–152. doi: 10.1159/000321756. [DOI] [PubMed] [Google Scholar]
- 31.Duquette D, Langbo C, Bach J, Kleyn M. Michigan BioTrust for Health: Public support for using residual dried blood spot samples for health research. Public Health Genomics. 2012;15(3-4):146–155. doi: 10.1159/000336565. [DOI] [PubMed] [Google Scholar]
- 32.Botkin JR, Rothwell E, Anderson R, Stark L, Goldenberg A, Lewis M, Burbank M, Wong B. Public Attitudes Regarding the use of Residual Newborn Screening Specimens for Research. Pediatrics. 2012;129(2):231–238. doi: 10.1542/peds.2011-0970. doi:10.1542/peds.2011-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothwell E, Anderson R, Goldenberg A, Lewis M, Stark L, Burbank M, Wong B, Botkin JR. Assessing public attitudes on the retention and use of residual newborn screening blood samples: A focus group study. Social Science & Medicine. 2012;74(8):1305–1309. doi: 10.1016/j.socscimed.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K. Public perspectives on informed consent for biobanking. American Journal of Public Health. 2009;99(12):2128–2134. doi: 10.2105/AJPH.2008.157099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute for Public Policy and Social Research [17 Mar 2014];State of the State Survey-60. 2011 retrieved from: http://www.ippsr.msu.edu/SOSS.
- 36.HHS.gov [24 Feb 2014];Regulatory changes in ANPRM. 2013 retrieved from: http://www.hhs.gov/ohrp/humansubjects/anprmchangetable.html.
- 37.Thiel DB, Platt T, Platt J, King SB, Kardia SLR. Community perspectives on public health biobanking: An analysis of community meetings on the Michigan BioTrust for health. J Community Genet. 2014;5:125–138. doi: 10.1007/s12687-013-0162-0. doi:10.1007/s12687-013-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nissenbaum H. Privacy as contextual integrity. [17 Mar 2014];Washington Law Review. 2004 79(1) Retrieved online at: http://ssrn.com/abstract=534622. [Google Scholar]
- 39.Shelton RH. Personal communication. 2014.
- 40.Gymrek M, McGuire AL, Golan D, Halperin E, Erlich Y. Identifying personal genomes by surname inference. Science. 2013;339(6117):321–324. doi: 10.1126/science.1229566. doi:10.1126/science.122956. [DOI] [PubMed] [Google Scholar]
- 41.Wendler D. One-time general consent for research on biological samples. BMJ. 2006;332(7540):544–547. doi: 10.1136/bmj.332.7540.544. doi:10.1136/bmj.332.7540.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henry J, Palmer B, Palinkas L, Glorioso DK, Caligiuri MP, Jeste DV. Reformed consent: Adapting to new media and research participant preferences. IRB. 2009;31(2):1–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Boenigk S, Leipnitz S, Scherhag C. Altruistic values, satisfaction and loyalty among first-time blood donors. International Journal of Nonprofit and Voluntary Sector Marketing. 2011;16(4):356–370. doi:10.1002/nvsm.43. [Google Scholar]
- 44.Kaufman D, Murphy J, Erby L, Hudson K, Scott J. Veterans' attitudes regarding a database for genomic research. Genetics in Medicine. 2009;11(5):329–337. doi: 10.1097/GIM.0b013e31819994f8. doi:10.1097/GIM.0b013e31819994f. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: A survey of public opinions about a large genetic cohort study. Genetics in Medicine. 2008;10(11):831–839. doi: 10.1097/GIM.0b013e31818bb3ab. doi:10.1097/GIM.0b013e31818bb3a. [DOI] [PubMed] [Google Scholar]
- 46.Kaye J, Whitley EA, Lund D, Morrison M, Teare H, Melham K. Dynamic consent: a patient interface for twenty-first century research networks European Journal of Human Genetics. 2014. advance online publication 7 May 2014; doi: 10.1038/ejhg.2014.71. [DOI] [PMC free article] [PubMed]